
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 21 November 2022
Sec. Pediatric Critical Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.918148
 Shane George1,2,3*
Shane George1,2,3* Megan Wilson4,5
Megan Wilson4,5 Susan Humphreys3,6
Susan Humphreys3,6 Kristen Gibbons3
Kristen Gibbons3 Elliot Long7,8,9
Elliot Long7,8,9 Andreas Schibler10,11
Andreas Schibler10,11
Objective: This review assesses the effect of apnoeic oxygenation during paediatric intubation on rates of hypoxaemia, successful intubation on the first attempt and other adverse events.
Data sources: The databases searched included PubMed, Medline, CINAHL, EMBASE and The Cochrane Library. An electronic search for unpublished studies was also performed.
Study selection: We screened studies that include children undergoing intubation, studies that evaluate the use of apnoeic oxygenation by any method or device with outcomes of hypoxaemia, intubation outcome and adverse events were eligible for inclusion.
Data extraction: Screening, risk of bias, quality of evidence and data extraction was performed by two independent reviewers, with conflicts resolved by a third reviewer where consensus could not be reached.
Data synthesis: From 362 screened studies, fourteen studies (N = 2442) met the eligibility criteria. Randomised controlled trials (N = 482) and studies performed in the operating theatre (N = 835) favoured the use of apnoeic oxygenation with a reduced incidence of hypoxaemia (RR: 0.34, 95% CI: 0.24 to 0.47, p < 0.001, I2 = 0% and RR: 0.27, 95% CI: 0.11 to 0.68, p = 0.005, I2 = 68% respectively). Studies in the ED and PICU were of lower methodological quality, displaying heterogeneity in their results and were unsuitable for meta-analysis. Among the studies reporting first attempt intubation success, there were inconsistent effects reported and data were not suitable for meta-analysis.
Conclusion: There is a growing body of evidence to support the use of apnoeic oxygenation during the intubation of children. Further research is required to determine optimal flow rates and delivery technique. The use of humidified high-flow oxygen shows promise as an effective technique based on data in the operating theatre, however its efficacy has not been shown to be superior to low flow oxygen in either the elective anesthetic or emergency intubation situations
Systematic Review Registration: This review was prospectively registered in the PROSPERO international register of systematic reviews (Reference: CRD42020170884, registered April 28, 2020).
Infants and children have, in comparison to adults, a lower tolerance for apnoea and become hypoxaemic faster (1). As a result, they are more likely to experience alveolar de-recruitment and significant oxygen desaturation during intubation. Hypoxaemia occurs for 1 in 8 children requiring emergency intubation (2). Previous studies have suggested that hypoxaemia during intubation may increase morbidity and mortality (3, 4).
The provision of apnoeic oxygenation has been proposed to reduce the risk of hypoxaemia during intubation in children. There are two major techniques that could provide apnoeic oxygenation to children undergoing intubation. The adult literature for emergency intubation suggests using 15 L/min of oxygen delivered via nasal cannulae during the apnoeic phase, a practice which has been taken up by many adult emergency physicians and has also been adopted in some paediatric institutions (5). Another delivery method using a Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE) technique has been suggested to prolong the safe apnoeic time in paediatric and adult patients, thus enabling unhurried intubation in child with an expected difficult airway (6, 7). THRIVE delivers high flow rates (≥2 L/kg/min) of 100% oxygen through specialised nasal cannulae. Its application for intubation in the emergency and critical care environment has not definitively been established.
This systematic review aims to analyse and compare existing studies to help inform clinical practice and to improve patient outcomes. We will assess the effect of using apnoeic oxygenation during the intubation of children aged 0–16 years on rates of hypoxaemia, successful intubation on the first attempt and other intubation related adverse events.
This review has considered studies that include children aged less than 16-years undergoing intubation either for elective or emergent reasons. Studies that evaluate the use of apnoeic oxygenation by any method or device were eligible for inclusion. This review has included studies that report the outcomes: hypoxaemia; number of intubation attempts; time to oxygen desaturation; and intubation related adverse events.
Apnoeic oxygenation is defined as the provision of oxygen through passive insufflation during the apnoeic phase of the intubation attempt.
The search strategy aimed to find both published and unpublished studies. The databases searched included: PubMed; Medline; CINAHL; EMBASE; and The Cochrane Library. The search for unpublished studies included: Clinical trial registries (Cochrane Central Register of controlled trials and ClinicalTrials.gov) to identify recent and ongoing studies; Google Scholar web search; and bibliographies from included studies, known reviews and text for additional citations.
Detailed search strings and results for each database are included in Supplementary Digital Content S1. The search was undertaken on 16 October 2019, all referenced studies at this date were included for screening.
Two review authors independently assessed titles and abstracts and, when needed, full texts of identified studies to determine eligibility for inclusion using the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Conflicts were resolved by discussion between reviewers and then by consulting with a third review author where a consensus could not be reached.
Full-text versions of all studies were obtained, and data extraction was completed using standardised data extraction form by two reviewers independently. Extracted data were compared for any differences, with conflict resolved by discussion between reviewers. Where consensus could not be reached a third reviewer was engaged. Extracted data were exported into Review Manager 5 (8) for processing, comparison and analysis.
Risk of bias was assessed according to the Cochrane Collaboration revised tool for assessing the risk of bias for randomised trials (ROB 2) (9) and the Risk Of Bias In Non-Randomized Studies - of Interventions (ROBINS-I) for non-randomised studies (10). The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach (11), was used to assess the quality of evidence for the following clinically relevant outcomes: hypoxaemia, first intubation attempt success rate; first attempt success rate for intubations without hypoxaemia; tracheal intubation related adverse events.
Evidence from RCTs was considered as high-quality but was downgraded by one level for serious (or two levels for very serious) limitations according to the following: design (risk of bias), consistency across studies, directness of evidence, precision of estimates, and presence of publication bias.
Children included in randomised studies were analysed on an intention-to-treat (ITT) basis. Assuming low levels of heterogeneity, analysed treatment effects in individual trials were initially analysed using a fixed-effect model for meta-analysis of combined data. If substantial heterogeneity was noted and there was no significant asymmetry in the associated funnel plot, analysis was undertaken using a random-effect model and we examined the potential cause of heterogeneity by performing subgroup and sensitivity analyses. If meta-analysis was inappropriate, we analysed and interpreted individual trials separately. The Mantel-Haenszel (MH) method was used for calculating estimates of risk ratio (RR) and are presented along with associated 95% confidence intervals (21, 22). For measured quantities, we used the inverse variance method. Appropriate graphical representation of the meta-analyses have been generated.
Heterogeneity of studies owing to the nature of the interventions provided is anticipated, this was assessed using χ2 and I2 statistics. Significant heterogeneity was investigated by performing subgroup analyses.
The systematic search returned 362 non-duplicate citations; an additional 6 citations were included in the manual reference check of included papers. After screening of title and abstract, 327 citations were excluded leaving 41 for full text review. A further 27 papers were excluded after full-text review. Fourteen studies remained meeting all inclusion and no exclusion criteria. There were 6 randomised controlled trials, 3 prospective observational study, 3 “before and after” observational studies and 2 retrospective observational studies. One ongoing randomised controlled trial was identified, however no interim data has been published (12).
A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart is presented in Figure 1.
Included studies provided a total of 2,442 patients for inclusion, with 1,159 children in operating theatre setting and 1,283 children being intubated in the ED or PICU environment. Characteristics of included studies and patient populations is included in Table 1. Risk of bias assessment for included studies is presented in Figure 2.
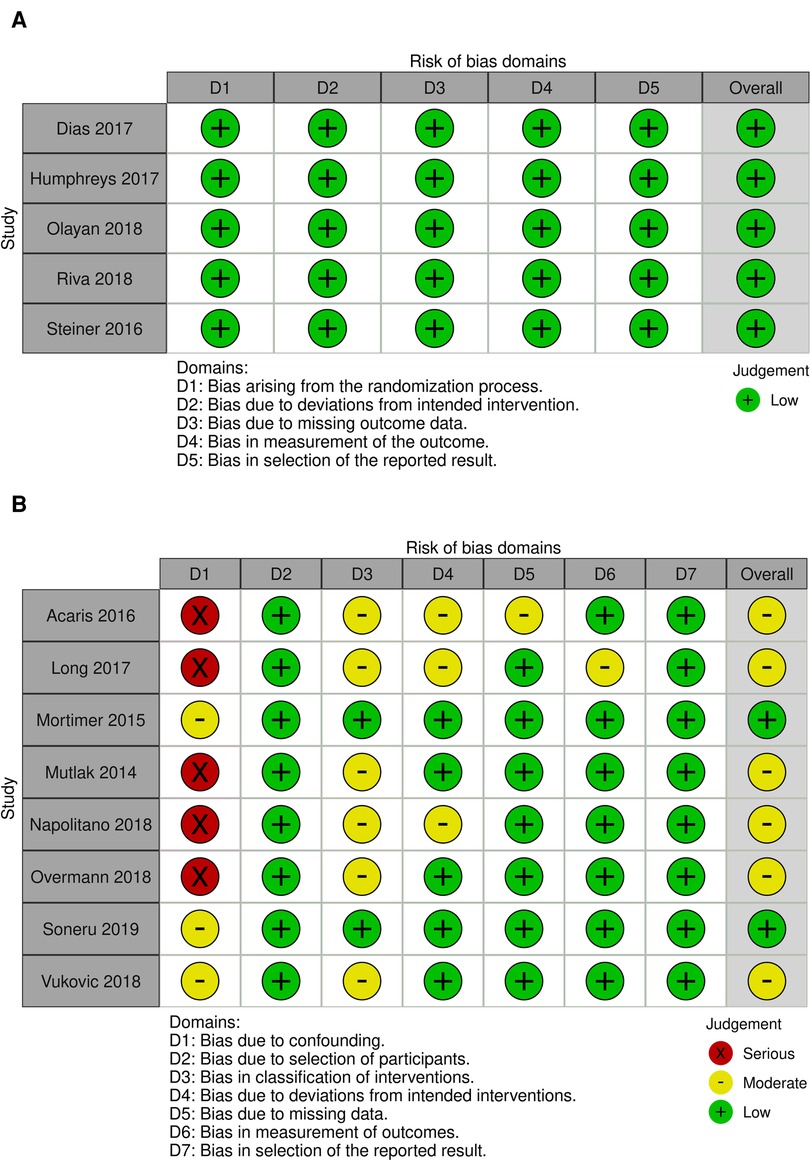
Figure 2. Risk of bias summary: review authors’ judgements about each risk of bias item for each included study. (A) Revised Cochrane risk of bias for randomised trails (ROB 2). (B) Risk of bias in non-randomised studies of interventions (ROBINS-I).
All studies included a measure of hypoxaemia as a primary or secondary outcome, however there was significant variation in the cut-off value used to define hypoxaemia between studies.
Eight studies (three RCTs, five observational studies) reported the outcome SpO2 < 90% as either a primary or secondary outcome with a total of 1,382 patients available for analysis. All RCTs included were conducted in the operating theatre (430 patients). Two observational studies were conducted in the operating theatre (421 patients) with the remaining three studies conducted in an ED (two studies, 398 patients) or PICU (one study, 149 patients). All included studies used oxygen at flow rates of <15 L/min (Table 1).
Overall, there was a decreased incidence of hypoxaemia in patients who receive apnoeic oxygenation (RR: 0.38, 95% CI: 0.31 to 0.48, p < 0.001); however, the included studies display significant heterogeneity (I2 = 84%) (Figure 3). Consistent findings were demonstrated when incorporating random effects (RR: 0.42, 95% CI: 0.22 to 0.82, p = 0.01) (Supplementary Figure S1).
Given the high degree of heterogeneity in the included studies, subgroup analysis was undertaken to investigate the effects in the high quality RCT studies and to also compare effects in the different patient groups of elective and emergent admissions. The three RCTs included were all performed on elective surgery patients in the operating theatre, with an ASA of I or II providing a more standardised group of patients at baseline. Meta-analysis of this data shows a reduced incidence of hypoxaemia in the apnoeic oxygenation group (RR: 0.34, 95% CI: 0.24 to 0.47, p < 0.001) when compared to control and no heterogeneity between studies (I2 = 0%) (Figure 4A).
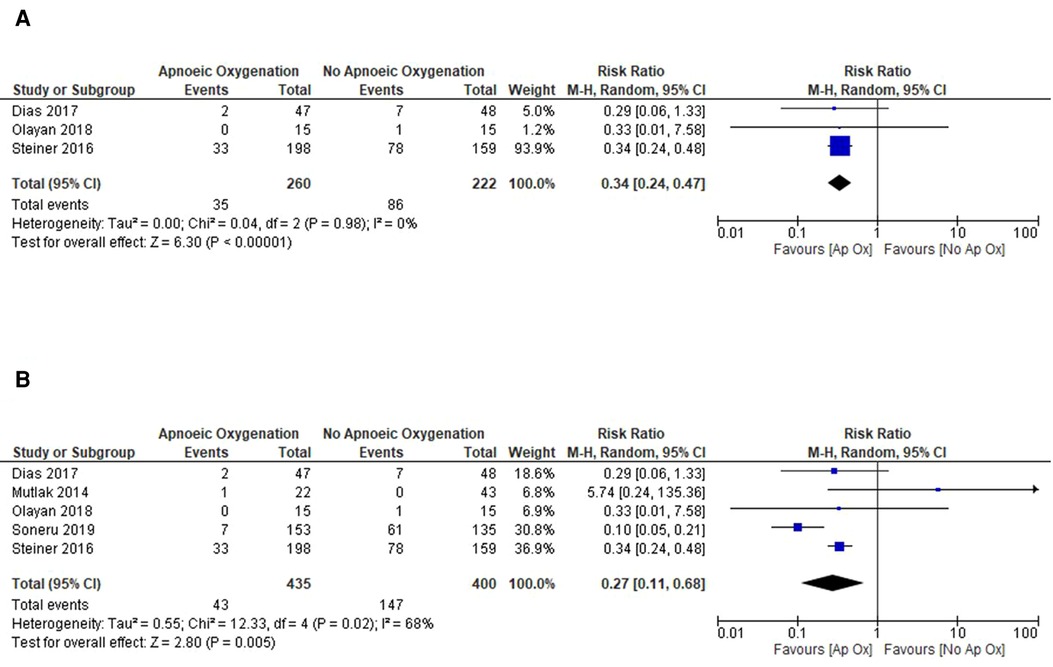
Figure 4. Subgroup analysis of included studies for outcome of SpO2 < 90%. (A) Forrest plot of included RCTs for outcome of SpO2 < 90%. (B) Forrest plot of included studies in the operating theatre setting for outcome of SpO2 < 90%.
The methodological quality was higher for the majority of studies in the operating theatre and meta-analysis of these studies also showed a significantly reduced risk of hypoxaemia with mild heterogeneity between studies (RR: 0.27, 95% CI: 0.11 to 0.68, p = 0.005) (Figure 4B). Studies performed in the ED and PICU were of lower methodological quality and displayed significant heterogeneity in their results and were thus unsuitable for meta-analysis. Given this, it is difficult to estimate the treatment effect in this cohort of patients.
Six included studies (1,000 patients) reported the lowest SpO2 during intubation as a primary or secondary outcome (13–18). There are conflicting results from these studies and methodological differences and bias make the data unsuitable for meta-analysis.
Four studies reported a small, but statistically significant, difference in the median lowest oxygen saturations between apnoeic oxygenation and control groups (13, 16–18). Three studies favoured the use of apnoeic oxygenation (13, 17, 18); however, all three studies report median/mean SpO2 of >95% with small IQR/SD making the clinical significance of this difference questionable. A single study reported a higher SpO2 in the no apnoeic oxygenation group, however this finding is difficult to interpret due to baseline differences in SpO2 prior to commencement of intubation potentially confounding this outcome (16).
Two studies reported no difference in the median lowest oxygen saturation during intubations when comparing apnoeic oxygenation to control (14, 15). Mutlak et al. report oxygen desaturation in a single patient in their cohort (N = 65), however the authors note this is likely due to inadequate preoxygenation. Overmann et al. report no significant difference in lowest SpO2, however they only report the lowest SpO2 for patients who experience a desaturation to < 90%. Data for the entire cohort is not presented.
Four included studies reported the number of intubation attempts as a primary or secondary outcome (15, 16, 19, 20). All studies report data as part of a departmental quality improvement initiative, one reports data for after the intervention, with no control for comparison (20). The remaining studies report conflicting outcomes, and display high risk of bias.
A study by Long et al. report no change in the first attempt success rate between groups (78% for both groups), however there was a difference in first pass success without hypoxaemia (78% post-intervention, 49% pre-intervention). Importantly all patients in the post-intervention group received apnoeic oxygenation, a significant number of the pre-intervention group (54%, 38/71) also received apnoeic oxygenation during their intubation attempt (19). Data is not presented on the proportion of pre-intervention patients without apnoeic oxygenation as a subgroup analysis. Overmann et al. (15) also report no difference in first pass success in their retrospective cohort. In contrast, Vukovic et al. also report that patients who receive apnoeic oxygenation had higher first attempt success (20/90 and 28/62, p = 0.0025) (16).
Given the high risk of bias and methodological variability meta-analysis was not performed.
Two RCTs have investigated the effect of humidified high flow nasal prong apnoeic oxygenation on the time to oxygenation desaturation. In an RCT comparing humidified high flow apnoeic oxygenation to control in an elective surgery setting Humphreys et al. demonstrated a significant increase (p < 0.001) in the time to desaturation across four age cohorts (0–6 months, 6–24 months, 2–5 years and 6–10 years) (21). Riva and colleagues also demonstrated a longer safe apnoea time in children receiving either low flow or humidified high flow apnoeic oxygenation through a composite end-point of hypoxaemia, duration of apnoea and transcutaneous CO2 (22).
In addition, two observational studies have reported the time taken for a child to experience hypoxaemia (as measured by a drop in SpO2). Soneru reports in a prospective cohort study an increase in the median time taken for a child's oxygen saturation to fall below 95% when apnoeic oxygenation is applied (202 s IQR 142–253 s) compared to no apnoeic oxygenation (127 s, IQR 83–165 s) (18). Conversely, in a retrospective observational study Overmann reported there was no significant difference in the median time to desaturation between the apnoeic oxygenation group (140 s, IQR 108–382 s) and the control group (125 s, IQR 83–272 s, p = 0.25) (15).
An alternative measure of time to desaturation was used by Steiner et al. where time to desaturation was defined as the time taken for a 1% absolute decrease in SpO2 from the pre-intubation level. The study compared two different methods of apnoeic oxygenation delivery with a control group. When compared to control (30 s, 95% CI: 24–39 s) the time to desaturation was longer for both apnoeic oxygenation groups (67 s, 95% CI: 35–149 s, p < 0.001 and 75 s, 95% CI: 37–122 s, p < 0.001) (23).
An observational study by Napolitano et al. reported the rate of TIAE between apnoeic oxygenation and control groups. The rate of adverse events was lower in the in the apnoeic oxygenation cohort when compared to control (6% and 10% respectively), but did not reach statistical significance (p = 0.14) (24).
There is an emerging body of literature around methods and application of apnoeic oxygenation during paediatric intubation, as demonstrated by the recency of publication for all included studies in this review. The heterogeneity of methods used to deliver apnoeic oxygenation, differences in characteristics of included patients and lack of consistency in outcome measures make it difficult to directly compare studies or make definitive treatment recommendations. Despite this, the available data from the operating theatre setting for elective procedures suggest a reduced incidence of hypoxemia with the provision of apnoeic oxygenation in this cohort.
Studies included in this review have examined two groups of patients which have significantly different baseline characteristics. One group of studies looks at patients undergoing elective medical procedures, who have no significant underlying medical conditions or pathologies while the other examines patients in the ED or PICU being intubated for a life-threatening critical illness with significant physiological compromise. Direct comparison of these two groups needs to be interpreted with caution and there is likely to be a differential effect of the intervention based on the severity of illness and presence (or absence) of respiratory failure at the time of intubation. Further research is required to better understand this relationship with an adequate sample size to power for this subgroup analysis.
The flow rates used to deliver apnoeic oxygenation are markedly variable between studies, making direct comparison difficult and estimation of the true effect size unreliable. The use of standard nasal cannulae is favoured by many clinicians as they are readily available, inexpensive, and simple to apply. However, to deliver high flow rates specialised high-flow nasal cannulae are required as the diameter of the nasal outlet is too narrow to accommodate higher flows. The use of higher flow rates of at least 2 L/kg/min through the THRIVE technique has gained increasing popularity in anesthesia despite limited understanding of the exact physiological mechanism of its demonstrated effect. To date there have been no studies published investigating the use of the THRIVE technique in the PICU or ED setting, despite promising pilot data for children in the operating theatre (21, 22).
The lack of standardised core outcome measures in airway management literature is a significant barrier to interpretation and application of the available data. The most consistent outcome measures reported are hypoxaemia, although the exact level varies, the number of intubation attempts required to successfully insert the endotracheal tube and the lowest SpO2 during the attempt. All of these outcome measures are objective, available and clinically relevant. While we are unlikely to achieve consensus on what level of hypoxaemia is clinically significant, the general standards of reporting mild (SpO2 81%–90%), moderate (SpO2 71%–80%) and severe (SpO2 < 70%) hypoxaemia would allow better comparison of the available data.
Some studies included in this review have reported statistically significant differences in the lowest SpO2 reported, with the values reported being >95% (13). While there may be a statistically significant difference the in values reported, the clinical significance of these differences is minimal.
The data available on the efficacy of apnoeic oxygenation in the paediatric population is more limited than that in the adult population, however there are similar methodological and physiological issues across both groups. In the adult literature there have been numerous systematic reviews published over recent years (25–30). In these reviews there is a consistent trend towards a reduction in the incidence of hypoxaemia and an increase in the proportion of intubations successful on first attempt but there is a paucity of high quality randomised controlled studies. Similar to the paediatric literature there is a lack of consensus on the optimal method of delivery of apnoeic oxygenation, as well contradictory reports on its efficacy in different patient populations, particularly patients with respiratory failure, and different treatment environments.
This literature review is confined to a relatively small pool of patients (n = 2,442) from studies of variable methodological quality and inconsistent outcome reporting. Two of the included studies were abstracts only (24, 31). Despite attempts to contact the authors, more detailed data could not be obtained. The review includes a number of retrospective observational studies limiting their internal validity and generalisability.
There is a growing body of evidence to support the use of apnoeic oxygenation during the intubation of children. Further research is required to determine optimal flow rates and delivery technique. The use of humidified high-flow oxygen shows promise as an effective technique based on data in the operating theatre, however its efficacy has not been shown to be superior to low flow oxygen in either the elective anesthetic or emergency intubation situations.
High quality randomised controlled trials with standardised outcome measures are required for both elective and emergent intubations, adequately powered for subgroup analysis for age, severity of illness and reason for intubation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
SG conceived the review question with input from EL, SH and AS. SG undertook the literature searches. SG, MW and SH undertook screening and assessment of identified papers. SG and MW performed data extraction, with input from SH and AS where discrepancies were identified. KG advised on statistical analyses and interpretations. SG prepared the initial manuscript with revisions and input from MW, SH, KG, EL and AS. All authors contributed to the article and approved the submitted version.
Support was provided solely from institutional and departmental sources.
The authors would like to acknowledge the assistance of the librarians at the University of Queensland Library for their assistance in designing and conducting the required database searches. We would also like to thank Vukovic and Prof Arnold of the Department of Paediatrics, Vanderbilt University for providing access to additional raw data for inclusion in this review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.918148/full#supplementary-material.
1. Patel R, Lenczyk M, Hannallah RS, McGill WA. Age and the onset of desaturation in apnoeic children. Can J Anaesth (1994) 41(9):771–4. doi: 10.1007/BF03011582
2. Li S, Hsieh TC, Rehder KJ, Nett S, Kamat P, Napolitano N, et al. Frequency of desaturation and association with hemodynamic adverse events during tracheal intubations in PICUs. Pediatr Crit Care Med. (2018) 19(1):e41–50. doi: 10.1097/PCC.0000000000001384
3. Parker MM, Nuthall G, Brown C 3rd, Biagas K, Napolitano N, Polikoff LA, et al. Relationship between adverse tracheal intubation associated events and PICU outcomes. Pediatr Crit Care Med. (2017) 18(4):310–8. doi: 10.1097/PCC.0000000000001074
4. Stinson HR, Srinivasan V, Topjian AA, Sutton RM, Nadkarni VM, Berg RA, et al. Failure of invasive airway placement on the first attempt is associated with progression to cardiac arrest in pediatric acute respiratory compromise. Pediatr Crit Care Med (2018) 19(1):9–16. doi: 10.1097/PCC.0000000000001370
5. Weingart SD, Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Ann Emerg Med (2012) 59(3):165–75.e1. doi: 10.1016/j.annemergmed.2011.10.002
6. Patel A, Nouraei SA. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. (2015) 70(3):323–9. doi: 10.1111/anae.12923
7. Bartlett RG Jr, Brubach HF, Specht H. Demonstration of aventilatory mass flow during ventilation and apnea in man. J Appl Physiol (1959) 14(1):97–101. doi: 10.1152/jappl.1959.14.1.97
8. Review manager (RevMan) [computer program]. The Cochrane Collaboration, editor. Copenhagen: The Nordic Cochrane Centre (2014).
9. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane (2022). p. 187–241. Available from: www.training.cochrane.org/handbook
10. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
11. Schünemann H, Brozek J, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October (2013).
12. George S, Humphreys S, Williams T, Gelbart B, Chavan A, Rasmussen K, et al. Transnasal humidified rapidinsufflation ventilatory exchange in children requiring emergent intubation (kids THRIVE): a protocol for a randomised controlled trial. BMJ Open. (2019) 9(2):e025997. doi: 10.1136/bmjopen-2018-025997
13. Dias R, Dave N, Chhabria R, Shah H, Garasia M. A randomised comparative study of miller laryngoscope blade versus oxiport® miller laryngoscope blade for neonatal and infant intubations. Indian J Anaesth. (2017) 61(5):404–9. doi: 10.4103/ija.IJA_86_17
14. Mutlak H, Rolle U, Rosskopf W, Schalk R, Zacharowski K, Meininger D, et al. Comparison of the TruView infant EVO2 PCD™ and C-MAC video laryngoscopes with direct macintosh laryngoscopy for routine tracheal intubation in infants with Normal airways. Clinics. (2014) 69(1):23–7. doi: 10.6061/clinics/2014(01)04
15. Overmann KM, Boyd SD, Zhang Y, Kerrey BT. Apneic oxygenation to prevent oxyhemoglobin desaturation during rapid sequence intubation in a pediatric emergency department. Am J Emerg Med. (2018) 37(8):1416–21. doi: 10.1016/j.ajem.2018.10.030
16. Vukovic AA, Hanson HR, Murphy SL, Mercurio D, Sheedy CA, Arnold DH. Apneic oxygenation reduces hypoxemia during endotracheal intubation in the pediatric emergency department. Am J Emerg Med (2018) 37(1):27–32. doi: 10.1016/j.ajem.2018.04.039
17. Olayan L, Alatassi A, Patel J, Milton S. Apnoeic oxygenation by nasal cannula during airway management in children undergoing general anaesthesia: a pilot randomised controlled trial. Perioper Med. (2018) 7:3. doi: 10.1186/s13741-018-0083-x
18. Soneru CN, Hurt HF, Petersen TR, Davis DD, Braude DA, Falcon RJ. Apneic nasal oxygenation and safe apnea time during pediatric intubations by learners. Paediatr Anaesth (2019) 29(6):628–34. doi: 10.1111/pan.13645
19. Long E, Cincotta DR, Grindlay J, Sabato S, Fauteux-Lamarre E, Beckerman D, et al. A quality improvement initiative to increase the safety of pediatric emergency airway management. Paediatr Anaesth. (2017) 27(12):1271–7. doi: 10.1111/pan.13275
20. Mortimer T, Burzynski J, Kesselman M, Vallance J, Hansen G. Apneic oxygenation during rapid sequence intubation in critically ill children. J Pediatr Intensive Care. (2015) 5(1):28–31. doi: 10.1055/s-0035-1568149
21. Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth (2017) 118(2):232–8. doi: 10.1093/bja/aew401
22. Riva T, Pedersen TH, Seiler S, Kasper N, Theiler L, Greif R, et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. (2018) 120(3):592–9. doi: 10.1016/j.bja.2017.12.017
23. Steiner JW, Sessler DI, Makarova N, Mascha EJ, Olomu PN, Zhong JW, et al. Use of deep laryngeal oxygen insufflation during laryngoscopy in children: a randomized clinical trial. Br J Anaesth. (2016) 117(3):350–7. doi: 10.1093/bja/aew186
24. Napolitano N, Laverriere E, Craig N, Snyder M, Thompson A, Davis D, et al. Apneic oxygenation as a quality improvement intervention in an academic PICU. Crit Care Med. (2018) 46:643. doi: 10.1097/01.ccm.0000529322.79113.ae
25. Binks MJ, Holyoak RS, Melhuish TM, Vlok R, Bond E, White LD. Apneic oxygenation during intubation in the emergency department and during retrieval: a systematic review and meta-analysis. Am J Emerg Med (2017) 35(10):1542–6. doi: 10.1016/j.ajem.2017.06.046
26. Binks MJ, Holyoak RS, Melhuish TM, Vlok R, Hodge A, Ryan T, et al. Apnoeic oxygenation during intubation in the intensive care unit: a systematic review and meta-analysis. Heart Lung. (2017) 46(6):452–7. doi: 10.1016/j.hrtlng.2017.08.001
27. Denton G, Howard L. BET 1: does apnoeic oxygenation reduce the risk of desaturation in patients requiring endotracheal intubation? Emerg Med J. (2016) 33(7):517–9. doi: 10.1136/emermed-2016-205965.1
28. Holyoak RS, Melhuish TM, Vlok R, Binks M, White LD. Intubation using apnoeic oxygenation to prevent desaturation: a systematic review and meta-analysis. J Crit Care. (2017) 41:42–8. doi: 10.1016/j.jcrc.2017.04.043
29. Pourmand A, Robinson C, Dorwart K, O'Connell F. Pre-oxygenation: implications in emergency airway management. Am J Emerg Med (2017) 35(8):1177–83. doi: 10.1016/j.ajem.2017.06.006
30. White LD, Melhuish TM, White LK, Wallace LA. Apnoeic oxygenation during intubation: a systematic review and meta-analysis. Anaesth Intensive Care. (2017) 45(1):21–7. doi: 10.1177/0310057X1704500104
Keywords: intubation, paediatric, apnoeic oxygenation, hypoxaemia, safe apnoeic period
Citation: George S, Wilson M, Humphreys S, Gibbons K, Long E and Schibler A (2022) Apnoeic oxygenation during paediatric intubation: A systematic review. Front. Pediatr. 10:918148. doi: 10.3389/fped.2022.918148
Received: 12 April 2022; Accepted: 20 October 2022;
Published: 21 November 2022.
Edited by:
Niranjan Kissoon, University of British Columbia, CanadaReviewed by:
Takanari Ikeyama, Aichi Child Health And Medical General Center, Japan© 2022 George, Wilson, Humphreys, Gibbons, Long and Schibler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shane George c2hhbmUuZ2VvcmdlQHVxLmVkdS5hdQ==
Specialty Section: This article was submitted to Pediatric Critical Care, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.