- 1Guangxi Key Laboratory of Major Infectious Disease Prevention Control and Biosafety Emergency Response, Guangxi Center for Disease Control and Prevention, Nanning, China
- 2The Second Affiliated Hospital of Guangxi Medical University, Nanning, China
- 3State Key Laboratory of Infectious Disease Prevention and Control (SKLID), Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Chinese Center for Disease Control and Prevention (China CDC), Beijing, China
- 4Department of Public Health, University of Tennessee, Knoxville, TN, United States
Background: The number of HIV infected children receiving antiviral treatment in Guangxi is increasing. Understanding factors and trends of mortality and attrition in HIV-infected children under antiretroviral therapy (ART) was an urgent need to improve treatment outcomes. This study aimed to estimate mortality and attrition rates and identify factors that were associated with mortality and attrition after ART initiation among children with HIV in Guangxi, China between 2004 and 2018.
Methods: Cohort study data were extracted from the National Free Antiretroviral Treatment Program (NFATP) database, which has standard guidelines for core treatment indicators and other data at all HIV/AIDS treatment facilities in Guangxi. A total of 901 HIV-infected children who have started ART were included in the study. The study collected the following data: age, gender, WHO clinic stages before ART, CD4 cell count before ART, Cotrimoxazole prophylaxis (CTX) use before ART, initial ART regimen, malnutrition before ART, abnormal liver function before ART, abnormal kidney function before ART, severe anemia before ART, and the time lag between an HIV diagnosis and ART initiation.
Results: HIV-infected children under ART had a mortality rate of 0.87 per 100 person-years [95% Confidence Interval (CI) 0.63–1.11], and an attrition rate of 3.02 per 100 person-years (95% CI 2.57–3.47). Mortality was lower among children with a CD4 count between 200 and 500 copies/ml [Adjusted Hazard Ratio (AHR) 0.22, 95% CI 0.09–0.55], and CD4 count ≥500 copies/ml (AHR 0.10, 95% CI 0.03–0.29); but higher among children with late ART initiation at 1–3 months (AHR 2.30, 95% CI 1.07–4.94), and at ≥3 months (AHR 2.22, 95% CI 1.04–4.74). Attrition was lower among children with a CD4 count ≥500 copies/ml (AHR 0.62, 95% CI 0.41–0.95), but higher among children with late ART initiation at 1–3 months (AHR 1.55, 95% CI 1.05–2.30).
Conclusion: Supportive programs are needed to educate children's families and parents on early ART, link HIV-infected children to care and retain them in care among other programs that treat and manage the medical conditions of HIV-infected children before ART initiation.
Introduction
Despite intensive efforts for the prevention of mother-to-child transmission (MTCT), HIV consistently impacts children. Globally 1.8 million children aged <15 years living with HIV/AIDS, ~150,000 children became newly infected and 95,000 children died of HIV/AIDS-related illnesses in 2019 (1). Children with HIV disproportionally impacted middle- and low-income countries where the HIV disease burden was high and resources were limited (2, 3). China's MTCT prevention programs have achieved full coverage in 2015, although significant improvement has been made, the MTCT rate was only 5.7% in 2016 (4, 5). Moreover, the leading causes of death among infectious diseases in Chinese students aged 6–22 years shifted from rabies and tuberculosis in 2008 to HIV/AIDS in 2017 (6).
Without timely antiretroviral therapy (ART), the mortality of HIV-infected young children significantly increased (52.5 vs. 7.6% uninfected at the age of 2) (7). On the other hand, early HIV diagnosis and early ART initiation substantially reduced infant mortality (8). ART was recommended by the World Health Organization (WHO) for children infected with HIV regardless of age, CD4 status, and clinical stage as of 2015 (9). However, children's ART coverage remained low worldwide, only ~53% of children aged <15 years have received ART compared to 67% of people aged 15 and older in 2019 (10, 11). A nationwide study reported that as of 2018, only 5,892/8,029 (73.4%) HIV-infected children ≤14 years have started ART in China (12). Although ART had significantly extended children's survival time (12), HIV-infected children had much lower rates of the receipt of ART compared to the national average ART coverage of 80.4% in China (13). Furthermore, HIV-infected children might encounter challenges such as parental death, school dropout, and stigma which in turn resulted in negative consequences (7).
Southern and southwestern China reported the highest HIV disease burden nationwide (14). Guangxi Zhuang Autonomous Region which is located in southern China sharing borders with Thailand, Laos, and Myanmar (Golden Triangle) ranked 3rd for HIV cases in 2018 (14, 15). It was one of six provinces and autonomous regions that had an HIV prevalence in pregnant women of over 50 per 100,000 population (14, 16). MTCT programs have been scaled up in Guangxi and contributed to a significant decrease in MTCT in recent years (17). Although early ART has been proven to be effective and beneficial for HIV-infected children, limited studies examined factors associated with death and attrition among HIV-infected children who were receiving ART. This retrospective study aimed to estimate mortality and attrition rates and identify factors that were related to death and attrition among a cohort of HIV-infected children who initiated ART. The findings of this study could be used to design programs with the goals to improve treatment outcomes and maximize the benefits of ART in HIV-infected children.
Materials and Methods
Study Design and Participants
The observational cohort study was conducted in Guangxi. Eligibility criteria included: (1) HIV positive children younger than 14 years old; (2) Enrolled in the National Free Antiretroviral Treatment Program (NFATP) between January 1, 2004, and December 31, 2018. All children or their parents or guardians were provided informed consent to the NFATP before they started the ART treatment. This NFATP informed consent also included encompassing their information in the NFATP database. NFATP included approximately all HIV-infected children who have been receiving ART in Guangxi. Furthermore, this study which only utilized de-identified data obtained approval from the institutional review board of the Guangxi Center for Disease Control and Prevention (GXIRB2016-0047-3). The study did not include participants who have not started ART.
Data Collection
Cohort study data were extracted from the NFATP database. The following baseline data of study participants were collected: age, gender, clinic stage before ART (using WHO's criteria), CD4 count before ART, cotrimoxazole prophylaxis (CTX) use before ART, initial ART regimen, malnutrition status before ART, liver function before ART, kidney function before ART, anemia status before ART, and time between an HIV diagnosis and ART initiation. Follow-up data that were collected included: death, cessation of ART, and loss to follow-up. The date of death was based on death certificate information. Attrition was defined as lost to follow-up or cessation of ART as recorded in the NFATP database. Lost to follow-up was defined as missing more than 90 days after the date of the last ART clinic visit, which was also defined as the date of ART cessation. More details could be found in a previously published article that used the Chinese national HIV treatment cohort study databases (18).
China has been providing free ART for all HIV-infected individuals. The most common ART regimens that were prescribed to HIV-infected children were zidovudine (AZT), lamivudine (3TC), and nevirapine (NVP)/efavirenz (EFV)/lopinavir/ritonavir (LPV/r); or abacavir (ABC), lamivudine (3TC), and nevirapine (NVP)/efavirenz (EFV)/lopinavir/ritonavir (LPV/r). We categorized ART regimens into the following three groups: containing AZT, containing ABC, and others (19). Malnutrition was defined as BMI-for-age Z-score (BMIZ) <-3SD (standard deviation) which was recommended by WHO for children and adolescents (20). Abnormal liver function was defined as either alanine transaminase (ALT) or aspartate transaminase (AST) was higher than lab references. A cutoff point for AST and ALT level was set at 40 IU/L (21). Abnormal kidney function was defined when serum creatinine (Scr) was higher than the lab reference. Scr thresholds using the enzymatic method were set up for different age groups: 0–7 days, 1.19 mg/ml; 7 days−1 month, 0.79 mg/ml; 1 month−1 year, 0.50 mg/ml; 1–10 years, 1.09 mg/ml; and 10–19 years, 1.29 mg/ml (22). Severe anemia was defined as a <8 g/dl of hemoglobin concentration value (23).
Statistical Analysis
We performed a time-to-event cohort analysis. The primary study endpoints were death and attrition. Mortality and attrition rates were calculated based on Poisson distributions and their 95% confidence intervals (CI) were assessed with incidence densities per 100 person-years at follow-up. Data were censored on Dec 31, 2019.
Cox proportional hazard models were performed to evaluate the treatment effect of initial ART regimens on death and attrition (cessation of ART or loss to follow-up) of HIV-infected children who started ART between 2004 and 2018, respectively. Competing risks for cause-specific hazard models were censored accordingly. The hazard ratios (HR) were generated through univariate regression models. Multivariate regression models were used to generate adjusted hazard ratios (AHR) with mortality and attrition, respectively. The following variables were included in the adjusted models: age before ART, gender, WHO stage before ART, CD4 count before ART, CTX use before ART, initial ART regimen, malnutrition before ART, abnormal liver function before ART, abnormal kidney function before ART, severe anemia before ART, time lag since HIV diagnosis and the start of ART, and year of ART initiation. A two-sided p-value of ≤0.05 was regarded as statistically significant. Statistical Analysis System (SAS 9.1™ for Windows; SAS Institute Inc., NC, USA) was used for all data analyses.
Results
Baseline Characteristics of Study Participants
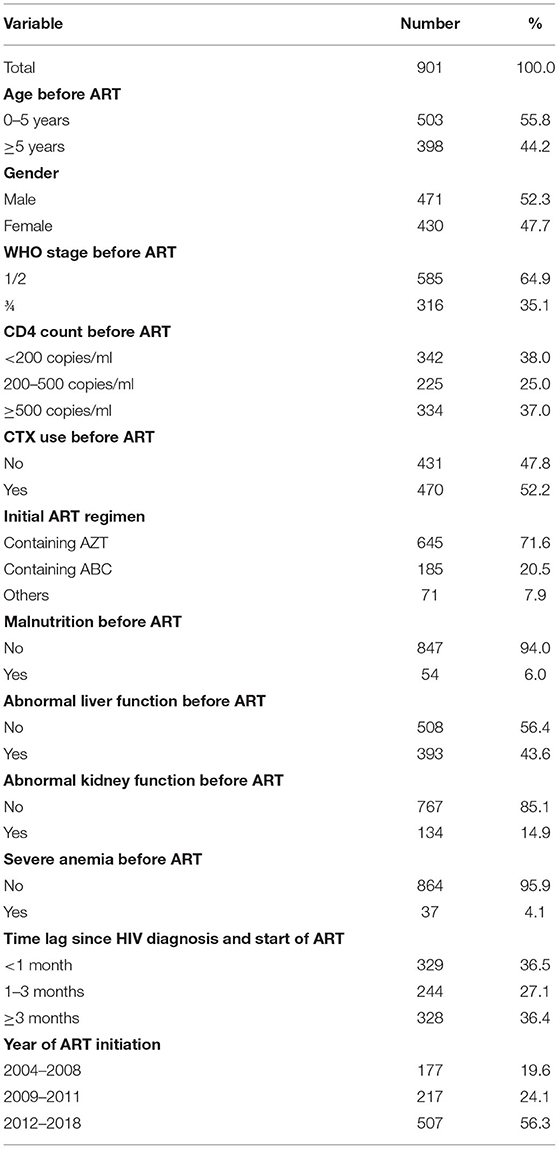
Of 911HIV-infected children who started ART from 2004 to 2018 in Guangxi, 10 participants were older than 14 years at ART initiation. A total of 901 study participants were included in the final cohort study analyses. The sample's mean age was 5.1 years with an SD of 3.2. Here was the age distribution of participants who were not older than 5 years, ≤1, 13.3% (67); 1–2, 20.9% (105); 2–3, 23.1% (116); 3–4, 21.4% (108); and 4–5, 21.3% (107). Regarding participants' characteristics at ART initiation, 55.8% of participants were <5 years old, more than half of participants were males (52.3%), and more than one-third were at a clinic stage of III or IV (35.1%, Table 1). In general, participants showed a low CD4 count level before ART initiation, many of them used CTX before ART initiation (52.2%), and the majority used ART regimens that contained AZT (71.6%). Furthermore, many HIV-infected children had medical conditions of malnutrition, abnormal liver function, abnormal kidney function, and severe anemia (6.0, 43.6, 14.9, and 4.1%, respectively). More than one-third of participants initiated ART within 1 month after receiving an HIV diagnosis (36.5%).
Mortality
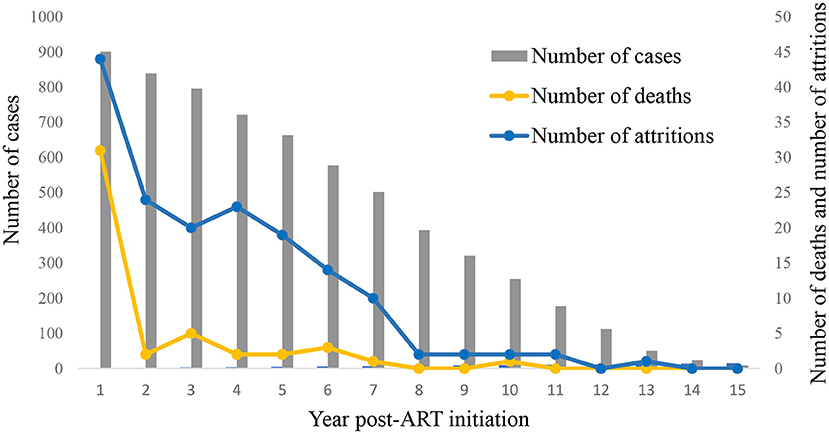
Among 901 HIV-infected children who started ART between 2004 and 2018, 47 of them died, the average mortality rate was 0.87 deaths per 100 person-years (95% CI 0.63–1.11). The mortality rate per 100 person-years was 3.65 in the first year of ART (95% CI 2.40–4.90), and it decreased thereafter (Figure 1 and Supplementary Table). Univariate Cox regression analyses indicated that several factors were significantly associated with mortality, including status before ART started such as disease severity (WHO clinic stage), CD4 count, malnutrition, abnormal liver function, initial ART regimen, and the time lag between an HIV diagnosis and ART initiation. In the multivariate model, higher levels of CD4 count before ART initiation were associated with reduced risks of mortality (200–500, AHR 0.22, 95% CI 0.09–0.55; ≥500, AHR 0.10, 95% CI 0.03–0.29, Table 2). Furthermore, a longer time lag between an HIV diagnosis and ART initiation was associated with increased risks of mortality (1–3 months, AHR 2.30, 95% CI 1.07–4.94; ≥3 months, AHR 2.22, 95% CI 1.04–4.74).
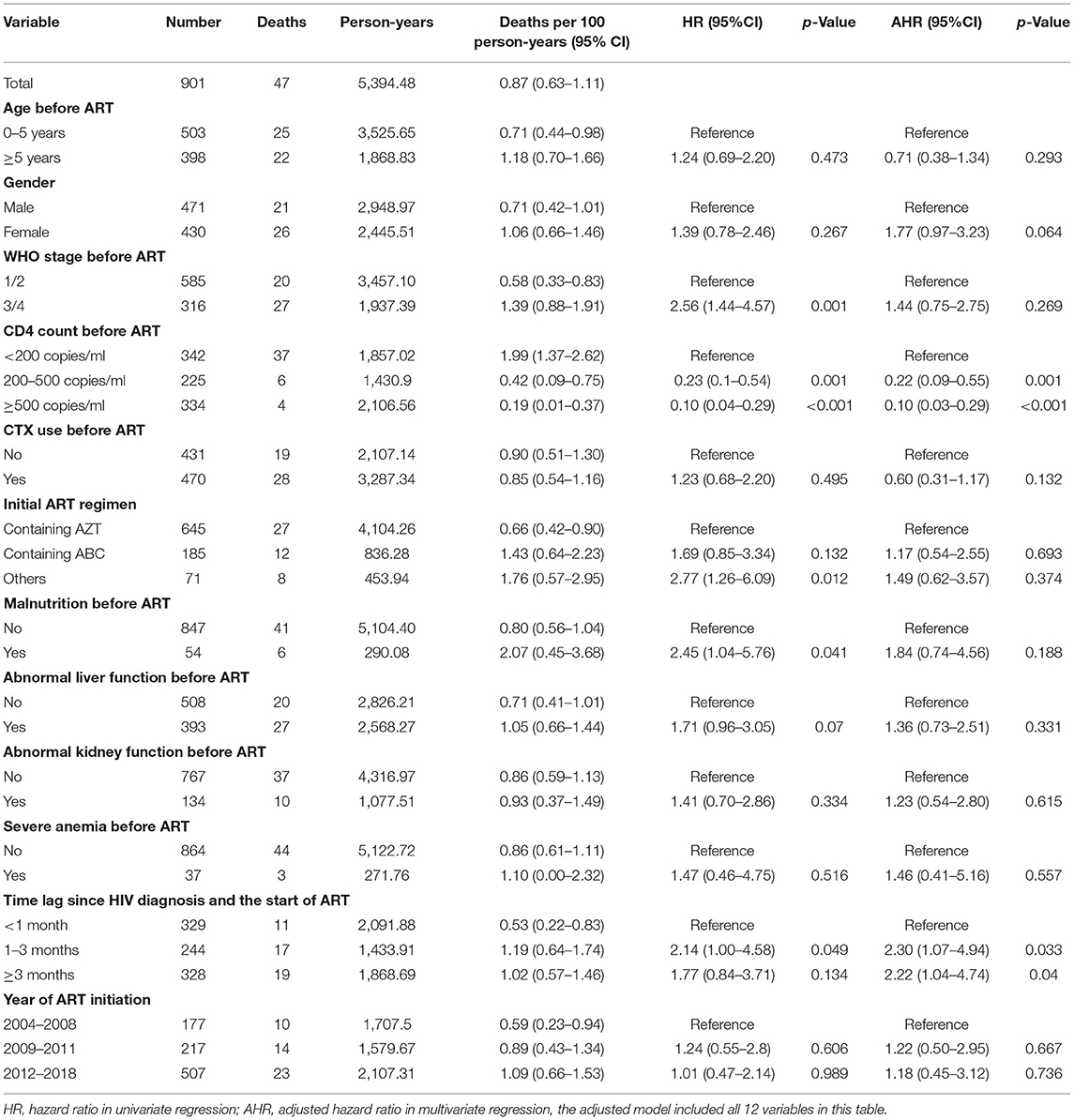
Table 2. Mortality rates and risk factors of HIV infected children started ART in Guangxi, 2004–2018.
Attrition
Among 901 HIV-infected children who started ART between 2004 and 2018, 163 attritions were reported, and the average attrition rate was 3.02 per 100 person-years (95% CI 2.57–3.47). The attrition rate per 100 person-years was 5.18 in the first year of ART (95% CI 3.69–6.67) and decreased after then (Figure 1 and Supplementary Table). We identified the following factors that were significantly associated with attrition using the univariate Cox regression model: age before ART, disease severity (WHO clinic stage) before ART, CD4 count before ART, initial ART regimen, and the time lag between an HIV diagnosis and ART initiation. In the multivariate model, a high CD4 level before ART initiation was associated with a reduced risk of attrition rate (≥500, AHR 0.62; CI 0.41–0.95, Table 3). On the other hand, delayed initiation of ART (1–3 months) was associated with an increased risk of attrition (AHR 1.55, CI 1.05–2.30).
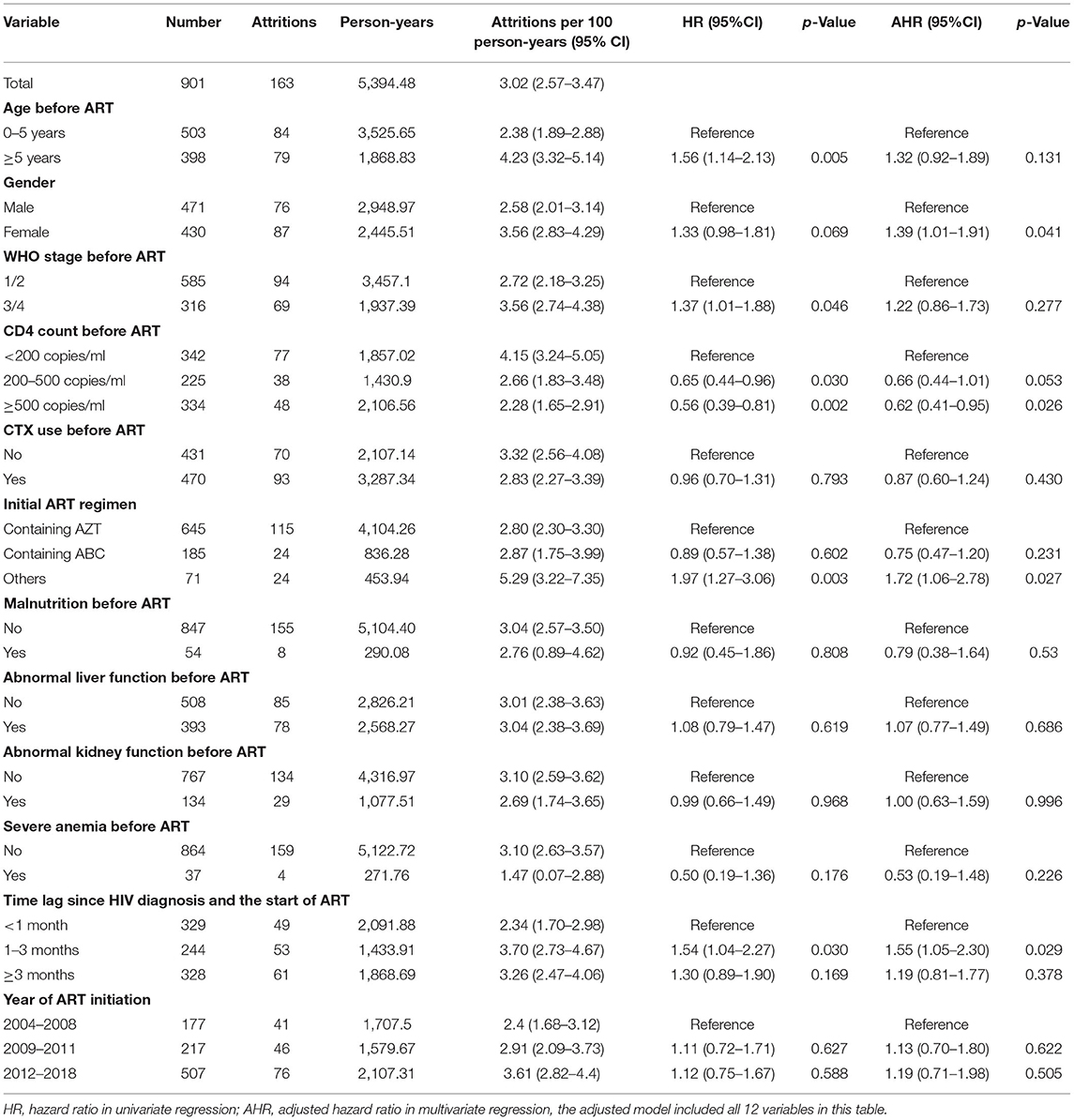
Table 3. Attrition rates and risk factors of HIV infected children started ART in Guangxi, 2004–2018.
Discussion
The study reported a mortality rate of 0.87 per 100 person-years in Guangxi HIV-infected children who have started ART. This result was lower than comparable reports from other Asian regions (2.1–2.86 per 100 person-years) for HIV-infected children (24, 25). Also, this mortality rate was much lower than in HIV-infected adults who were receiving ART in Guangxi (5.94 per 100 person-years) (26, 27). Moreover, mortality reached the peak of 3.65 per 100 person-years within the first year, dramatically reduced from the 2nd year, and reached nearly 0 at the 8th year of ART initiation. The trends were consistent with previous studies that reported generally higher mortalities in the first 3–6 months of ART initiation and decreased over time (24, 28). It could be explained that infections and other complications related to advanced HIV disease and late clinic stages were the possible major causes of the first-year deaths because medication-related deaths were generally low (28).
ART has significantly reduced HIV-infected children's mortality in this sample and initiating ART at a high CD4 level of ≥500 contributed to the lowest mortality. On the other hand, low CD4 count before ART initiation and late ART initiation resulted in increased mortality. In addition to a weakened immune system and its consequences, previous studies reported that a higher CD4 count before ART initiation could reduce the odds of virologic failure and HIV drug resistance mutations (29). However, 63.5% of participants have started ART after 1 month of an HIV diagnosis, and 38% started ART with a very low CD4 count (<200 copies/ml) in this sample. And at the time of initiating ART, many children were with other medical conditions, such as malnutrition, abnormal liver function, abnormal kidney function, and severe anemia. It suggested that supportive programs, such as nutritional support, routine examination, and lab test are needed in addition to ART promotion.
The overall attrition rate was 3.02 per 100 person-years in this study (18.1%). The result was consistent with the reports of Thailand (2.9 per 100 person-years) and Zambia (16%) for HIV-infected children (25, 30), but much lower than HIV-infected adults who started ART in Guangxi (10.86 per 100 person-years) (26). More than 25% of attritions occurred in the first year of ART initiation. High attrition rates can result in suboptimal treatment outcomes, increased mortality, increased cost of care, and excess infections (31). Therefore, future interventions can provide retention support, such as case management, and follow-ups to help retain HIV-infected children in care. Moreover, health education and linkage to care services are needed to educate families with HIV-infected children to start ART as early as possible and remove possible barriers for ART initiation.
One strength of the study was it utilized a database that included approximately all HIV-infected children who were under ART in Guangxi. The study provided a profile of this group of children and assessed factors associated with mortality and attrition. The study also confirmed the effectiveness of pediatric ART. Therefore, the findings have important real-world implications to reduce mortality and attrition in HIV-infected children. The study has limitations. First, the study included HIV-infected children who were in NFATP between 2004 and 2018 in Guangxi, some groups were not covered such as HIV-infected children who were not on ART. Therefore, the results cannot be generalized to these groups. Second, this sample and the number of events were relatively small (47 deaths), which possibly could cause the overfitting of the Cox model to predict risk factors for mortality. Third, the retrospective study was limited by the existing information in the database. Some information that might be helpful to explain the study results was lacking, such as children's viral load, parental HIV status, and parental or families' attitudes or sociodemographic characteristics. It is possible the lack of these variables results in unexpected results such as the higher mortality/attrition rates for the 1–3 months of ART initiation group than the >3 months group.
Conclusion
MTCT prevention efforts and ART have significantly reduced HIV-infected children's mortality although first-year mortality and attrition were relatively high after ART initiation. Low CD4 count and late ART initiation (>1 month) were associated with increased mortality and attrition. The findings have implications to design interventions and programs that support linkage to care, early ART initiation, and retention in care.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions. Data were extracted from the National Free Antiretroviral Treatment Program database. The original database was not available to the public due to institutional regulations. Requests to access these datasets should be directed to JZha, emhhbmdqaWFuZmVuZ0BzdHUuZ3htdS5lZHUuY24=.
Ethics Statement
This study was approved by the Institutional Review Board of the Guangxi Center for Disease Control and Prevention (GXIRB2016-0047-3). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
XZe, JZha, and YR were responsible for study design and planning. QZ, ZS, GL, and HC contributed to the data collection and management. XZh, JZhu, and YR contributed to the data analyses. XZe, JZha, YS, XZh, and YR contributed to the interpretation of the study results. XZe, JZha, XZh, and YR contributed to writing the report. All authors reviewed and revised the manuscript and approved the final version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82160636 and 11971479), Guangxi Natural Science Foundation Project (grants 2020GXNSFAA159020), Guangxi Key Laboratory of AIDS Prevention Control and Translation (ZZH2020010), Ministry of Science and Technology of China (2018ZX10721102-006 and 2018ZX10715008), Guangxi Bagui Honor Scholarship, and Chinese State Key Laboratory of Infectious Disease Prevention and Control. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, and writing of the paper. The corresponding author (JZha) has full access to all data in the study and takes final responsibility for the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We collaborated with Guangxi Center for Disease Control and Prevention (CDC) on the data collection. We are thankful to Guangxi CDC and all study participants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.916740/full#supplementary-material
Abbreviations
AHR, adjusted hazard ratio; HR, hazard ratio.
References
1. UNAIDS. UNAIDS data. (2020). Available online at: https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf (accessed May 13, 2021).
2. Iyun V, Technau K-G, Vinikoor M, Yotebieng M, Vreeman R, Abuogi L, et al. Variations in the characteristics and outcomes of children living with HIV following universal ART in sub-Saharan Africa (2006–17): a retrospective cohort study. Lancet HIV. (2021) 8:e353–62. doi: 10.1016/S2352-3018(21)00004-7
3. Rujumba J, Ndeezi G. Considerations for strengthening ART scale-up for children. Lancet HIV. (2021) 8:e313–4. doi: 10.1016/S2352-3018(21)00052-7
4. Dong Y, Guo W, Gui X, Liu Y, Yan Y, Feng L, et al. Preventing mother to child transmission of HIV: lessons learned from China. BMC Infect Dis. (2020) 20:1–10. doi: 10.1186/s12879-020-05516-3
5. China National Working Committee on Children and Women under State Council. National mother-to-child transmission rate of HIV fell to 5.7% in 2016. Available online at: http://www.nwccw.gov.cn/2017-12/04/content_187271.htm (accessed May 13, 2021).
6. Dong Y, Wang L, Burgner DP, Miller JE, Song Y, Ren X, et al. Infectious diseases in children and adolescents in China: analysis of national surveillance data from 2008 to 2017. BMJ. (2020) 369:m1043. doi: 10.1136/bmj.m1043
7. Newell M-L, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet HIV. (2004) 364:1236–43. doi: 10.1016/S0140-6736(04)17140-7
8. Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. (2008) 359:2233–44. doi: 10.1056/NEJMoa0800971
9. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs For Treating and Preventing HIV Infection: Recommendations For a Public Health Approach - Second Edition. (2016). Available online at: https://www.who.int/hiv/pub/arv/arv-2016/en/ (accessed May 13, 2021).
10. UNICEF. Paediatric care and treatment. (2020). Available online at: https://data.unicef.org/topic/hivaids/paediatric-treatment-and-care/ (accessed May 15, 2021).
11. UNAIDS. Global HIV & AIDS statistics-2020 fact sheet (2021). Available onine at: https://www.unaids.org/en/resources/fact-sheet (accessed February 14, 2021).
12. Yin H, Ma Y, Yang X, Zhao H, Han M. Survival analysis on HIV-infected children aged 14 years old and younger in China. Chin J Epidemiol. (2020) 41:850–5. doi: 10.3760/cma.j.cn112338-20191129-00844
13. UNAIDS. Country progress report-China. (2018). Available online at: https://www.unaids.org/sites/default/files/country/documents/CHN_2018_countryreport.pdf (accessed May 13, 2021).
14. Wu Z, Chen J, Scott SR, McGoogan JM. History of the HIV epidemic in China. Curr HIV/AIDS Rep. (2019) 16:458–66. doi: 10.1007/s11904-019-00471-4
15. Cao B, Saha PT, Leuba SI, Lu H, Tang W, Wu D, et al. Recalling, sharing and participating in a social media intervention promoting HIV testing: a longitudinal analysis of HIV testing among MSM in China. AIDS Behav. (2019) 23:1240–9. doi: 10.1007/s10461-019-02392-0
16. Jiang J, Zhou Y, Li H, Gao Y, Zhang Y, Luo S, et al. HIV epidemic among pregnant women in China, 2016: trend and spatial analysis. Chin Med J. (2018) 98:3360–4. doi: 10.3760/cma.j.issn.0376-2491.2018.41.014
17. Zhao J, Chen Q, Fu C, Qin Q, Huang H, Feng Y, et al. Rate of the HIV transmission and associated factors among HIV-exposed infants in Guangxi, China: 2014–2019. AIDS Res Hum Retroviruses. (2020) 36:647–55. doi: 10.1089/aid.2020.0073
18. Huang X, Xu L, Sun L, Gao G, Cai W, Liu Y, et al. Six-year immunologic recovery and virological suppression of HIV patients on LPV/r-based second-line antiretroviral treatment: a multi-center real-world cohort study in China. Front Pharmacol. (2019) 10:1455. doi: 10.3389/fphar.2019.01455
19. Guo P-L, He H-L, Chen X-J, Chen J-F, Chen X-T, Lan Y, et al. Antiretroviral long-term efficacy and resistance of lopinavir/ritonavir plus lamivudine in HIV-1-infected treatment-Naïve patients (ALTERLL): 144-week results of a randomized, open-label, non-inferiority study from Guangdong, China. Front Pharmacol. (2020) 11:569766. doi: 10.3389/fphar.2020.569766
20. Kigaru DMD, Ndung'u ZW, Macharia-Mutie CW. Application of stable isotope dilution techniques to assess body fat and comparison with WHO BMI-for-age classification as a measure of obesity among schoolchildren in Nairobi, Kenya. Public Health Nutr. (2021) 24:3587–91. doi: 10.1017/S1368980020001950
21. Tadesse BT, Foster BA, Kabeta A, Ayalew F. H/Meskel G, Jerene D, et al. Hepatic and renal toxicity and associated factors among HIV-infected children on antiretroviral therapy: a prospective cohort study. HIV Med. (2019) 20:147–56. doi: 10.1111/hiv.12693
22. Beng H, Rakhmanina N, Moudgil A, Tuchman S, Ahn S-Y, Griffith C, et al. HIV-Associated CKDs in children and adolescents. Kidney Int Rep. (2020) 5:2292–300. doi: 10.1016/j.ekir.2020.09.001
23. Nguyen RN, Ton QC, Luong MH, Le LHL. Long-term outcomes and risk factors for mortality in a cohort of HIV-infected children receiving antiretroviral therapy in Vietnam. HIV/AIDS. (2020) 12:779. doi: 10.2147/HIV.S284868
24. Moy FS, Fahey P, Nik Yusoff NK, Razali KA, Nallusamy R, TREAT Asia Pediatric HIV Observational database. Outcomes of human immunodeficiency virus-infected children after anti-retroviral therapy in Malaysia. J Paediatr Child Health. (2015) 51:204–8. doi: 10.1111/jpc.12712
25. Teeraananchai S, Kerr SJ, Puthanakit T, Bunupuradah T, Ruxrungtham K, Chaivooth S, et al. Attrition and mortality of children receiving antiretroviral treatment through the universal coverage health program in Thailand. J Pediatr. (2017) 188:210–6. e1. doi: 10.1016/j.jpeds.2017.05.035
26. Zhu J, Yousuf MA, Yang W, Zhu Q, Shen Z, Lan G, et al. Mortality and attrition rates within the first year of antiretroviral therapy initiation among people living with HIV in Guangxi, China: an observational cohort study. Biomed Res Int. (2021). https://doi.org/10.1155/2021/6657112. doi: 10.1155/2021/6657112
27. UNICEF. Global and regional trends. (2020). Available online at: https://data.unicef.org/topic/hivaids/global-regional-trends/ (accessed May 13, 2021).
28. Walker AS, Prendergast AJ, Mugyenyi P, Munderi P, Hakim J, Kekitiinwa A, et al. Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis. (2012) 55:1707–18. doi: 10.1093/cid/cis797
29. Muri L, Gamell A, Ntamatungiro AJ, Glass TR, Luwanda LB, Battegay M, et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS. (2017) 31:61. doi: 10.1097/QAD.0000000000001273
30. Mutanga JN, Mutembo S, Ezeamama AE, Song X, Fubisha RC, Mutesu-Kapembwa K, et al. Long-term survival outcomes of HIV infected children receiving antiretroviral therapy: an observational study from Zambia (2003–2015). BMC Public Health. (2019) 19:1–12. doi: 10.1186/s12889-019-6444-7
Keywords: HIV, children, mortality, attrition, antiretroviral therapy
Citation: Zeng X, Chen H, Zhu Q, Shen Z, Lan G, Liang J, Liang F, Zhu J, Xing H, Shao Y, Ruan Y, Zhang J and Zhang X (2022) Treatment Outcomes of HIV Infected Children After Initiation of Antiretroviral Therapy in Southwest China: An Observational Cohort Study. Front. Pediatr. 10:916740. doi: 10.3389/fped.2022.916740
Received: 20 April 2022; Accepted: 13 June 2022;
Published: 12 July 2022.
Edited by:
Luis Escosa-García, University Hospital La Paz, SpainReviewed by:
Carlos Grasa, University Hospital La Paz, SpainSara Guillén, Hospital de Getafe, Spain
Copyright © 2022 Zeng, Chen, Zhu, Shen, Lan, Liang, Liang, Zhu, Xing, Shao, Ruan, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfeng Zhang, emhhbmdqaWFuZmVuZ0BzdHUuZ3htdS5lZHUuY24=; Xiangjun Zhang, enhqQG5ldmFkYS51bnIuZWR1
 Xiaoliang Zeng1,2
Xiaoliang Zeng1,2 Zhiyong Shen
Zhiyong Shen Yuhua Ruan
Yuhua Ruan Jianfeng Zhang
Jianfeng Zhang Xiangjun Zhang
Xiangjun Zhang