- 1Neonatal Intensive Care Unit, South Tees Hospitals National Health Service (NHS) Foundation Trust, James Cook University Hospital, Middlesbrough, United Kingdom
- 2Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, United Kingdom
- 3School of Biomedical Engineering, Czech Technical University in Prague, Prague, Czechia
Oxygen is the most common drug used in the neonatal intensive care. It has a narrow therapeutic range in preterm infants. Too high (hyperoxemia) or low oxygen (hypoxemia) is associated with adverse neonatal outcomes. It is not only prudent to maintain oxygen saturations in the target range, but also to avoid extremes of oxygen saturations. In routine practice when done manually by the staff, it is challenging to maintain oxygen saturations within the target range. Automatic control of oxygen delivery is now feasible and has shown to improve the time spent with in the target range of oxygen saturations. In addition, it also helps to avoid extremes of oxygen saturation. However, there are no studies that evaluated the clinical outcomes with automatic control of oxygen delivery. In this narrative review article, we aim to present the current evidence on automatic oxygen control and the future directions.
Introduction
Oxygen is a drug with a narrow therapeutic range in vulnerable preterm neonates. Avoiding both hypoxemia and hyperoxemia is important especially in neonates as both are associated with short-term and long-term adverse outcomes (1, 2). Hypoxemia causes cellular damage, and this may be associated with poor outcomes such as death or disability (3–5). Hyperoxemia causes oxygen toxicity and oxidative stress that has been implicated in the development of bronchopulmonary dysplasia (BPD) and retinopathy of prematurity (ROP) (6–8). Although, there are recommendations for the oxygen saturation (SpO2) targeting in preterm infant, it is challenging in the routine practice to keep the SpO2 in the prescribed target range (TR) (9, 10). Traditionally the Fractional inspired oxygen (FiO2) is often needed to be titrated by the bedside staff (Manual control, M-FiO2) to try to maintain the SpO2 in the TR. The compliance with manual control is hugely variable across centers (11). Studies have shown that M-FiO2 results in a considerable proportion of time spent outside of the TR of SpO2. The preterm infants in view of their cardiorespiratory instabilities and apnea of prematurity, are prone to fluctuations in SpO2 and intermittent episodes of hypoxemia (12). In a prospective study, reporting achieved vs. intended SpO2 targets in preterm infants <28 weeks, only 16–64% of time infants were in the intended range and above the range 20–73% of time (11). With the advent of automated system in oxygen delivery (A-FiO2), the challenges to maintain the SpO2 in the TR has been overcome to an extent. A-FiO2 uses real time continuous SpO2 data to makes necessary adjustments in FiO2 based on algorithms that differ with devices and systems.
Studies using A-FiO2 have consistently shown to improve the proportion of time spent in the TR of SpO2, reduce hypoxemia and hyperoxemia in preterm infants on non-invasive or invasive respiratory support. Whilst the A-FiO2 systems have been commercially available, it has not yet established itself in the routine care in the neonatal ICU (2, 13). This is indicative of the challenges with its use, and more importantly the lack of clinical outcome data with the use of A-FiO2.
In this review article we will make a case for importance of SpO2 targeting in preterm infants, clinical implications of intermittent hypoxemia/hyperoxemia, current evidence for the use of A-FiO2, the types of algorithms available in clinical practice, challenges in implementation of technology and the future directions.
SpO2 Targeting in Preterm Infants
In the last decade, five large randomized controlled trials (14–18) were conducted to evaluate the optimal SpO2 TR in preterm infants. Following this, three systematic reviews (19–21) including Cochrane review (9) and one individual patient meta-analysis (10) have been published. There was no difference in the primary composite outcome of death or major disability at 18–24 months corrected age between the lower SpO2 group (85–89%) and higher SpO2 group (91–95%). The lower SpO2 group was associated with higher risk of mortality and NEC. The risk of ROP was higher in the higher SpO2 group. However, there was no difference in the rates of severe visual impairment (22). Interestingly, the separation of SpO2 between the two groups in these studies was less than expected with significant overlap in SpO2 in the two groups. The current recommendations by international bodies suggest the use of 90–95% as SpO2 TR in preterm infants until 36 weeks Post menstrual age (20, 23).
Effects of Hypoxemia and Hyperoxemia
In post-hoc analysis of Canadian oxygen trial (COT study), intermittent prolonged hypoxemia (SpO2 < 80%) for at least 1 min was associated with increase in composite outcome of death after 36 weeks or major neuro-disability (RR 1.66, 95% CI: 1.35–2.05) at 18 months corrected age (5). Jensen et al. in their post-hoc analysis also showed increased risk of severe BPD with both the frequency of severe hypoxemic episodes and duration of hypoxemia (4). Compared with infants with lowest decile of hypoxemic episodes, infants with highest number of hypoxemic episodes (10th decile) had an adjusted relative risk of 20.40 (95% CI: 12.88–32.32) for severe BPD.
Oxygen supplementation and hyperoxemia, whilst on supplemental oxygen, has been associated with ROP, BPD and PVL (24, 25). Hyperoxemia, mostly an overshoot to the oxygen supplementation following a hypoxemic event, is a preventable by strict adherence to the SpO2 target or by the use of A-FiO2.
Whilst it is important to maintain the SpO2 in TR for preterm infants, it is equally imperative to avoid hypoxemia and hyperoxemia. Hence it is essential to choose and adhere to the appropriate alarm limits for the SpO2 TR (26). A-FiO2 studies have shown an advantage of A-FiO2 over M-FiO2in reducing extremes of SpO2.
Algorithms for A-FiO2
The A-FiO2 works on the principles of continuous SpO2 monitoring using pulse oximeter, regular feedback into the rule-based algorithms and changes in FiO2 delivery based on this feedback. The algorithms vary in designs and hence the frequency and magnitude of changes to FiO2 is variable across the various A-FiO2 devices. The designs include on adaptive model control algorithms, proportional integral differential algorithm and state machine control algorithm (27).
The state machine control algorithm is based on a set of rules. The algorithm uses the difference between the desired and the actual SpO2, its velocity and acceleration as input. The incorporated rules then set out a FiO2 change by the controller. In the proportional integral differential algorithms, the controller calculates the difference between the desired and the actual SpO2 (error), integrates over time and velocity and determines the oxygen output. The adaptive model algorithms consider the infant's physiology that may have the effect on oxygen dissociation curve. A non-linear model is created based on FiO2-oxygen saturation relationship. The controller adjusts its model of this relationship to achieve target saturations (28).
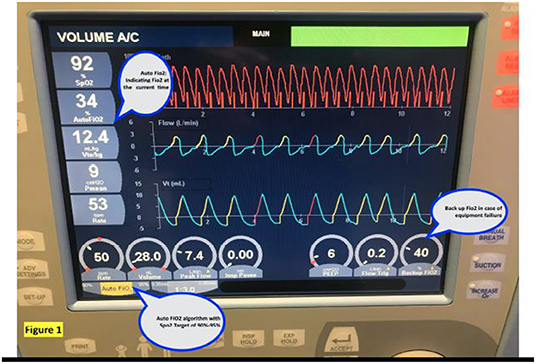
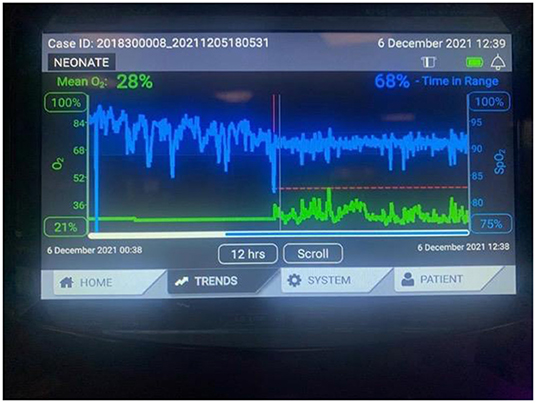
The currently available algorithms include integral to the AveaⓇ infant ventilator (Figure 1), CLAC (Closed Loop automatic oxygen control) incorporated into the Leoni ventilator, IntellO2™ in the Oxygen assist module in Vapotherm Precision Flow (Figure 2), OxyGenie on SLE6000 ventilator, PRICO on the Fabian acutronic ventilators and SPOC on Sophie neonatal ventilator (MEDACX).
Current Available Evidence for the Use of A-FiO2 in Neonates
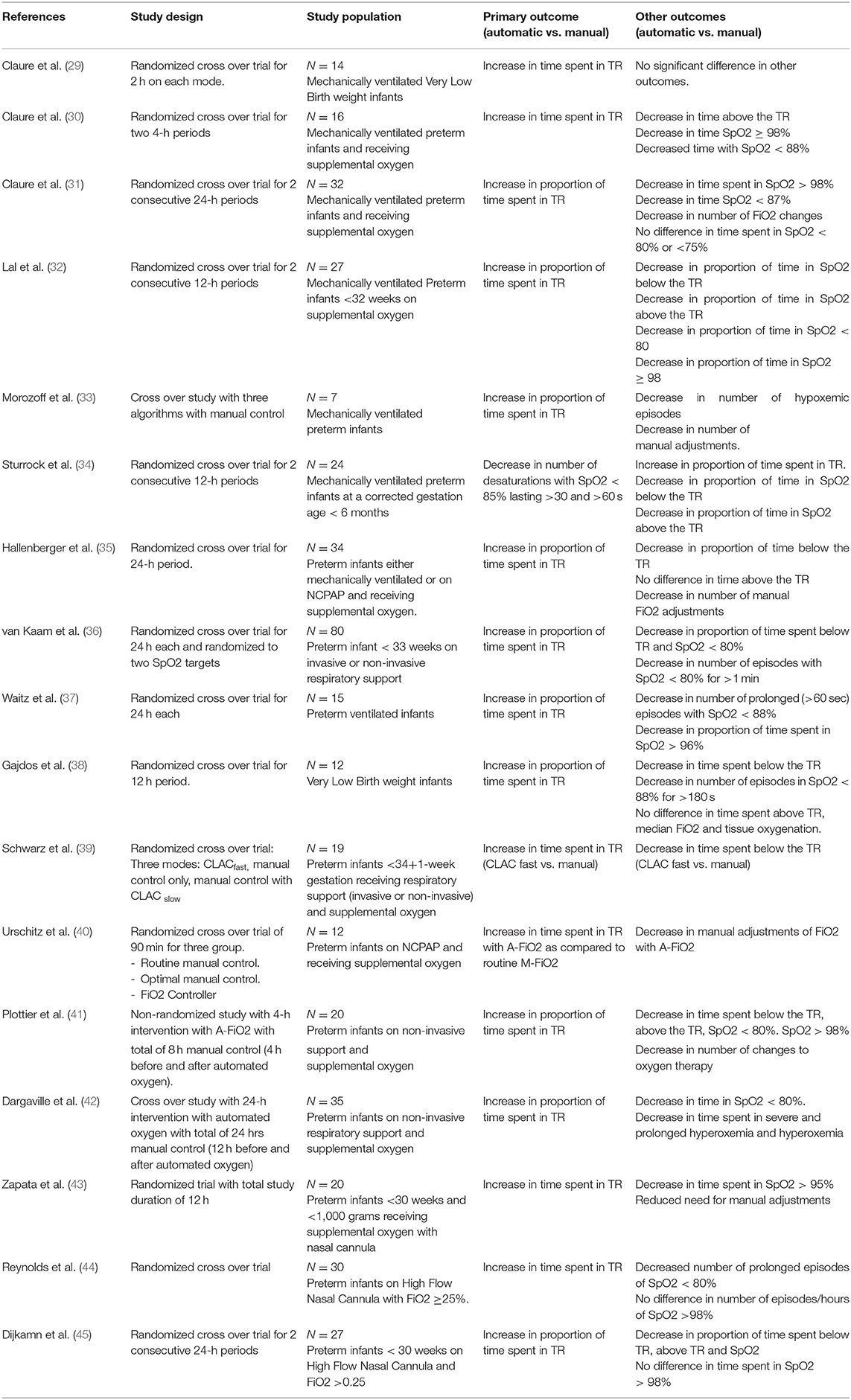
The details of the currently available studies are shown in Table 1 (29–45). Majority of these studies were cross-over RCT. The SpO2 targets used were variable across the studies, as were the post-natal age at entry and the algorithms used. All the studies were of short duration varying from 2 to 48 h. Six of these studies included infants on invasive ventilation, another six used a combination of invasive ventilation and nasal continuous positive airway pressure (NCPAP), and further six studies only included infants on non-invasive respiratory support (NCPAP or High Flow therapy). The primary outcome in most was the proportion of time in SpO2 TR. The studies consistently reported significantly higher proportion of time in SpO2 TR, lower proportion of time below & above the SpO2 TR and reduced need for manual adjustments with A-FiO2. In a recent systematic review with 13 studies, A-FiO2 resulted in increased time spent in target SpO2 of 85–96% [MD = 8.96; 95% CI (6.26, 11.67), p < .00001], and 90–95% [MD = 18.25; 95% CI (4.58, 31.65), p = 0.008] (46). A-FiO2 reduced the time in hypoxemia [SpO2 < 85%; MD = −1.24; 95% CI (−2.05, −0.43), p = 0.003] and hyperoxemia [SpO2 > 98%; MD = −0.99; 95% CI (−1.74, −0.25), p = 0.009].
Various algorithms are available with A-FiO2. Only two studies compared different A-FiO2 algorithms. Schwarz et al. compared fast and slow CLAC algorithms (39) and Salverda et al. compared OxyGenie controller (SLE6000 ventilator) with CLiO2 controller (AVEA ventilator) in randomized cross over trial (47). In the latter study 15 preterm infants received each intervention for 24 h in a cross over fashion. Time spent in the SpO2 TR were higher with OxyGenie with median time of 80.2 (72.6–82.4) % vs. 68.5% (56.7–79.3%) in CLiO2 algorithm. With OxyGenie time spent above the TR were lower (6.3 vs. 15.9%, p < 0.005) and time spent below the TR (14.7 vs. 9.3%, p < 0.05) were higher as compared to CLiO2. The difference in the hypoxemia and hyperoxemia episodes may be related to the different design of the algorithm.
Although A-FiO2 has consistently shown to be superior to the M-FiO2 in maintaining the SpO2 in the TR, we do not know if this physiological benefit is associated with improved clinical benefits. It can be hypothesized that better control in maintaining SpO2 in TR, reduction in hypoxemia and hyperoxemia may concomitantly result in improved short- and long-term clinical outcomes. There are currently no studies available that has looked at use of A-FiO2 to improve clinical outcomes. For a clinical outcome study with A-FiO2, it is imperative that parallel arm RCT design is chosen. The study should also capture the entire period on respiratory support and supplemental oxygen. A large RCT with aim to recruit 2,340 preterm infants (<28 weeks) is currently underway (NCT03168516) (48). In this clinical outcome study, infants are randomized to either A-FiO2 or M-FiO2, continue to be in randomized arm as much as time possible without any crossover. Primary outcome of this RCT is composite outcome of death or severe ROP, BPD or NEC. This study has another primary outcome of composite of death or any of the following: language or cognitive delay, motor impairment, severe visual impairment or hearing impairment all assessed at 2 years of age.
An improvement in saturation targeting with A-FiO2 was not associated with improved tissue oxygenation in studies by Dani et al. and Waitz et al. (37, 49).
Alarms are necessary evils in any intensive care units (50). Alarm overloads can result in fatigue and desensitization among staff which in turn could pose a clinical risk. Studies with A-FiO2 have shown a significant lower alarm rate as compared to M-FiO2 (32). The frequency of alarms in A-FiO2 can be further reduced with much looser alarm limits (51). The reduction in alarm frequency may help in reducing the nursing workload and possibly increase cognitive attention. However, it is imperative to consider the appropriate alarm threshold for SpO2 and FiO2 so as to alert the caregivers of a deterioration.
Few centers have implemented A-FiO2 for routine care of preterm infants. Van Zanten et al. reported outcomes of before and after implementation of A-FiO2 (52). Although there was a significant improvement in time spent in the SpO2 TR, there was no difference in duration of respiratory support and mortality. Salverda et al. also reported pre (2012–2015; N = 293) and post (2015–2018; N = 295) implementation of A-FiO2 in preterm infants (53). There was no difference in any of the clinical outcomes like ROP, NEC, BPD, and duration of hospital stay. Both these studies by the nature of their design were not powered for these outcomes.
Van Zanten et al. also reported that the staff were reluctant to go back to M-FiO2 after implementation of A-FiO2 as this reduced their workload (52). To our knowledge, there are no studies reporting parental experience with use of A-FiO2 either in clinical or research set-up.
In summary, currently there is good evidence to show that A-FiO2 is superior to M-FiO2 in maintaining SpO2 in TR and reducing extremes of SpO2 in preterm infants. However, there are no studies to support the clinical benefits of A-FiO2.
What Is the Current Position of A-FiO2 in NICUs
Recent survey among UK neonatal units (192 units), showed that around 19 neonatal units (9.9%) units used A-FiO2 (54). Sixty-eight percent of the users used it in extreme preterm infants <26 weeks. Most responders to the survey reported higher ability to achieve proportion of time within the target SpO2 range and reduced need for manual adjustments. 89% of responders did not report any adverse outcomes. There were two reports that A-FiO2 resulted in inadvertent higher FiO2 when the probe was displaced and one report of masking event of desaturations.
The main challenges to implementation of A-FiO2 in NICU are lack of devices delivering A-FiO2, unfamiliarity with the devices and the lack of clinical outcome studies. Most of the new neonatal ventilators have A-FiO2 options on them. However, without appropriate expertise and training, the introduction and implementation of any change can be a failure. There are few reports that A-FiO2 can result in inadvertent higher FiO2 when the probe was displaced and mask desaturations.
Potential and/or Perceived Barriers and Opportunities
Masking of Clinical Deterioration
One of the concerns with regards to use of A-FiO2 is that it may mask clinical deteriorations. A-FiO2 is better than M-FiO2 at reducing hypoxemic episodes by automatically increasing the FiO2. However, the hypoxemic events may occur in relation to clinical deterioration like sepsis and just by increasing the FiO2 during these episodes, such events may be masked. This is generally not an issue especially if the staffing level is such that there is continuous close observation of these infants. This can also be overcome by appropriate staff training and using appropriate FiO2 alarms. In our unit we have addressed this by staff education and training. The CLiO2 system provides base FiO2 which is a trend, and a trend upwards may be indicative of deteriorating clinical condition. There is continuous scrutiny and medical staff are alerted when the there is an upwards trend of more than 5%.
Hypoxemic Events Related to Apnea
Another potential limitation with A-FiO2 is its inability to differentiate hypoxemic events secondary to apneic episodes. A-FiO2 would provide sufficient oxygen to keep the SpO2 in TR, whereas with severe apneic episodes the infant may need other intervention like stimulation and positive pressure support. This issue can be overcome again by close observation of the infant and appropriate vital parameter alarm limits. Again, in these scenarios the role of staff education and training cannot be over emphasized.
Average FiO2
It is often perceived at the bedside that FiO2 tends to be higher with A-FiO2 than M-FiO2. Some cross over studies with A-FiO2, did not show any statistical difference in the median FiO2 (32, 36, 42), was lower in A-FiO2 arm in Claure et al.'s study (31) and higher in Dijkman et al.'s study using PRICO (45).
Lower SpO2 Median
Whilst on M-FiO2, the staff proactively intervene for hypoxia than hyperoxia episodes (11). Also, in a M-FiO2 set-up there is a tendency to keep the SpO2 in the upper range of the target (closer to 95%), whereas automated oxygen devices tend to target middle of SpO2 TR (close to 92–93%). This could potentially lead to lower mean/median SpO2 with A-FiO2. Whether this would have any impact on clinical outcomes needs to be studied and if needed this issue could be tackled with changes in algorithm. Further if such subtles of control are found to be warranted, shifting of A-FiO2 TR and alarm limits can be implemented.
Disparity in SpO2 Readings Between the Monitors
In most of the A-FiO2 devices, the SpO2 can be monitored on the device in which the algorithm is incorporated. Some of the A-FiO2 devices albeit having the monitoring functionality does not have SpO2 alarms incorporated. This necessitates having additional SpO2 monitoring system with alarms to alert the staff of the deviation from TR. Despite using the same SpO2 technology, on occasions there seems to be discrepancy in SpO2 between the two monitoring devices. In our practice, we instruct our nursing staff to reposition/replace SpO2 probe which seems to resolve this discrepancy on most occasions. Resolution of discrepancy on most occasions reassures us that this discrepancy is not to the extent of clinical significance (hypoxia/hyperoxia), still it could result in staff and parental anxiety. However, this can be overcome by incorporating SpO2 monitoring with alarm limits on the same device.
Cost-Effective and Staff Workload
Cost of the equipment is reported as another major limitation. Although, most of the newer neonatal ventilators are equipped with A-FiO2, the older versions may not have this facility. The discussion around cost-effectiveness should consider the clinical benefits with this technology. However, we are clearly lacking clinical studies looking at the short- and long-term outcomes of A-FiO2. When staff work load is considered, A-FiO2 has shown to be associated with significant reduction in the number of manual adjustments required thus allowing staff to focus on other aspects of clinical care (55).
Customizing TR in Preterm Infants
Not all neonates of the exact same maturity are alike. The recent AAP guidelines recommends TR between 90 and 95%. However, it also underlines that there is no ideal TR and that it is patient specific and vary with gestation, chronological age and the underlying condition (56). Studies have shown that SGA are more susceptible to lower SpO2 (57). Also, the outcome data from individual centers may influence the TR used (58). A-FiO2 offers the potential to individualize TR according to the needs of the infant.
Role of A-FiO2 in Neonatal Resuscitation
At birth, preterm infants slowly transition from fetal to neonatal life and often need interventions to support with this transition. Oxygen supplementation is often needed for these infants to maintain recommended SpO2 levels in the first 10 min of life. Hence use of pulse oximetry is recommended by the resuscitation council to monitor and titrate oxygen supplementation (59). With particular focus on reducing hyperoxemia and hypoxemia, most resuscitation councils recommended use of oxygen ranging from 21 to 30% for preterm infants at birth (59, 60).
Even with advances in neonatal resuscitation it remains a challenge to meet the SpO2 targets during the first 10 min of life. In a study with preterm infants ≤30 weeks the median percentages of time spent above and below the target were 44 and 51%, respectively (61). A-FiO2 could be one of the solutions to achieve the SpO2 targets at the time of birth. A study in ventilated preterm lambs showed a significant reduction in time spent above the SpO2 TR with the use of A-FiO2 using PRICO technology at birth (3). Use of A-FiO2 in resuscitation could be potentially useful and needs further research.
Future Directions
• Need for RCTs that are adequately powered for short term and long-term outcomes. These studies should also report the cost effectiveness of the intervention, considering all the health outcomes and staff workload. The future studies should consider recruitment as soon as possible after birth to limit extremes of oxygenation during early period of the life.
• Studies are needed with characterization of all the existing algorithms with both invasive and non-invasive respiratory support.
• Innovations are needed to provide commercial algorithms that could support moving SpO2 targets (like during first 10 min of birth).
• Role of automated oxygen during elective neonatal intubation and reduction in hypoxemia during these procedures.
• Use of automated oxygen in preterm infants receiving nasal cannula low flow oxygen.
• Establish a role of A-FiO2 in low resource-staff limited settings.
Conclusions
There is overwhelming evidence that A-FiO2 achieves higher proportion of time in SpO2 TR, reduces duration and episodes of hypoxemia and hyperoxemia. Although the impact on clinical outcomes associated with A-FiO2 is yet to be proven, from the available studies we can presume that there is no harm. Merely adopting the recommendations of targeting SpO2 (90–95%) will not suffice. It is essential that this is achieved. If not, this will be justice half done and infact we may not see the actual clinical benefits of SpO2 targeting. A-FiO2 is a promising technology that helps to achieve this target. However, the clinical benefits of it are still unknown.
Author Contributions
VN, PL, and ML conceptualized the review, drafted the initial manuscript, reviewed, and revised the manuscript. TB reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tan A, Schulze A, O'Donnell CP, Davis PG. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev. (2005) 2005:Cd002273. doi: 10.1002/14651858.CD002273.pub2
2. Dargaville PA, Marshall AP, McLeod L, Salverda HH, Te Pas AB, Gale TJ. Automation of oxygen titration in preterm infants: current evidence and future challenges. Early Hum Dev. (2021) 162:105462. doi: 10.1016/j.earlhumdev.2021.105462
3. Hütten MC, Goos TG, Ophelders D, Nikiforou M, Kuypers E, Willems M, et al. Fully automated predictive intelligent control of oxygenation (PRICO) in resuscitation and ventilation of preterm lambs. Pediatr Res. (2015) 78:657–63. doi: 10.1038/pr.2015.158
4. Jensen EA, Whyte RK, Schmidt B, Bassler D, Vain NE, Roberts RS. Association between intermittent hypoxemia and severe bronchopulmonary dysplasia in preterm infants. Am J Respir Crit Care Med. (2021) 204:1192–9. doi: 10.1164/rccm.202105-1150OC
5. Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA. (2015) 314:595–603. doi: 10.1001/jama.2015.8841
6. Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. (1995) 346:1464–5. doi: 10.1016/S0140-6736(95)92475-2
7. Laughon M, Allred EN, Bose C, O'Shea TM, Van Marter LJ, Ehrenkranz RA, et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. (2009) 123:1124–31. doi: 10.1542/peds.2008-0862
8. Saugstad OD. Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol. (2001) 13:147–53. doi: 10.1097/00001703-200104000-00009
9. Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M, et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev. (2017) 4:Cd011190. doi: 10.1002/14651858.CD011190.pub2
10. Askie LM, Darlow BA, Finer N, Schmidt B, Stenson B, Tarnow-Mordi W, et al. Association between oxygen saturation targeting and death or disability in extremely preterm infants in the neonatal oxygenation prospective meta-analysis collaboration. JAMA. (2018) 319:2190–201. doi: 10.1001/jama.2018.5725
11. Hagadorn JI, Furey AM, Nghiem TH, Schmid CH, Phelps DL, Pillers DA, et al. Achieved versus intended pulse oximeter saturation in infants born less than 28 weeks' gestation: the AVIOx study. Pediatrics. (2006) 118:1574–82. doi: 10.1542/peds.2005-0413
12. Martin RJ, Wang K, Köroglu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. (2011) 100:303–10. doi: 10.1159/000329922
13. Beddis IR, Collins P, Levy NM, Godfrey S, Silverman M. New technique for servo-control of arterial oxygen tension in preterm infants. Arch Dis Child. (1979) 54:278–80. doi: 10.1136/adc.54.4.278
14. Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. (2010) 362:1959–69. doi: 10.1056/NEJMoa0911781
15. Darlow BA, Marschner SL, Donoghoe M, Battin MR, Broadbent RS, Elder MJ, et al. Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. J Pediatr. (2014) 165:30–5.e2. doi: 10.1016/j.jpeds.2014.01.017
16. Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. (2013) 309:2111–20. doi: 10.1001/jama.2013.5555
17. Tarnow-Mordi W, Stenson B, Kirby A, Juszczak E, Donoghoe M, Deshpande S, et al. Outcomes of two trials of oxygen-saturation targets in preterm infants. N Engl J Med. (2016) 374:749–60. doi: 10.1056/NEJMoa1514212
18. Vaucher YE, Peralta-Carcelen M, Finer NN, Carlo WA, Gantz MG, Walsh MC, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. (2012) 367:2495–504. doi: 10.1056/NEJMoa1208506
19. Manja V, Lakshminrusimha S, Cook DJ. Oxygen saturation target range for extremely preterm infants: a systematic review and meta-analysis. JAMA Pediatr. (2015) 169:332–40. doi: 10.1001/jamapediatrics.2014.3307
20. Manja V, Saugstad OD, Lakshminrusimha S. Oxygen saturation targets in preterm infants and outcomes at 18-24 months: a systematic review. Pediatrics. (2017) 139:e20161609. doi: 10.1542/peds.2016-1609
21. Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. (2014) 105:55–63. doi: 10.1159/000356561
22. Stenson BJ. Achieved oxygenation saturations and outcome in extremely preterm infants. Clin Perinatol. (2019) 46:601–10. doi: 10.1016/j.clp.2019.05.011
23. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of respiratory distress syndrome - 2016 update. Neonatology. (2017) 111:107–25. doi: 10.1159/000448985
24. Gantz MG, Carlo WA, Finer NN, Rich W, Faix RG, Yoder BA, et al. Achieved oxygen saturations and retinopathy of prematurity in extreme preterms. Arch Dis Child Fetal Neonat Ed. (2020) 105:138–44. doi: 10.1136/archdischild-2018-316464
25. Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W. NeOProM: neonatal oxygenation prospective meta-analysis collaboration study protocol. BMC Pediatr. (2011) 11:6. doi: 10.1186/1471-2431-11-6
26. Schmidt B, Whyte RK, Roberts RS. Trade-off between lower or higher oxygen saturations for extremely preterm infants: the first benefits of oxygen saturation targeting (BOOST) II trial reports its primary outcome. J Pediatr. (2014) 165:6–8. doi: 10.1016/j.jpeds.2014.03.004
27. Salverda HH, Cramer SJE, Witlox R, Dargaville PA, Te Pas AB. Automated oxygen control in preterm infants, how does it work and what to expect: a narrative review. Arch Dis Child Fetal Neonatal Ed. (2021) 106:215–21. doi: 10.1136/archdischild-2020-318918
28. Morozoff EP, Smyth JA. Evaluation of three automatic oxygen therapy control algorithms on ventilated low birth weight neonates. Annu Int. (2009) 2009:3079–82. doi: 10.1109/IEMBS.2009.5332532
29. Claure N, Gerhardt T, Everett R, Musante G, Herrera C, Bancalari E. Closed-loop controlled inspired oxygen concentration for mechanically ventilated very low birth weight infants with frequent episodes of hypoxemia. Pediatrics. (2001) 107:1120–4. doi: 10.1542/peds.107.5.1120
30. Claure N, D'Ugard C, Bancalari E. Automated adjustment of inspired oxygen in preterm infants with frequent fluctuations in oxygenation: a pilot clinical trial. J Pediatr. (2009) 155:640–5.e1-2. doi: 10.1016/j.jpeds.2009.04.057
31. Claure N, Bancalari E, D'Ugard C, Nelin L, Stein M, Ramanathan R, et al. Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics. (2011) 127:e76–83. doi: 10.1542/peds.2010-0939
32. Lal M, Tin W, Sinha S. Automated control of inspired oxygen in ventilated preterm infants: crossover physiological study. Acta Paediatr. (2015) 104:1084–9. doi: 10.1111/apa.13137
33. Morozoff E, Smyth JA, Saif M. Applying computer models to realize closed-loop neonatal oxygen therapy. Anesth Analg. (2017) 124:95–103. doi: 10.1213/ANE.0000000000001367
34. Sturrock S, Ambulkar H, Williams EE, Sweeney S, Bednarczuk NF, Dassios T, et al. A randomised crossover trial of closed loop automated oxygen control in preterm, ventilated infants. Acta Paediatr. (2021) 110:833–7. doi: 10.1111/apa.15585
35. Hallenberger A, Poets CF, Horn W, Seyfang A, Urschitz MS. Closed-loop automatic oxygen control (CLAC) in preterm infants: a randomized controlled trial. Pediatrics. (2014) 133:e379–85. doi: 10.1542/peds.2013-1834
36. van Kaam AH, Hummler HD, Wilinska M, Swietlinski J, Lal MK, te Pas AB, et sal. Automated versus manual oxygen control with different saturation targets and modes of respiratory support in preterm infants. J Pediatr. (2015) 167:545–50.e1-2. doi: 10.1016/j.jpeds.2015.06.012
37. Waitz M, Schmid MB, Fuchs H, Mendler MR, Dreyhaupt J, Hummler HD. Effects of automated adjustment of the inspired oxygen on fluctuations of arterial and regional cerebral tissue oxygenation in preterm infants with frequent desaturations. J Pediatr. (2015) 166:240–4.e1. doi: 10.1016/j.jpeds.2014.10.007
38. Gajdos M, Waitz M, Mendler MR, Braun W, Hummler H. Effects of a new device for automated closed loop control of inspired oxygen concentration on fluctuations of arterial and different regional organ tissue oxygen saturations in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F360–5. doi: 10.1136/archdischild-2018-314769
39. Schwarz CE, Kidszun A, Bieder NS, Franz AR, König J, Mildenberger E, et al. Is faster better? A randomised crossover study comparing algorithms for closed-loop automatic oxygen control. Arch Dis Child Fetal Neonat Ed. (2020) 105:369–74. doi: 10.1136/archdischild-2019-317029
40. Urschitz MS, Horn W, Seyfang A, Hallenberger A, Herberts T, Miksch S, et al. Automatic control of the inspired oxygen fraction in preterm infants: a randomized crossover trial. Am J Respir Crit Care Med. (2004) 170:1095–100. doi: 10.1164/rccm.200407-929OC
41. Plottier GK, Wheeler KI, Ali SK, Fathabadi OS, Jayakar R, Gale TJ, et al. Clinical evaluation of a novel adaptive algorithm for automated control of oxygen therapy in preterm infants on non-invasive respiratory support. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F37–43. doi: 10.1136/archdischild-2016-310647
42. Dargaville PA, Marshall AP, Ladlow OJ, Bannink C, Jayakar R, Eastwood-Sutherland C, et al. Automated control of oxygen titration in preterm infants on non-invasive respiratory support. Arch Dis Child Fetal Neonatal Ed. (2022) 107:39–44. doi: 10.1136/archdischild-2020-321538
43. Zapata J, Gómez JJ, Araque Campo R, Matiz Rubio A, Sola A. A randomised controlled trial of an automated oxygen delivery algorithm for preterm neonates receiving supplemental oxygen without mechanical ventilation. Acta Paediatr. (2014) 103:928–33. doi: 10.1111/apa.12684
44. Reynolds PR, Miller TL, Volakis LI, Holland N, Dungan GC, Roehr CC, et al. Randomised cross-over study of automated oxygen control for preterm infants receiving nasal high flow. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F366–71. doi: 10.1136/archdischild-2018-315342
45. Dijkman KP, Mohns T, Dieleman JP, van Pul C, Goos TG, Reiss IK, et al. Predictive intelligent control of oxygenation (PRICO) in preterm infants on high flow nasal cannula support: a randomised cross-over study. Arch Dis Child Fetal Neonatal Ed. (2021) 106:621–6. doi: 10.1136/archdischild-2020-320728
46. Abdo M, Hanbal A, Asla MM, Ishqair A, Alfar M, Elnaiem W, et al. Automated versus manual oxygen control in preterm infants receiving respiratory support: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. (2021) 1−8. doi: 10.1080/14767058.2021.1904875
47. Salverda HH, Cramer SJE, Witlox R, Gale TJ, Dargaville PA, Pauws SC, et al. Comparison of two devices for automated oxygen control in preterm infants: a randomised crossover trial. Arch Dis Child Fetal Neonat Ed. (2022) 107:20–5. doi: 10.1136/archdischild-2020-321387
48. Maiwald CA, Niemarkt HJ, Poets CF, Urschitz MS, König J, Hummler H, et al. Effects of closed-loop automatic control of the inspiratory fraction of oxygen (FiO(2)-C) on outcome of extremely preterm infants - study protocol of a randomized controlled parallel group multicenter trial for safety and efficacy. BMC Pediatr. (2019) 19:363. doi: 10.1186/s12887-019-1735-9
49. Dani C, Pratesi S, Luzzati M, Petrolini C, Montano S, Remaschi G, et al. Cerebral and splanchnic oxygenation during automated control of inspired oxygen [FiO(2)] in preterm infants. Pediatr Pulmonol. (2021) 56:2067–72. doi: 10.1002/ppul.25379
50. Li T, Matsushima M, Timpson W, Young S, Miedema D, Gupta M, et al. Epidemiology of patient monitoring alarms in the neonatal intensive care unit. J Perinatol. (2018) 38:1030–8. doi: 10.1038/s41372-018-0095-x
51. Warakomska M, Bachman TE, Wilinska M. Evaluation of two SpO(2) alarm strategies during automated FiO(2) control in the NICU: a randomized crossover study. BMC Pediatr. (2019) 19:142. doi: 10.1186/s12887-019-1496-5
52. Van Zanten HA, Kuypers K, Stenson BJ, Bachman TE, Pauws SC, Te Pas AB. The effect of implementing an automated oxygen control on oxygen saturation in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F395–f9. doi: 10.1136/archdischild-2016-312172
53. Salverda HH, Oldenburger NJ, Rijken M, Pauws SC, Dargaville PA, Te Pas AB. The effect of automated oxygen control on clinical outcomes in preterm infants: a pre- and post-implementation cohort study. Eur J Pediatr. (2021) 180:2107–13. doi: 10.1007/s00431-021-03982-8
54. Kaltsogianni O, Dassios T, Belbal R, Greenough A. Survey of closed-loop automated oxygen control systems in neonatal intensive care units. Acta Paediatr. (2022) 111:1002–3. doi: 10.1111/apa.16239
55. Sturrock S, Williams E, Dassios T, Greenough A. Closed loop automated oxygen control in neonates-A review. Acta Paediatr. (2020) 109:914–22.
56. Cummings JJ Polin RA Committee Committee on Fetus and Newborn. Oxygen targeting in extremely low birth weight infants. Pediatrics. (2016) 138:e20161576. doi: 10.1542/peds.2016-1576
57. Di Fiore JM, Martin RJ, Li H, Morris N, Carlo WA, Finer N, et al. Patterns of oxygenation, mortality, and growth status in the surfactant positive pressure and oxygen trial cohort. J Pediatr. (2017) 186:49–56.e1. doi: 10.1016/j.jpeds.2017.01.057
58. Schmidt B, Whyte RK. Oxygen saturation target ranges and alarm settings in the NICU: what have we learnt from the neonatal oxygenation prospective meta-analysis (NeOProM)? Semin Fetal Neonatal Med. (2020) 25:101080. doi: 10.1016/j.siny.2020.101080
59. Pemberton C, Howarth C. Resuscitation Council UK: review of updated 2021 neonatal life support guideline. Arch Dis Child Educ Pract Ed. (2022). doi: 10.1136/archdischild-2021-323277. [Epub ahead of print].
60. Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. (2020) 142(16_suppl_2):S524–50. doi: 10.1161/CIR.0000000000000902
Keywords: automated oxygen, hyperoxemia, hypoxemia, oxygen saturation, preterm
Citation: Nair V, Loganathan P, Lal MK and Bachman T (2022) Automated Oxygen Delivery in Neonatal Intensive Care. Front. Pediatr. 10:915312. doi: 10.3389/fped.2022.915312
Received: 07 April 2022; Accepted: 20 May 2022;
Published: 22 June 2022.
Edited by:
Charles Christoph Roehr, University of Oxford, United KingdomReviewed by:
Tomasz Szczapa, Poznan University of Medical Sciences, PolandJanneke Dekker, Leiden University, Netherlands
Copyright © 2022 Nair, Loganathan, Lal and Bachman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mithilesh Kumar Lal, bS5sYWwmI3gwMDA0MDtuaHMubmV0
 Vrinda Nair
Vrinda Nair Prakash Loganathan1
Prakash Loganathan1 Mithilesh Kumar Lal
Mithilesh Kumar Lal Thomas Bachman
Thomas Bachman