- Department of Pediatric Surgery, Charité - Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
One of the most common clinical pictures has become the focus of attention during the COVID-19 pandemic: acute appendicitis with the associated diagnostics and therapy. The aim of the work is to show inconsistencies with regard to epidemiology, pathophysiology and therapy against the background of the pandemic with special attention to the conditions for children and to explain the pathophysiological processes that are likely to underlie the disease based on scientifically plausible models.
Introduction
The Covid-19 pandemic has often been referred to metaphorically as a magnifying glass for various social, political and medical problems and peculiarities. It is not surprising that one of the most common clinical pictures has also become the focus of attention during the pandemic. However, the history of appendicitis is primarily a history of perception. Compared to pre-pandemic times, three fundamental aspects have come to the fore: pathophysiology, epidemiology and clinical aspects (diagnostics and therapy). With regard to all these aspects, various connections with pandemic conditions have been seen or at least claimed. In the current situation it is interesting that these three aspects are reflected in a condensed manner, allowing for a summary discussion of the same.
The epidemiology of acute appendicitis under pandemic conditions
One of the first and most profound observations under pandemic conditions regarding acute appendicitis was a substantial change of the frequencies of affected patients. In one of the first works published on the subject in the title it was asked: Where did the patients go (1)? This was followed by the question in another publication: Where did all the appendicitis go (2)? The central observation in these published studies was that of a general decrease of the incidence of acute appendicitis during the pandemic compared with pre-pandemic times. This observation was repeatedly made in other studies (3–6). However, the central message in other studies was different: there it was particularly found that the rates of clinically complicated forms of the disease had increased during the pandemic. These results were primarily attributed to a delay in diagnostics and treatment of the patients, affecting adults and children (7–11). Thus, there is a clear difference between the statements of these studies. Even if all this work seems to provide new insights against the background of the pandemic, it reflects an old discussion that has not yet found a conclusion.
The central observation is that the total number of patients with acute appendicitis decreased irrespective of age group (1–6). As the number of patients with appendicitis in the particular years varies over the years – especially in bigger cities with more than one hospital -, information on total numbers is not easy to get: it is either necessary to collect data within a multicenter approach (1, 2, 5, 6) or to use huge central data bancs e.g., from health insurances (4). The second observation regarding the perception of an increase of patients with complicated appendicitis is based on proportions and not on total numbers. First, it has to be mentioned that particularly definitions are a crucial point here. In most of the studies on appendicitis definitions of complicated appendicitis seem to be similar, but in fact differ. Criteria in the particular studies included CT findings of phlegmon or abscess, intraoperative findings of perforation or abscess (1), rates of open appendectomy, percutaneous drainage, shock, mortality, hospital length of stay (2) or gangrene, perforation or peri-appendicular abscess (6), and others referred to the general surgical Clavien-Dindo classification (5). The most widely used definition of complicated appendicitis is given by the appearance of appendiceal perforation: a macroscopically visible transmural defect of the appendix wall. That is understandable: appendiceal perforation has been associated with a worse outcome and a mortality rate of up to 5% (12). This definition does most probably not resemble the most reasonable differentiation criterion on a pathophysiological level – as will be demonstrated later in this article -, but at least it does not leave room for interpretation. One analysis revealed an increase in pediatric perforated appendicitis within the COVID-19 outbreak: compared with a historic baseline cohort patients a significant relative increase of perforations was found (37.6% vs. 22%) (8). The paper has to be interpreted against the background of the observation of a general decrease of uncomplicated cases, which has been made particularly in multi-center studies (1, 2, 5, 6), and leads back to an observation that was already made a few decades ago. In a very broad study including more than 56,000 patients Andersson has demonstrated that the incidence per 100,000 inhabitants of non-perforating appendicitis was strongly associated with age, the analyzed time period, diagnostic accuracy and the negative appendectomy rate, while the incidence of perforation appendicitis was stable without any of those associations (13). This led to the conclusion that a huge proportion of acute appendiceal inflammations resolves by itself. The observation was later qualified as “disconnect between incidence of nonperforated and perforated appendicitis” in another comprehensive study (14).
Compared to most other studies, the two latter are distinguished by two characteristics: first, they include a huge number of patients, which, second, allows the reliable analysis on the basis of absolute numbers, in contrast to relative numbers, which represent a quotient of two variables both influencing independently the result: according to Andersson, it can only be the number of uncomplicated appendicitis that leads to different results, since the absolute number of perforating appendicitis - within certain limits - remains stable. This finding is supported by a multicenter study with data collected during a 10-week interval within the COVID-19 lockdown 2020 in Germany (15). Data where compared with those from the same period in the same hospitals in 2019. The analysis of 1,915 appendectomies from 41 surgical departments revealed that the rate of complicated inflammation increased while the absolute number even slightly decreased. Morbidity and mortality were not affected. This observation of decreased incidence of uncomplicated appendicitis without an accompanying increase in complicated disease during the COVID-19 pandemic has also been made by others and led to the familiar conclusion that particularly spontaneous resolution of uncomplicated might have been responsible (1, 3, 16).
But how can this observation be explained on a pathophysiological level? Are there really two independent entities or is there a link between complicated and uncomplicated appendicitis? Might COVID-19 itself influence the course of the disease?.
In the following all these issues will be addressed based on the most conclusive information and studies. The resulting construct does not claim to reflect the absolute truth but represents a hypothesis that is able to resolve some contradictions against the background of a well-founded chain of causality.
The physiological function of the vermiform appendix
First fundamental theories on the formation of acute appendicitis have been naturally been driven by, again, the perception of the course of the disease, which can take on dramatic proportions. And it is these dramatic trajectories that understandably have driven theories of the disease's pathophysiology. It is no coincidence that the first theories on the emergence and course of acute appendicitis and even treatment recommendations came from pathologists (17). Aware of the course of the disease, which can lead to death, a pattern of explanation emerges from the microscopic comparison of differently inflamed appendices: that of tissue inflammation that begins mildly and then necessarily ends in perforation, which causes severe disease. The recommendation: emergency appendectomy.
However, when considering the pathophysiology, it is worth taking a step back to the physiological function of the vermiform appendix. The normal function of the appendix is not easy to prove, but there are some compelling indications. The first question which has to be addressed is if the vermiform appendix fulfills any relevant function at all. A study including 57,261 children who had been undergoing appendectomy revealed that children who received appendectomy had a 2.38 times higher risk to develop sepsis (18). This finding fits into a fundamental theory of the physiological function of the appendix: the “safe house” theory. According to this theory, the appendix serves as a refuge area for the bacterial local flora of the intestine, through which the intestine can be repopulated after viral gastrointestinal infections (19, 20). Indeed, the vermiform is histopathologically distinguished from other parts of the colon by particular accumulations of lymphatic tissue. Interestingly, these accumulations consist of certain T- and B-cell populations, which is very well compatible with theories on antiviral functions (21). And even further: these cell populations can already be found in the fetal stage of development, which shows that the accumulation of these cell types is not the result of infection but is most probably constitutive and provides the prerequisite for the physiological function of appendix. All this information taken together might lead to the conclusion that the persistence of the commensal bacterial flora of the gut is widely dependent on the antiviral function of the appendix. Disturbance of this balance e.g., by surgical appendectomy might lead to an increased risk for non-physiological bacterial overgrowth and might explain the increased risk for the development of sepsis. Thus, the answer seems to be: yes, the appendix has a function.
The inflammation of the vermiform appendix
The emergence of acute appendicitis has been attributed to most different reasons like obstruction – particularly by fecaliths – (22), infectious agents (23), hygienic factors (24), dietary aspects (25), ischemia (26), traumatic causes (27), genetic factors (28) and allergy (29). However, the pathophysiology of acute appendicitis can most probably be explained against the background of the physiological function of this part of the bowel: a constitutively antiviral function. Indeed, there is evidence pointing at viral infections as possible cause for the development of symptomatic appendicitis (23, 30, 31).
This evidence leads back to the epidemiological observation of inflammatory entities presenting with independent courses with different consequences for affected patients (13, 14). The challenge is to provide pathophysiological explanatory patterns for these observations. In an attempt to provide pathophysiological footing for their own epidemiological study results, the group around Roland Andersson performed immunological research in patients with acute appendicitis. The central finding of a comprehensive cytokine-based investigation was that patients with histopathologically gangrenous appendicitis where characterized by a substantial increase of highly inflammatory markers which are particularly associated with Th1 and Th17 cells (32, 33). IL-17 leads to rapid recruitment of neutrophils to sites of infection by promoting epithelial, endothelial, and stromal cells, each of which produce activating cytokines and chemokines. The relevance of the IL-17 immune response in patients is also reflected in other observations in patients with appendiceal inflammation (34). It has been demonstrated that IL-17 is particularly rapidly induced in infection with Escherichia coli, which represents the most commonly detected bacterial finding in acute complicated appendicitis (31). Appendectomy at a young age protects against inflammatory bowel disease, which has been attributed to suppression of the IL-23/Th17 pathway (35). Thus, some clinically relevant connections have been previously suggested.
The crucial point in the studies which had been performed by Andersson and Rubér was that the relevant discrimination criterion was not perforation but transmural necrosis within gangrenous appendicitis with the risk for bacterial transmigration and progression to perforation (32, 33). Indeed, gangrenous appendicitis is clinically associated with significant complication rates as abscess formation, wound infection and bowel dysmotility compared with non-necrotic phlegmonous appendicitis (26, 36, 37). The mortality rate is 6 times as high (12). Analysis of differential blood counts in patients with gangrenous appendicitis showed a time stable increase of neutrophils, which are highly associated with Th17 and Interleukin-(IL-)17 dependent pathways (38). Furthermore, inflammatory course in patients with gangrenous appendicitis was characterized by a steady increase and then stabilization of c-reactive protein – in contrast to phlegmonous appendicitis with a moderate increase and then spontaneous resolution (38, 39). The histopathological features of phlegmonous and gangrenous appendicitis are demonstrated in Figure 1.
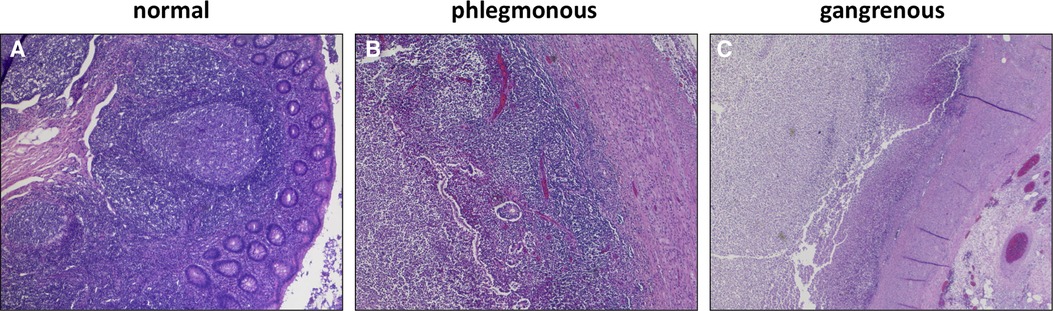
Figure 1. Histopathological features of the vermiform appendix (40): (A) Normal lymphofollicular structures of uninflamed appendix. (B) transmural granulocyte infiltration and oedema in phlegmonous appendicitis. (C) transmural necrosis and fibrinous-purulent inflammation in gangrenous appendicitis.
Research has so far made no substantial progress with regard to the pathophysiological explanation of acute appendicitis. Next to the disagreement regarding the relevant differentiation criteria, the investigation methods were previously defined by assumptions and correspondingly limited methodological approaches. Particularly, the RNA based genome-wide gene expression analysis enables a comprehensive consideration of basic biological processes with good temporal resolution by quantifying almost all transcriptional processes of protein biosynthesis (41). Hopeful diagnostic approaches have emerged with the use of the method, e.g., for the prediction of perioperative sepsis (42).
The method has also given interesting insights into the differential pathophysiology of histopathologically phlegmonous and gangrenous appendicitis in children. In a recent study genome-wide gene expression analysis was followed by specific immunological pathway analysis (40). The difference to previous gene expression analyses in patients with acute appendicitis was the differential investigation of necrotizing gangrenous and non-necrotizing phlegmonous appendicitis, which was assumed to be the essential difference (43, 44). Gene expression in patients with gangrenous appendicitis was particularly characterized by increase of gene expression associated with neutrophil and monocyte function. The role of the previously relevantly shown Th17-associated mechanisms in gangrenous appendicitis was particularly demarcated by the highly differential regulation of cytokines and transcription factors of the IL-23/IL-17 pathway: IL-17A, IL-23A, IL-23R, IL-27 and SOCS3 (40, 45).
Phlegmonous appendicitis was accompanied by an expression pattern suggesting a specific immunological synapse, which is essential for induction of an antiviral humoral response: the overexpression of mRNA of proteins of the T cell-CD3 complex and of co-stimulatory CD40l and CD2 (40). CD40l has independently been shown to be a predictor of acute appendicitis (46). The expression patterns of T cell receptor subunits are of particular importance. 33 T cell receptor subunits (alpha and beta subtypes) are exclusively significantly overexpressed in patients with phlegmonous appendicitis, representing 11% of the 100 top differentially expressed genes alone (40). Next to the observation of the virus associated immunological synapse this finding supports the theory of virus induced phlegmonous appendicitis, as the modulation of T cell receptor signaling and expression is a specific viral function (47). Figure 2 demonstrates the significantly overexpressed genes related to surface proteins and transcription factors associated with neutrophilic granulocytes and monocytes in patients with gangrenous appendicitis and show significantly overexpressed genes associated with B and T cells in patients with phlegmonous appendicitis after immunological pathway analysis as heat map (40). Figures 3, 4 show the corresponding proteins in their physiological context.
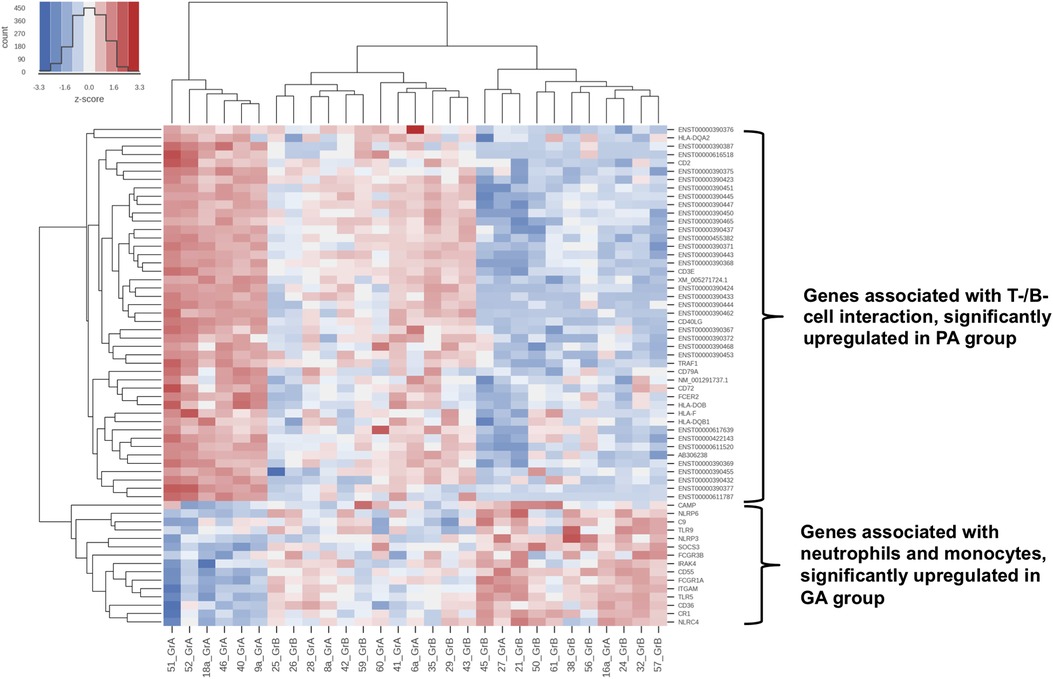
Figure 2. Heat map: cluster formation of samples with similar immunological patterns within genome wide gene expression analysis with RNA-microarray (40). Differentially expressed pathways according to the Generally Applicable Gene-set Enrichment (GAGE) method by Luo et al. using the “gage” package in R (48).: Toll and lmg signaling pathway, Antigen processing and presentation, NOD-like receptor signaling pathway, Hematopoietic cell lineage, Natural killer cell-mediated cytotoxicity, TNF signaling pathway and Intestinal immune network for IgA production, Complement and coagulation pathways, RIG-I-like receptor signaling pathway, Cytosolic DNA-sensing pathway, C-type lectin receptor signaling pathway, T-cell receptor signaling pathway, Th1 and Th2 cell differentiation, Th17 cell differentiation, IL-17 signaling pathway, Fc epsilon RI signaling pathway, Fc gamma R-mediated phagocytosis and Chemokine signaling pathway. (A) samples from patients with phlegmonous appendicitis (PA); (B) samples from patients with gangrenous appendicitis (GA); red colour: signal above mean signal, blue colour: below mean signal.
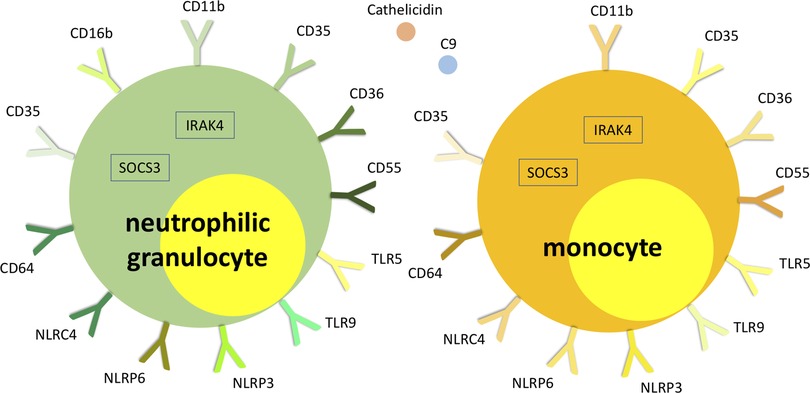
Figure 3. Visualization of significantly overexpressed genes related to neutrophilic granulocyte and monocyte surface proteins and transcription factors in patients with gangrenous appendicitis (40).
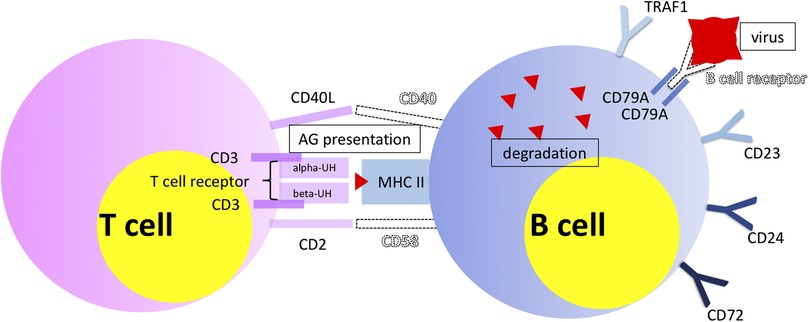
Figure 4. Visualization of significantly overexpressed genes associated with B and T cells, demonstrating particularly their interaction (40). Objects shown dashed and virus symbol: added for demonstration purposes.
It has been proposed to establish gene expression based signatures for diagnostic purposes based on the smallest possible set of genes with the best possible diagnostic or predictive performance (41). By using modern methods of artificial intelligence and machine learning it was possible to define a gene expression based biomarker signature consisting of 4 genes best performing for differentiation of phlegmonous and gangrenous appendicitis in children within an experimental approach (49).
Thus, substantial evidence qualifies necrotic gangrenous appendicitis as a bacterial and non-necrotic phlegmonous appendicitis as viral disease – the latter particularly against the background of its probable originate function as antiviral unit.
The difficult question remains whether there is an association between uncomplicated and complicated appendicitis. The above suggests two completely independent entities. There is the frequent perception that obstruction of the appendix lumen might be a leading course of acute appendicitis (26). In fact obstruction appears in only a minority of patients with acute appendicitis patients and especially fecaliths are regular findings in normal appendices without inflammation (26, 50). On the other hand, particularly perforated appendicitis has been associated with the presence of fecaliths (51), while in a recent analysis on the discriminatory capacity of ultrasound the presence of a fecalith showed a low specificity for presence of gangrenous appendicitis (52). However, as one study encounters fecaliths as incidental findings in one third of autopsies (53), it is theoretically conceivable that the constant incidence of the presence of fecaliths justifies a constant incidence of complicated appendicitis on the basis of a significantly larger number of phlegmonous appendicitis.
Are there clinical implications for treatment of acute appendicitis in children within the COVID-19 pandemic?
Although a pathophysiological correlation of acute appendicitis with Covid-19 associated pediatric pathologies like Kawasaki Disease and Pediatric Multisystem Inflammatory Syndrome has been discussed in single case reports (54–56), a causal relationship between those two is not likely – especially due to the fact that conditions of complicated appendicitis remained most probably stable and the incidence of uncomplicated inflammation decreased (1–5). Possibly there might be even a negative correlation, as there was a particular decrease of viral infections during the pandemic, which might have influenced the incidence of acute appendicitis in general and specifically of phlegmonous inflammation (57). This hypothesis is supported by the decrease of hospital admissions of children with (other) virally triggered conditions like asthma (58).
A central clinical aspect which had been addressed frequently during the pandemic is a possible delay of diagnosis and related deterioration of the affected patientś clinical conditions (9, 10). Especially children have been reported to be affected (8, 11). Again, these results can be looked at against the background of pre-existing studies on the influence of a delay of diagnosis and treatment of acute appendicitis. Existing evidence contradicts the thesis that the timing of intervention has a relevant influence on the outcome or length of stay irrespective of age group, at least if the delay does not exceed 24 h (59–61). In accordance with these results several studies could not confirm the observation of an average late presentation of patients with acute appendicitis within the COVID-19 pandemic. Even if delayed treatment was found, the treatment results were not affected compared to pre-pandemic times (2, 4, 5, 7, 62).
In summary, especially against the background of existing evidence it can be said that the COVID-19 pandemic has most likely no causal relationships with acute appendicitis in children and adults that go beyond the described. However, current changes in epidemiological conditions within the pandemic allow for a discussion about basic conditions and prerequisites of the disease. It remains to be seen whether the insights gained during the pandemic will actually have an impact on future therapeutic modalities, especially with regard to conservative treatment strategies (16, 63).
Author contributions
MR conceived, wrote the manuscript, contributed to the article, and approved the submitted version.
Conflict of interest
The author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Neufeld MY, Bauerle W, Eriksson E, Azar FK, Evans HL, Johnson M, et al. Where did the patients go? Changes in acute appendicitis presentation and severity of illness during the coronavirus disease 2019 pandemic: a retrospective cohort study. Surgery. (2021) 169(4):808–15. doi: 10.1016/j.surg.2020.10.035
2. Rosenthal MG, Fakhry SM, Morse JL, Wyse RJ, Garland JM, Duane TM, et al. Where did all the appendicitis go? Impact of the COVID-19 pandemic on volume, management, and outcomes of acute appendicitis in a nationwide, multicenter analysis. Annals of Surgery Open. (2021) 2(1):e048. doi: 10.1097/AS9.0000000000000048
3. Scheijmans JCG, Borgstein ABJ, Puylaert CAJ, Bom WJ, Bachiri S, van Bodegraven EA, et al. Impact of the COVID-19 pandemic on incidence and severity of acute appendicitis: a comparison between 2019 and 2020. BMC Emerg Med. (2021) 21(1):61. doi: 10.1186/s12873-021-00454-y
4. Köhler F, Acar L, van den Berg A, Flemming S, Kastner C, Müller S, et al. Impact of the COVID-19 pandemic on appendicitis treatment in Germany—a population-based analysis. Langenbeck's Arch Surg. (2021) 406(2):377–83. doi: 10.1007/s00423-021-02081-4
5. Ceresoli M, Coccolini F, Magnone S, Lucianetti A, Bisagni P, Armao T, et al. The decrease of non-complicated acute appendicitis and the negative appendectomy rate during pandemic. Eur J Trauma Emerg Surg. (2021) 47(5):1359–65. doi: 10.1007/s00068-021-01663-7
6. Tankel J, Keinan A, Blich O, Koussa M, Helou B, Shay S, et al. The decreasing incidence of acute appendicitis during COVID-19: a retrospective multi-centre study. World J Surg. (2020) 44(8):2458–63. doi: 10.1007/s00268-020-05599-8
7. Sheath C, Abdelrahman M, MacCormick A, Chan D. Paediatric appendicitis during the COVID-19 pandemic. J Paediatr Child Health. (2021) 57(7):986–9. doi: 10.1111/jpc.15359
8. Schäfer FM, Meyer J, Kellnar S, Warmbrunn J, Schuster T, Simon S, et al. Increased incidence of perforated appendicitis in children during COVID-19 pandemic in a bavaria multi-center study. Front Pediatr. (2021) 9:683607. doi: 10.3389/fped.2021.683607
9. Orthopoulos G, Santone E, Izzo F, Tirabassi M, Pérez-Caraballo AM, Corriveau N, et al. Increasing incidence of complicated appendicitis during COVID-19 pandemic. Am J Surg. (2021) 221(5):1056–60. doi: 10.1016/j.amjsurg.2020.09.026
10. Burgard M, Cherbanyk F, Nassiopoulos K, Malekzadeh S, Pugin F, Egger B. An effect of the COVID-19 pandemic: significantly more complicated appendicitis due to delayed presentation of patients!. PLoS One. (2021) 16(5):e0249171. doi: 10.1371/journal.pone.0249171
11. Gerall CD, DeFazio JR, Kahan AM, Fan W, Fallon EM, Middlesworth W, et al. Delayed presentation and sub-optimal outcomes of pediatric patients with acute appendicitis during the COVID-19 pandemic. J Pediatr Surg. (2021) 56(5):905–10. doi: 10.1016/j.jpedsurg.2020.10.008
12. Di Saverio S, Podda M, de Simone B, Ceresoli M, Augustin G, Gori A, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. (2020) 15(1):27. doi: 10.1186/s13017-020-00306-3
13. Andersson R, Hugander A, Thulin A, Nystrom PO, Olaison G. Indications for operation in suspected appendicitis and incidence of perforation. Br Med J. (1994) 308(6921):107–10. doi: 10.1136/bmj.308.6921.107
14. Livingston EH, Woodward WA, Sarosi GA, Haley RW. Disconnect between incidence of nonperforated and perforated appendicitis. Ann Surg. (2007) 245(6):886–92. doi: 10.1097/01.sla.0000256391.05233.aa
15. Willms AG, Oldhafer KJ, Conze S, Thasler WE, von Schassen C, Hauer T, et al. Appendicitis during the COVID-19 lockdown: results of a multicenter analysis in Germany. Langenbecks Arch Surg. (2021) 406(2):367–75. doi: 10.1007/s00423-021-02090-3
16. Köhler F, Müller S, Hendricks A, Kastner C, Reese L, Boerner K, et al. Changes in appendicitis treatment during the COVID-19 pandemic – A systematic review and meta-analysis. Int J Surg. (2021) 95:106148. doi: 10.1016/j.ijsu.2021.106148
17. Fitz RH. Perforating inflammation of the vermiform appendix: with special reference to its early diagnosis and treatment. Am J Sci. (1886) 92:321–102.
18. Liao T-H, Lin C-L, Lin C-H, Wu M-C, Wei J C-C. Children with appendectomy have increased risk of future sepsis: real-world data in Taiwan. Int J Clin Pract. (2021) 75(12):e14912. doi: 10.1111/ijcp.14912
19. Laurin M, Everett Ml, Parker W. The cecal appendix: one more immune component with a function disturbed by post-industrial culture. Anat Rec (Hoboken). (2011) 294(4):567–79. doi: 10.1002/ar.21357
20. Randal Bollinger R, Barbas AS, Bush EL, Lin SS, Parker W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol. (2007) 249(4):826–31. doi: 10.1016/j.jtbi.2007.08.032
21. Bhide SA, Wadekar KV, Koushik SA. Peyer's patches are precocious to the appendix in human development. Dev Immunol. (2001) 8(2):159–66. doi: 10.1155/2001/71685
23. Andersson R, Hugander A, Thulin A, Nyström PO, Olaison G. Clusters of acute appendicitis: further evidence for an infectious aetiology. Int J Epidemiol. (1995) 24(4):829–33. doi: 10.1093/ije/24.4.829
24. Barker DJ, Osmond C, Golding J, Wadsworth ME. Acute appendicitis and bathrooms in three samples of British children. Br Med J. (1988) 296(6627):956–8. doi: 10.1136/bmj.296.6627.956
25. Barker DJ, Morris J, Nelson M. Vegetable consumption and acute appendicitis in 59 areas in England and Wales. Br Med J. (1986) 292(6525):927–30. doi: 10.1136/bmj.292.6525.927
26. Carr NJ. The pathology of acute appendicitis. Ann Diagn Pathol. (2000) 4(1):46–58. doi: 10.1016/s1092-9134(00)90011-x
27. Serour F, Efrati Y, Shikar S, Weinberg M, Vinograd I. Acute appendicitis following abdominal trauma. Arch Surg. (1996) 131(7):785–6. doi: 10.1001/archsurg.1996.01430190107026
28. Hiraiwa H, Umemoto M, Take H. Prevalence of appendectomy in Japanese families. Acta Paediatr Jpn. (1995) 37(6):691–3. doi: 10.1111/j.1442-200x.1995.tb03405.x
29. Aravindan KP. Eosinophils in acute appendicitis: possible significance. Indian J Pathol Microbiol. (1997) 40(4):491–8.
30. Alder AC, Fomby TB, Woodward WA, Haley RW, Sarosi G, Livingston EH. Association of viral infection and appendicitis. Arch Surg. (2010) 145(1):63–71. doi: 10.1001/archsurg.2009.250
31. Richardsen I, Schöb DS, Ulmer TF, Steinau G, Neumann UP, Klink CD, et al. Etiology of appendicitis in children: the role of bacterial and viral pathogens. J Invest Surg. (2016) 29(2):74–9. doi: 10.3109/08941939.2015.1065300
32. Rubér M, Berg A, Ekerfelt C, Olaison G, Andersson RE. Different cytokine profiles in patients with a history of gangrenous or phlegmonous appendicitis. Clin Exp Immunol. (2006) 143(1):117–24. doi: 10.1111/j.1365-2249.2005.02957.x
33. Rubér M, Andersson M, Petersson BF, Olaison G, Andersson RE, Ekerfelt C. Systemic Th17-like cytokine pattern in gangrenous appendicitis but not in phlegmonous appendicitis. Surgery. (2010) 147(3):366–72. doi: 10.1016/j.surg.2009.09.039
34. Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1 + γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. (2007) 178(7):4466–72. doi: 10.4049/jimmunol.178.7.4466
35. Sarra M, Pallone F, MacDonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. (2010) 16(10):1808–13. doi: 10.1002/ibd.21248
36. Romano A, Parikh P, Byers P, Namias N. Simple acute appendicitis versus non-perforated gangrenous appendicitis: is there a difference in the rate of post-operative infectious complications? Surg Infect. (2014) 15(5):517–20. doi: 10.1089/sur.2013.106
37. Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT. Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet. (2015) 386(10000):1278–87. doi: 10.1016/S0140-6736(15)00275-5
38. Reismann J, Schädlich D, Minderjahn MI, Rothe K, Reismann M. Eosinophilia in pediatric uncomplicated appendicitis is a time stable pattern. Pediatr Surg Int. (2019) 35(3):335–40. doi: 10.1007/s00383-018-4423-1
39. Minderjahn MI, Schädlich D, Radtke J, Rothe K, Reismann M. Phlegmonous appendicitis in children is characterized by eosinophilia in white blood cell counts. World J Pediatr. (2018) 14(5):504–9. doi: 10.1007/s12519-018-0173-3
40. Kiss N, Minderjahn M, Reismann J, Svensson J, Wester T, Hauptmann K, et al. Use of gene expression profiling to identify candidate genes for pretherapeutic patient classification in acute appendicitis. BJS Open. (2021) 5(1):zraa045. doi: 10.1093/bjsopen/zraa045
41. Gliddon HD, Herberg JA, Levin M, Kaforou M. Genome-wide host RNA signatures of infectious diseases: discovery and clinical translation. Immunol. (2018) 153(2):171–8. doi: 10.1111/imm.12841
42. Hinrichs C, Kotsch K, Buchwald S, Habicher M, Saak N, Gerlach H, et al. Perioperative gene expression analysis for prediction of postoperative sepsis. Clin Chem. (2010) 56(4):613–22. doi: 10.1373/clinchem.2009.133876
43. Murphy CG, Glickman JN, Tomczak K, Wang YY, Beggs AH, Shannon MW, et al. Acute appendicitis is characterized by a uniform and highly selective pattern of inflammatory gene expression. Mucosal Immunol. (2008) 1(4):297–308. doi: 10.1038/mi.2008.13
44. Arlt A, Bharti R, Ilves I, Häsler R, Miettinen P, Paajanen H, et al. Characteristic changes in microbial community composition and expression of innate immune genes in acute appendicitis. Innate Immun. (2015) 21(1):30–41. doi: 10.1177/1753425913515033
45. McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23–IL-17 immune pathway. Trends Immunol. (2006) 27(1):17–23. doi: 10.1016/j.it.2005.10.003
46. Huang W-Y, Chen C-Y, Chang Y-J, Lee E-P, Wu H-P. Serum soluble CD40 ligand in predicting simple appendicitis and complicated appendicitis at different time points in children. Front Pediatr. (2021) 9:676370. doi: 10.3389/fped.2021.676370
47. Jerome KR. Viral modulation of T-cell receptor signaling. J Virol. (2008) 82(9):4194–204. doi: 10.1128/JVI.00059-08
48. Luo W, Friedman MS, Shedden K, Hankenson KD, Woolf PJ. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics. (2009) 10:161. doi: 10.1186/1471-2105-10-161
49. Reismann J, Kiss N, Reismann M. The application of artificial intelligence methods to gene expression data for differentiation of uncomplicated and complicated appendicitis in children and adolescents - a proof of concept study. BMC Pediatr. (2021) 21(1):268. doi: 10.1186/s12887-021-02735-8
50. Chang AR. An analysis of the pathology of 3003 appendices. ANZ J Surg. (1981) 51(2):169–78. doi: 10.1111/j.1445-2197.1981.tb05932.x
51. Miyauchi H, Okata Y, Hatakeyama T, Nakatani T, Nakai Y, Bitoh Y. Analysis of predictive factors for perforated appendicitis in children. Pediatr Int. (2020) 62(6):711–5. doi: 10.1111/ped.14148
52. Rawolle T, Reismann M, Minderjahn MI, Bassir C, Hauptmann K, Rothe K, et al. Sonographic differentiation of complicated from uncomplicated appendicitis. Br J Radiol. (2019) 92(1099):20190102. doi: 10.1259/bjr.20190102
53. Andreou P, Blain S, Du Boulay CE. A histopathological study of the appendix at autopsy and after surgical resection. Histopathol. (1990) 17(5):427–31. doi: 10.1111/j.1365-2559.1990.tb00763.x
54. Kuroda K, Stottlemyre M. Acute appendicitis associated with kawasaki disease: case report and review of the literature. Cureus. (2021) 13(10):e18997. doi: 10.7759/cureus.18997
55. Ahsanuddin S, Elfituri M, Diaz E, Volkin Y. A case of multisystem inflammatory syndrome in a pediatric patient with acute appendicitis. Cureus. (2021) 13(8):e17084. doi: 10.7759/cureus.17084
56. Anderson J, Bhisitkul D, Pham T, Wilson K, Barbera AR. Multisystem inflammatory syndrome presenting as early acute appendicitis. Cureus. (2021) 13(12):e20200. doi: 10.7759/cureus.20200
57. Wilder JL, Parsons CR, Growdon AS, Toomey SL, Mansbach JM. Pediatric hospitalizations during the COVID-19 pandemic. Pediatrics. (2020) 146(6):e202005983. doi: 10.1542/peds.2020-005983
58. Chavasse RJ. COVID-19: reduced asthma presentations in children. Br Med J. (2020) 370:m2806. doi: 10.1136/bmj.m2806
59. Clyde C, Bax T, Merg A, MacFarlane M, Lin P, Beyersdorf S, et al. Timing of intervention does not affect outcome in acute appendicitis in a large community practice. Am J Surg. (2008) 195(5):590–2. doi: 10.1016/j.amjsurg.2008.01.005
60. Gurien LA, Wyrick DL, Smith SD, Dassinger MS. Optimal timing of appendectomy in the pediatric population. J Surg Res. (2016) 202(1):126–31. doi: 10.1016/j.jss.2015.12.045
61. Almström M, Svensson JF, Patkova B, Svenningsson A, Wester T. In-hospital surgical delay does not increase the risk for perforated appendicitis in children. Ann Surg. (2017) 265(3):616–21. doi: 10.1097/SLA.0000000000001694
62. Turanli S, Kiziltan G. Did the COVID-19 pandemic cause a delay in the diagnosis of acute appendicitis? World J Surg. (2021) 45(1):18–22. doi: 10.1007/s00268-020-05825-3
Keywords: acute appendicitis, children, COVID-19, pathophysiology, clinical implications
Citation: Reismann M (2022) A concise pathophysiological model of acute appendicitis against the background of the COVID-19 pandemic. Front. Pediatr. 10:908524. doi: 10.3389/fped.2022.908524
Received: 30 March 2022; Accepted: 26 September 2022;
Published: 13 October 2022.
Edited by:
Janne Suominen, Hospital District of Helsinki and Uusimaa, FinlandReviewed by:
Ernesto Leva, University of Milan, ItalyKrystian Toczewski, Wroclaw Medical University, Poland
© 2022 Reismann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Reismann bWFyYy5yZWlzbWFubkBjaGFyaXRlLmRl
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 Marc Reismann
Marc Reismann