- 1Pediatric Palliative Care Service, Department of Pediatric Subspecialities, KK Women's and Children's Hospital, Singapore, Singapore
- 2Children's Intensive Care Unit, Department of Pediatric Subspecialties, KK Women's and Children's Hospital, Singapore, Singapore
Palliative care (PC) is an integral component of optimal critical care (CC) practice for pediatric patients facing life-threatening illness. PC acts as an additional resource for patients and families as they navigate through critical illness. Although PC encompasses end of life care, it is most effective when integrated early alongside disease-directed and curative therapies. PC primarily focuses on improving quality of life for patients and families by anticipating, preventing and treating suffering throughout the continuum of illness. This includes addressing symptom distress and facilitating communication. Effective communication is vital to elicit value-based goals of care, and to guide parents through patient-focused and potentially difficult decision-making process which includes advanced care planning. A multidisciplinary approach is most favorable when providing support to both patient and family, whether it is from the psychosocial, practical, emotional, spiritual or cultural aspects. PC also ensures coordination and continuity of care across different care settings. Support for family carries on after death with grief and bereavement support. This narrative review aims to appraise the current evidence of integration of PC into pediatric CC and its impact on patient- and family-centered outcomes. We will also summarize the impact of integration of good PC into pediatric CC, including effective communication with families, advanced care planning, withholding or withdrawal of life sustaining measures and bereavement support. Finally, we will provide a framework on how best to integrate PC in PICU. These findings will provide insights on how PC can improve the quality of care of a critically ill child.
Introduction
Palliative care (PC) is recognized to be an integral component of optimal critical care (CC) practice for children facing life-threatening illness (LTI) or life limiting illness (LLI). Although it encompasses end of life (EOL) care, PC is most effective when integrated early alongside disease-directed and curative therapies. Professional organizations such as World Health Organization and the American Academy of Pediatrics endorse early integration of PC in management of seriously ill children, regardless of whether the patient is receiving disease-directed therapy and their expected outcome (1, 2). The primary goal of PC is to enhance quality of life, reduce suffering, optimize function and support both patient and families.
Pediatric intensive care units (PICUs) care for children with serious illnesses, complex medical conditions and technology dependence. While overall PICU mortality is low and declining, up to 80% of all inpatient pediatric deaths occurs in the PICU setting, often preceded by withdrawal of life-sustaining therapy (3–5). Improved PICU survival rate has also resulted in more acquired morbidities and chronic complex conditions (CCC) in survivors, shifting the focus of CC from aggressive life-sustaining therapy to one that maintains comfort and preserves quality of life in this group of patients (6). Timely and optimal management of distressing symptoms is important to reduce patient's suffering. It is also imperative to address families' emotional, psychological and spiritual distress while they make difficult decisions for their critically ill child. For these reasons, early integration of PC with CC is recommended (7). Despite its established benefits, PC utilization for critically ill children remains low with considerable variability across institutions (8, 9). This highlights the need to standardize integration and utilization of PC into CC.
This narrative review aims to summarize current literature to describe different models for integrating PC into CC and its impact on patient and family-centered outcomes. We will highlight the skills in PC that is required in PICU and the range of needs which arise from these children and their families. Finally, we will provide a framework on how best to integrate PC in the CC setting.
Challenges of Pediatric Palliative Care
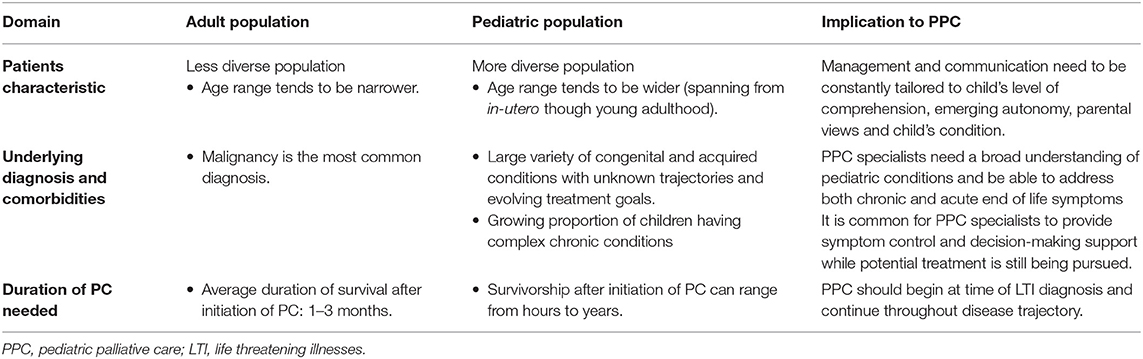
Pediatric palliative care (PPC) is a multidisciplinary clinical approach which delivers patient and family centered care to children with LLI or LTI to minimize suffering while maximizing quality of life (1, 10). Although PPC is a rapidly growing field, PC for adults is comparatively far more established. There are several fundamental differences between the pediatric and adult population which preclude the generalisability of adult PC on children. Table 1 summarizes the differences between adult and palliative population and its impact on PPC (6, 11–14).
The death of a child has been described as the most stressful of life events with significant implications (15). Parental grief after the loss of a child is more intense and prolonged compared to grief experienced by adults who has suffered the loss of a spouse or parent (16). Many studies reported that many bereaved parents suffer from long-standing mental health issues such as complicated grief, depression and post-traumatic stress disorder (PTSD) (17–20). Bereaved parents are also shown to have increased health risk for cancers, type 2 diabetes, myocardial infarction and acute illnesses (21–24). For these reasons, early PPC is advocated to provide additional support for both patient and families.
A growing body of literature describe direct benefits of PPC for patients, families and staff. Despite this, the adoption of PPC is still suboptimal. A prospective international multicenter study on PICU mortality showed that patients who died in PICU were less likely to have a DNR or PC consult compared to patients who died in another inpatient setting (25). Systemic integration of PPC into CC practice will likely improve this situation. This is demonstrated in a retrospective study in Taiwan where a standardized approach to EOL care resulted in increased willingness to accept withdrawal of life-sustaining interventions and lowered PICU care intensity, such as PICU utilization and use of catecholamines infusion in patients with the DNR status (26).
Impact of Palliative Care Interventions
Pain and Symptom Management
Pain, from the disease or interventions, is the most common symptom experienced by critically ill children in PICU (27, 28). Other commonly experienced symptoms are nausea, dyspnea and delirium. There are numerous barriers that may contribute to under-reporting of these symptoms. This includes communication difficulties by PICU patients due to severity of illness, unrecognized delirium, neurocognitive impairment and presence of invasive support such as endotracheal tube. Pain assessment in the pediatric population is also more challenging than in adults because patients of different age groups express pain differently. A wide range of pain rating scales for different age groups and verbal skills are readily available to achieve consistency of pain assessment (29). Initiatives to improve EOL care in PICU should include raising awareness of pain as a vital sign and standardizing guidelines for symptoms management.
The management of distressing EOL symptoms is of upmost importance in PC. Retained memories of unrelieved EOL symptoms have negative impacts on bereaved parents and siblings (30). A high index of suspicion and close monitoring assist with identification of symptoms, which can be quickly followed by aggressive interventions in collaboration with subspecialties like acute pain team and PC team. Early engagement of PC team positively impact patient and family-centered outcomes by facilitating better pain and EOL symptoms management. Alleviation of physical EOL symptoms also enables PC team to form a good rapport and better negotiate domains of psychological and spiritual care with the family and patient.
Effective Communication
Effective communication is an essential pillar of good pediatric CC. Parents of PICU patients are often overwhelmed with medical concepts and uncertainties and are required to make high-stake decisions for their child. Many PICU physicians and subspecialists function on a roster basis, making continuity of care challenging (7). In a qualitative study, many parents reported that the sheer number of physicians and the coordination of communication added on to their emotional burden and eroded their confidence as they needed to seek clarifications (31). PC specialists can act as a constant and strengthen the team's ability for effective communication in such instances.
The high stress environment of the PICU may predispose to conflicts between physician-family, among physicians and within family (32). Conflicts compromise quality of care and contributes to physicians' burnout (33). Commonly cited reasons for physician-family conflict are disagreement over care plans and poor communication (32, 34). Sources of conflict among physicians include disagreement in medical decisions such as pain management, lack of leadership and undervaluing each other's role in a multidisciplinary team, all of which fall under the umbrella of poor communication (35). Palliative specialists can help neutralize tension between all parties and redirect the focus toward advocating for the child's best interest.
End of life discussion in PICU is a delicate and challenging process for physicians, with uncertainty around prognosis of many pediatric conditions adding to the complexity of it. Even though communication is one of the core skills of PICU physicians, many are uncomfortable with EOL discussion and may delay these important conversations (36). This delay can result in missed opportunities for identification of emotional issues and negatively impair healing for the family (37). A cross-sectional study of family conferences held in the PICU of Children's National Hospital, United States reported that nearly three quarters of family conferences and 79% of physician speech was medically focused (38). This study also reported that a higher patient-centeredness score was associated with higher patient satisfaction (38). The family-centered model of PC helps forge a beneficial and supportive partnership between families and physicians.
Advanced Care Planning
Advanced Care Planning (ACP) aims to facilitate early planning of treatment goals, including EOL care, through professionally facilitated discussions with patients and families (39). Positive impacts of pediatric ACP include higher rating of EOL care in patients, decrease negative emotions in parents and enabling parents to be better informed and certain about their decisions (40, 41). A study on bereaved parents reported that all parents felt that ACP was important even though only 61% of the parents had finalized ACP prior to their child's death (42).
The answer to when, how and who to initiate ACP remains controversial. A study on clinical providers' attitudes on ACP identified unrealistic parent expectations, differences between clinical and patient/parent understanding of prognosis and lack of parent readiness as the top 3 barriers to ACP discussion (43). Despite having clarity of the barriers, 71% of the respondents believed that ACP happened too late in the patient's clinical course (43). Naturally, many physicians feel insecure about discussing ACP as they are worried about burdening families and destroying the therapeutic alliance with parents (44). PC specialists can step in to share this burden and ensure timely discussion of ACP in a sensitive manner. It is, however, important that ACP discussion should not be owned by a particular physician and should be shared by the entire medical care team.
Withdrawal, Withholding of Life Sustaining Therapy or Non-escalation
A framework by the Royal College of Pediatrics and Child Health, United Kingdom states that there are three sets of circumstances when withholding, withdrawal or non-escalation of life sustaining interventions (WWNLST) can be considered (i) when life is limited in quantity, (ii) when life is limited in quality, (iii) lack of ability to benefit (45). Transition in goals of care from curative to comfort should be made by clinical teams in partnership, and with the agreement of, the parents and patient. PPC can work together with PICU physicians to identify patients suitable for WWNLST and aid in timely open discussion with the family to achieve consensus. A large single center retrospective study in Spain reported that WWNLST was more frequently facilitated in suitable patients after the development of a PC unit (46).
The practice of WWNLST remains highly variable despite many published recommendations (47, 48). Poorly handled WWNLST can lead to confusion and distress for the patient, family and medical staff. The role of PC is to formulate a carefully thought-out plan, from planning to post withdrawal of care, to ensure a smooth process and an optimal experience for all involved stakeholders.
Compassionate extubation at home (CEAH) is a valuable service that PICU can offer and facilitate. The familiarity and comfort of home help families achieve a higher level of satisfaction and comfort with their child's EOL care (49). Medical staff involved in CEAH also reported it to be valuable despite its complex orchestration (50). Despite being resource intensive and logistically challenging, reports have reaffirmed the feasibility of CEAH in the pediatric population with positive outcomes (51, 52). A recently published framework detailing processes from preparation to follow through acts as a good reference for PICU intensivists in their provision of CEAH as an option of EOL care (53).
Bereavement Care Services
The death of a child can lead to long-term adverse effects on parental and siblings' physical and psychological health (54, 55). Data also suggest that bereaved parents have higher mortality rates (56). The goals of bereavement support are to facilitate healing and adjustment of bereaved parents after the death or their child so that they can continue to live normal and meaningful lives, and also to carry out early intervention for individuals at risk of negative bereavement reactions (57). Despite the established benefits, hospitals lack coordinated and standardized bereavement programs (58).
A systematic review identified five key components of pediatric bereavement: (i) acknowledgment of parenthood and child's life; (ii) establishing keepsakes, (iii) follow-up contact, (iv) education and information, and (v) remembrance activities (59). However, only four out of 12 studies reported interventions that commenced before the death of the child, inconsistent with bereavement theories of facilitating the transition of parents toward a new reality (59). A qualitative study conducted in the United States reported that five out of nine bereaved parents experienced feelings of abandonment by the medical team after the death of their child, with some parents verbalizing their wish for follow up meeting or support (57). These highlight the need for improvement and standardization of bereavement care.
An anecdotal report by a PPC physician on primary medical providers after redirection of patient's care plan toward comfort stated “sudden loss of power of prescription” and “assumption that bereavement should be delegated to other team members” as challenges primary providers faced (60). Hence, PC specialists can help to ensure that important components of pediatric bereavements are met and to empower primary physicians in providing bereavement care, reducing the risks of adverse effects associated with the death of a child.
Other Outcomes
A systematic review of adult controlled trials reported a reduction in relative risk of ICU admission and ICU length of stay by 37 and 26%, respectively, in patients who received PC interventions and ACP discussion (61). A pediatric retrospective study conducted at St.Jude Hospital, United States reported that children who received PC intervention were significantly less likely to die in PICU and to receive invasive treatment (62). These outcomes have important economic implications and reduce the financial burden of some families. Indeed, an adult cohort study demonstrated that patients who received PC incurred significantly lower costs as a result of reduced length of hospital stay and number of investigations performed compared to patients who received usual care (63).
Integrating Palliative Care Into PICU: Models of Care
The integration of PPC in PICU is largely extrapolated from adult models. The traditional models for PC-CC integration can be broadly classified into “integrative,” “consultative,” and “mixed” models (64). The integrative model embeds standardized PC principles and interventions into daily CC practice by the ICU team for all patients and families facing critical illness. The consultative model incorporates the involvement of specialist PC team on a needs basis, reserved for those at highest risk for poor outcomes. Successful implementation of this model includes the use of clinical triggers for expert PC consult. Mixed models would feature aspects of both integrative and consultative models (Figure 1).
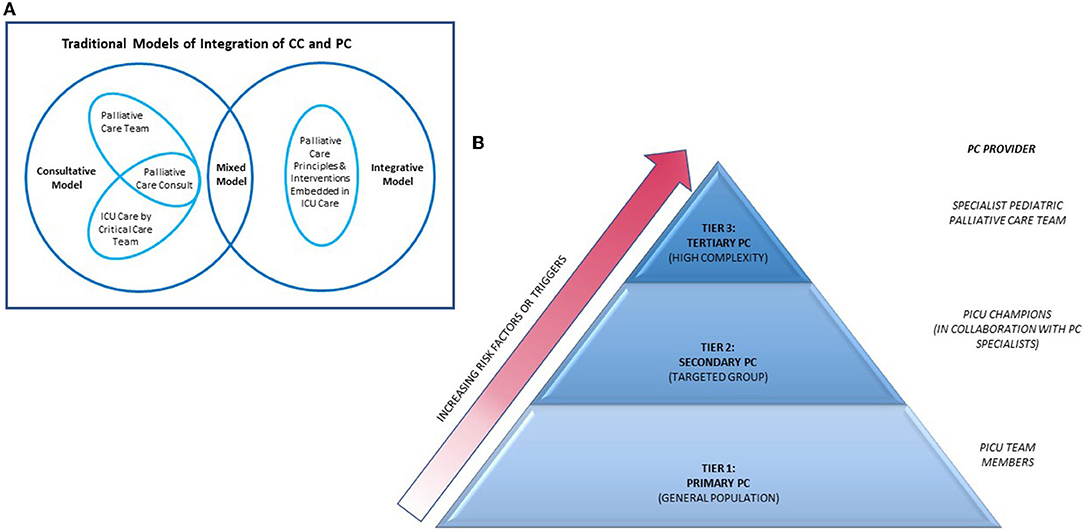
Figure 1. Models of integration of PC into ICU. PC, palliative care; CC, critical care; ICU, intensive care unit. (A) Diagram depicting interactions of traditional models of integration of PC into ICU. Adapted from Nelson et al. (64). (B) Pyramid model of integrating of PC into ICU. Source—Public domain and adapted from Rothschild et al. (65).
More recently, a tiered approach for PC-CC integration has been described (Figure 1) (65). In this model, interventions are categorized into primary, secondary and tertiary PC, with increasing PC specialists involvement across the levels. Primary PC is the provision of evidence-based PC interventions by critical care physicians. This is useful in institutions where dedicated PC teams are not available. Examples of primary PC interventions can be found in the Initiative for Pediatric Palliative Care (IPPC) curriculum which highlights six core constituents of quality PPC: holistic care of the child, support of family unit, involvement of the family and child in decision-making, communication and planning of care, treatment of pain and other symptoms, continuity of care and support of grief and bereavement (66). PICU physicians are also equipped with knowledge about EOL issues including pronouncing death and discussing need for autopsies. However, delivery of primary PC can be highly variable and is dependent on resources, manpower and critical care physicians' knowledge on PC. An international multicentre cross-sectional study including 34 PICUs of varying socio-economic settings reported heterogenous and incomplete fulfillment of IPPC domains in their delivery of primary PC, with better adherence in higher income groups and units with shorter shift lengths (67).
Secondary PC uses ICU-based champions who receive additional PC training through courses and subspecialty rotations. These ICU champions strengthen the delivery of PC in ICU by spearheading PC-based training for other ICU staff, advocating for earlier PC subspecialty involvement in suitable patients and also improving PC via quality improvement initiatives and protocols development. A recent report describes the integration of a pediatric palliative care-champion (PPCC) based model into the cardiac ICU in Boston Children's Hospital, United States (68). The PPCC model is expected to be more sustainable than other PC-CC integration models as the workload is shared with overextended subspecialty PC services, hence relieving the strain on PC teams while allowing early integration of PC principles in the ICU. However, provision of secondary PC will require commitment from ICU providers and a robust PPC team to support program development and education.
Tertiary PC involves consultation of a subspecialty PC team as an additional resource. This is helpful in specific situations where PC team can facilitate more difficult communications, support complex decision making in the face of uncertainty or conflict while providing both emotional and spiritual support for both patient and family and assist with managing difficult symptoms. Added benefits include ensuring continuity in goals of care and care coordination across multiple providers and settings.
Defining clinical triggers for PC consultations ensures that palliative consults are made appropriately. In PICUs, common triggers criteria include baseline patient characteristics (e.g., extreme prematurity), selected acute or life-limiting diagnoses (e.g., severe traumatic brain injury, Trisomy 13), resource utilization based criteria (e.g., ECMO duration, number of ICU admissions over time), social risk factors or failure of initial ICU efforts to address PC needs of patients and families (64). However, variability in resources and systems of care limits the use of a fixed set of trigger criteria across institutions. Adaptation of triggers mapped to institution resources and needs is a more logical approach (69).
Choosing to adopt any of the above models can be an important initial step toward an initiative to incorporate PC practice into the PICU. Both primary and secondary PC have the same characteristics as the integrative model while tertiary PC is most alike to the consultative model. In reality, there is usually a large degree of overlap between models and no one model can suit the demands of all institutions. Careful and realistic assessment of available resources, attitude of stakeholders, cultural and value system of the institution would be required to find the best fit.
Conclusion
The integration of PC to CC has many positive impacts on patient and family-centered outcomes and is becoming the standard for high-quality care of critically ill children. PC also ensures both coordination and continuity of care across different care settings are met. Several models of integration have been proposed but the model of choice should be tailored to available resources, attitude of stakeholders, cultural context and value system of the institution.
Author Contributions
SB and SL wrote sections of the manuscript. YM, JL, and YC contributed additional resources/journals and advice regarding content. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Palliative care. Geneva: WHO (2015). Available onlie at: http://www.who.int/cancer/palliative/en (accessed March 03, 2022).
2. Soha care. Pediatric palliative care and hospice care commitments, guidelines, and recommendations. Pediatrics. (2013) 132:966–72. doi: 10.1542/peds.2013
3. Burns JP, Sellers DE, Meyer EC, Lewis-Newby M, Truog RD. Epidemiology of death in the PICU at five U.S teaching hospitals*. Crit Care Med. (2014) 42:2101–8. doi: 10.1097/CCM.0000000000000498
4. Fraser LK, Parslow R. Children with life-limiting conditions in paediatric intensive care units: a national cohort, data linkage study. Arch Dis Child. (2018) 103:540–7. doi: 10.1136/archdischild-2017-312638
5. Meert KL, Keele L, Morrison W, Berg RA, Dalton H, Newth CJ, et al. End-of-life practices among tertiary care PICUs in the United States: a multicenter study. Pediatr Crit Care Med. (2015) 16:e231–8. doi: 10.1097/PCC.0000000000000520
6. DeCourcey DD, Silverman M, Oladunjoye A, Balkin EM, Wolfe J. Patterns of care at the end of life for children and young adults with life-threatening complex chronic conditions. J Pediatr. (2018) 193:196–203.e2. doi: 10.1016/j.jpeds.2017.09.078
7. Boss R, Nelson J, Weissman D, Campbell M, Curtis R, Frontera J, et al. Integrating palliative care into the PICU: a report from the Improving Palliative Care in the ICU Advisory Board. Pediatr Crit Care Med. (2014) 15:762–7. doi: 10.1097/PCC.0000000000000209
8. Boss RD, Falck A, Goloff N, Hutton N, Miles A, Shapiro M, et al. Low prevalence of palliative care and ethics consultations for children with chronic critical illness. Acta Paediatr. (2018) 107:1832–3. doi: 10.1111/apa.14394
9. O'Keefe S, Maddux AB, Bennett KS, Youngwerth J, Czaja AS. Variation in pediatric palliative care allocation among critically ill children in the United States. Pediatr Crit Care Med. (2021) 22:462–73. doi: 10.1097/PCC.0000000000002603
10. Lizzie C. A Guide to Children's Palliative Care (Fourth Edition). Together for Short Lives, England. (2018). Bristol: Together for Short Lives.
11. Feudtner C, Kang TI, Hexem KR, Friedrichsdorf SJ, Osenga K, Siden H, et al. Pediatric palliative care patients: a prospective multicenter cohort study. Pediatrics. (2011) 127:1094–101. doi: 10.1542/peds.2010-3225
12. Feudtner C, Rosenberg AR, Boss RD, Wiener L, Lyon ME, Hinds PS, et al. Challenges and priorities for pediatric palliative care research in the u.s. and similar practice settings: report from a pediatric palliative care research network workshop. J Pain Symptom Manage. (2019) 58:909–17.e3. doi: 10.1016/j.jpainsymman.2019.08.011
13. Lagman RL, Walsh D, Davis MP, Young B. All patient refined-diagnostic related group and case mix index in acute care palliative medicine. J Support Oncol. (2007) 5:145–9.
14. Osta BE, Palmer JL, Paraskevopoulos T, Pei BL, Roberts LE, Poulter VA, et al. Interval between first palliative care consult and death in patients diagnosed with advanced cancer at a comprehensive cancer center. J Palliat Med. (2008) 11:51–7. doi: 10.1089/jpm.2007.0103
15. Rubin S, Malkinson R. Parental response to child loss across the life cycle: Clinical and research perspectives. In: Stroebe MS, Hansson RO, Stroebe W, Schut H, editors. Handbook of Bereavement Research: Consequences, Coping and Care. Washington, DC: American Psychological Association (2001), pp. 219–40.
16. Middleton W, Raphael B, Burnett P, Martinek N. A longitudinal study comparing bereavement phenomena in recently bereaved spouses, adult children and parents. Aust N Z J Psychiatry. (1998) 32:235–41. doi: 10.3109/00048679809062734
17. Li J, Laursen TM, Precht DH, Olsen J, Mortensen PB. Hospitalization for mental illness among parents after the death of a child. N Engl J Med. (2005) 352:1190–6. doi: 10.1056/NEJMoa033160
18. Meert KL, Shear K, Newth CJ, Harrison R, Berger J, Zimmerman J, et al. Follow-up study of complicated grief among parents eighteen months after a child's death in the pediatric intensive care unit. J Palliat Med. (2011) 14:207–14. doi: 10.1089/jpm.2010.0291
19. Morris S, Fletcher K, Goldstein R. The grief of parents after the death of a young child. J Clin Psychol Med Settings. (2019) 26:321–38. doi: 10.1007/s10880-018-9590-7
20. Suttle M, Hall MW, Pollack MM, Berg RA, McQuillen PS, Mourani PM, et al. Complicated grief, depression and post-traumatic stress symptoms among bereaved parents following their child's death in the pediatric intensive care unit: a follow-up study. Am J Hosp Palliat Care. (2022) 39:228–36. doi: 10.1177/10499091211015913
21. Li J, Johansen C, Hansen D, Olsen J. Cancer incidence in parents who lost a child: a nationwide study in Denmark. Cancer. (2002) 95:2237–42. doi: 10.1002/cncr.10943
22. Li J, Hansen D, Mortensen PB, Olsen J. Myocardial infarction in parents who lost a child: a nationwide prospective cohort study in Denmark. Circulation. (2002) 106:1634–9. doi: 10.1161/01.CIR.0000031569.45667.58
23. Olsen J, Li J, Precht DH. Hospitalization because of diabetes and bereavement: a national cohort study of parents who lost a child. Diabet Med. (2005) 22:1338–42. doi: 10.1111/j.1464-5491.2005.01642.x
24. Brooten D, Youngblut JM, Caicedo C, Del Moral T, Cantwell GP, Totapally B. Parents' acute illnesses, hospitalizations, and medication changes during the difficult first year after infant or child NICU/PICU death. Am J Hosp Palliat Care. (2018) 35:75–82. doi: 10.1177/1049909116678597
25. Nicoll J, Dryden-Palmer K, Frndova H, Gottesman R, Gray M, Hunt EA, et al. Death and dying in hospitalized pediatric patients: a prospective multicenter, multinational study. J Palliat Med. (2022) 25:227–33. doi: 10.1089/jpm.2021.0205
26. Wu ET, Wang CC, Huang SC, Chen CH, Jou ST, Chen YC, et al. End-of-life care in taiwan: single-center retrospective study of modes of death. Pediatr Crit Care Med. (2021) 22:733–42. doi: 10.1097/PCC.0000000000002715
27. Thomas R, Phillips M, Hamilton RJ. Pain management in the pediatric palliative care population. J Nurs Scholarsh. (2018) 50:375–82. doi: 10.1111/jnu.12389
28. LaFond CM, Hanrahan KS, Pierce NL, Perkhounkova Y, Laures EL, McCarthy AM. Pain in the pediatric intensive care unit: how and what are we doing? Am J Crit Care. (2019) 28:265–73. doi: 10.4037/ajcc2019836
29. Manworren RC, Stinson J. Pediatric pain measurement, assessment, and evaluation. Semin Pediatr Neurol. (2016) 23:189–200. doi: 10.1016/j.spen.2016.10.001
30. Worden JW, Monahan JR. Caring for bereaved parents. In Armstrong-Daily A, Zarbock S, editors. Hospice Care for Children, end ed. Oxford: Oxford University Press (2001), p. 137–56.
31. Meyer EC, Ritholz MD, Burns JP, Truog RD. Improving the quality of end-of-life care in the pediatric intensive care unit: parents' priorities and recommendations. Pediatrics. (2006) 117:649–57. doi: 10.1542/peds.2005-0144
32. Studdert DM, Burns JP, Mello MM, Puopolo AL, Truog RD, Brennan TA. Nature of conflict in the care of pediatric intensive care patients with prolonged stay. Pediatrics. (2003) 112:553–8. doi: 10.1542/peds.112.3.553
33. Embriaco N, Azoulay E, Barrau K, Kentish N, Pochard F, Loundou A, et al. High level of burnout in intensivists: prevalence and associated factors. Am J Respir Crit Care Med. (2007) 175:686–92. doi: 10.1164/rccm.200608-1184OC
34. Breen CM, Abernethy AP, Abbott KH, Tulsky JA. Conflict associated with decisions to limit life-sustaining treatment in intensive care units. J Gen Intern Med. (2001) 16:283–9. doi: 10.1046/j.1525-1497.2001.00419.x
35. Studdert DM, Mello MM, Burns JP, Puopolo AL, Galper BZ, Truog RD, et al. Conflict in the care of patients with prolonged stay in the ICU: types, sources, and predictors. Intensive Care Med. (2003) 29:1489–97. doi: 10.1007/s00134-003-1853-5
36. Contro NA, Larson J, Scofield S, Sourkes B, Cohen HJ. Hospital staff and family perspectives regarding quality of pediatric palliative care. Pediatrics. (2004) 114:1248–52. doi: 10.1542/peds.2003-0857-L
37. Hutton N. Pediatric palliative care: the time has come. Arch Pediatr Adolesc Med. (2002) 156:9–10. doi: 10.1001/archpedi.156.1.9
38. October TW, Hinds PS, Wang J, Dizon ZB, Cheng YI, Roter DL. Parent satisfaction with communication is associated with physician's patient-centered communication patterns during family conferences. Pediatr Crit Care Med. (2016) 17:490–7. doi: 10.1097/PCC.0000000000000719
39. Rietjens JAC, Sudore RL, Connolly M, van Delden JJ, Drickamer MA, Droger M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol. (2017) 18:e543–51. doi: 10.1016/S1470-2045(17)30582-X
40. Lyon ME, Garvie PA, Briggs L, He J, McCarter R, D'Angelo LJ. Development, feasibility, and acceptability of the Family/Adolescent-Centered (FACE) advance care planning intervention for adolescents with HIV. J Palliat Med. (2009) 12:363–72. doi: 10.1089/jpm.2008.0261
41. Lyon ME, Garvie PA, McCarter R, Briggs L, He J, D'Angelo LJ. Who will speak for me? Improving end-of-life decision-making for adolescents with HIV and their families. Pediatrics. (2009) 123:e199–206. doi: 10.1542/peds.2008-2379
42. DeCourcey DD, Silverman M, Oladunjoye A, Wolfe J. Advance care planning and parent-reported end-of-life outcomes in children, adolescents, and young adults with complex chronic conditions. Crit Care Med. (2019) 47:101–8. doi: 10.1097/CCM.0000000000003472
43. Durall A, Zurakowski D, Wolfe J. Barriers to conducting advance care discussions for children with life-threatening conditions. Pediatrics. (2012) 129:e975–82. doi: 10.1542/peds.2011-2695
44. Lotz JD, Jox RJ, Borasio GD, Führer M. Pediatric advance care planning from the perspective of health care professionals: a qualitative interview study. Palliat Med. (2015) 29:212–22. doi: 10.1177/0269216314552091
45. Larcher V, Craig F, Bhogal K, Wilkinson D, Brierley J. Health RCoPaC. Making decisions to limit treatment in life-limiting and life-threatening conditions in children: a framework for practice. Arch Dis Child. (2015) 100(Suppl 2):s3–23. doi: 10.1136/archdischild-2014-306666
46. Bobillo-Perez S, Segura S, Girona-Alarcon M, Felipe A, Balaguer M, Hernandez-Platero L, et al. End-of-life care in a pediatric intensive care unit: the impact of the development of a palliative care unit. BMC Palliat Care. (2020) 19:74. doi: 10.1186/s12904-020-00575-4
47. Downar J, Delaney JW, Hawryluck L, Kenny L. Guidelines for the withdrawal of life-sustaining measures. Intensive Care Med. (2016) 42:1003–17. doi: 10.1007/s00134-016-4330-7
48. Paruk F, Kissoon N, Hartog CS, Feldman C, Hodgson ER, Lipman J, et al. The Durban World Congress Ethics Round Table conference report: III. Withdrawing Mechanical ventilation–the approach should be individualized. J Crit Care. (2014) 29:902–7. doi: 10.1016/j.jcrc.2014.05.022
49. Nelson H, Mott S, Kleinman ME, Goldstein RD. Parents' experiences of pediatric palliative transports: a qualitative case series. J Pain Symptom Manage. (2015) 50:375–80. doi: 10.1016/j.jpainsymman.2015.04.004
50. Raed M, Grossoehme DH, Brown M, Friebert S. Hospital to home transport at end of life: survey of clinician experience. Palliat Med. (2020) 34:424–9. doi: 10.1177/0269216319870641
51. Postier A, Catrine K, Remke S. Interdisciplinary pediatric palliative care team involvement in compassionate extubation at home: from shared decision-making to bereavement. Children. (2018) 5:37. doi: 10.3390/children5030037
52. Menon AP, Mok YH, Loh LE, Lee JH. Pediatric palliative transport in critically Ill children: a single center's experience and parents' perspectives. J Pediatr Intensive Care. (2020) 9:99–105. doi: 10.1055/s-0039-3401009
53. Woodruff AG, Bingham SB, Jarrah RJ, Bass AL, Nageswaran S. A framework for pediatric intensivists providing compassionate extubation at home. Pediatr Crit Care Med. (2021) 22:454–61. doi: 10.1097/PCC.0000000000002655
54. Rostila M, Saarela J, Kawachi I. The forgotten griever: a nationwide follow-up study of mortality subsequent to the death of a sibling. Am J Epidemiol. (2012) 176:338–46. doi: 10.1093/aje/kws163
55. Dias N, Brandon D, Haase JE, Tanabe P. Bereaved parents' health status during the first 6 months after their child's death. Am J Hosp Palliat Care. (2018) 35:829–39. doi: 10.1177/1049909117744188
56. Li J, Precht DH, Mortensen PB, Olsen J. Mortality in parents after death of a child in Denmark: a nationwide follow-up study. Lancet. (2003) 361:363–7. doi: 10.1016/S0140-6736(03)12387-2
57. Morris SE, Dole OR, Joselow M, Duncan J, Renaud K, Branowicki P. The development of a hospital-wide bereavement program: ensuring bereavement care for all families of pediatric patients. J Pediatr Health Care. (2017) 31:88–95. doi: 10.1016/j.pedhc.2016.04.013
58. Wiener L, Rosenberg AR, Lichtenthal WG, Tager J, Weaver MS. Personalized and yet standardized: an informed approach to the integration of bereavement care in pediatric oncology settings. Palliat Support Care. (2018) 16:706–11. doi: 10.1017/S1478951517001249
59. Kochen EM, Jenken F, Boelen PA, Deben LMA, Fahner JC, van den Hoogen A, et al. When a child dies: a systematic review of well-defined parent-focused bereavement interventions and their alignment with grief- and loss theories. BMC Palliat Care. (2020) 19:28. doi: 10.1186/s12904-020-0529-z
60. Levy C, Drouin K, Dorsett A, Sood E. Supporting transition to the bereaved community after the death of a child. Pediatrics. (2021) 148:e2021052943. doi: 10.1542/peds.2021-052943
61. Khandelwal N, Kross EK, Engelberg RA, Coe NB, Long AC, Curtis JR. Estimating the effect of palliative care interventions and advance care planning on ICU utilization: a systematic review. Crit Care Med. (2015) 43:1102–11. doi: 10.1097/CCM.0000000000000852
62. Snaman JM, Kaye EC, Lu JJ, Sykes A, Baker JN. Palliative care involvement is associated with less intensive end-of-life care in adolescent and young adult oncology patients. J Palliat Med. (2017) 20:509–16. doi: 10.1089/jpm.2016.0451
63. Morrison RS, Dietrich J, Ladwig S, Quill T, Sacco J, Tangeman J, et al. Palliative care consultation teams cut hospital costs for Medicaid beneficiaries. Health Aff. (2011) 30:454–63. doi: 10.1377/hlthaff.2010.0929
64. Nelson JE, Bassett R, Boss RD, Brasel KJ, Campbell ML, Cortez TB, et al. Models for structuring a clinical initiative to enhance palliative care in the intensive care unit: a report from the IPAL-ICU Project (Improving Palliative Care in the ICU). Crit Care Med. (2010) 38:1765–72. doi: 10.1097/CCM.0b013e3181e8ad23
65. Rothschild CB, Derrington SF. Palliative care for pediatric intensive care patients and families. Curr Opin Pediatr. (2020) 32:428–35. doi: 10.1097/MOP.0000000000000903
66. Browning DM, Solomon MZ. Team IfPPCII. The initiative for pediatric palliative care: an interdisciplinary educational approach for healthcare professionals. J Pediatr Nurs. (2005) 20:326–34. doi: 10.1016/j.pedn.2005.03.004
67. Grunauer M, Mikesell C, Bustamante Callejas G, Group P-MR. Primary palliative care integrated model in paediatric ICU: an international cross-sectional study. BMJ Support Palliat Care. (2021). doi: 10.1136/bmjspcare-2020-002627. [Epub ahead of print].
68. Moynihan KM, Snaman JM, Kaye EC, Morrison WE, DeWitt AG, Sacks LD, et al. Integration of pediatric palliative care into cardiac intensive care: a champion-based model. Pediatrics. (2019) 144:e20190160. doi: 10.1542/peds.2019-0160
Keywords: pediatric intensive care unit (PICU), integrative models, critical care, palliative care, framework
Citation: Buang SNH, Loh SW, Mok YH, Lee JH and Chan YH (2022) Palliative and Critical Care: Their Convergence in the Pediatric Intensive Care Unit. Front. Pediatr. 10:907268. doi: 10.3389/fped.2022.907268
Received: 29 March 2022; Accepted: 05 May 2022;
Published: 10 June 2022.
Edited by:
Niranjan Kissoon, University of British Columbia, CanadaReviewed by:
Yves Ouellette, Mayo Clinic, United StatesCopyright © 2022 Buang, Loh, Mok, Lee and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siti Nur Hanim Buang, c2l0aS5udXIuaGFuaW1Ac2luZ2hlYWx0aC5jb20uc2c=
 Siti Nur Hanim Buang
Siti Nur Hanim Buang Sin Wee Loh
Sin Wee Loh Yee Hui Mok2
Yee Hui Mok2 Jan Hau Lee
Jan Hau Lee