- 1Department of Child and Adolescent Health, University of the West Indies, Kingston, Jamaica
- 2Department of Child and Adolescent Health (Neurology), University Hospital of the West Indies, Kingston, Jamaica
- 3Department of Child and Adolescent Health (Adolescent Health), University Hospital of the West Indies, Kingston, Jamaica
- 4Department of Microbiology (Virology), University of the West Indies, Kingston, Jamaica
- 5Global Virus Network, Baltimore, MD, United States
- 6Centers for Diseases Control and Prevention, Caribbean Regional Office, Kingston, Jamaica
- 7Department of Child and Adolescent Health (Infectious Diseases), University Hospital of the West Indies, Kingston, Jamaica
Objectives: COVID-19 in children was initially mild until the emergence of Multisystem Inflammatory Syndrome in Children (MIS-C). We describe pediatric COVID-19 in a developing country within the Caribbean.
Methods: Jamaican children who were hospitalized with SARS-CoV-2 infection, in one Caribbean regional academic referral center from April 2020 through June 2021 were included. Prospective surveillance and pediatric infectious disease consultations were performed using the CDC's MIS-C case definition. Data were extracted from patients' hospital charts using WHO's reporting form, entered into the RedCap database, and SPSS 28 was used for analysis. MIS-C and non-MIS-C patients were compared using independent sample t-tests for continuous variables and Fisher's exact test for categorical variables, p values < 0.05 were statistically significant.
Results: Seventy-nine children with COVID-19 with/without MIS-C presented to UHWI. Thirty-eight (48%) were mild ambulatory cases. Hospitalizations occurred in 41 (52%) children, with median age of 10 years. SARS-CoV-2 RT-PCR positivity was present in 26 (63%), Immunoglobulin M, or Immunoglobulin G (IgM/IgG) positivity in 8 (20%), with community exposures in 7 (17%). Eighteen (44%) MIS-C positive patients were significantly more likely than 23 MIS-C negative patients (56%) to present with fever (94% vs. 30%; p < 0.001), fatigue/lethargy (41% vs. 4%; p = 0.006), lymphadenopathy (33% vs. 0%; p = 0.003), elevated neutrophils (100% vs. 87%; p = 0.024), and ESR (78% vs. 9%; p = 0.002). Involvement of > two organ systems occurred more frequently in MIS-C positive cases (100% vs. 34%; p < 0.001), including gastrointestinal (72% vs. 17%; p < 0.001); vomiting/nausea (39% vs. 9%; p < 0.028); hematological/coagulopathic (67% vs. 4%; p < 0.001); dermatologic involvement (56% vs. 0%; p < 0.001); and mucositis (28% vs. 0%; p = 0.001). MIS-C patients had Kawasaki syndrome (44%), cardiac involvement (17%), and pleural effusions (17%). MIS-C patients had >4 abnormal inflammatory biomarkers including D-dimers, C-reactive protein, ESR, ferritin, troponins, lactate dehydrogenase, neutrophils, platelets, lymphocytes, and albumen (72%). MIS-C patients were treated with intravenous immune gamma globulin (78%), aspirin (68%), steroids (50%), and non-invasive ventilation (11%). None required inotropes/vasopressors. MIS-C negative patients received standard care. All recovered except one child who was receiving renal replacement therapy and developed myocardial complications.
Conclusions: In this first report of COVID-19 from the Caribbean, children and adolescents with and without MIS-C were not very severe. Critical care interventions were minimal and outcomes were excellent.
Background
There were no reported cases of the novel coronavirus in children aged less than 18 years until ten weeks into the epidemic in China (1). Subsequently, child and adolescent cases were reported from China, Italy, Iceland, and the United States (1–4). Initially, COVID-19 in children was either asymptomatic or had a milder course with a better prognosis than in adults (5). By April 2020, the post-infectious multisystem inflammatory syndrome in children (MIS-C), the Pediatric Inflammatory Multisystem Syndrome (PIMS-Ts), was being recognized in children with COVID-19 (6–10). Case definitions for MIS-C/PIMS-Ts were first created by the USA's Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and Royal College of Pediatrics and Child Health (RCPCH) (6–10). The American Academy of Pediatrics and American College of Rheumatology later recommended a treatment approach for children with MIS-C (11, 12).
The pediatric COVID-19 pandemic has since expanded globally (13–17). The severity of illness and outcomes in children vary based on demographic factors, circulating SARS-CoV-2 variants of concern, patient age, comorbid illnesses, a diagnosis of MIS-C, and timely access to optimal medical care (13–19). In the USA, during the SARS-CoV-2 Omicron outbreak, children aged <4 years were being hospitalized at > 5 times the observed rate during the peak of the Delta variant predominance (19). As in adults, children and adolescents are also experiencing the effects of “Long COVID-19” (18). Globally, access to COVID-19 vaccination in adolescents has lagged behind adult immunizations and is disproportionate in developing as compared to developed countries (20). Also, COVID-19 vaccines are not routinely recommended for children without comorbidities, and vaccine hesitancy is accentuated by increased reports of adverse events of myocarditis and pericarditis in youth (20). Indirect adverse effects of the global pandemic on children and adolescents have included mental health concerns, school closures, loss of educational opportunities, missed vaccinations, and death of their caregivers (21).
The Caribbean has a population of about 44.2 million residents in 700 islands, comprising 44 countries, or territories that are bordered by the land masses of North, Central, and South America and the waters of the Caribbean Sea, the Gulf of Mexico, and the Atlantic Ocean (22). The Caribbean is diverse in its ethnicity, culture, socioeconomic status, and language and is highly dependent on tourism to sustain its economic viability (22). Jamaica, with a Gross National Income (GNI) per capita of USD 4,990 (2018, World Bank) is an upper middle-income, developing, Caribbean Island nation of 2.9 million inhabitants, which diagnosed its first imported case of COVID-19 on March 10, 2020, one day before COVID-19 was declared a pandemic by the WHO (22, 23). “Face-to-face” school attendance was suspended, and virtual classes began approximately five weeks later. Varied public health measures were implemented. Community transmission was declared in September 2020 with test positivity rates fluctuating between 8 and 41%, peaking in March 2021. By June 1st, 2021, Jamaica reported 48,639 COVID-19 confirmed cases, of these 10.3% (4999) were aged <18 years. Of these 4,999 confirmed cases, five percent (250) were under one year old, 10.4% (520) were aged 1–2 years, 13.1% (653) 3–5 years, 36.1% (1,807) 6–12 years, and 35.4% (1769) 13–17 years old. Deaths were reported in 0.1% and 0.2% in those aged <10 years and 10–19 years old, respectively (23). These national data were gleaned from the Jamaican Ministry of Health and Wellness for patients presenting for treatment and care in hospitals and primary care settings. The Caribbean Public Health Agency reported the B.1.1.7 (Alpha) SARS-CoV-2 as the only “variant of concern” identified in Jamaica through the third week of June 2021 (24). According to sequencing data that were reported by the United States' Centers for Disease Control and Prevention, among samples that were selected by Jamaica's National Public Health Laboratory, the SARS-CoV-2 “Delta” variant was first seen in a sample that was collected on 11 February, 2021 (gisaid.org) and reported 23 June, 2021 (25). While “Omicron” and its sub-variants were first seen 21 December, 2021 (gisaid.org) and they have been circulating in Jamaica since then (26).
The University Hospital of the West Indies (UHWI), Jamaica, is a tertiary academic institution that accepts referred and ambulatory adult and pediatric patients island-wide and throughout the Caribbean for hospitalization including intensive care. The UHWI has approximately 120 inpatient beds for patients aged 0–16 years. SARS-CoV-2 RT-PCR nasopharyngeal testing is performed on every hospitalized patient. SARS-CoV-2 antibody testing is done when indicated, especially in patients suspected with MIS-C, who test negative for SARS-CoV-2. Although SARS-CoV-2 RT-PCR has very high analytical sensitivity, diagnostic sensitivity is imperfect and false negatives range from 1.8 to 58% (27). The use of SARS-CoV-2 RT-PCR followed by antibody testing in those with a presumed diagnosis of MIS-C increases the diagnostic sensitivity for this disease, which usually occurs up to 6 weeks or more after the virus is cleared from the upper respiratory tract (28).
This report documents COVID-19 in children presenting during the first 15 months of the global pandemic in a resource-constrained single-center teaching hospital in Jamaica and is the first of its kind from a developing country within the Caribbean region. It provides historical context and also informs clinicians in similar settings as the pandemic rapidly evolves. We describe the clinical characteristics, investigations, therapy, and outcomes in all children aged <16 years old who were hospitalized at UHWI with acute SARS-CoV-2 infection, and/or MIS-C from March 2020 through June 2021 of the global COVID-19 pandemic. The clinical characteristics and outcomes between patients presenting with MIS-C and those without MIS-C were compared.
Methods
This descriptive ambispective cohort study included all admitted patients, aged <16 years, with SARS-CoV-2 infection presenting to the UHWI from all over the island from April 1, 2020, through June 30, 2021. Patients who were hospitalized at the UHWI, Jamaica, who met the CDC's case definition for MIS-C and non-MIS-C patients were further analyzed in detail for this report (9). The MIS-C diagnostic criteria included fever for >24 h, laboratory evidence of inflammation with at least one of the following criteria: elevated C-reactive protein, erythrocyte sedimentation rate (ESR), fibrinogen, procalcitonin, D-dimer, ferritin, lactic acid dehydrogenase, interleukin-6, elevated neutrophils, or reduced lymphocytes and or albumin and clinically severe illness requiring hospitalization, with multisystem (>2) organ involvement, including cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, or neurological; no alternative diagnoses; evidence of current, or recent SARS-CoV-2 infection by RT-PCR, serology, or antigen test; or exposure to a suspected, or confirmed COVID-19 case in the 4 weeks prior (9). Organ system involvement was assessed with supporting clinical features and laboratory and other related investigations. Children meeting full, or partial criteria for Kawasaki disease, or a pediatric death from SARS-CoV-2 infection are also considered MIS-C (9).
Children were managed using treatment guidelines as they evolved (8, 12). Children with MIS-C received intravenous gamma globulin (2 g/kg as a single infusion over 10–12 h), steroids (methylprednisolone intravenously 2 mg/kg/day in two divided doses, transitioned to oral prednisolone, or prednisone which is tapered over two to four weeks), acetyl salicylic acid [aspirin] (80–100 mg/kg per day divided into four doses), and anticoagulants (such as enoxaparin). Other supporting modalities included blood and blood products, antibiotics, fluids, oxygen, and other treatment modalities, as appropriate. Those without MIS-C received standard care, which included investigations of complete blood count, electrolytes, renal function, blood and urine culture, antipyretics, fluids, oxygen, dexamethazone, anti-thrombotic agents and antibiotics, if needed. All patients were treated for their underlying comorbidities, as deemed appropriate.
Hospitalized patients have mandatory nasopharyngeal RT-PCR testing for SARS-CoV-2.
All suspected and confirmed hospitalized COVID-19 patients had written Pediatric Infectious Diseases consultation(s) in accordance with UHWI's COVID-19 hospital policy (all on file). Upon hospital discharge, the patients were reviewed and followed by the pediatric inpatient or outpatient department. SARS-CoV-2 infection was confirmed by real-time RT-PCR positivity of nasopharyngeal samples at the National Influenza Center, which is housed at the UHWI's Diagnostic Virology Laboratory, the presence of IgG and/or IgM antibodies (Abbott Architect), and/or a confirmed epidemiological link, or presumed contact with an infected person during the community outbreak. SARS-CoV-2 antibody testing was performed in those with suspected MIS-C but with negative RT-PCR tests. Electronic medical and laboratory records and paper clinical records were reviewed by senior researchers and a clinical research assistant. Results of clinical features, investigations, treatments, and outcomes were extracted from patients' hospital charts using a modified WHO case report form, and data were entered into RedCap database by senior clinical researchers.
Statistical analyses were performed using IBM SPSS version 28 [International Business Machines Corporation (IBM), Armonk, New York, USA]. Descriptive statistics were obtained for clinical characteristics and outcomes in children aged <16 years old on admission using mean and standard deviation for continuous variables and number and percent for categorical variables. Comparison of differences between children with or without MIS-C was computed using independent samples t-tests for continuous variables and Fisher's exact test for categorical variables. A p-value < 0.05 was considered statistically significant.
Results
During April 2020 to June 2021, 79 children and adolescents aged <16 years presented through the UHWI's Emergency Room with SARS-CoV-2 RT-PCR positive status, or COVID-19, 41 (52%) were hospitalized and 18 (44%) of these met the CDC's case definition for MIS-C.
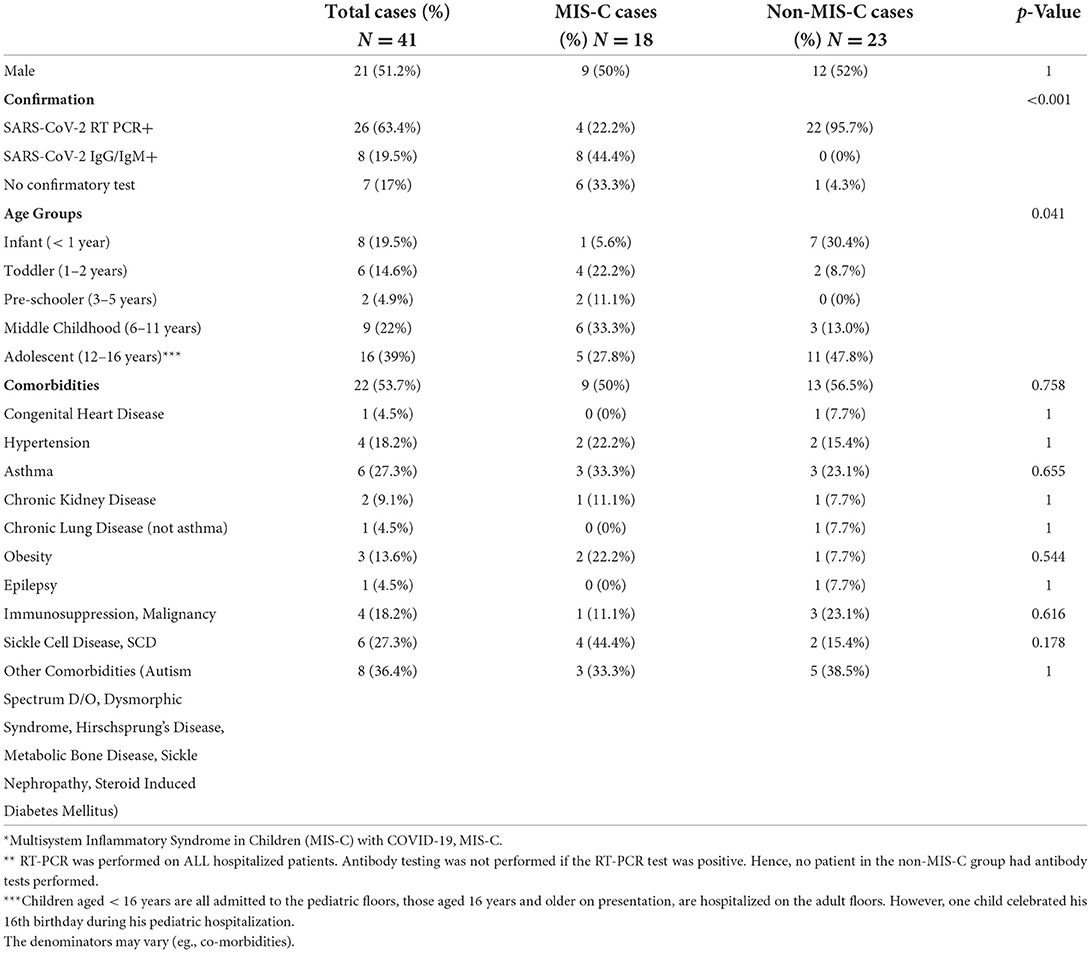
Among 41 (52%) patients who were hospitalized, the median age was 10.5 years (range 0–16 years); SARS-CoV-2 RT-PCR positivity was present in 26 (63.4%) and SARS-CoV-2 IgM/IgG positivity in 8 (20%), the rest 7 (17%) had community exposures (Table 1). Twenty-one (51.2%) were males and 40 (98%) were of mixed Afro-Caribbean ethnicity. Twenty-two (53.7%) had comorbidities; of these, Sickle cell disease (27.3%), asthma (27.3%), and immunodeficiency (18.2%) were the most common. The prevalence of comorbidities was similar in patients with MIS-C and without MIS-C (Table 1).
Twenty-two of the 23 non-MIS-C patients (95.7%) were RT-PCR positive, 11 (47.8%) were asymptomatic, 12 (52.2%) had mild symptoms, including fever (30.4%), cough (18.2%), and abdominal pain (21.7%) (Tables 1, 2A). Sickle cell disease and asthma were the most common comorbidities. In symptomatic acute cases, it was clinically impossible to ascribe the cough and respiratory distress to these diseases or to the acute SARS-CoV-2 infection. Similarly, in those with acute appendicitis, it was challenging to ascribe the abdominal pain to appendicitis or acute COVID-19. Reasons for hospitalization included bony fracture, hematocolpus from an imperforate hymen, Sickle cell disease, chronic kidney disease for hemodialysis, malignancy, appendicitis, diabetic ketoacidosis, and four well neonates whose mothers were RT-PCR positive within 48 h of delivery.
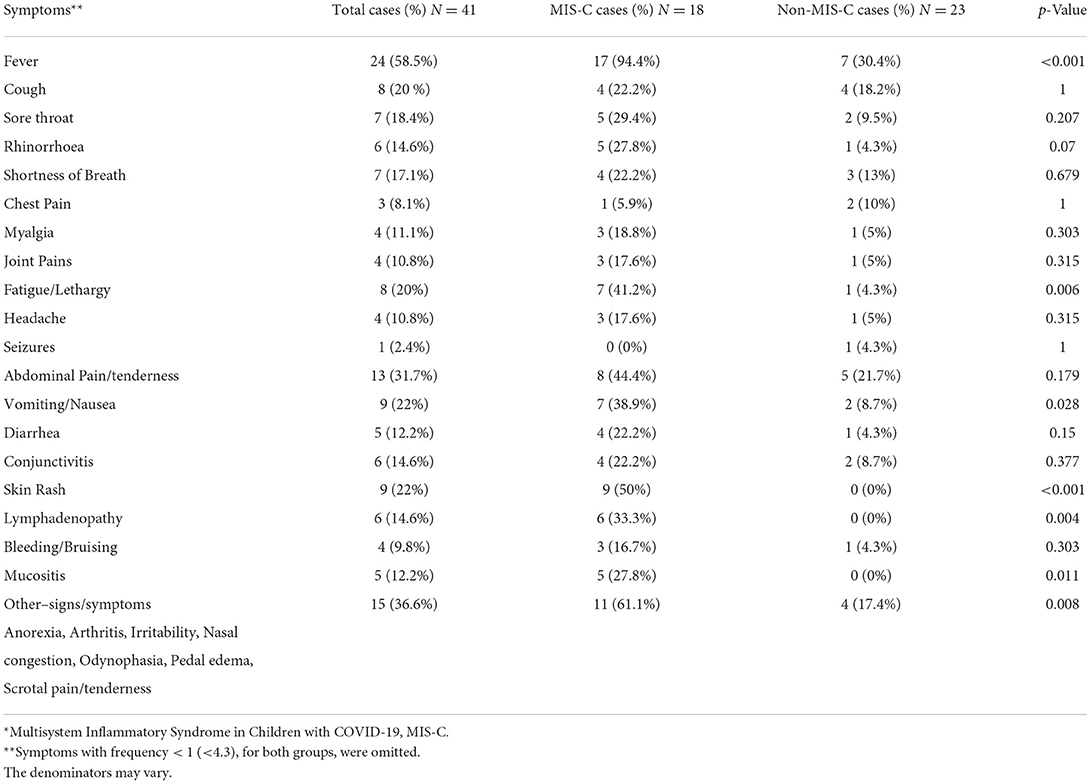
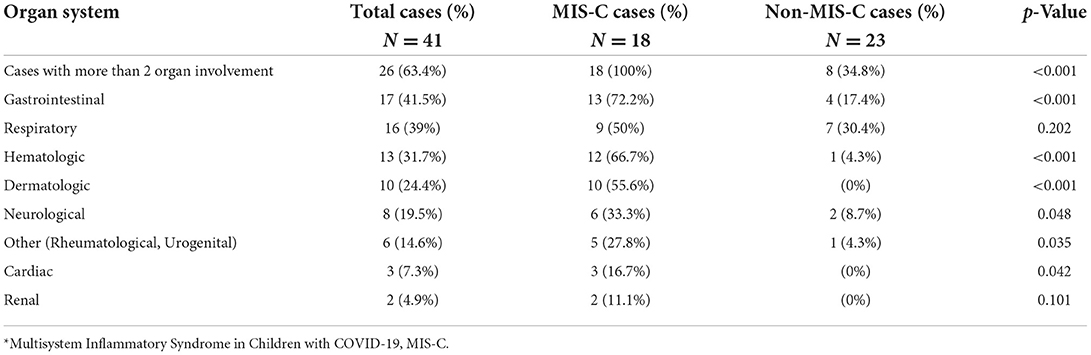
Eighteen (44%) hospitalized patients were diagnosed with MIS-C: 4 (22%) were SARS-CoV-2 RT-PCR positive, 8 (44%) were SARS-CoV-2 IgM/IgG positive, and 6 (33%) had a presumed household or community contact to someone with SARS-CoV-2 infection (Table 1). Seventeen MIS-C patients had a history of fever, or documented fever, while the one remaining patient met the MIS-C criteria due to demise with COVID-19 (9). The 18 MIS-C patients compared to 23 non-MIS-C patients presented with documented fever in hospital for >24 h (94% vs. 30%, p < 0.001), fatigue/lethargy (41% vs. 4%; p = 0.006), and lymphadenopathy (33% vs. 0%; p = 0.004). Other signs and symptoms are shown (Table 2A). All MIS-C patients had >2 organ systems involved (100% vs. 34.8%, p < 0.001) (Table 2B); with gastrointestinal symptoms (72% vs. 17%; p < 0.001) including/nausea (39% vs. 9%; p = 0.028), dermatological symptoms (56% vs. 0%; p < 0.001) with mucositis (28% vs. 0%; p = 0.011); neurological 33.3%, hematological/coagulopathic (67% vs. 4%; p < 0.001), respiratory 50% with pleural effusions (17% vs. 0%; p = 0.077) and cardiac symptoms (17% vs. 0%; p = 0.077) (Tables 2A, B), with elevated troponin-T (17% MIS-C), myocardial dysfunction (5.6%), and coronary arteritis (5.6%) (Tables 3, 4). Of the 18 MIS-C patients, 44% (8) fulfilled the criteria for Kawasaki disease; one was SARS-CoV-2 RT PCR positive, four were SARS-CoV-2 IgG/IgM positive, and the remaining three were serologically negative with a history of community or household contact. There was the one sudden death (5.6%) in a child who was SARS-CoV-2 RT-PCR positive with a comorbidity.

Table 2B. Description of mucocutaneous manifestations in the hospitalized children and adolescents with MIS-C.
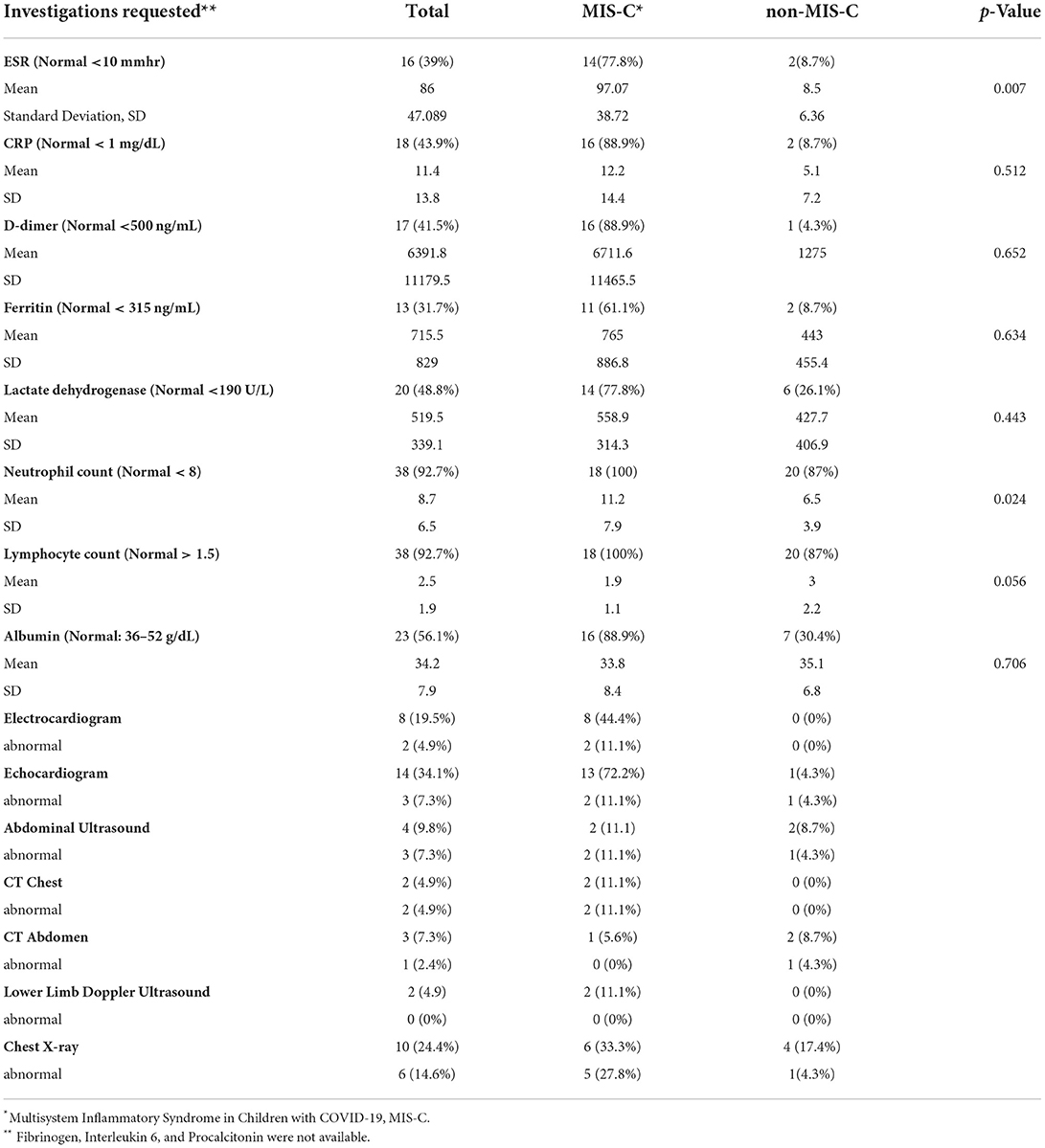
Thirteen (72%) MIS-C cases had ≥4 abnormal inflammatory biomarkers, including D-dimers, C-reactive protein, ESR, ferritin, troponins, lactate dehydrogenase, neutrophils, platelets, lymphocytes, and albumen (Tables 4, 5). The MIS-C group's inflammatory markers were significantly more deranged than the non-MIS-C group, which included neutrophilia, anemia, and elevated ESR (Tables 4, 5). MIS-C cases had more abnormal radiological features, including pulmonary embolism, coronary arteritis, lung consolidation, pleural effusion, and pulmonary opacities than those presenting without MIS-C (Table 5). Thirteen (72.2%) echocardiograms were done in the MIS-C group, and 11.1% had abnormalities including coronary arteritis, mild mitral regurgitation, elevated pulmonary pressures, and a small pericardial effusion in a patient with Sickle cell disease (Table 4).
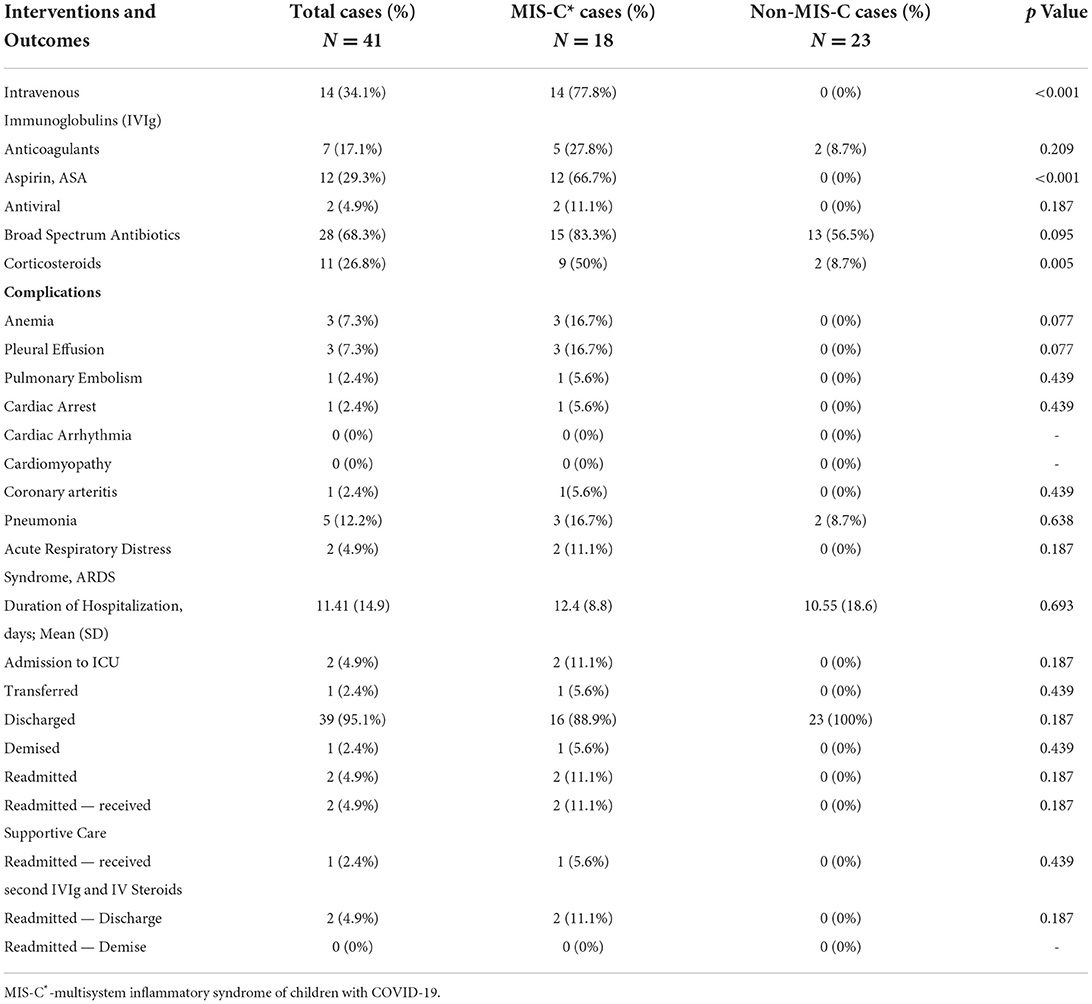
Table 5. Medical Intervention and outcomes of children hospitalized with COVID-19, MIS-C* vs. non-MIS-C.
Patients from both groups were treated for their presenting complaints (Table 5). Fourteen of the MIS-C group received intravenous immune gammaglobulin (77.8%), 12 (66.7%) received aspirin, and 9 (50%) high-dose corticosteroids. Other treatments included appendectomy in 2 (8.7%) non-MIS-C patients who presented with acute appendicitis. Antibiotics were administered in both MIS-C and non-MIS-C patients (Table 5).
Thirty-nine (95.1%) of 41 hospitalized children were discharged to follow-up. One (2.4%) patient with MIS-C commenced IVIgG, corticosteroids, and aspirin and was transferred to home country and successfully completed treatment. Readmissions occurred in three children. The first was a child with homozygous sickle cell disease (HbSS) who was hospitalized and treated for MIS-C with IVIgG, intravenous steroids, aspirin and improved. Two weeks later, she was readmitted with headaches, unilateral weakness of the lower limb, and worsening systemic inflammation requiring exchange blood transfusion, the second course of IVIgG, intravenous corticosteroids, and long-term anticoagulation, with the resolution of weakness by day 3. Another child with leukemia was readmitted ten days after initial hospital discharge and treatment for MIS-C, with altered mental status and delirium, which also resolved by day three. The third was a neonate who was born to a SARS-CoV-2 positive mother who was admitted with prolonged respiratory distress and small for gestational age. She was readmitted with significant neurodevelopmental challenges and autoimmune myasthenia gravis. There was one death (2.4%) from sudden myocardial dysfunction 13 days after testing SARS-CoV-2 RT-PCR positive, in a child who had Hemoglobin SC disease (HbSC) disease and was receiving hemodialysis for preexisting chronic kidney and bony metabolic disease.
Discussion
This is the first formal report of COVID-19 in children from the Caribbean, in the multiethnic Jamaican subpopulation, describing the rate of admission, severity of illness, outcomes, and the differences between those children who had MIS-C and those who were acutely ill without MIS-C throughout various age ranges and comorbidities. These children presented in the first 15 months of the novel pandemic, when pediatric COVID-19 clinical epidemiology, case definitions, and treatment guidelines, were being defined globally, including here in Jamaica. Historically, it is important to track this disease in the Caribbean that is also an important subregion of the world. Our series is also different in that all hospitalized children were carefully identified and evaluated prospectively “case by case” for SARS-CoV-2 infection and MIS-C. The CDC published publicly available diagnostic criteria to identify MIS-C cases and also provided the resources to perform the relevant diagnostic investigations and administer early treatment with IVIgG. Training and active case surveillance may have contributed to identifying children and adolescents with a wider spectrum of diseases, including milder less severe cases, which were diagnosed and treated earlier in the illness with immune-modulating and anti-inflammatory agents, which probably aborted the inflammatory process earlier and likely contributed to our documented better outcomes.
This ambispective cohort study has also enabled improved clinical identification and development of best practices for SARS-CoV-2 infection in children and contributes to public health policy in Jamaica and treatment of children with SARS-CoV-2 infection and MIS-C from the Caribbean and similar developing countries. During the first 15 months of the pandemic, 79 children and adolescents who presented through UHWI's emergency room were later found to have evidence of SARS-CoV-2 infection and MIS-C. Forty-one of these 79 children were hospitalized, and all had written infectious diseases clinical consultations. Eighteen (44%) of the 41 hospitalized patients fulfilled criteria for MIS-C when the CDC's case definition was consistently and conscientiously applied. These data suggest that MIS-C may be more common than is recognized. Children from all age groups studied presented with SARS-CoV-2 infection. These cases occurred while SARS-CoV-2 infections in children, MIS-C, and the clinical and laboratory diagnostic criteria were being globally defined and as the treatment recommendations evolved. This case series captures children hospitalized during the COVID-19 pandemic in the first 15 months of its evolution in one regional academic medical center in a developing Caribbean island nation.
Overall, the patients did well, with or without MIS-C. They did not have fluid refractory shock or require inotropes, vasopressors, volume expanders, or invasive ventilation. Only 11.1% (2) MIS-C vs. 0% non-MIS-C patients required intensive care for noninvasive ventilation. This is significantly less than reported previously. In contrast, a meta-analysis of MIS-C patients revealed that 65.8% developed shock, 79.1% required admission to the intensive care unit (ICU), and 33.0% required mechanical ventilation (29). In Southern Turkey, Spain, and England, it was reported that 17.3, 84.4, and 50% of children with MIS-C presented with shock, respectively; while 28.8, 13.3, and 79% required ICU admission (30–32). In the USA, 51.0% received vasopressors and 15.0% mechanical ventilation in New York, and a national surveillance study in the US of children with MIS-C reported 45.1% had shock and required vasopressors and 9.4% required invasive ventilation over the first three waves of the pandemic (33, 34). While a pediatric case series in Qatar reported 71.4% requiring inotropes while in intensive care (35). In the developing world, reports from Mexico and Pakistan reported 23 and 25% of children with MIS-C in shock, respectively (36, 37).
The significantly improved outcomes that were observed in our study may be multifactorial. There was increased surveillance with a high index of suspicion by clinicians, widespread educational sessions on COVID-19 and MIS-C, early and throughout the pandemic. This led to increased knowledge and application of the CDC's MIS-C case definition which were simple to interpret and implement. Case surveillance and pediatric infectious disease consultations led to early referrals to and within our regional academic hospital, prompt diagnosis and aggressive treatment of MIS-C with IV immunoglobulin, steroids, and aspirin, as well as monitoring of inflammatory markers, which likely led to more favorable outcomes with successful hospital discharge rates for patients with MIS-C (94.4%) and without MIS-C (100%). In our study, IV immunoglobulin was given to 77.8% of our MIS-C patients and 50% also received IV glucocorticoids. This may be beneficial in resource-constrained settings, such as Jamaica. However, IV immunoglobulin is costly and may affect accessibility and outcomes in children as the pandemic progresses. A study from the United States reported initial treatment with IV immunoglobulin and IV glucocorticoids at diagnosis of MIS-C led to reduced left ventricular dysfunction and need for vasopressors than if IV immunoglobulin was administered alone (38). Measures must be implemented to ensure that all children have equal access to these lifesaving medications once MIS-C is diagnosed. WHO has since recommended steroids for the treatment of MIS-C in developing countries and other resource-constrained settings, where IV immunoglobulin may be unavailable.
The outcomes in our study may be linked primarily to the B.1.1.7 (Alpha) SARS-CoV-2 “variant of concern,” the ancestral strain, and other minor variants, which were circulating in Jamaica during the period of our study (24–26). This may be equated to the experiences in other countries, although most reported more severe MIS-C outcomes during the same 15-month period of the global pandemic.
The majority of our patients were “Afro-Caribbean.” The ethnically diverse nature of being “Caribbean” may also be protective. A prospective cohort study in the US reported “Black” and “Hispanic” race as predictors for hospitalization in children with COVID-19; 19.5% of hospitalized children required intensive care (39). Apolipoprotein E4 (apoE4) occurs high in frequency in individuals of African descent as compared to Europeans or Asians and was considered to be a predictor of rapid and severe illness in COVID-19, as it leads to robust immune responses (40, 41). Jamaica, a Caribbean Island, is considered ethnically-diverse (National Motto – ‘Out of Many, One People') because of the many populations that initially settled in the region, including mixes of European, African, Asian, Indian, Indonesian Javanese, Chinese, and Aboriginal Indians (42).
Children with MIS-C had a wide array of organ-system involvement and abnormal inflammatory markers. Both simple, more accessible inflammatory markers, such as complete blood count, lactate dehydrogenase, ESR, and CRP, as well as the more costly and less accessible markers in the developing world, such as D-dimers, ferritin, and procalcitonin, were made available to our patients and were abnormal. These are all characteristic of the multisystemic inflammatory nature of MIS-C (41–48). Just under a quarter of the MIS-C cases were SARS-CoV-2 RT-PCR positive and of the RT-PCR negative MIS-C cases, 44.4% had positive IgG/IgM antibodies and the remaining 33.3% had confirmed, or presumed contact usually with infected household family members, especially during the periods of widespread and intense community transmission. This is congruent with the post-infectious nature of MIS-C, which develops up to six weeks or more after initial exposure to SARS-CoV-2. Many institutions in Jamaica do not have access to SARS-CoV-2 antibody testing, despite its availability in our academic center. The lack of testing could lead to evidence of SARS-CoV-2 being missed and families' unawareness of their child's initial exposure, or acute infection, which may be asymptomatic. Despite these challenges, conscientious application of the CDC's case definition during intense community transmission, assisted clinical diagnosis and implementing treatment promptly, since community transmission increases the likelihood of SARS-CoV-2 exposure. Antibody testing and high index of clinical suspicion also improved MIS-C diagnosis and facilitated treatment. A study from the USA reported that MIS-C followed their COVID-19 community peaks in two to five weeks (49). All patients in our study who were identified clinically as MIS-C without serological evidence (33%), demonstrated multisystem involvement and abnormal inflammatory markers, without an alternate etiology to explain their symptoms. All children treated for MIS-C had improved clinical findings and inflammatory markers once therapy was started.
Cardiac decompensation, though present in under one-fifth of our sample population, was not a significant feature in our study, in contradistinction to other published reports (29, 31, 39, 41–46, 48). Our findings may be attributed to a disease that was not very severe, associated with prompt diagnosis and treatment. The variant alpha strain or the ethnicity may also be significant (23, 24, 41, 42). Challenges in accessing echocardiograms and/or electrocardiograms in the acute setting in our developing country may have also led to radiological under-representation. However, our patients did not have clinical cardiac decompensation, and troponins, when performed, were not frequently abnormal. One patient, with multiple comorbidities including Hemoglobin SC disease and chronic renal failure requiring hemodialysis, had sudden cardiac dysfunction and died 13 days after his positive SARS-CoV-2 RT-PCR result. The prevalence of comorbidities was similar in our MIS-C and non MIS-C patients. Globally, comorbidities have been noted to lead to more severe outcomes (30–33, 36–39, 49, 50). Children and adolescents should therefore be prioritized for COVID-19 vaccinations within their age bands, once authorized and accessible. However, COVID-19 vaccines may be challenging to access for pediatric cases in developing countries and the elderly and those with comorbidities are at higher risk for death from COVID-19 and are usually prioritized ahead of children (20).
Laboratory confirmation of SARS-CoV-2 infection was not uniformly available throughout the study for a small minority of the patients, as the global pandemic evolved, laboratory investigations were being refined and community exposures were uniformly accepted in all of the diagnostic criteria (8–12). MIS-C, a post-inflammatory response, occurs up to six weeks or more after the initial SARS-CoV-2 exposure, which may be associated with an initial symptomatic or asymptomatic infection. Other investigations were not readily available acutely, in our developing country setting. However, 72% of patients with MIS-C obtained echocardiograms later and few had cardiac involvement. A few patients did not return or respond to telemedicine follow-up visits to determine post-hospital ambulatory outcomes.
In conclusion, of 79 children and adolescents who presented with SARS-CoV-2 infection and/or MIS-C to UHWI, the regional academic Medical Center in Jamaica during the first 15 months of the global pandemic, 52% were hospitalized and of these 44% were diagnosed with MIS-C when the CDC's case definition was uniformly and consistently applied. MIS-C seemed more common than is recognized. Non-MIS-C patients were mild and were treated successfully with standard care. In symptomatic non-MIS-C patients, it was often challenging to ascribe overlapping clinical features to the comorbidities or to their acute SARS-CoV-2 infection (e.g., Sickle cell disease, asthma, appendicitis). Ninety-four percent of MIS-C patients were successfully treated promptly with IV gamma globulin, steroids, and/or aspirin most likely expediting their recovery before cardiopulmonary decompensation and the need for inotropes, vasopressors, or invasive ventilation. One death from myocardial dysfunction occurred in a child with premorbid homozygous sickle cell disease and chronic renal failure who was receiving renal replacement therapy. While MIS-C cases were not very severe in this series, the high index of suspicion, widespread training (51–54), active surveillance, and correct application of the CDC's MIS-C diagnostic criteria, with prompt diagnosis and treatment likely contributed to early diagnosis of more cases and improved outcomes in children in this resource-constrained multi-ethnic Caribbean developing country.
Author's note
An abstract was published, and a poster presentation of this manuscript has been displayed and was virtually presented by Crista-Lee Shahine Berry to the American Academy of Pediatrics (AAP), National Conference and Exhibition, on 8–12 October 2021 in the Section on “Global Health Program” (VH1309), at the Philadelphia Convention Center, Pennsylvania, USA. The paper was also uploaded to the preprint server on 26 November, 2021 viz: Berry C, Melbourne-Chambers R, Harrison A. Anzinger J. Gordon Johnson K., Deyde V, Christie CDC. “COVID-19 Clinical Characteristics and Outcomes in Children Aged <16 years at the University Hospital of the West Indies, Jamaica in 2020-2021.” Preprint–medRxiv 2021.11.26.21266916; doi: 10.1101/2021.11.26.21266916.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Mona Campus Research Ethics Committee of the University of the West Indies and protocol clearance by the Division of Global HIV and Tuberculosis (DGHT) Science Integrity Branch of the United States Centers for Disease Control and Prevention (CDC). Clinical data were collected from clinical records only (and in the context of an epidemic), hence there was exemption for consent/assent from patients, parents and guardians. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
C-LB, RM-C, AH, JA, and CC acquired the data. C-LB, K-AG-J, and VD performed data analysis, and everyone commented on the proposal, the work and data interpretation throughout. C-LB and CC concepted, designed the work, drafted and revised the work. All authors contributed to the process throughout and approved the final version submitted for publication.
Funding
The project described was supported by the Centers for Disease Control and Prevention CDC under the terms of project number: CDC#19JM3710P1001.
Acknowledgments
The authors acknowledge the support Donovan McGrowder and the Chemical Pathology Laboratory of the University Hospital of the West Indies for performing highly specialized investigations, including inflammatory markers. Reayah Francis is acknowledged for assistance with data entry and Captain Douan Kirivong (CDC) and also Charmaine Neath-Hewitt, Jacqueline McLean, and Trudy-Ann Blair-Wallace (UWI, Mona Campus) for administrative support. We thank the medical records department for their support. We are grateful and appreciative to the entire health care team of the University Hospital of the West Indies, especially the pediatric consultants, residents, nurses, pharmacists, and laboratory staff who ably assisted in providing holistic care to these children. We thank the affected children and families and hope our interventions enabled them to improve their quality of life. Finally, we are grateful to the editor and reviewers whose expertise assisted in improving the quality of the final manuscript as published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies.
References
1. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). Available online at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed August 16, 2022).
2. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. (2020) 323:1335. doi: 10.1001/jama.2020.4344
3. Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. (2020) 382:2302–15. doi: 10.1056/NEJMoa2006100
4. Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T. Coronavirus Disease 2019 in Children — United States, February 12–April 2, 2020 MMWR Morb Mort Wkly Rep. (2020) 69:422–26. doi: 10.15585/mmwr.mm6914e4
5. Ludvigsson J. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. (2020) 109:1088–95. doi: 10.1111/apa.15270
6. Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis K. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. (2020) 159:1571–74. doi: 10.1053/j.gastro.2020.05.079
7. West DW. Acute Care Exclusive: National Alert as ‘Coronavirus-Related Condition May be Emerging in Children. HSJ for Healthcare Leaders. Available online at: https://www.hsj.co.uk/acute-care/exclusive-national-alert-as-coronavirus-related-condition-may-be-emerging-in-children/7027496.article (accessed August 16, 2022).
8. Royal College of Paediatrics Child Health. Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated With COVID-19. www.Rcpch.Ac.Uk. Available online at: https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf May 2020.
9. Centers for Disease Control Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated With Coronavirus Disease 2019 (COVID-19). May 14, 2020. Available online at: https://emergency.cdc.gov/han/2020/han00432.asp (accessed February 2, 2022).
10. World Health Organization b. Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. Available online at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed August 16, 2022).
11. American Academy of Pediatrics. What is the Case Definition of Multisystem Inflammatory Syndrome in Children (MISC)? Available online at: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance/ February 10, 2021.
12. American College of Rheumatology. Clinical Guidance for Pediatric Patients With Multisystem Inflammatory Syndrome in Children (MIS-C) Associated With SARS-CoV-2 and Hyperinflammation in COVID-19. Available online at: https://www.rheumatology.org/Portals/0/Files/ACR-COVID-19-Clinical-Guidance-Summary-MIS-C-Hyperinflammation.pdf October 19, 2021 (accessed 2 February, 2022).
13. Martin B, DeWitt PE, Russell S, Anand A, Bradwell KR, Bremer C, et al. Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US National COVID cohort collaborative. JAMA Network Open. (2022) 5:e2143151. doi: 10.1001/jamanetworkopen.2021.43151
14. Götzinger F Santiago-García B Noguera-Julián A Lanaspa M Lancella L Calò Carducci FI et al for the ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. (2020) 4:653–61. doi: 10.1016/S2352-4642(20)30177-2
15. Nachega JB, Sam-Agudu NA, Machekano RN, Rabie H, van der Zalm MM, Redfern A, et al. for the African Forum for Research and Education in Health (AFREhealth) COVID-19 Research Collaboration on Children and Adolescents. Assessment of clinical outcomes among children and adolescents hospitalized with COVID-19 in 6 Sub-Saharan African Countries. JAMA Pediatr. (2022) 176:e216436. doi: 10.1001/jamapediatrics.2021.6436
16. López-Medina E, Camacho-Moreno G, Brizuela ME, Dávalos DM, Torres JP, Ulloa-Gutierrez R, et al. Factors associated with hospitalization or intensive care admission in children with COVID-19 in Latin America. Front Pediatr. (2022). doi: 10.3389/fped.2022.868297
17. Nayak S, Panda PC, Biswal B, Agarwalla SK, Satapathy AK, Jena PK, et al. For the EICOMISC Study Group. Eastern India Collaboration on Multisystem Inflammatory Syndrome in Children (EICOMISC): a multicenter observational study of 134 cases. Front Pediatr. (2022) 10:834039. doi: 10.3389/fped.2022.834039
18. Berg SK, Palm P, Nygaard U, Bundgaard H, Petersen MNS, Rosenkilde S, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. (2022) 6:240–48. doi: 10.1016/S2352-4642(22)00004-9
19. Marks KJ, Whitaker M, Agathis NT, Anglin O, Milucky J, et al. Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19 — COVID-NET, 14 states, march 2020–february 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:429–36. doi: 10.15585/mmwr.mm7111e2
20. WHO. Strategy to Achieve Global Covid-19 Vaccination by mid-2022. Available online at: https://cdn.who.int/media/docs/default-source/immunization/covid-19/strategy-to-achieve-global-covid-19-vaccination-by-mid-2022.pdf?sfvrsn=5a68433c_5&download=true (accessed August 16, 2022).
21. UNICEF. Impact of COVID-19 on Children and Families in Latin America and the Caribbean. Available online at: https://www.unicef.org/lac/media/14381/file/UNICEF_LACRO_COVID19_impact.pdf (accessed August 16, 2022).
22. Christie CDC, Thompson T, Webster-Kerr K. COVID-19 was games in the caribbean-round one. Covid-19 Pand: Case Stud. Opin. (2020) 1:68-76. Available online at: https://researchinfotext.com/article-details/COVID-19-War-Games-in-the-Caribbean–ndash–Round-One
23. COVID-19 COVID-19 Ministry of Health Jamaica 2020 and 2021. Available online at: https://jamcovid19.moh.gov.jm/ (accessed August 16, 2022).
24. Ministry Ministry of Health Wellness Jamaica. COVID-19 UK Variant Confirmed in Local Samples Tested. Available online at: https://jis.gov.jm/covid-19-uk-variant-confirmed-in-local-samples-tested/ Jamaica Information Service, 1 May, 2021
25. Ministry Ministry of Health Wellness Jamaica. COVID-19 Delta Variant Present in Jamaica. Available online at: https://jis.gov.jm/covid-19-delta-variant-present-in-jamaica/ Jamaica Information Service, 18 August, 2021
26. Jamaican Ministry of Health Wellness. Omicron BA2 variant now in Jamaica. Available online at: https://www.moh.gov.jm (accessed August 16, 2022).
27. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, Zambrano-Achig P, Del Campo R, Ciapponi A, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS ONE. (2020) 15:e0242958. doi: 10.1371/journal.pone.0242958
28. Wang P. Combination of serological total antibody and RT-PCR test for detection of SARS-CoV-2 infections. J Virol Methods. (2020) 283:113919. doi: 10.1016/j.jviromet.2020.113919
29. Yasuhara J, Watanabe K, Takagi H, Sumitomo N. Kuno, T. COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatric Pulmonol. (2021) 56:837–48. doi: 10.1002/ppul.25245
30. Tolunay O, Çelik O, Arslan U, Orgun I, Demir A, Demir H, et al. (2021) Multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19: a case series experience in a Tertiary Care Hospital of Southern Turkey. Jour Trop Pediatr. (2021) 67:fmab050. doi: 10.1093/tropej/fmab050
31. García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, Balcells Ramírez J, Slöcker Barrio M, Leóz Gordillo I, et al. for the Spanish Pediatric Intensive Care Society working group on SARS-CoV-2 infection. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care. (2020) 24:666. doi: 10.1186/s13054-020-03332-4
32. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. for the PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259–69. doi: 10.1001/jama.2020.10369
33. Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem inflammatory syndrome in children associated with severe acute Respiratory syndrome Coronavirus 2 Infection (MIS-C): a multi-institutional study from New York City. J Pediatr. (2020) 224:24–9. doi: 10.1016/j.jpeds.2020.06.045
34. Miller AD, Zambrano LD, Yousaf AR, Abrams JY, Meng L, Wu MJ, et al. Multisystem inflammatory syndrome in Children-United States, February 2020-July 2021. Clin Infect Dis. (2021) 5:ciab1007. doi: 10.1093/cid/ciac253
35. Hasan MR, Al Zubaidi K, Diab K, Hejazi Y, Bout-Tabaku S, Al-Adba B et al. COVID-19 related multisystem inflammatory syndrome in children (MIS-C): a case series from a tertiary care pediatric hospital in Qatar. BMC Pediatr. (2021) 21:267. doi: 10.1186/s12887-021-02743-8
36. Macias-Parra M, Fortes-Gutierrez S, Aguilar-Gomez N, Diaz-Garcia L, Otero-Mendoza F, Arias De La Garza E, et al. Clinical and epidemiological characteristics of pediatric patients diagnosed with COVID-19 in a Tertiary Hospital in Mexico City. J Tropical Pediatr. (2021) 67:fmab025. doi: 10.1093/tropej/fmab025
37. Sadiq M, Aziz OA, Kazmi U, Hyder N, Sarwar M, Sultana N, Bari A, et al. Multisystem inflammatory syndrome associated with COVID-19 in children in Pakistan. Lancet Child Adolesc Health. (2020) 4:e36–7. doi: 10.1016/S2352-4642(20)30256-X
38. Son MBF, Murray N, Friedman K, Young CC, Newhams MM, Feldstein LR, et al. Multisystem inflammatory syndrome in children — initial therapy and outcomes. N Engl J Med. (2021) 385:23–34. doi: 10.1056/NEJMoa2102605
39. Howard LM, Garguilo K, Gillon J, LeBlanc K, Seegmiller AC, Schmitz JE, et al. The first 1000 symptomatic pediatric SARS-CoV-2 infections in an integrated health care system: a prospective cohort study. BMC Pediatr. (2021) 21:403. doi: 10.1186/s12887-021-02863-1
40. Goldstein MR, Poland GA, Graeber ACW. Does apolipoprotein E genotype predict COVID-19 severity? QJM. (2020) 113:529–30. doi: 10.1093/qjmed/hcaa142
41. Goldstein MR, Poland GA, Graeber CW. Coronavirus disease 2019, multisystem inflammatory syndrome in children, apolipoprotein E4, and race. J Peds. (2020) 29:313–14. doi: 10.1016/j.jpeds.2020.10.072
42. Premdas RR. Identity, ethnicity, and the Caribbean homeland in an era of globalization. Social Identities. (2011) 17:811–32. doi: 10.1080/13504630.2011.606676
43. Aronoff SC, Hall A, Del Vecchio M. The natural history of severe acute respiratory Syndrome Coronavirus 2–related multisystem inflammatory syndrome in children: a systematic review. J Pediatr Infect Dis Soc. (2020) 9:746–51. doi: 10.1093/jpids/piaa112
44. Ahmed M, Advani SA, Moreira AM, Zoretic SZ, Martinez JM, Chorath KC, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. (2020) 26:100527. doi: 10.1016/j.eclinm.2020.100527
45. Consiglio CR, Cotugno N, Sardh F, Pou C, Landegren N, Palma P, et al. The Immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. (2020) 183:968–981.e7. doi: 10.1016/j.cell.2020.09.016
46. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. for the Overcoming COVID-19 Investigators and the CDC COVID-19 Response Team. Multisystem inflammatory syndrome in US children and adolescents. N Engl Jour Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
47. Hoang A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F, et al. COVID-19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. (2020) 24:100433. doi: 10.1016/j.eclinm.2020.100433
48. Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. (2021) 38:51–7. doi: 10.1016/j.prrv.2020.08.001
49. Belay ED, Abrams J, Oster ME, Giovanni J, Pierce T, Meng L, et al. Trends in geographic and temporal distribution of US Children with multisystem inflammatory syndrome during the COVID-19 Pandemic. JAMA Pediatr. (2021) 175:837–45. doi: 10.1001/jamapediatrics.2021.0630
50. Woodruff RC, Campbell AP, Taylor CA, Chai CJ, Kawasaki B, Meek J, et al. for the COVID-NET Surveillance Team. Risk factors for severe COVID-19 in children. Pediatrics. (2021) 149:e2021053418. doi: 10.1542/peds.2021-053418
51. “COVID-19, Pandemic Preparedness Conference”. COVID-19 Conference Series - #1, Faculty of Medical Sciences Teaching and Research Complex, University of the West Indies, Mona Campus, Lecture Theatre 3, streamed live on Thursday, 5 March, 2020. Available online at: https://www.youtube.com/watch?v=i0Z9Za6ofi8; >28,000 views (accessed August 1, 2022).
52. “COVID-19:, Approaching Code Red Teleconference”. COVID-19 Conference Series - #2, Faculty of Medical Sciences, University of the West Indies, Mona Campus, streamed live on 17 April, 2020. Available online at: https://www.youtube.com/watch?v=KnO-FB8nDT8; 25,147 views (accessed August 1, 2022).
53. “COVID-19: “COVID-19: Impact on Children and Families in the Caribbean Now and Beyond”; COVID-19 Conference Series - #3 Faculty Faculty of Medical Sciences University University of the West Indies Mona Campus streamed streamed live on 16 May 2020. Available online at: https://www.youtube.com/watch?v=22isFWHKYuw&t=11s; > 7,800 views (accessed August 1, 2022).
54. “COVID-19:, Making New Year Resolutions Teleconference”. COVID-19 Conference Series - #4, Faculty of Medical Sciences, University of the West Indies, Mona Campus, streamed live on January 24, 2021. Available online at: https://www.youtube.com/results?search_query=COVID-19%3A+Making+New+Year+Resolutions+telecon; > 3,200 views (accessed August 1, 2022).
Keywords: COVID-19 outcomes in Jamaican Children, COVID-19, children, adolescents, multisystem inflammatory syndrome of children, MISC, Jamaica, Caribbean
Citation: Berry C-LS, Melbourne-Chambers RH, Harrison AN, Anzinger JJ, Gordon-Johnson K-AM, Deyde VM and Christie CDC (2022) Hospitalized children with SARS-CoV-2 infection and MIS-C in Jamaica: A dive into the first 15 months of the novel pandemic. Front. Pediatr. 10:904788. doi: 10.3389/fped.2022.904788
Received: 04 April 2022; Accepted: 03 August 2022;
Published: 07 September 2022.
Edited by:
Kenneth E. Remy, Case Western Reserve University, United StatesReviewed by:
Nerea Domínguez-Pinilla, University Hospital, SpainMonty Mazer, Rainbow Babies and Children's Hospital, United States
Copyright © 2022 Berry, Melbourne-Chambers, Harrison, Anzinger, Gordon-Johnson, Deyde and Christie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Celia Dana Claire Christie, Q2VsaWEuQ2hyaXN0aWVTYW11ZWxzQHV3aW1vbmEuZWR1Lmpt; Y2VsaWFjaHJpc3RpZTAyQGdtYWlsLmNvbQ==
 Crista-Lee Shahine Berry
Crista-Lee Shahine Berry Roxanne Helene Melbourne-Chambers
Roxanne Helene Melbourne-Chambers Abigail Natalie Harrison
Abigail Natalie Harrison Joshua James Anzinger
Joshua James Anzinger Kelly-Ann Maxorinthia Gordon-Johnson6
Kelly-Ann Maxorinthia Gordon-Johnson6 Varough Mohamed Deyde
Varough Mohamed Deyde Celia Dana Claire Christie
Celia Dana Claire Christie