
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 07 July 2022
Sec. Pediatric Critical Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.899193
Background: The benefits of breast milk oral care in mechanically ventilated preterm infants remain controversial. This study aimed to systematically review the evidence on the benefits of breast milk oral care in mechanically ventilated preterm infants.
Methods: The randomized controlled trials of breast milk oral care for mechanically ventilated preterm infants were searched in EMBASE, PubMed, Cochrane Library, Web of Science, WANFANG Date and China National Knowledge Infrastructure databases. The retrieval language was limited to Chinese and English, and the final search was conducted until March 2022. Outcome measures included ventilator-associated pneumonia (VAP), mechanical ventilation time (MVT), length of stay (LOS), necrotizing enterocolitis (NEC), late-onset sepsis, mortality during hospitalization, time of full intestinal feeding and time of full oral feeding. Two researchers independently screened the literature, extracted the data, and conducted the literature quality assessment. Meta-analysis was mainly performed using RevMan 5.3.
Results: Eight articles involving 1,046 preterm infants were included. Our meta-analysis showed that compared with the control group, breast milk oral care could reduce the incidence of VAP [RR = 0.41, 95% CI (0.23, 0.75), P = 0.003] and NEC [RR = 0.54, 95% CI (0.30, 0.95), P = 0.03], and shorten the MVT [MD = −0.45, 95% CI (−0.73, −0.18), P = 0.001] and LOS [MD = −5.74, 95% CI (−10.39, −1.10), P = 0.02]. There were no significant differences in the mortality during hospitalization [RR = 0.94, 95% CI (0.67, 1.33), P = 0.74], the incidence of late-onset sepsis [RR = 0.79, 95% CI (0.40, 1.59), P = 0.51], the time of full intestinal feeding [MD = −2.42, 95% CI (−5.37, 0.52), P = 0.11] and the time of full oral feeding [MD = −3.40, 95% CI (−10.70, 3.91), P = 0.36] between the two groups.
Conclusions: Oral care of breast milk can reduce the incidence of VAP and NEC, shorten MVT and LOS in mechanically ventilated preterm infants. However, due to the quality and quantity limitations of the included studies, larger sample size and more strictly designed clinical trials are still needed in the future to further confirm the findings of this study.
Preterm birth is defined as being born before 37 weeks, and more than 41,000 babies worldwide are born before this gestational age every day (1). Preterm infants have immature lungs, lack surfactant, and immature respiratory control mechanisms, and mechanical ventilation (MV) is often required, which plays a vital role in reducing the early mortality of this population (2, 3). However, the establishment of artificial airway destroys the normal protective mechanism of respiratory tract in preterm infants, and the aspiration of oropharyngeal pathogenic bacteria is easy to cause ventilator-associated pneumonia (VAP) (4). Several studies have shown that strict and effective oral care can prevent oropharyngeal bacterial colonization and reduce the incidence of VAP (5–7). Therefore, oral care for mechanically ventilated preterm infants is particularly important to the prevention of VAP.
Breast milk is rich in immune active factors, which may be absorbed by the oropharyngeal mucosa of preterm infants, and can effectively inhibit the activity of oropharyngeal pathogens (8). Studies have shown that the use of breast milk as oral care solution is safe and effective, conducive to the rehabilitation of preterm infants, and can reduce the length of stay (LOS) (9). Recently, a meta-analysis published by Ma et al. (6) showed that oral administration of colostrum had a positive effect on reducing the incidence of VAP and necrotizing enterocolitis (NEC) in preterm infants and shortening the time of full intestinal feeding. However, this meta-analysis is mainly aimed at preterm infants (whether with or without MV), and the evidence for the effects of breast milk oral care (BMOC) on mechanically ventilated preterm infants remains insufficient. In addition, there is still controversy about the impact of BMOC on mechanically ventilated preterm infants, such as mechanical ventilation time (MVT) and LOS (6, 9–11). Therefore, this study focused on mechanically ventilated preterm infants and explored the impact of BMOC intervention on mechanically ventilated preterm infants through systematic evaluation and meta-analysis, so as to provide the scientific basis for clinical nursing.
This systematic review with meta-analysis was conducted based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary File 1). The research protocol was registered and updated with PROSPERO under the registration number CRD42021273155.
The databases, including Embase, PubMed, Cochrane Library, Web-of-Science, WANFANG Data and China National Knowledge Infrastructure (CNKI) databases were systematically searched up to March 2022, with Chinese and English language restrictions. At the same time, the references in the included literature and the articles quoting the included literature were traced to supplement and obtained the relevant literature. Medical subject words (MESH) and free words were used for retrieval, and the search words included: “Milk, Human,” “Colostrum,” “Intubation, Intratracheal,” “Ventilators, Negative Pressure,” “Ventilators, Mechanical,” “Child,” “Infant, Newborn,” “Infant, Premature,” and “Oral Hygiene.” We adjusted the search strategy and the full search strategy was presented in a supplemental document (Supplementary File 2).
Endnote software was used for literature management and screening of duplicate studies. Qualified studies were independently identified and cross-checked by two researchers according to the inclusion and exclusion criteria below, and consulted by a third reviewer when necessary.
1) Population (P): Mechanically ventilated preterm infants. To improve the homogeneity and comparability of the study, we adjusted the study population from mechanically ventilated infants to mechanically ventilated preterm infants.
2) Intervention (I): Use BMOC.
3) Comparison (C): Use non-BMOC products (normal saline or sterile water) or blank control.
4) Outcome (O): Clinical treatment related indicators of children, including VAP, MVT, LOS, NEC, late-onset sepsis, mortality during hospitalization, time of full intestinal feeding, and time of full oral feeding.
5) Study design (S): Randomized controlled trial (RCT).
1) Preterm infants undergoing surgery.
2) The experimental group adopted BMOC combined with other intervention measures.
3) Retrospective studies, reviews, systematic evaluations, case reports, letters, reviews, or editorials.
4) Unable to obtain complete data.
Ambiguities about data extraction were resolved after discussion and consulting a third reviewer when necessary. The data extracted included: (1) Study title and country; (2) The first author and year of publication; (3) Research design; (4) Sample size; (5) Gestational age and birth weight; (6) MV baseline; (7) Method, time and frequency of intervention; (8) Outcomes: VAP, MVT, LOS, NEC, late-onset sepsis, mortality during hospitalization, time of full intestinal feeding, and time of full oral feeding.
The quality of the included studies was evaluated and cross-checked by two researchers, respectively, according to the Cochrane Risk of Bias Tool (12), and consulted by a third reviewer when necessary. There are seven bias risk items. Make a judgment of “low risk,” “high risk,” and “unclear risk,” for each criterion, respectively. The included studies were rated as low bias risk if all of their bias risk items were rated as “low risk.” The included studies were rated as unclear bias risk if their bias risk items were rated as “low risk” and “risk unclear,” or all were rated as “unclear risk.” The included studies were rated as high bias risk if all of their bias risk items were rated as “high risk.”
RevMan 5.3 software was used for statistical analysis, and P < 0.05 was considered to be statistically significant. Statistical data such as the incidence of VAP and NEC, mortality during hospitalization, and late-onset sepsis used relative risk (RR) and 95% confidence intervals (CI) as effect statistics, while quantitative data such as MVT, LOS, time of full intestinal feeding, and time of full oral feeding used selective mean difference (MD) and 95% CI as effect statistics.
The heterogeneity among the included studies was represented by the I2 value. If the heterogeneity among the included studies was not significant (I2 < 50%, P > 0.1), the fixed-effect model was used to calculate the combined statistics. If there was significant heterogeneity among studies (I2 ≥ 50%, P ≤ 0.1), the random-effect model was used for analysis. Sensitivity analysis was performed by changing the effect model and using a one-by-one elimination method to evaluate the stability and reliability of the results. In addition, publication bias was detected by funnel plot.
A database search identified 385 studies and a manual search included 1 study (13). One hundred and nineteen duplicated studies were excluded, and a total of 267 studies needed preliminary screening. After deleting obviously irrelevant literature by reading the title and abstract, the remaining 24 studies needed to be read in full. After reading the full text, 8 studies were finally identified for inclusion in this review (9, 10, 13–18). The process and results of literature screening are shown in Figure 1.
The risk of bias for all included studies is shown in Figures 2, 3. Among the 8 included studies, 1 study was evaluated as “low risk of bias” (13), 1 as “unclear risk of bias” (18), and 6 as “high risk of bias” (9, 10, 14–17).
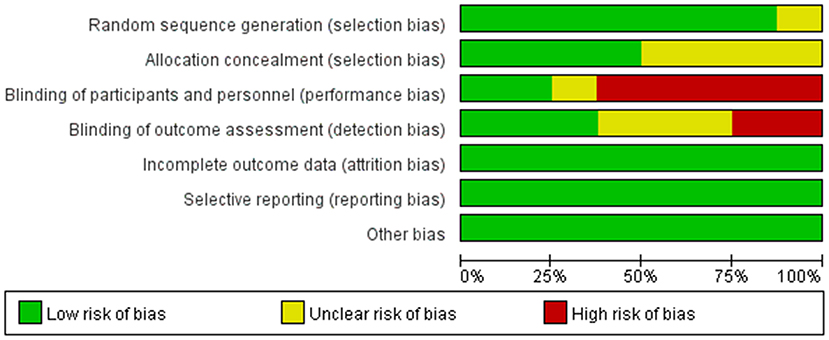
Figure 3. Authors' judgments about each risk of bias item presented as percentages across all included studies.
The study characteristics are shown in Table 1. The study covered the period from 2015 to 2021. Sample sizes ranged from 12 to 260 (1,046 in total). Among the 8 included studies, 6 were published in English (9, 10, 13–16), and 2 in Chinese (17, 18); 3 studies were conducted in China (16–18), 2 in India (9, 14), 1 in Egypt (10), 1 in South Korea (13), and 1 in the United States (15). Six studies used breast milk drop as an intervention (9, 10, 13–16), and two studies used breast milk scrub as an intervention (17, 18). The frequency of interventions ranged from every 2–8 h per day. There was no significant difference in gestational age and birth weight among the study groups (P > 0.05), which were comparable in baseline characteristics.
The incidence of VAP was reported in 6 studies (9, 10, 13–15, 18), with low heterogeneity among studies (I2 = 0%, P = 0.47) and using a fixed-effect model analysis. The results showed that the incidence of VAP in the BMOC intervention group was significantly lower than in the control group [RR = 0.41, 95% CI (0.23, 0.75), P = 0.003] (Figure 4A). By changing the effect model for sensitivity analysis, the results did not change significantly, suggesting that the results were relatively stable [RR = 0.41, 95% CI (0.22, 0.77), P = 0.005].
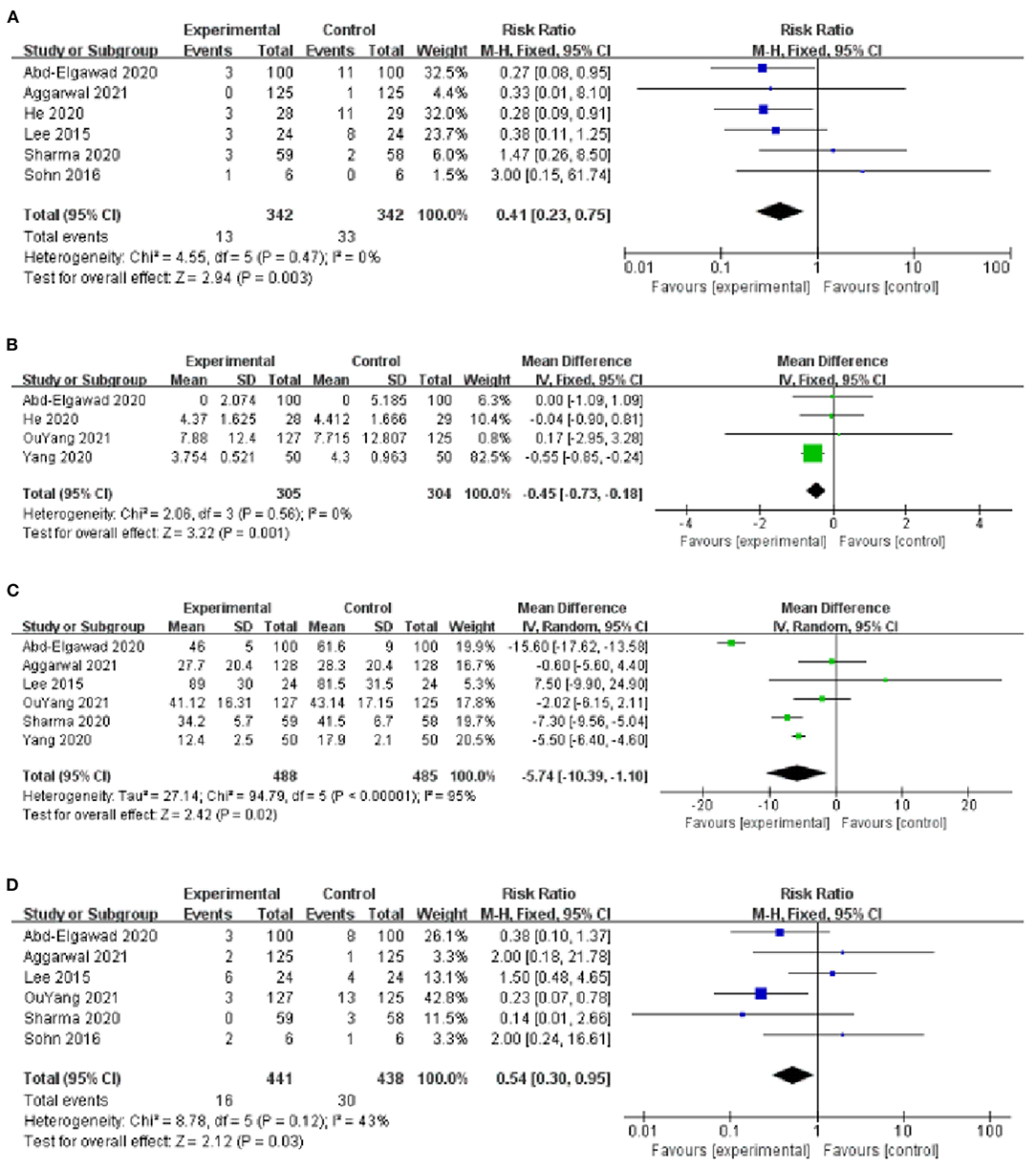
Figure 4. (A) Forest plot of the incidence of VAP. (B) Forest plot of the MVT. (C) Forest plot of the LOS. (D) Forest plot of the incidence of NEC.
Four studies provided data on MVT (10, 16–18), with low heterogeneity among studies (I2 = 0%, P = 0.56) and using a fixed-effect model analysis. The results showed that the MVT of the BMOC intervention group was shorter than the control group [MD = −0.45, 95% CI (−0.73, −0.18), P = 0.001] (Figure 4B). By changing the effect model for sensitivity analysis, the results did not change, suggesting that the results were relatively stable [MD = −0.45, 95% CI (−0.73, −0.18), P = 0.001].
The LOS was reported in 6 studies (9, 10, 13, 14, 16, 17), with significant heterogeneity among studies (I2 = 95%, P < 0.001) and using a random-effect model analysis. The results showed that the LOS of BMOC intervention group was shorter than the control group [MD = −5.74, 95% CI (−10.39, −1.10), P = 0.02] (Figure 4C). Sensitivity analysis was performed by using the one-by-one elimination method. After excluding the study of Abd-Elgawad et al. (10), the combined results did not change significantly [MD = −4.49, 95% CI (−6.83, −2.16), P < 0.001], but the heterogeneity decreased (I2 = 64%, P = 0.03), indicating that this study may be the source of heterogeneity (Table 2).
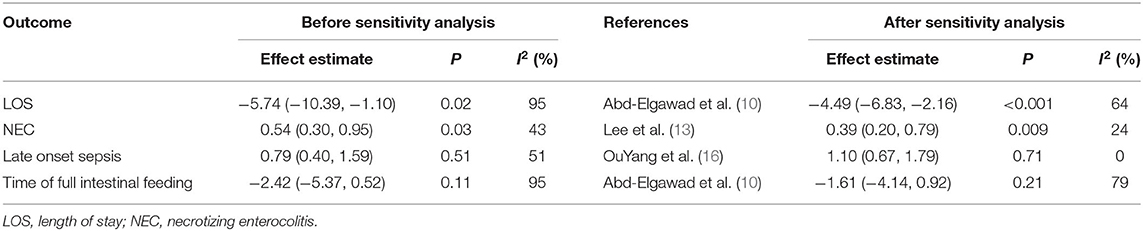
Table 2. The sensitivity analysis results of LOS, NEC, late onset sepsis and time of full intestinal feeding.
Six studies provided data on the incidence of NEC (9, 10, 13–16), with moderate heterogeneity among studies (I2 = 43%, P = 0.12) and using a fixed-effect model analysis. The results showed that the incidence of NEC in the BMOC intervention group was lower than in the control group [RR = 0.54, 95% CI (0.30, 0.95), P = 0.03] (Figure 4D). Sensitivity analysis was performed by using the one-by-one elimination method. After excluding the study of Lee et al. (13), the combined results did not change significantly [RR = 0.39, 95% CI (0.20, 0.79), P = 0.009], but the heterogeneity decreased (I2 = 24%, P = 0.26), indicating that this study may be the source of heterogeneity (Table 2).
Six studies evaluated the effect of BMOC on the mortality during hospitalization (9, 10, 13–15, 17), with low heterogeneity among studies (I2 = 0%, P = 0.64), and the fixed-effect model analysis was used to consolidate the effect value. The results showed that there was no significant difference in mortality during hospitalization between the two groups [RR = 0.94, 95% CI (0.67, 1.33), P = 0.74] (Figure 5A). By changing the effect model for sensitivity analysis, the results did not change significantly [RR = 0.93, 95% CI (0.61, 1.44), P = 0.76], suggesting that the results were relatively stable.
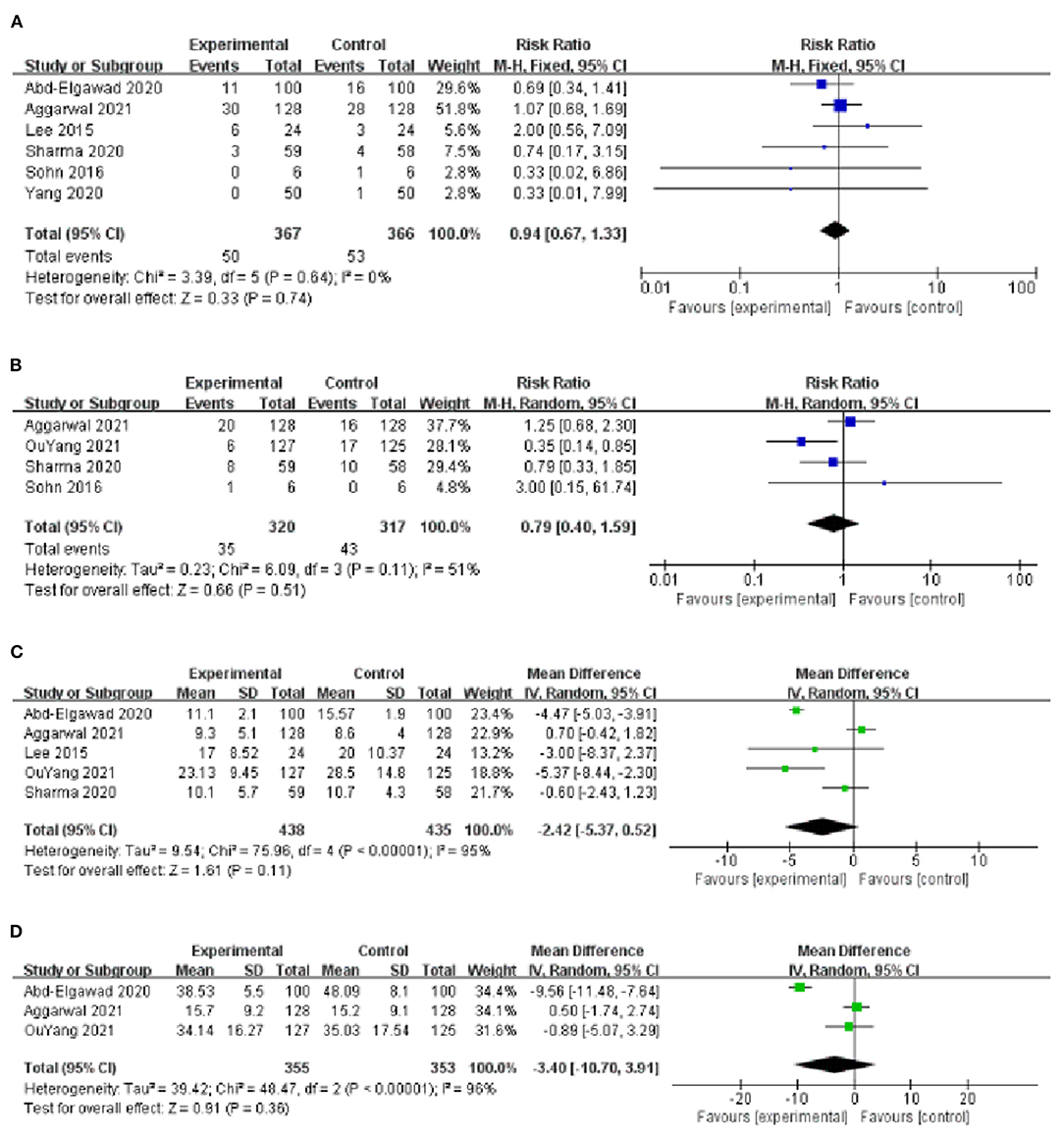
Figure 5. (A) Forest plot of the mortality during hospitalization. (B) Forest plot of the late-onset sepsis. (C) Forest plot of the time of full intestinal feeding. (D) Forest plot of the time of full oral feeding.
Four studies reported the effect of BMOC on late-onset sepsis (9, 14–16), with moderate heterogeneity among studies (I2 = 51%, P = 0.11) and using a random-effect model analysis. The results showed that there was no significant difference in late-onset sepsis between the two groups [RR = 0.79, 95% CI (0.40, 1.59), P = 0.51] (Figure 5B). Sensitivity analysis was performed by using the one-by-one elimination method. After excluding the study of Yang et al. (16), the combined results did not change significantly [RR = 1.10, 95% CI (0.67, 1.79), P = 0.71], but the heterogeneity decreased (I2 = 0%, P = 0.55), indicating that this study may be the source of heterogeneity (Table 2).
Five studies reported the effect of BMOC on the time of full intestinal feeding (9, 10, 13, 14, 16), with significant heterogeneity among studies (I2 = 95%, P < 0.001) and using a random effect model analysis. The results showed that there was no significant difference in the time of full intestinal feeding between the two groups [MD = −2.42, 95% CI (−5.37, 0.52), P = 0.11] (Figure 5C). Sensitivity analysis was performed by using the one-by-one elimination method. After excluding the study of Abd-Elgawad et al. (10), the combined results did not change significantly [MD = −1.61, 95% CI (−4.14, 0.92), P = 0.21], but the heterogeneity decreased (I2 = 79%, P = 0.002), indicating that this study may be the source of heterogeneity (Table 2).
Three studies reported the effect of BMOC on the time of full oral feeding (10, 14, 16), with significant heterogeneity among studies (I2 = 96%, P < 0.001) and using a random-effect model analysis. The results showed that there was no significant difference in the time of full oral feeding between the two groups [MD = −3.40, 95% CI (−10.70, 3.91), P = 0.36] (Figure 5D).
The funnel plots of VAP, NEC and the mortality during hospitalization were visually symmetrical and did not show a significant risk of publication bias (Figures 6A–C). The funnel plot of LOS was visually asymmetric, suggesting the possibility of publication bias (Figure 6D).
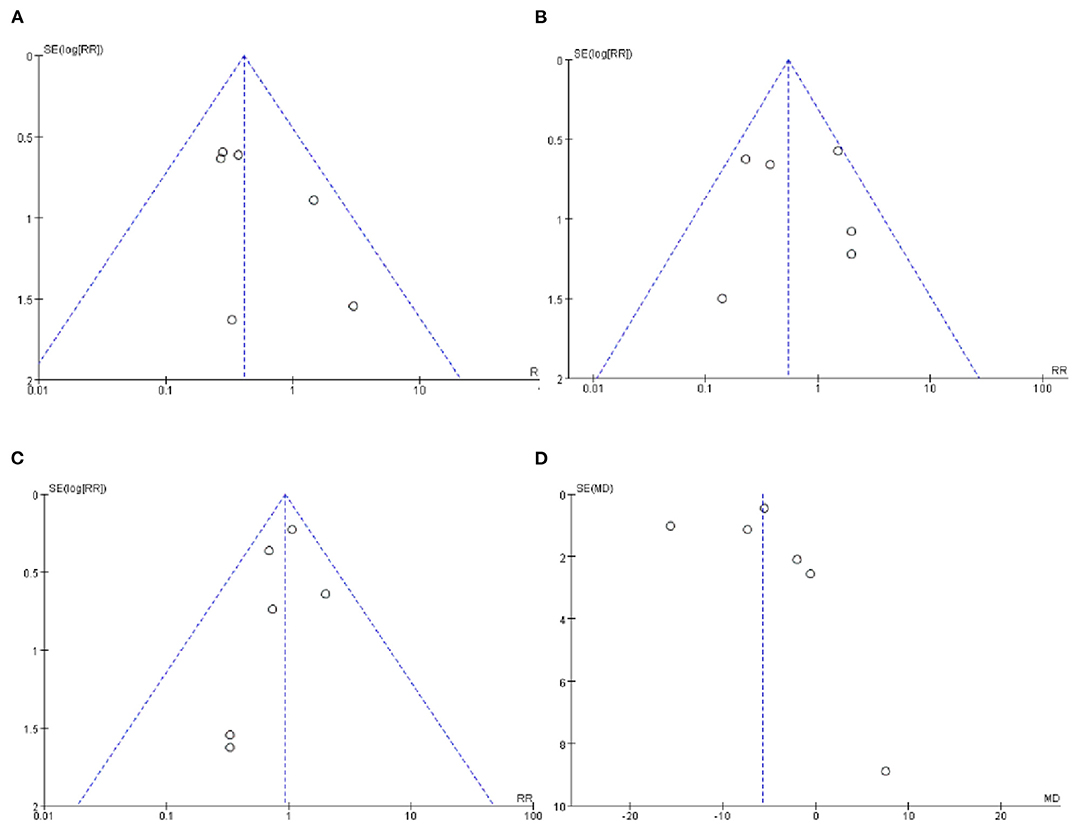
Figure 6. (A) Funnel plot of the incidence of VAP. (B) Funnel plot of the incidence of NEC. (C) Funnel plot of the mortality during hospitalization. (D) Funnel plot of the LOS.
In recent years, BMOC is reported to be beneficial to infant health, which is safe, feasible, and cost effective (19). This systematic review and meta-analysis identified 8 studies involving 1,046 preterm infants, and the results showed that BMOC had a positive effect on reducing the incidence of VAP and NEC and shortening MVT and LOS for mechanically ventilated preterm infants, however, no effect was observed on late-onset sepsis, mortality during hospitalization, time of full intestinal feeding and time of full oral feeding between the two groups.
VAP is one of the most common hospital infections in the neonatal intensive care unit, and the main reason is that the artificial airway destroys the natural mechanical barrier of oral and nasal mucosa against pathogens, which provides a direct and rapid way for oropharyngeal colonization bacteria to enter the lower respiratory tract (4). Once VAP occurs in mechanically ventilated preterm infants, it is extremely easy to cause difficulty in weaning, prolong MVT and LOS, and increase hospitalization cost and mortality (20–22). Studies have shown that poor oral hygiene or dry mouth is one of the common factors affecting bacterial colonization, and effective oral care can prevent the colonization of pathogenic microorganisms in the upper respiratory tract and reduce the incidence of VAP (23). At present, the commonly used oral care solutions in clinical practice are normal saline (24), chlorhexidine (25) and sodium bicarbonate (26), etc., but all of them have various deficiencies. Research have shown that secretory IgA (sIgA), lactoferrin, and other active components in breast milk can be absorbed through oropharyngeal mucosa, activate the infant immune system, and kill pathogenic microorganisms such as streptococcus pneumonia and chlamydia spores. At the same time, it can form a protective layer on the oral surface of preterm infants, block bacterial colonization of mucosa and play a first-line defense role (27, 28). Oral care with breast milk can exert the immune effect of breast milk and reduce the incidence of VAP by reducing oropharyngeal and endotracheal pathogens in children with MV (29), which is consistent with the results of this study. As an objective indicator, VAP has small heterogeneity and high credibility and therefore highlights the usefulness of BMOC to prevent the occurrence of VAP among mechanically ventilated preterm infants.
The results of this study showed that BMOC can shorten MVT and LOS, which is closely related to the reduction of VAP. This result is consistent with the view of Li et al. (30), who found that MVT and LOS in patients without VAP infection were significantly lower than in those with VAP infection. Breast milk is the most natural, safest, and completely natural food for the growth of infants (31). It is the best choice for newborns, especially for preterm infants. It can not only provide nutrients such as amino acids but also enhance their resistance to pathogenic microorganisms, which is conducive to the rehabilitation of mechanically ventilated newborns.
We also found that BMOC can reduce the incidence of NEC. NEC is the most common and serious cause of gastrointestinal-related incidence rate and mortality in preterm infants (32). Probiotics such as bifidobacteria in breast milk can help preterm infants establish intestinal flora, improve the capacity of the intestinal immune system, and protect intestinal mucosa from excessive stimulation (33). A recent study had shown that sIgA in breast milk could prevent the development of NEC in preterm infants (34), Rodriguez et al. (27) inhaled colostrum into the oral cavity of preterm infants through syringes, and found sIgA and lactoferrin in their urine and tracheal aspirates, suggesting that breast milk could be absorbed by mucosa, which could reasonably explain our results.
However, no significant difference in the mortality during hospitalization was observed between the two groups. The reason may be that the mortality of preterm infants was affected by many factors, such as gestational age, weight, infectious diseases, etc. (35). In our study, we observed no significant difference in late-onset sepsis between the two groups, and the reason may be that the stimulation effect of breast milk on lactoferrin was not persistent (36). The existing evidence in this study cannot prove that BMOC has an impact on the time to reach full intestinal feeding and full oral feeding, which may be related to the different gestational age or birth weight of preterm infants, resulting in the difference of enteral feeding tolerance (37). In addition, there are differences in feeding strategies implemented in different hospitals, which may also affect the results (38).
Although we adjusted the study population from mechanically ventilated infants to mechanically ventilated preterm infants to improve the homogeneity and comparability of the study, there are still some limitations in this systematic review and meta-analysis. First, only 6 databases were retrieved in this meta-analysis, and the language was limited to Chinese and English, so there may be incomplete retrieval. Second, there was heterogeneity, due to differences between studies in the implementation of BMOC, such as intervention methods (including drop and scrub), intervention start time, intervention end time, intervention frequency and dose. However, we were unable to conduct subgroup analysis due to the small number of included studies. It is suggested that clinical medical staff should pay attention to the effects of different BMOC time, methods, frequency, and doses on mechanically ventilated preterm infants, to seek the best intervention scheme. Third, the funnel plot of LOS suggested a possible risk of publication bias, so caution should be taken in interpreting the results. In the future, large sample and high-quality RCTs are still needed to further verify the safety and effectiveness of BMOC.
According to the summary analysis of the currently available data, the use of BMOC is helpful to reduce the incidence of VAP and NEC, and shorten MVT and LOS in mechanically ventilated preterm infants. The results should be treated cautiously as the differences in the intervention schemes of different studies. In the future, it is still necessary to carry out large sample and high-quality RCT studies to further clarify the application effect of BMOC on mechanically ventilated preterm infants.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
MC and YL: research design. MC: later stages of the design and writing of this paper. MC and LL: identifying and screening of the included randomized controlled trials. MC and YP: data analysis and evaluation. YL and LC: manuscript revision. All authors suggestions to the data analysis, helping in interpretation of results, and final manuscript reading and approval.
This study was supported by the Fujian Provincial Finance Special Project (2020CZ011), the Guiding Project of Fujian Science and Technology (2021Y0023), and Fujian Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors gratefully thank the editors and reviewers for their suggestions as well as all the experts and members of our group for their help and advice. We confirm that all listed authors meet the authorship criteria, and all authors are in agreement with the content of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.899193/full#supplementary-material
1. Platt MJ. Outcomes in preterm infants. Public Health. (2014) 128:399–403. doi: 10.1016/j.puhe.2014.03.010
2. Barreira ER, Munoz GO, Cavalheiro PO, Suzuki AS, Degaspare NV, Shieh HH, et al. Epidemiology and outcomes of acute respiratory distress syndrome in children according to the Berlin definition: a multicenter prospective study. Crit Care Med. (2015) 43:947–53. doi: 10.1097/CCM.0000000000000866
3. Bresesti I, Agosti M, Lakshminrusimha S, Lista G. Synchronized invasive mechanical ventilation. Clin Perinatol. (2021) 48:813–24. doi: 10.1016/j.clp.2021.07.008
4. Soussan R, Schimpf C, Pilmis B, Degroote T, Tran M, Bruel C, et al. Ventilator-associated pneumonia: the central role of transcolonization. J Crit Care. (2019) 50:155–61. doi: 10.1016/j.jcrc.2018.12.005
5. Hu JM. Research Progress on the effect of oral and pharyngeal administration of breast milk on preterm infants. Chin J Nurs. (2017) 52:107–10. doi: 10.3761/j.issn.0254-1769.2017.01.023
6. Ma A, Yang J, Li Y, Zhang X, Kang Y. Oropharyngeal colostrum therapy reduces the incidence of ventilator-associated pneumonia in very low birth weight infants: a systematic review and meta-analysis. Pediatr Res. (2021) 89:54–62. doi: 10.1038/s41390-020-0854-1
7. Fernandez Rodriguez B, Peña Gonzalez L, Calvo MC, Chaves Sanchez F, Pallas Alonso CR, de Alba Romero C. Oral care in a neonatal intensive care unit. J Matern Fetal Neonatal Med. (2017) 30:953–7. doi: 10.1080/14767058.2016.1192599
8. Lewis ED, Richard C, Larsen BM, Field CJ. The importance of human milk for immunity in preterm infants. Clin Perinatol. (2017) 44:23–47. doi: 10.1016/j.clp.2016.11.008
9. Sharma D, Kaur A, Farahbakhsh N, Agarwal S. Role of oropharyngeal administration of colostrum in very low birth weight infants for reducing necrotizing enterocolitis: a randomized controlled trial. Am J Perinatol. (2020) 37:716–21. doi: 10.1055/s-0039-1688817
10. Abd-Elgawad M, Eldegla H, Khashaba M, Nasef N. Oropharyngeal administration of mother's milk prior to gavage feeding in preterm infants: a pilot randomized control trial. JPEN J Parenter Enteral Nutr. (2020) 44:92–104. doi: 10.1002/jpen.1601
11. Thibeau S, Boudreaux C. Exploring the use of mothers' own milk as oral care for mechanically ventilated very low-birth-weight preterm infants. Adv Neonatal Care. (2013) 13:190–7. doi: 10.1097/ANC.0b013e318285f8e2
12. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
13. Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, et al. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. (2015) 135:e357–66. doi: 10.1542/peds.2014-2004
14. Aggarwal R, Plakkal N, Bhat V. Does oropharyngeal administration of colostrum reduce morbidity and mortality in very preterm infants? A randomised parallel-group controlled trial. J Paediatr Child Health. (2021) 57:1467–72. doi: 10.1111/jpc.15529
15. Sohn K, Kalanetra KM, Mills DA, Underwood MA. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol. (2016) 36:106–11. doi: 10.1038/jp.2015.157
16. OuYang X, Yang CY, Xiu WL, Hu YH, Mei SS, Lin Q. Oropharyngeal administration of colostrum for preventing necrotizing enterocolitis and late-onset sepsis in preterm infants with gestational age ≤ 32 weeks: a pilot single-center randomized controlled trial. Int Breastfeed J. (2021) 16:59. doi: 10.1186/s13006-021-00408-x
17. Yang XJ, Pan GF, Wang PL, Wu YJ. Effect of oral nursing with breast milk on preventing nosocomial infection of newborns. Int J Nurs. (2020) 39:3559–62. doi: 10.3760/cma.j.cn221370-20190527-01096
18. He L, Xiao J, Wen EY. Clinical randomized controlled study of colostrum oral immune therapy to prevent ventilator-associated pneumonia in premature infants. Chongqing Med. (2020) 49:2751–4. doi: 10.3969/j.issn.1671-8348.2020.16.033
19. Li DF, Shi CX, Zhao L, Shi FZ, Jiang ML, Kang WQ. Prevention of neonatal ventilator-associated pneumonia through oral care with the combined use of colostrum and sodium bicarbonate. Eur Rev Med Pharmacol Sci. (2021) 25:2361–6. doi: 10.26355/eurrev_202103_25275
20. Hu XH, Wang J. Analysis of factors related to ventilator associated pneumonia in neonates. Sichuan Med J. (2015) 36:p646–649. doi: 10.16252/j.cnki.issn1004-0501-2015.04.020
21. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. (2013) 60:49–74. doi: 10.1016/j.pcl.2012.10.002
22. Sen AC, Morrow DF, Balachandran R, Du X, Gauvreau K, Jagannath BR, et al. Postoperative infection in developing world congenital heart surgery programs: data from the international quality improvement collaborative. Circ Cardiovasc Qual Outcomes. (2017) 10:e002935. doi: 10.1161/CIRCOUTCOMES.116.002935
23. Almirall J, Boixeda R, de la Torre MC, Torres A. Aspiration pneumonia: a renewed perspective and practical approach. Respir Med. (2021) 185:106485. doi: 10.1016/j.rmed.2021.106485
24. Su LD. Effect of oral care intervention on prevention of neonatal thrush. Chin J Nurs. (2010) 16:2771–2. doi: 10.3760/cma.j.issn.1674-2907.2010.23.017
25. Kusahara DM, Peterlini MA, Pedreira ML. Oral care with 0.12% chlorhexidine for the prevention of ventilator-associated pneumonia in critically ill children: randomised, controlled and double blind trial. Int J Nurs Stud. (2012) 49:1354–63. doi: 10.1016/j.ijnurstu.2012.06.005
26. Liu M. Application of sodium bicarbonate oral nursing in the prevention of ventilator-associated pneumonia in neonates. Nurs Pract Res. (2018) 15:75–6. doi: 10.3969/j.issn.1672-9676.2018.11.032
27. Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother's colostrum to extremely low-birth-weight infants. Adv Neonatal Care. (2010) 10:206–12. doi: 10.1097/ANC.0b013e3181e94133
28. Kell DB, Heyden EL, Pretorius E. The biology of Lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front Immunol. (2020) 11:1221. doi: 10.3389/fimmu.2020.01221
29. Banupriya B, Biswal N, Srinivasaraghavan R, Narayanan P, Mandal J. Probiotic prophylaxis to prevent ventilator associated pneumonia (VAP) in children on mechanical ventilation: an open-label randomized controlled trial. Intensive Care Med. (2015) 41:677–85. doi: 10.1007/s00134-015-3694-4
30. Li Y, Liu C, Xiao W, Song T, Wang S. Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: a meta-analysis. Neurocrit Care. (2020) 32:272–85. doi: 10.1007/s12028-019-00773-w
31. Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: a review on its composition and bioactivity. Early Hum Dev. (2015) 91:629–35. doi: 10.1016/j.earlhumdev.2015.08.013
32. Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res. (2008) 63:117–23. doi: 10.1203/PDR.0b013e31815ed64c
33. Brandtzaeg P. (2010) The mucosal immune system and its integration with the mammary glands. J Pediatr. 156:S8–S15. doi: 10.1016/j.jpeds.2009.11.014
34. Gopalakrishna KP, Macadangdang BR, Rogers MB, Tometich JT, Firek BA, Baker R, et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat Med. (2019) 25:1110–5. doi: 10.1038/s41591-019-0480-9
35. Cheng DX, Zhang Q, Lu HR, Liu HF, Zhao SH. Neonatal mortality and death causes in hospital patients from 2012 to 2016. J Xi'an Jiaotong Univ. (2018) 39:106–110. doi: 10.7652/jdyxb201801023
36. Moreno-Fernandez J, Sánchez-Martínez B, Serrano-López L, Martín-Álvarez E, Diaz-Castro J, Peña-Caballero M, et al. Enhancement of immune response mediated by oropharyngeal colostrum administration in preterm neonates. Pediatr Allergy Immunol. (2019) 30:234–41. doi: 10.1111/pai.13008
37. Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, et al. Stereotypic immune system development in newborn children. Cell. (2018) 174:1277–92.e14. doi: 10.1016/j.cell.2018.06.045
Keywords: breast milk, oral care, mechanical ventilation, preterm infants, meta-analysis
Citation: Cai M, Lin L, Peng Y, Chen L and Lin Y (2022) Effect of Breast Milk Oral Care on Mechanically Ventilated Preterm Infants: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pediatr. 10:899193. doi: 10.3389/fped.2022.899193
Received: 18 March 2022; Accepted: 07 June 2022;
Published: 07 July 2022.
Edited by:
Jos M. Latour, University of Plymouth, United KingdomReviewed by:
Christoph E. Schwarz, University College Cork, IrelandCopyright © 2022 Cai, Lin, Peng, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjuan Lin, Zmp4aHlqbEAxNjMuY29t; Liangwan Chen, Y2hlbmxpYW5nd2FuQHRvbS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.