
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 15 August 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.894526
This article is part of the Research Topic Women in Neonatology: 2021 View all 9 articles
 Serena Xodo1*
Serena Xodo1* Giulia Trombetta1
Giulia Trombetta1 Lisa Celante1
Lisa Celante1 Carla Pittini2
Carla Pittini2 Lorenza Driul1,3
Lorenza Driul1,3 Angelo Cagnacci4
Angelo Cagnacci4 Ambrogio P. Londero4
Ambrogio P. Londero4Introduction: This study aimed to compare the outcomes of preterm infants given 12 vs. 24mg of betamethasone prophylaxis to understand whether a partial course of antenatal corticosteroids (CCS) could prevent or mitigate the major preterm birth complications.
Methods: This is a retrospective single-center cohort study including neonates born between 24 and 34 weeks of gestation from 2001 to 2019 at the University Hospital of Udine. The study population was divided into two groups: one group received 12 mg, and another received a 24mg dose of betamethasone before the delivery. A separate analysis was performed for single and multiple pregnancies. The two groups were evaluated for various neonatal outcomes.
Results: The study population included a total of 1,258 pregnancies and 1,543 neonates delivered between 24 and 34 weeks of gestation, of which 1,022 (803 single and 219 multiple pregnancies) were exposed to the complete CCS prophylaxis, whereas 236 (192 single and 44 multiple pregnancies) received the incomplete CCS prophylaxis. In single pregnancies, as for maternal characteristics, the most significant differences observed between the two groups are the following: a higher prevalence of spontaneous vaginal deliveries in the incomplete CCS prophylaxis (36.46 vs. 23.91%) and, by contrast, a higher prevalence of cesarean deliveries in the complete CCS prophylaxis group (75.72 vs. 63.02%). As for neonatal outcomes, the low Apgar score in the first and fifth min was significantly more prevalent in the incomplete CCS prophylaxis group compared with the complete CCS prophylaxis group. The group of incomplete CCS prophylaxis reported a higher occurrence of the following outcomes: IVH grade 3-4 (7.81 vs. 3.74%, p < 0.05), PVL (7.29 vs. 1.99% p < 0.05), ROP (23.96 vs. 18.06% p = 0.062), and RDS (84.38 vs. 78.83% p = 0.085). After adjusting for covariates, the complete CCS prophylaxis group in single pregnancies was significantly protective for IVH grade 3-4, PVL, and low Apgar's scores. Similar results were found in multiple pregnancies except for RDS.
Discussion: This retrospective single-center cohort study found that, compared with preterm infants treated with 24mg betamethasone in utero, those given half course of betamethasone had a significantly higher prevalence of IVH grade 3-4, PVL, RDS, and lower Apgar scores at 1 and 5 min. In conclusion, the evidence from this single-center retrospective study supports the preference for the complete CCS prophylaxis in women at risk of preterm birth because of its beneficial effect on the main adverse outcomes.
The ability of corticosteroids (CCS) to induce fetal lung maturation was accidentally demonstrated in 1969 by Liggins while testing the effect of dexamethasone on premature parturition of lambs (1). In 1972 Liggins and Howie finalized the first randomized controlled trial (RCT) in humans, demonstrating the effectiveness of two maternal injections of 12 mg betamethasone given 24 h apart in reducing the incidence of respiratory distress syndrome (RDS) (2). Since then, the administration of either betamethasone or dexamethasone to women at risk of imminent preterm labor has become a milestone in preventing the complications of preterm birth. The most recent Cochrane review on this issue reported a significant decrease in the risk of perinatal death, neonatal death, RDS, and intraventricular hemorrhage (IVH) with CCS given before an anticipated childbirth (3).
Recently, the scientific community raised concerns that glucocorticoid therapy during pregnancy could potentially have adverse long-term effects on infants. Two retrospective studies found that using CCS antenatally increased the risk of neurodevelopmental disorders (4, 5). However, the minimum dose required for the appearance of significant beneficial effects in preterm neonates has not yet been determined. Moreover, preterm birth could be precipitous in some circumstances, thus hampering the completion of the antenatal CCS course. In this scenario, whether or not the complete course of antenatal CCS is as effective as the partial course is still questionable.
Hence, in this current study, we decided to retrospectively compare the outcomes of preterm infants given 12 mg (incomplete CCS prophylaxis) vs. 24 mg (complete CCS prophylaxis) of betamethasone prophylaxis to understand whether a partial course of antenatal CCS could prevent or mitigate the major preterm birth complications.
This study was a retrospective single-center cohort investigation including women who delivered between January 1, 2001, and December 31, 2019, at the Department of Obstetrics and Gynecology of the University Hospital of Udine. This center is a tertiary-level maternity hospital with a neonatal intensive care unit and advanced medical facilities for the mother and the neonate. The internal review board approved the present study, which followed the dictates of the general authorization produced by the Italian Data Protection Authority in matter of processing data for scientific research. Furthermore, all ethical principles of the Helsinki Declaration were heeded.
The target population was the total of preterm newborns eligible for CCS prophylaxis. All the consecutive neonates born between 24 and 34 weeks of gestation after preterm labor with intact membranes, preterm premature rupture of membranes and medically indicated delivery were included. The exclusion criteria were gestational age equal to or >34 weeks of gestation and incomplete data about CCS prophylaxis. Population selection is described in Figure 1A.
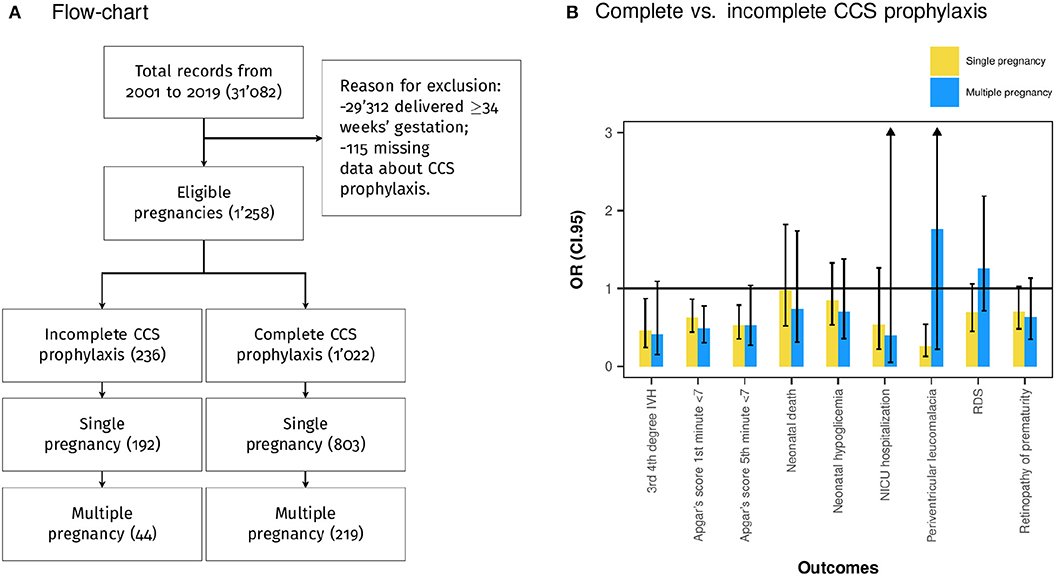
Figure 1. (A) Population selection flowchart. (B) Bivariate logistic regression analysis considering as independent variable complete corticosteroid (CCS) prophylaxis and dependent variables the assessed outcomes. The plot shows the OR and the 95% confidence interval (CI.95) pairwise for single and multiple pregnancies.
The exposure assessed in the present study was the completeness of CCS prophylaxis. Following the aim of the study, the exposure variable was coded into two levels according to the dose of betamethasone administered before delivery: one level represented the group of incomplete CCS prophylaxis (12 mg), and the other level the group of complete CCS prophylaxis (24 mg). The considered outcomes were: Apgar score (at 1st and 5th min), neonatal intensive care unit (NICU) hospitalization, neonatal hypoglycemia, RDS, periventricular leukomalacia (PVL), 3rd 4th-degree IVH, retinopathy of prematurity (ROP), and neonatal death. The covariates were: mother age, parity, mode of conception, pregnancy-related hypertensive disorders (PRHDs), gestational age at delivery, delivery mode, multiple births, neonatal sex, and neonatal weight.
A low Apgar score was expressed as <7 at the first and fifth min (6). Respiratory distress syndrome was clinically identified by the need for mechanical ventilation and oxygen for at least 48 h and the presence of radiographic chest findings (air bronchograms and reticulogranular appearance of the pulmonary parenchyma). Intraventricular hemorrhage grades were classified according to Papile's classification (7, 8). Moreover, PVL, ROP, and neonatal hypoglycemia were defined as previously described (7, 9, 10). Perinatal death was defined as death occurring <28 days of age (11). PRHDs were classified as previously described, including gestational hypertension, preeclampsia, preeclampsia superimposed on chronic hypertension, and eclampsia (12). The neonatal weight multiple of the median (MoM), also known as birth weight ratio, is the ratio between the observed birth weight and the 50th percentile of birth weight (sex-specific) at the same gestational age (7, 12, 13). Sex-specific neonatal anthropometric charts of the Italian population were used to calculate neonatal weight MoMs (14). Lastly, gestational age was determined by the last menstrual period, confirmed by ultrasound examination during the first and second trimester, and expressed in weeks.
Data were analyzed through R (version 4.2.0) (15), considering significant a two-tailed p-value < 0.05. The statistical analysis was conducted after a Kolmogorov-Smirnoff test application to assess whether the distribution of continuous variables was parametric. An independent analysis was performed for singleton and multiple pregnancies. In singleton pregnancies, where the complete CCS group consisted of 803 pregnancies, a minimum of 33 pregnancies were required in the incomplete CCS group to detect a medium effect size (0.5) in comparing the proportions of the selected outcomes with a power of 80% and significance level of 0.05 (16). In multiple pregnancies, where the complete CCS group consisted of 459 newborns, a minimum of 34 pregnancies were required in the incomplete CCS group to detect a medium effect size (0.5), comparing proportions, with a power of 80% and significance level of 0.05 (16). Bivariate analysis for continuous variables was performed, as appropriate, using the Wilcoxon test (non-parametric variables) or t-test (parametric variables). For categorical variables, the differences were tested, as appropriate, with the Chi-square test or Fisher's exact test. Bivariate and multivariate logistic regressions were also performed. The previously selected outcomes were settled as dependent variables. Meanwhile, the exposure and the covariates were settled as independent variables. The initial multivariate model included all possible variables known to be possible confounders or moderators and had a p < 0.200 at the bivariate analysis. Interaction terms were maintained in the final multivariate model only if significant, and the NA values were considered missing.
The study population included a total of 1,258 pregnancies and 1,543 neonates delivered between 24 and 34 weeks of gestation, of which 1,022 were exposed to 2 doses of betamethasone 24 h apart (group of complete CCS prophylaxis), whereas 236 received one dose of betamethasone before delivery (group of incomplete CCS prophylaxis) (Figure 1A). In the group of complete CCS prophylaxis 803 single pregnancies and 219 multiple pregnancies were included. Meanwhile, in the group of incomplete CCS prophylaxis 192 single pregnancies and 44 multiple pregnancies (Figure 1A) were enclosed. The median maternal age at delivery was 33.00 years (IQR 29.00–36.00), and the median gestational age at delivery was 31.00 weeks (IQR 28.00–32.00) (Table 1). The population characteristics of singleton and multiple pregnancies are also shown in Table 1.
Table 2 shows the differences between the complete and incomplete CCS prophylaxis among singleton pregnancies. As for maternal outcomes, the most significant differences observed between the two groups are the following: a higher prevalence of spontaneous vaginal deliveries in the incomplete CCS prophylaxis (36.46 vs. 23.91%) and, by contrast, a higher prevalence of cesarean deliveries (CD) in the complete CCS prophylaxis group (75.72 vs. 63.02%). As for neonatal characteristics, the median birthweight was 1,355 g (IQR 988-1770) in the complete CCS prophylaxis group vs. 1,299 g (IQR 941-1780) in the incomplete CCS prophylaxis group (p = 0.611). The median Apgar score at the first min was significantly lower in the incomplete CCS prophylaxis group compared with the complete CCS prophylaxis group (Table 2). And a high prevalence of low pH at the fifth min was present in the incomplete than in the complete CCS prophylaxis group (21.35 vs. 12.45%, p < 0.05). The group of incomplete CCS prophylaxis reported a higher occurrence of the following outcomes: IVH grade 3–4 (7.81 vs. 3.74%, p < 0.05), PVL (7.29 vs. 1.99% p < 0.05), ROP (23.96 vs. 18.06% p = 0.062), and RDS (84.38 vs. 78.83% p = 0.085) (Table 2). No significant differences were retrieved for the following outcomes: admission to neonatal intensive unit care, the necessity of neonatal cardiopulmonary reanimation, and neonatal death. Table 3 shows the bivariate and multivariate logistic regression analysis. After adjusting for covariates, the complete CCS prophylaxis group was significantly protective for IVH grade 3–4, PVL, low Apgar's scores, ROP, and RDS.
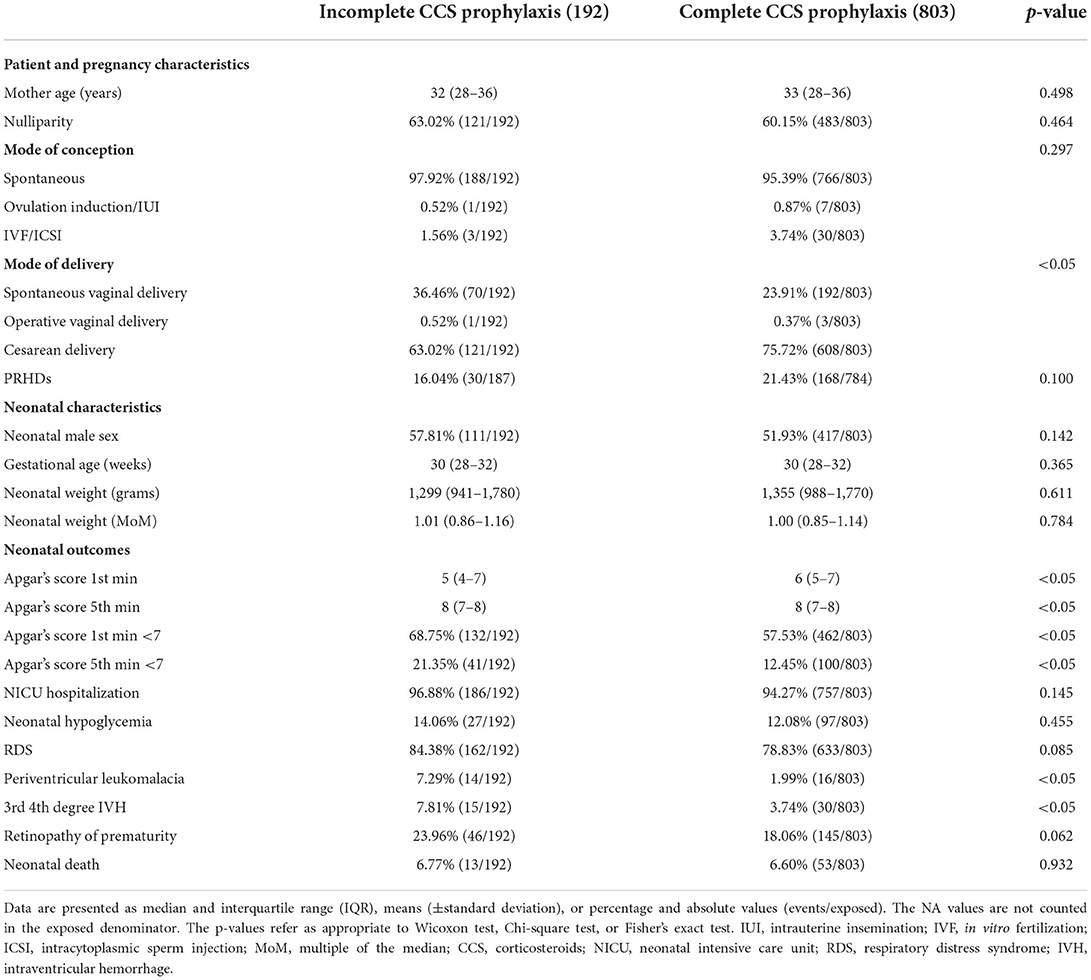
Table 2. Population description of singleton pregnancies stratified by complete or incomplete corticosteroid (CCS) prophylaxis.
Table 1 shows the characteristics of the multiple pregnancies sub-group, and Table 4 shows the differences between complete and incomplete CCS prophylaxis. In addition, Figure 1B shows pairwise the bivariate logistic regression of singleton and multiple pregnancies. The effect of complete CCS prophylaxis is similar in single and multiple pregnancies except for RDS. Furthermore, complete CCS prophylaxis was associated with lower prevalence of low Apgar score at first (p < 0.05) and fifth (p = 0.060) min, and 3rd 4th-degree IVH (p = 0.065) (Table 4).
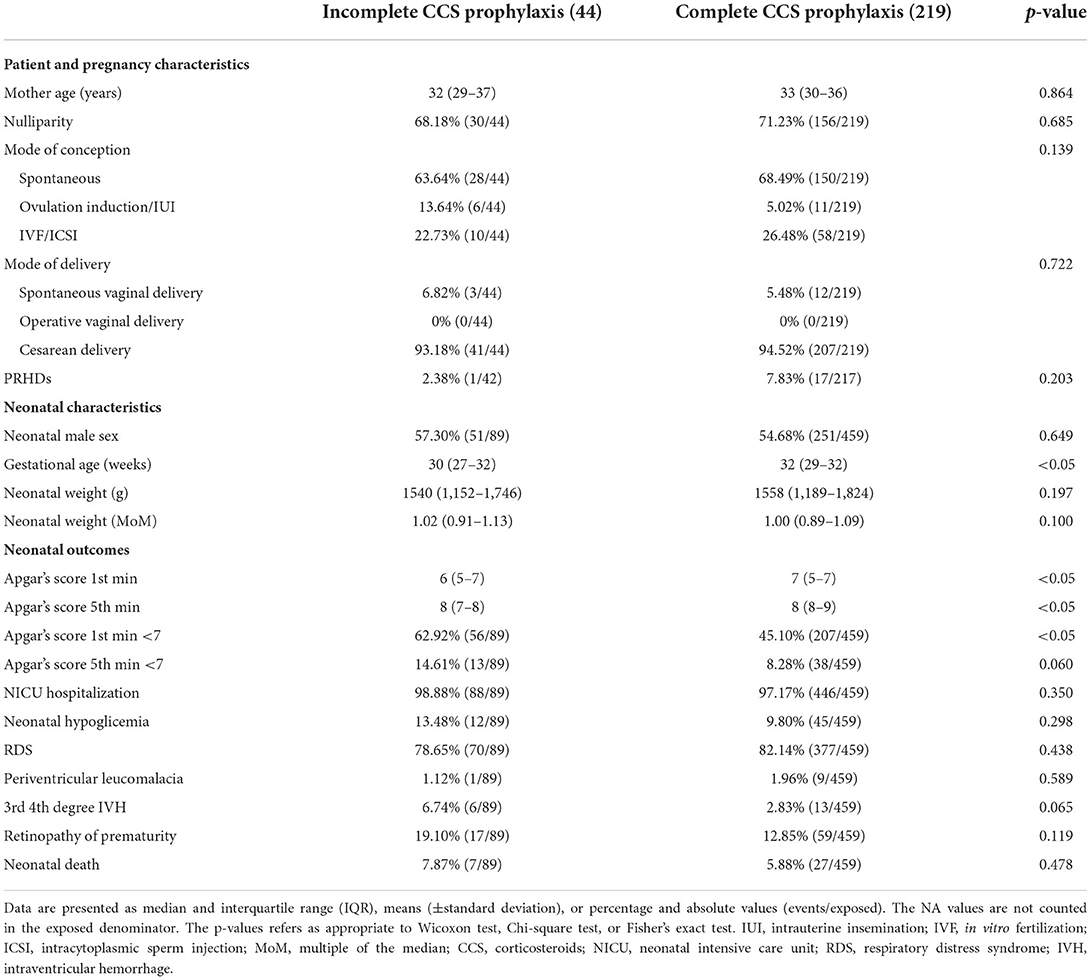
Table 4. Population description of multiple pregnancies stratified by complete or incomplete corticosteroid (CCS) prophylaxis.
This retrospective single-center cohort study found that, compared with preterm infants treated with 24 mg betamethasone in utero, in singleton pregnancies, those given half-courses of betamethasone had a significantly higher prevalence of low Apgar scores at first and fifth min, IVH grade 3–4, PVL, and RDS. Similar results were observed in multiple pregnancies except for RDS. Moreover, spontaneous vaginal delivery occurred more frequently in the group of incomplete CCS prophylaxis, whereas CD was significantly higher in the group of complete CCS prophylaxis.
The original regimen used by Liggins and Howie in their first RCT was a mixture of betamethasone phosphate 6 mg and betamethasone acetate 6 mg in 2 doses given 24 h apart (2). The phosphate preparation guarantees rapid exposure to the drug, while the acetate form offers a more sustained exposure (17). This regimen raises the 75% occupancy level of glucocorticoid receptors, providing near-maximal induction of associated genes in fetal tissues. The Authors found in another clinical trial that doubling the dose of betamethasone did not further improve the beneficial effects in preterm neonates (17). From then on, CCS has become the mainstay of prophylactic treatment in preterm birth.
Only a few studies in the literature explored the effects of different dose regimens of antenatal CCS prophylaxis, yielding conflicting results. Studies comparing half course of CCS with no CCS therapy (18–20), showed that the exposure to an incomplete course of prophylaxis decreases the rate of severe neonatal outcomes, especially at very early gestational weeks (25–27 weeks) and in non-tertiary hospitals (18). Other observational studies compared the administration of an incomplete CCS course vs. a complete CCS course (21–24) demonstrating a significant improvement of the neonatal clinical outcome with the half dose exposure. However, a discrepancy between the studies is seen for severe IVH since a significantly lower rate of severe IVH was observed in the incomplete antenatal steroids group in the oldest study (21). At the same time, this trend was not confirmed in the most recent studies (23, 24). The impact of the incomplete course of CCS prophylaxis on severe IVH rate has still to be clarified. Our results suggest that, compared with complete exposure to CCS, an incomplete exposure leads to a significantly higher rate of this unfavorable outcome. Antenatal steroid therapy has been shown to decrease the vulnerability of the germinal matrix vasculature to hemorrhage (25). It could be speculated that the steroid-induced molecular and morphological changes are dose-dependent, thus explaining why compared with the incomplete course of CCS, the complete course significantly lowers the IVH rate. According to our study, the complete CCS prophylaxis significantly decreased the prevalence of PVL, irrespective of the gestational age, as shown in the multivariate regression analysis. It has been postulated that CCS might suppress the upregulation of inflammatory cytokines, thus preventing the injury of preoligodendrocytes through the activation of microglia within the immature white matter (26). Again, the superiority of the complete CCS course over the incomplete one in reducing PVL prevalence may suggest a dose-dependent effect of CCS action.
Surprisingly, our data show that half the standard regimen given to patients with threatened preterm labor might be sufficient to gain benefits on respiratory outcomes in twin pregnancies. A recent metanalysis of 16 observational studies has shown that antenatal CCS may be beneficial in reducing the risk of RDS, mortality, IVH and PVL among preterm twin pregnancies. A sub-analysis found that subjects exposed to the complete course of steroid prophylaxis had a lower OR of RDS (27). However, our study may suggest that fetal lung maturation might be accelerated in twin pregnancies compared to singleton pregnancies. Historically, fetal lung maturity has been extensively investigated, arising conflicting results (27–30). It might be speculated that the accelerated pulmonary maturity in twins could be due to the antenatal competitive environment to which twins are exposed. Since maternal resources have to be shared in multiple pregnancies, twin fetuses respond with a slower rate of fetal growth during the third trimester, and possibly reaching the lung maturation earlier when compared with singleton fetuses.
Additionally, our study revealed that neonates treated with the incomplete CCS prophylaxis had significantly lower mean Apgar scores in the first and fifth min. Both antenatal and postnatal factors are involved in the assignment of a low Apgar score. The score may reflect an injury that occurred before birth or the adequacy of resuscitation. Moreover, Apgar score's effectiveness has been questioned in preterm neonates since their scores could be depressed by their immaturity (31). Interestingly, even after adjustment for gestational age, the Apgar score at the first and fifth min was significantly lower in the group of incomplete CCS prophylaxis, thus hinting at a potential protective role of CCS.
The higher prevalence of spontaneous vaginal deliveries in the group of incomplete CCS prophylaxis in our population reflects the rampage of preterm labor in those cases, leaving no time for the second drug injection. By contrast, the group of complete CCS prophylaxis experienced a higher prevalence of cesarean births, suggesting that they mainly account for iatrogenic preterm births. Compared to vaginal deliveries, CDs are undeservedly thought to be less traumatic for very premature infants because of the avoidance of prolonged labor. A growing body of evidence in the literature shows that compared to vaginal delivery, the CD is not protective in the peri-viable period. For example, a retrospective cohort study on 271 singleton pregnancies delivered between 22 to 29 weeks revealed that CD performed for standard obstetrical indications is associated with an increase in immediate survival, but it is not associated with an overall decrease in morbidity or mortality (32). Another study found no differences in neurodevelopment at 2 years of age in 158 neonates delivered through vaginal delivery and CD at the time of peri-viability (33). Our data confirm that CD was not protective against severe neonatal outcomes such as IVH. In addition, we found CD to be associated in multivariate logistic regression with increased odds of ROP, low Apgar's score at the first min, and RDS.
The retrospective design of our study is its main limitation. Second, there is an inherent danger in extrapolating conclusions from a cohort study that spans over a decade, given that significant advances in neonatal care have been established over the last few decades, thus hampering the generalizability of our findings. The main strength of this study is the topic considered in light of the scantiness of data in the literature. Another strength of this study is the sample size considered, which is higher than the number of patients included in other retrospective studies.
Our study might have a relevant clinical implication. In light of our results, women with threatened preterm birth should be counseled on the superiority of the complete course of CCS over the incomplete one in preventing the major complications related to preterm birth. Additionally, women with twin pregnancy and an imminent delivery should be informed that half a course of antenatal CCS might potentially improve the respiratory outcomes of preterm babies.
Further data would be required to clarify whether half the course of antenatal CCS is sufficient to improve the respiratory outcomes in twin pregnancies, as suggested by our study. If these findings would be confirmed it could be interesting to realize which is the physiological explanation behind this observation.
In conclusion, the evidence from this single-center retrospective study supports the preference for the complete CCS prophylaxis in women at risk of preterm birth because of its beneficial effect on the main adverse outcomes.
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available, but restrictions apply to the availability of these data. These data were used under license for the current study, and so are not publicly available. However, data are available upon reasonable request to the authors and with permission of the Internal Review Board. Requests to access these datasets should be directed to SX, c2VyZW5heG9kb0B5YWhvby5pdA==.
The studies involving human participants were reviewed and approved by the Internal Review Board of the Department of Medical Area (University of Udine). The present study was conducted in accordance with Helsinki Declaration and it followed the dictates of the general authorization to process personal data for scientific research purposes by the Italian Data Protection Authority. The need for an informed consent, according with national legislation, was waived by the IRB listed above because this was a retrospective cohort study. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Substantial contributions to conception and design or acquisition of data or to analysis and interpretation of data: SX, GT, LC, CP, LD, AC, and APL. Drafting the article or revising it critically for important intellectual content: SX, CP, LD, AC, and APL. All authors have read and approved the final manuscript.
The authors would like to thank the whole staff collaborating in article collection, selection, reading, and in paper writing and reviewing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CCS, corticosteroids; CD, cesarean delivery; CI.95, 95% confidence interval; ICSI, intracytoplasmic sperm injection; IQR, interquartile range; IUI, intrauterine insemination; IVF, in vitro fertilization; IVH, intraventricular hemorrhage; MoM, multiple of the median; NICU, neonatal intensive care unit; OR, odds ratio; PRHD, pregnancy-related hypertensive disorder; PVL, periventricular leucomalacia; RCT, randomized controlled trial; RDS, respiratory distress syndrome; ROP, retinopathy of prematurity.
1. Liggins GC. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. (1969) 45:515–23. doi: 10.1677/joe.0.0450515
2. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. (1972) 50:515–25. doi: 10.1542/peds.50.4.515
3. McGoldrick E, Stewart F, Parker R, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. (2020) 12:CD004454. doi: 10.1002/14651858.CD004454.pub4
4. Melamed N, Asztalos E, Murphy K, Zaltz A, Redelmeier D, Shah BR, et al. Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: a population-based study. BMJ Open. (2019) 9:e031197. doi: 10.1136/bmjopen-2019-031197
5. Räikkönen K, Gissler M, Kajantie E. Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA. (2020) 323:1924–33. doi: 10.1001/jama.2020.3937
6. Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg. (1953) 32:260–7. doi: 10.1213/00000539-195301000-00041
7. Londero AP, Rossetti E, Pittini C, Cagnacci A, Driul L. Maternal age and the risk of adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. (2019) 19:261. doi: 10.1186/s12884-019-2400-x
8. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
9. Visentin S, Londero AP, Grumolato F, Trevisanuto D, Zanardo V, Ambrosini G, et al. Timing of delivery and neonatal outcomes for small-for-gestational-age fetuses. J Ultrasound Med. (2014) 33:1721–8. doi: 10.7863/ultra.33.10.1721
10. Xodo S, Londero AP, D'Agostin M, Novak A, Galasso S, Pittini C, et al. Is glycated hemoglobin a1c level associated with adverse pregnancy outcomes of women affected by pre-gestational diabetes? Medicina. (2021) 57:461. doi: 10.3390/medicina57050461
11. Orsaria M, Liviero S, Rossetti E, Pittini C, Driul L, Londero AP, et al. Placental acute inflammation infiltrates and pregnancy outcomes: a retrospective cohort study. Sci Rep. (2021) 11:24165. doi: 10.1038/s41598-021-03655-4
12. Londero AP, Orsaria M, Parisi N, Tassi A, Pittini C, Driul L, et al. In vitro fertilization is associated with placental accelerated villous maturation. Int J Clin Exp Pathol. (2021) 14:734–40.
13. Kramer MS, Platt R, Yang H, McNamara H, Usher RH. Are all growth-restricted newborns created equal(ly)? Pediatrics. (1999) 103:599–602. doi: 10.1542/peds.103.3.599
14. Bertino E, Spada E, Occhi L, Coscia A, Giuliani F, Gagliardi L, et al. Neonatal anthropometric charts: the Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. (2010) 51:353–61. doi: 10.1097/MPG.0b013e3181da213e
15. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria (2022).
16. Cohen J. Statistical Power Analysis for the Behavioral Sciences2nd ed. Hillsdale, NJ: L Erlbaum Associates (1988).
17. Ballard PL, Ballard RA. Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am J Obstet Gynecol. (1995) 173:254–62. doi: 10.1016/0002-9378(95)90210-4
18. Costa S, Zecca E, De Luca D, De Carolis MP, Romagnoli C. Efficacy of a single dose of antenatal corticosteroids on morbidity and mortality of preterm infants. Eur J Obstet Gynecol Reprod Biol. (2007) 131:154–7. doi: 10.1016/j.ejogrb.2006.05.006
19. Elimian A, Figueroa R, Spitzer AR, Ogburn PL, Wiencek V, Quirk JG. Antenatal corticosteroids: are incomplete courses beneficial? Obstet Gynecol. (2003) 102:352–5. doi: 10.1097/00006250-200308000-00025
20. Saldaña-García N, Espinosa-Fernández MG, Gómez-Robles C, Postigo-Jiménez AJ, Bello N, Rius-Díaz F, et al. Benefits of a single dose of betamethasone in imminent preterm labour. J Clin Med. (2021) 11:20. doi: 10.3390/jcm11010020
21. Sen S, Reghu A, Ferguson SD. Efficacy of a single dose of antenatal steroid in surfactant-treated babies under 31 weeks' gestation. J Matern Fetal Neonatal Med. (2002) 12:298–303. doi: 10.1080/jmf.12.5.298.303
22. Sehdev HM, Abbasi S, Robertson P, Fisher L, Marchiano DA, Gerdes JS, et al. The effects of the time interval from antenatal corticosteroid exposure to delivery on neonatal outcome of very low birth weight infants. Am J Obstet Gynecol. (2004) 191:1409–13. doi: 10.1016/j.ajog.2004.06.055
23. Chawla S, Natarajan G, Shankaran S, Pappas A, Stoll BJ, Carlo WA, et al. Association of neurodevelopmental outcomes and neonatal morbidities of extremely premature infants with differential exposure to antenatal steroids. JAMA Pediatr. (2016) 170:1164–72. doi: 10.1001/jamapediatrics.2016.1936
24. Wong D, Abdel-Latif M, Kent A, NICUS Network. Antenatal steroid exposure and outcomes of very premature infants: a regional cohort study. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F12–20. doi: 10.1136/archdischild-2013-304705
25. Vinukonda G, Dummula K, Malik S, Hu F, Thompson CI, Csiszar A, et al. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. (2010) 41:1766–73. doi: 10.1161/STROKEAHA.110.588400
26. Hershkovich Shporen C, Reichman B, Zaslavsky-Paltiel I, Lerner-Geva L, Flidel-Rimon O, Israel Neonatal Network. Antenatal corticosteroid therapy is associated with a lower risk of cystic periventricular leukomalacia. Acta Paediatr. (2021) 110:1795–802. doi: 10.1111/apa.15772
27. Winn HN, Romero R, Roberts A, Liu H, Hobbins JC. Comparison of fetal lung maturation in preterm singleton and twin pregnancies. Am J Perinatol. (1992) 9:326–8. doi: 10.1055/s-2007-999256
28. Lin D, Fan D, Chen G, Luo C, Guo X, Liu Z. Association of antenatal corticosteroids with morbidity and mortality among preterm multiple gestations: meta-analysis of observational studies. BMJ Open. (2021) 11:e047651. doi: 10.1136/bmjopen-2020-047651
29. McElrath TF, Norwitz ER, Robinson JN, Tanasijevic MJ, Lieberman ES. Differences in TDx fetal lung maturity assay values between twin and singleton gestations. Am J Obstet Gynecol. (2000) 182:1110–2. doi: 10.1067/mob.2000.105437
30. Vaz A, Malheiro MF, Severo M, Rodrigues T, Guimarães H, Montenegro N. Effect of antenatal corticosteroids on morbidity and mortality of preterm singletons and twins. J Matern Fetal Neonatal Med. (2018) 31:754–60. doi: 10.1080/14767058.2017.1297408
31. Lee HC, Subeh M, Gould JB. Low Apgar score and mortality in extremely preterm neonates born in the United States. Acta Paediatr. (2010) 99:1785–9. doi: 10.1111/j.1651-2227.2010.01935.x
32. Zahedi-Spung LD, Raghuraman N, Macones GA, Cahill AG, Rosenbloom JI. Neonatal morbidity and mortality by mode of delivery in very preterm neonates. Am J Obstet Gynecol. (2022) 226:114. doi: 10.1016/j.ajog.2021.07.013
Keywords: betamethasone, antenatal corticosteroid prophylaxis, preterm birth, respiratory distress syndrome, intraventricular hemorrhage
Citation: Xodo S, Trombetta G, Celante L, Pittini C, Driul L, Cagnacci A and Londero AP (2022) Partial vs. complete course of antenatal corticosteroid prophylaxis: An Italian single center retrospective study. Front. Pediatr. 10:894526. doi: 10.3389/fped.2022.894526
Received: 11 March 2022; Accepted: 27 July 2022;
Published: 15 August 2022.
Edited by:
MaryAnn Volpe, Tufts University School of Medicine, United StatesReviewed by:
Jonathan Michael Davis, Tufts University, United StatesCopyright © 2022 Xodo, Trombetta, Celante, Pittini, Driul, Cagnacci and Londero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serena Xodo, c2VyZW5heG9kb0B5YWhvby5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.