- 1Pediatrics Department, King Khalid University Hospital, Riyadh, Saudi Arabia
- 2Clinical Practice Guidelines and Quality Research Unit, Quality Management Department, King Saud University Medical City, Riyadh, Saudi Arabia
- 3Research Chair for Evidence-Based Health Care and Knowledge Translation, King Saud University, Riyadh, Saudi Arabia
- 4Alexandria Center for Evidence-Based Clinical Practice Guidelines, Alexandria University, Alexandria, Egypt
- 5Adaptation Working Group, Guidelines International Network, Perth, Scotland
- 6Pediatrics Department, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 7Neonatal Intensive Care Unit, King Saud University Medical City, Riyadh, Saudi Arabia
- 8Saudi Neonatology Society (SNS), Riyadh, Saudi Arabia
- 9Neonatology Department, Ministry of Health, Assir, Saudi Arabia
- 10Neonatology Department, King Abdulaziz Hospital, Ministry of Health, Jeddah, Saudi Arabia
- 11Clinical Pharmacy Department, Pharmacy Services, Second Health Cluster in Central Region, Riyadh, Saudi Arabia
- 12Pharmacy Department, King Fahad Medical City, Ministry of Health, Riyadh, Saudi Arabia
- 13Obstetrics and Gynecology Department, King Fahad Medical City, Ministry of Health, Riyadh, Saudi Arabia
- 14College of Nursing, King Saud University, Riyadh, Saudi Arabia
- 15Saab Medical Library, University Libraries, American University of Beirut, Beirut, Lebanon
- 16Wegner Health Sciences Library, University of South Dakota, Sioux Falls, SD, United States
- 17Department of Pediatrics, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
- 18Morbidity and Mortality Unit, King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia
- 19Public Health and Community Medicine Department, Theodor Bilharz Research Institute (TBRI), Academy of Scientific Research, Cairo, Egypt
- 20Neonatal Intensive Care Unit, Infectious Diseases Unit, Pediatrics Department, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
Background and objective: Neonatal sepsis (NS) continues to be a critical healthcare priority for the coming decades worldwide. The aim of this study was to critically appraise the quality of recent clinical practice guidelines (CPGs) for neonatal sepsis and to summarize and compare their recommendations.
Methods: This study involves a systematic review of CPGs. We identified clinical questions and eligibility criteria and searched and screened for CPGs using bibliographic and CPG databases and professional societies. Each included CPG was assessed by four independent appraisers using the Appraisal of Guidelines for REsearch & Evaluation II (AGREE II) instrument. We summarized the recommendations in a comparison practical table. The systematic review was drafted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement. Its protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews (ID: CRD42021258732).
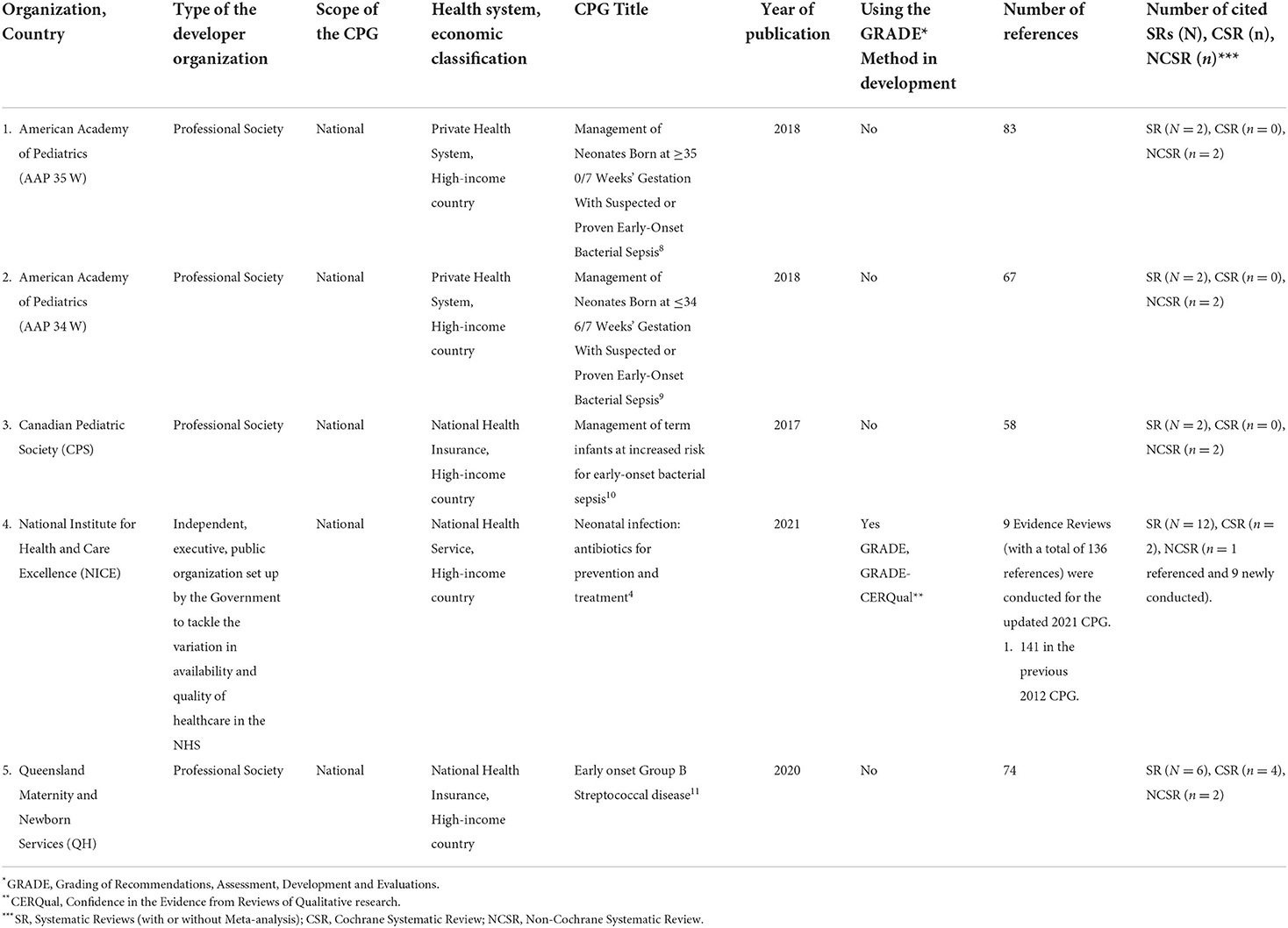
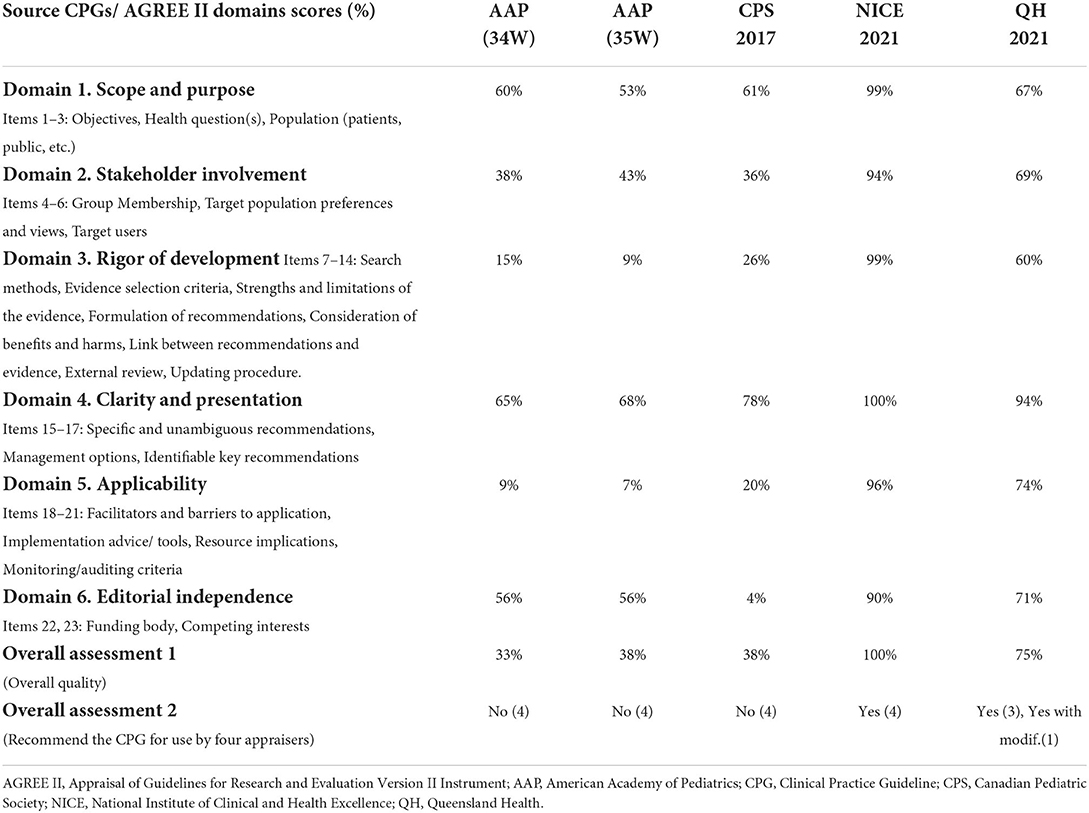
Results: Our search retrieved 4,432 citations; of which five CPGs were eligible and appraised: American Academy of Pediatrics (AAP 2018) (35 and 34 weeks); Canadian Pediatric Society (CPS 2017); National Institute for Health and Care Excellence (NICE 2021); and Queensland Maternity and Neonatal Services (QH 2020). Among these, the overall assessment of two evidence-based CPGs scored > 70% (NICE and QH), which was consistent with their higher scores in the six domains of the AGREE II instrument. In domain 3 (rigor of development), NICE and QH scored 99 and 60%, respectively. In domain 5 (applicability), they scored 96 and 74%, respectively, and in domain 6 (editorial independence), they scored 90 and 71%, respectively.
Conclusion: The methodological quality of the NICE CPG was superior followed by the QH CPG with relevant recommendations for use in practice.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021258732, PROSPERO (CRD42021258732).
Introduction
Neonatal sepsis (NS) continues to pose significant morbidity and mortality despite the continued advancement in neonatal care (1, 2). Neonatal sepsis is classified into early- and late-onset depending on the timing of infection in days after birth (3). Another classification includes hospital-acquired vs. community-acquired (4, 5).
The global incidence of NS varies, with a population-level estimate of 2,202 per 100,000 live births, with mortality rates ranging from 11 to 19% in high- and middle-income countries (6) and 2.9 to 24 per 1,000 live births in low-income countries (7). Advancement in obstetrical care and universal screening for Group B Streptococcus (GBS) to stratify risk for NS has helped reduce the incidence of sepsis even further (8). Despite the reduction in NS in many countries, it still possesses a serious threat to neonates (9). Neonatal bacterial infection affecting neonates admitted to the neonatal intensive care unit (NICU) further complicates their course in the hospital and increases the risk of morbidity and mortality (10).
Identifying infants at risk for sepsis is crucial to reducing the complications of neonatal sepsis (11). Many organizations have developed clinical practice guidelines (CPGs), protocols, and policies to try to minimize neonatal sepsis (12). Due to the lack of high-quality studies in the management of NS, most of the published CPGs are based on expert opinions and do not really provide clear guidance for the physician taking care of vulnerable neonates (13). The developed CPGs concentrate mostly on early NS risk assessment and provide no guidance to late-onset sepsis including the AAP and CPS CPGs (14).
At present, there is no unified national CPG in Saudi Arabia for the management of sepsis (15). Furthermore, GBS screening for pregnant women is not a standard of care in Saudi Arabia yet (16). A recent cohort study identified the risk for early NS in Saudi Arabia to be 0.5/1,000 live births (17). A unified management plan of at-risk neonates can help further reduce the risk of complications posed by having an early neonatal infection (18). CPGs have been recognized with their potential to improve clinical practice and patient outcomes (19).
In 2021, the Saudi Neonatology Society (SNS) launched a number of projects to adapt national evidence-based CPGs for the management of high-priority health topics in neonatal healthcare, with the goal of providing evidence-based guidance and recommendations to neonatologists and pediatricians across the country. The “KSU-Modified-ADAPTE” as a formal CPG adaptation methodology consisting of three phases, namely, setup, adaptation, and finalization, has guided these projects (20–23).
The Appraisal of Guidelines for REsearch and Evaluation (AGREE II) instrument is the gold standard for assessing the quality of CPGs. AGREE II is a CPG appraisal tool that has been cited and endorsed by a number of healthcare organizations (24–26). AGREE II identifies components that CPGs should address in order to improve their quality and dependability and achieve positive patient outcomes (24–26).
Because systematic reviews of CPGs using AGREE II is a critical step in the CPG adaptation process, we have dedicated this study to report the results of this systematic review and AGREE II assessment of the recently published CPGs for the management (i.e., diagnosis and treatment) of neonatal sepsis (21, 27, 28). This CPG adaptation project was registered in the PREPARE (Practice guideline REgistration for trancPAREncy) platform, University of Lanzhou, Lanzhou, China http://www.guidelines-registry.org/ (Registration Number: IPGRP-2021CN383) (29).
Both the PIPOH (P, Population; I, Intervention; P, Professionals; O, Outcomes; and H, Healthcare setting) and PICAR [P: Population, clinical indication(s), and condition(s), I: Intervention(s), C: Comparator(s), Comparison(s), and (key) Content, A: Attributes of eligible CPGs, and R: Recommendation characteristics] models were used to guide the search strategy (21, 27).
Methods
The protocol for this study was registered in PROSPERO (International Prospective Register of Systematic Reviews) (Protocol ID: CRD42021258732).
Our Guidelines Review Group (GRG) included seven expert consultant neonatologists: one of them with expertise in infectious diseases, one with expertise in systematic reviews, a consultant in Obstetrics and Gynecology, a clinical pharmacist with expertise in neonatal medication management, a specialized nurse with relevant expertise, a medical and healthcare librarian, and a CPG methodologist with a background in pediatrics.
Data sources and search strategy
The librarian systematically searched MEDLINE, EMBASE, and CINAHL databases for relevant CPGs using the Ovid platform and hand-searched the relevant CPG databases and repositories for eligible CPGs (refer to search strategy in Supplement 1).
Two reviewers (LS and AH) independently screened the titles and abstracts of CPGs and articles that met the inclusion criteria. Three different reviewers checked the screening and full-text review (YSA, JA, and NA). After retrieving and reviewing the full-text articles or full CPG documents, disagreements were resolved through focus group discussions.
Inclusion and exclusion criteria
The following were the NS CPGs eligibility requirements: (1) evidence-based, with a clear detailed record of the CPG development methodology. (2) English or Arabic language. (3) original source CPGs (de novo development). (4) national or international scope, and (5) published by an organization or group authorship and accessible from a CPG database or peer-reviewed journal or relevant professional society website. Each source CPG was only appraised in its most recent version. Both organism-specific and nonspecific were considered in the search. CPGs that were published prior to 2016, were not in English or Arabic, were adapted from other CPGs, were presented as consensus or expert-based statements, or had a single author were excluded.
AGREE II instrument training workshop
The CPG methodologist led a capacity-building workshop for the GRG, which included hands-on sessions on evidence-based CPG standards and using the AGREE II instrument. Following that, four reviewers were assigned to score the CPGs that were included. Each CPG was critically appraised independently by each of the four reviewers. All appraisers read the full CPG documents, including any updates with relevant supplementary information or links to online web pages related to CPG methods or implementation tools. The AGREE II appraisers were instructed to record the justifications for their scores in the “Comments” section for each item or question (28).
AGREE II assessment of NS CPGs
The AGREE II instrument (www.agreetrust.org) has 23 items or questions divided into six domains, namely, scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence. Using a 7-point Likert scale, each item was scored. The AGREE II evaluation was guided by its online version, “My AGREE PLUS,” which allows for the creation of a CPG appraisal group for each CPG and compiles and calculates the item ratings into domain ratings and comments. Each CPG was critically appraised by four AGREE II raters who were members of the GRG including four clinicians, and one of them was a methodologist (24, 25, 28).
Large discrepancies in the assessors' scores of items or questions (i.e., a difference of more than 3) were resolved through discussion with the GRG. The standardized AGREE domain scores or ratings were calculated automatically by the online My AGREE PLUS. For each AGREE standardized domain score or rating, we agreed on a cutoff point of 70% since the AGREE II does not provide a specific cutoff point to define high- vs. low-quality CPGs, and several cutoff points have been proposed by different CPG appraisal groups. Following the appraisal, greater emphasis was placed on the scores of domains 3 and 5 in order to facilitate the filtration and final evaluation of the reporting quality of the included CPGs. Identical cutoff values have been reported (28, 30–32).
Data analysis plan
Using the methods recommended by the AGREE II instrument, we calculated standardized scores ranging from 0 to 100% for each AGREE II domain. The key recommendations of the eligible CPGs were summarized in a comparative tabular format. The quality of CPGs was classified based on the rating of domain 3 (rigor of development), with a high-quality CPG receiving a standardized domain rating of more than or equal to 70%, a moderate-quality CPG receiving a domain rating of 40–69%, and a low-quality CPG receiving a domain rating of <40% (28).
Inter-rater analysis
We used inter-rater reliability tests to determine the degree of agreement between raters (IRR) using a percent agreement inter-rater reliability assessment test for each question in each area of the five appraised CPGs to determine the level of agreement among the four raters. In addition to the percent agreement in the first overall assessment (OA1), we also investigated the consistency of ratings or the capacity for datasets that were gathered as clusters or sorted into clusters using intra-class correlation in the second overall assessment (OA2). Intra-class correlation is a popular IRR approach (ICC). We use this when there are more than two raters. A strong intra-class correlation coefficient (kappa) around 1 suggested that standards from the same set were quite comparable. A low kappa value around 0 indicated that standards from the same set were not similar. We used ANOVA “One-Way Random” on SPSS Statistics, version 21, since we had inconsistent raters/rates. We picked ICCC because of the wide range of numerical data from groups or clusters. This helped us determine the repeatability and how closely peers resembled one another in terms of certain features or attributes. We investigated how well two ordinal scale categories agreed with one another.
We used weighted kappa since the data came from an ordered scale (quadratic weights). The weights are calculated as follows: Cohen's kappa notation is used. Because the difference between the first and second categories was comparable with the difference between the second and third categories, and so on, we chose linear weights. To quantify agreement, the kappa (K) statistic is used (32, 33): When there is total agreement between the categorization systems, K = 1 when there is no agreement larger than chance, and K is negative when there is agreement poorer than chance. Supplement 3.1. Table illustrates how the K value might be interpreted (34).
Results
Identification of neonatal sepsis CPGs
A total of 4,432 records were retrieved 469 records were duplicates 3,916 records were excluded by title and abstract review using Rayyan https://www.rayyan.ai/ (35), and 41 records were excluded after full-text review according to the health questions and the eligibility criteria. Only five source original CPGs were found to be eligible for the quality assessment step, namely, Management of Neonates Born at ≥35 0/7 Weeks' Gestation With Suspected or Proven Early-Onset Bacterial Sepsis (AAP 2018) (36), Management of Neonates Born at ≤ 34 6/7 Weeks' Gestation With Suspected or Proven Early-Onset Bacterial Sepsis (AAP2 2018) (37), Canadian Pediatric Society: Management of Term Infants at Increased Risk for Early-Onset Bacterial Sepsis (CPS 2017) (14), National Institute for Health and Care Excellence. Neonatal Infection: Antibiotics for Prevention and Treatment (NICE 2021) (38), and Queensland Health: Early Onset Group B Streptococcal Disease (QH 2020) (39). Two reviewers conducted the screening (LS, AH), and two additional reviewers (JA, YSA) resolved any discrepancies by discussions. The PRISMA flowchart was reported in the online Supplementary material (Supplement 2. Figure).
Key characteristics of neonatal sepsis CPGs
Table 1 highlights the characteristics of all eligible CPGs. The CPG developer organizations were reference, professional organizations in pediatrics, neonatology, or general non-specialized including AAP, CPS, NICE, and QH. All organizations were from high-income countries.
Reporting the quality of NS CPGs
The AGREE II standardized domain ratings are summarized in Table 2.
Domain 1: Scope and purpose
The range of domain 1 was between 65 and 99%. The score of one CPG was > 70% (NICE = 99%). NICE presented its 13 health questions in the online full CPG document and appendices. The overall objective of the NICE CPG was clearly stated.
Domain 2: Stakeholder involvement
The range of domain 2 was between 36 and 94%. The score of one CPG was > 70% (NICE = 94%) where the guideline development group was properly reported and included a multidisciplinary team representing all related disciplines to the health topic of neonatal sepsis.
Domain 3: Rigor of development
The range of domain 3 was between 9 and 99%. The score of one CPG was > 70% (NICE = 99%). The NICE CPG followed the NICE manual for CPG development that uses the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) method. It takes account of both clinical and economic evidence in the formulation of the recommendations. Full GRADE and GRADE-CERQual Evidence Tables were provided including the assessment of the five modified GRADE domains (i.e., risk of bias, inconsistency, indirectness, imprecision, and publication bias). The QH CPG reported an overall CPG development process. Both CPGs considered benefits and harms during the formulation of their recommendations.
Domain 4: Clarity of presentation
The range of domain 4 was between 65 and 100%. The score of three CPGs were > 70% (NICE = 100%, QH = 94%, CPS = 78%). The three CPGs presented a clear summary of their key recommendations.
Domain 5: Applicability
The range of domain 5 was between 7 and 96%. Two CPGs scored > 70% (NICE = 96%, QH = 74%). The NICE CPG reported a full package of CPG implementation tools including clinical pathways, quality standards, baseline assessment tool, visual summary versions of the CPG, a link to the online Kaiser Permanente neonatal sepsis calculator, patient health education information, and a shared learning experience from the United Kingdom. Furthermore, the QH CPG provided a variety of implementation tools like flowcharts, an implementation section, quality measures, safety and quality table, education, consumer information, a set of online learning resources, and audit items.
Domain 6: Editorial independence
The range of domain 6 was between 4 and 90%. The scores of two CPGs were > 70% in domain 6 (NICE = 90%, QH = 71%). Documenting the funding body and the conflicts of interest were included in both CPGs.
Overall assessment
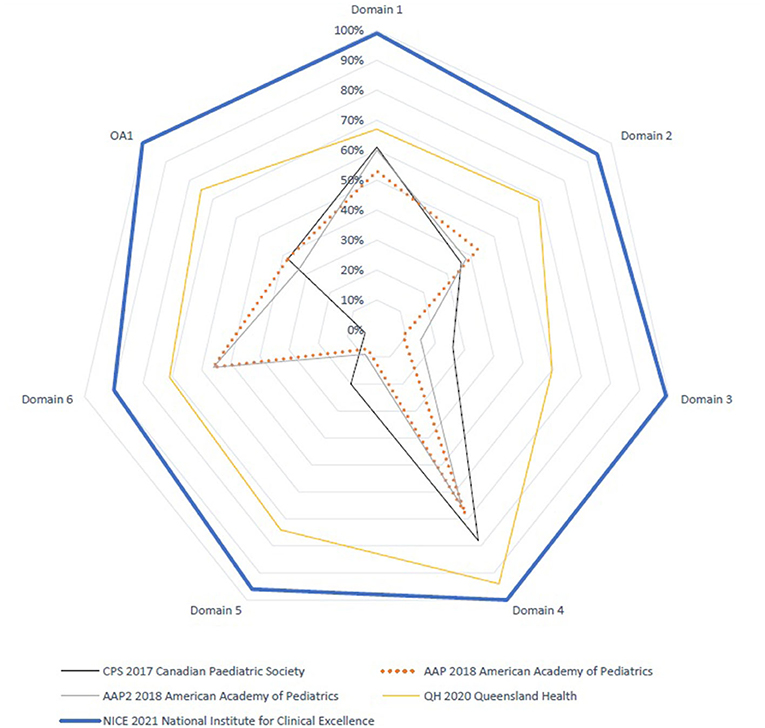
The AGREE II standardized domain scores for the first overall assessment ranged from 33 to 100%. Two CPGs scored > 70% (NICE and QH), which was consistent with the high scores in the six AGREE II domains. Figures 1 and 2 display the AGREE II domain scores that were generated. These two radar maps show the appraised CPGs' final percentage scores for each of the six domains and each of the 23 questions in Figures 1 and 2, respectively.
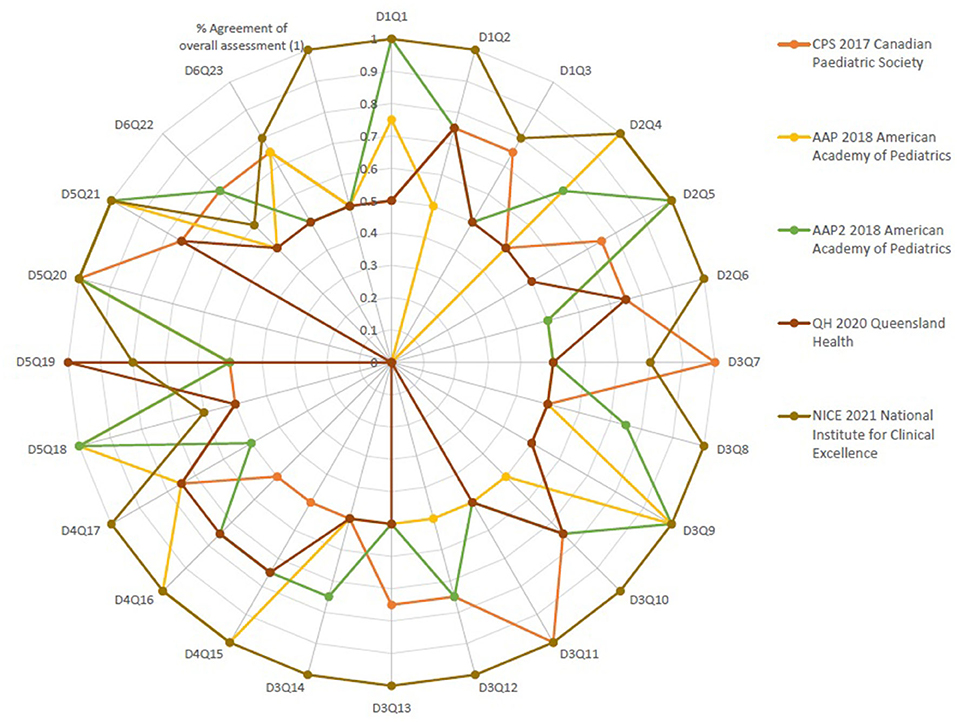
Figure 2. Percent agreement among raters for the five source neonatal sepsis practice guidelines focusing on every question in every domain of the AGREE II Instrument.
Recommending the neonatal sepsis CPGs for use in neonatal practice
The second (overall) assessment (i.e., the recommendation for using the CPG in practice) revealed a consensus between the reviewers on recommending the use of two CPGs (NICE and QH).
Inter-rater analysis
Table 2 shows the AGREE II group appraisal of the five eligible source CPGs. We calculated the percentage of agreement between raters. The findings of the inter-rater reliability tests revealed a high level of agreement among the four raters for every question in every area in the six domains, as well as the overall assessment's percent agreement. Figure 2, Supplement 3.1. Table and 3.2. Table show that the majority of the kappa scores ranged from 0.50 to 1.00, indicating a good to excellent agreement.
Three assessments, presented in Figure 2, namely, One in AAP 2018 American Academy of Pediatrics and two assessments in QH 2020 Queensland Maternity and Neonatal Clinical Guidelines, found a low level of agreement (K = 0.0). No questions in any of the guidelines have a fair degree of agreement. There was very good agreement in 10 questions in CPS 2017 Canadian Pediatric Society, 12 questions in AAP 2018 American Academy of Pediatrics, 9 questions in AAP2 2018 American Academy of Pediatrics guideline, 14 questions in QH 2020 Queensland Maternity and Neonatal Clinical Guidelines, and 2 questions in NICE 2021. There was an excellent degree of agreement in 3 questions in CPS 2017 Canadian Pediatric Society, 8 questions in AAP 2018 American Academy of Pediatrics, 6 questions in AAP2 2018 American Academy of Pediatrics guideline, 1 question in QH 2020 Queensland Maternity and Neonatal Clinical Guidelines, and 18 questions in NICE 2021. Regarding the summation of scores, NICE 2021 showed the highest score of 786 and also 35 was the OA1 score. Overall assessment of NICE 2021 was “Excellent” (weighted kappa score = 1). The intraclass correlation coefficient (kappa value) among raters of the four recommendations for the overall assessment (2)showed that the number of observed agreements is six (61.26% of the observations); 8 agreements are predicted by chance (75% of the observations). Kappa = 0.837; kappa SE = 0.761; 95% confidence interval: weighted kappa = 0.091 for values ranging from 0.214 to 0.617.
Discussion
Despite the large volume of national and international neonatal CPGs that are continuously published, there exists the challenge of variability of their quality and evidence base. To the best of our knowledge, this review is novel in that it systematically evaluates the quality of recently published CPGs of neonatal sepsis using the AGREE II instrument as a part of a national CPG adaptation initiative (40–43).
Five source CPGs addressing the management of neonatal sepsis were assessed using the AGREE II instrument. This AGREE II assessment highlighted several areas of improvement in the methodological rigor of the included CPGs. Although the assessment of overall guideline quality and the recommendation for use are standard components of AGREE II, it is possible that they are underreported in the documented methodology of the published CPGs.
In this review, the scores of only two CPGs (NICE and QH) were ≥ 60% in domain 3 (rigor of development) that has been identified as the strongest indicator of the quality and evidence base of a CPG more than the other five domains (30). A comparison or a recommendation matrix table was summarized for the five included assessed source CPGs in Table 3.
The Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) Study conducted an international prospective observational cohort study across 12 clinical sites that highlighted the burden and high mortality of neonatal sepsis among facility-born neonates in low-income and middle-income countries (44). Based on data from the Global Burden of Disease Study 2019, similar results were obtained (45).
Our study is the first systematic critical appraisal of CPGs with diagnostic and therapeutic recommendations for newborns with sepsis that we are aware of. Strengths of our study included using a comprehensive PRISMA-compliant systematic review methodology to identify potentially relevant CPGs and performed quality assessment using the AGREE II instrument by a multidisciplinary expert team of neonatology clinicians and methodologists.
Nevertheless, some limitations were identified in our work. Earlier disadvantages of the AGREE II instrument have been addressed in the “AGREE-REX” (Recommendation EXcellence) tool, which addresses the clinical credibility of the CPG recommendations (46, 47). Language limitation (i.e., searching only English or Arabic language CPGs) may have resulted in the exclusion of relevant neonatal sepsis CPGs that were intended for use in non-English-speaking and non-Arabic contexts.
Implications for practice: Guidance for clinical guideline uptake
The findings of this review can be further used to inform and support any relevant CPG development or adaptation project for neonatal sepsis.
We recommend including the AGREE II criteria in the capacity building of clinicians to guide their decisions in selecting high-quality and evidence-based CPGs for use in their daily practice through evidence scouting and searching for similar published AGREE II assessments of CPGs in their needed neonatology health topic.
Furthermore, we recommend building a recommendation map (or RecMap) for high-priority health topics or, if possible, for all published neonatology CPGs similar to RecMap initiative for COVID-19 CPGs and for tuberculosis CPGs to increase the accessibility of pre-appraised and living specialized CPGs by the professionals, parents, carers, and the public (48, 49).
Implications for future CPG research
We recommend conducting research projects to further explore the impact of high or low quality of the NS CPGs on their implementability and implementation including facilitators and barriers in different healthcare contexts, especially in low-resource settings.
Conclusion
The methodological quality of the NICE and QH CPGs was superior, followed by CPS and AAP CPGs. Recommendations included identification of risk factors, initial assessment, investigations, antibiotic therapy, and treatment of the two main types of neonatal sepsis (i.e., early onset and late onset).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YA and JA conceptualized and designed the study. LH, YA, LS, AH, JA, and NA contributed to the search, screening, review, and critical appraisal of guidelines. YA, LS, and AE wrote the first draft of the manuscript. YA and AE analyzed and interpreted the data. YA and JA supervised the procedures in the study and reviewed the drafts and final version of this manuscript. All authors have made substantial contributions and provided final approval for the conception, drafting, and final version of this manuscript. All authors have read and approved the final version of the manuscript.
Funding
This study was funded by the Saudi Neonatology Society (SNS).
Acknowledgments
We would like to thank the Saudi Neonatology Society (SNS) for the logistic support in the parallel training workshops conducted as part of this project and financial support for the publication fees of this article. The society did not influence any phase of this research project. This study was supported by King Saud University, Deanship of Scientific Research, Research Chair for Evidence-Based Health Care and Knowledge Translation, Riyadh, Saudi Arabia. Furthermore, we would like to extend our thanks to the King Saud University Medical City for logistics, databases access, and relevant resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.891572/full#supplementary-material
Abbreviations
AGREE II, Appraisal of Guidelines for REsearch and Evaluation (Version 2); AAP, American Academy of Pediatrics; CPG, Clinical Practice Guideline; CPS, Canadian Pediatric Society; GRADE, Grading of Recommendations; Assessment; Development and Evaluations; GRADE-CERQual, Confidence in the Evidence from Reviews of Qualitative research; NICE, National Institute for Health and Care Excellence; NICU, Neonatal Intensive Care Unit; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses Statement; PROSPERO, International Database of Prospectively Registered Systematic Reviews with a Health Related Outcome; RecMap, Recommendation Map; QH, Queensland Health (maternity and newborn).
References
1. Singh M, Alsaleem M, Gray CP. Neonatal Sepsis. In: StatPearls. Treasure Island (FL): StatPearls Publishing (202).
2. Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. (2010) 29:315–48. doi: 10.3109/08830181003792803
3. Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F257–63. doi: 10.1136/archdischild-2014-306213
4. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. (2017) 390:1770–80. doi: 10.1016/S0140-6736(17)31002-4
5. Markwart R, Saito H, Harder T, Tomczyk S, Cassini A, Fleischmann-Struzek C, et al. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: a systematic review and meta-analysis. Intensive Care Med. (2020) 46:1536–51. doi: 10.1007/s00134-020-06106-2
6. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6:223–30. doi: 10.1016/S2213-2600(18)30063-8
7. Huynh BT, Padget M, Garin B, Herindrainy P, Kermorvant-Duchemin E, Watier L, et al. Burden of bacterial resistance among neonatal infections in low income countries: how convincing is the epidemiological evidence? BMC Infect Dis. (2015) 15:127. doi: 10.1186/s12879-015-0843-x
8. Le Doare K, Heath PT, Plumb J, Owen NA, Brocklehurst P, Chappell LC. Uncertainties in screening and prevention of group B streptococcus disease. Clin Infect Dis. (2019) 69:720–5. doi: 10.1093/cid/ciy1069
9. Mukhopadhyay S, Puopolo KM. Risk assessment in neonatal early onset sepsis. Semin Perinatol. (2012) 36:408–15. doi: 10.1053/j.semperi.2012.06.002
10. Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am. (2013) 60:367–89. doi: 10.1016/j.pcl.2012.12.003
11. Downey LC, Smith PB, Benjamin DK Jr. Risk factors and prevention of late-onset sepsis in premature infants. Early Hum Dev. (2010) 86 Suppl 1:7–12. doi: 10.1016/j.earlhumdev.2010.01.012
12. Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. (2015) 61:1–13. doi: 10.1093/tropej/fmu079
13. Kristóf K, Kocsis E, Nagy K. Clinical microbiology of early-onset and late-onset neonatal sepsis, particularly among preterm babies. Acta Microbiol Immunol Hung. (2009) 56:21–51. doi: 10.1556/AMicr.56.2009.1.2
14. Jefferies AL. Management of term infants at increased risk for early-onset bacterial sepsis. Paediatr Child Health. (2017) 22:223–8. doi: 10.1093/pch/pxx023
15. Health sector MOH. Scientific Team. Covid-19 guidelines. Ministry Of Health Saudi Arabia. Available online at: https://www.moh.gov.sa/en/Ministry/MediaCenter/Publications/Pages/covid19.aspx (accessed August 2, 2022).
16. Alamri Y, Albasri S, Abduljabbar GH, Alghamdi H, Balkhair AM, AlAam R. Awareness of pregnancy screening for group B streptococcus infection among women of reproductive age and physicians in Jeddah, Saudi Arabia. Cureus. (2021) 13:e18765. doi: 10.7759/cureus.18765
17. Huseynova R, Bin Mahmoud L, Hamad Aljobair F, et al. Use of early-onset sepsis risk calculator for neonates ≥ 34 weeks in a large tertiary Neonatal Centre, Saudi Arabia. Cureus. (2021) 13:e14620. doi: 10.7759/cureus.14620
18. Polin RA. Committee on fetus and newborn management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. (2012) 129:1006–15. doi: 10.1542/peds.2012-0541
19. Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet. (2017) 390:415–23. doi: 10.1016/S0140-6736(16)31592-6
20. Saudi Neonatology Society (SNS). Home - Saudi Neonatology Society (SNS). (2022). Available online at: https://sns.med.sa/?lang=en (accessed August 2, 2022).
21. Amer YS, Wahabi HA, Abou Elkheir MM, Bawazeer GA, Iqbal SM, Titi MA, et al. Adapting evidence-based clinical practice guidelines at university teaching hospitals: a model for the eastern Mediterranean Region. J Eval Clin Pract. (2019) 25:550–60. doi: 10.1111/jep.12927
22. Amer YS, Elzalabany MM, Omar TI, Ibrahim AG, Dowidar NL. The'Adapted ADAPTE': an approach to improve utilization of the ADAPTE guideline adaptation resource toolkit in the Alexandria Center for Evidence-Based Clinical Practice Guidelines. J Eval Clin Pract. (2015) 21:1095–106. doi: 10.1111/jep.12479
23. Wang Z, Norris SL, Bero L. The advantages and limitations of guideline adaptation frameworks. Implement Sci. (2018) 13:72. doi: 10.1186/s13012-018-0763-4
24. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ Can Med Assoc J. (2010) 182:E839–42. doi: 10.1503/cmaj.090449
25. Brouwers MC, Spithoff K, Lavis J, Kho ME, Makarski J, Florez ID. What to do with all the AGREEs? The AGREE portfolio of tools to support the guideline enterprise. J Clin Epidemiol. (2020) 125:191–7. doi: 10.1016/j.jclinepi.2020.05.025
26. Siering U, Eikermann M, Hausner E, Hoffmann-Eßer W, Neugebauer EA. Appraisal tools for clinical practice guidelines: a systematic review. PLoS ONE. (2013) 8:e82915. doi: 10.1371/journal.pone.0082915
27. Johnston A, Kelly SE, Hsieh SC, Skidmore B, Wells GA. Systematic reviews of clinical practice guidelines: a methodological guide. J Clin Epidemiol. (2019) 108:64–76. doi: 10.1016/j.jclinepi.2018.11.030
28. Login - AGREE Enterprise website. Agreetrust.org. Available online at: https://www.agreetrust.org/my-agree/ (accessed August 2, 2022).
29. Chen Y, Guyatt GH, Munn Z, Florez ID, Marušić A, Norris SL, et al. Clinical practice guidelines registry: toward reducing duplication, improving collaboration, and increasing transparency. Ann Intern Med. (2021) 174:705–7. doi: 10.7326/M20-7884
30. Amer YS, Titi MA, Godah MW, Wahabi HA, Hneiny L, et al. International alliance and AGREE-ment of 71 clinical practice guidelines on the management of critical care patients with COVID-19: a living systematic review. J Clin Epidemiol. (2021) 142:333–70. doi: 10.1016/j.jclinepi.2021.11.010
31. Hoffmann-Eßer W, Siering U, Neugebauer EA, Lampert U, Eikermann M. Systematic review of current guideline appraisals performed with the appraisal of guidelines for research & evaluation II instrument—a third of AGREE II users apply a cut-off for guideline quality. J Clin Epidemiol. (2018) 95:120–7. doi: 10.1016/j.jclinepi.2017.12.009
32. Cohen JA. Coefficient of agreement for nominal scales. Educ Psychol Measur. (1960) 20:37–46. doi: 10.1177/001316446002000104
33. Fleiss JL, Shrout PE. Approximate interval estimation for a certain intraclass correlation coefficient. Psychometrika. (1978) 43:259–62. doi: 10.1007/BF02293867
34. Bland JM, Altman DG. Statistics Notes: Measurement error and correlation coefficients. BMJ. (1996) 313:41–2. doi: 10.1136/bmj.313.7048.41
35. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:1–0. doi: 10.1186/s13643-016-0384-4
36. Puopolo K, Benitz W, Zaoutis T. Management of neonates born at ≥35 0/7 Weeks' Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics. (2018) 142:e20182894. doi: 10.1542/9781610023047-part05-management
37. Puopolo K, Benitz W, Zaoutis T. Management of neonates born at ≤ 34 6/7 Weeks' Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics. (2018) 142:e20182896. doi: 10.1542/9781610023047-part05-management_of_neonates
38. Overview Neonatal infection: antibiotics for prevention treatment Guidance NICE. National Institute for Health and Care Excellence. Nice.org.uk (2021). Available online at: https://www.nice.org.uk/guidance/ng195 [cited 7 March 2022].
39. Queensland Clinical Guidelines. Early Onset Group B Streptococcal disease. Guideline No. MN16.20-V4-R21. Queensland Health. 2020. Available online at: https://www.health.qld.gov.au/qcg/publications#neonatal.
40. Yeo KT, Oei JL, De Luca D, Schmölzer GM, Guaran R, Palasanthiran P, et al. Review of guidelines and recommendations from 17 countries highlights the challenges that clinicians face caring for neonates born to mothers with COVID-19. Acta Paediatr. (2020) 109:2192–207. doi: 10.1111/apa.15495
41. Balice-Bourgois C, Zumstein-Shaha M, Vanoni F, Jaques C, Newman CJ, Simonetti GD, et al. systematic review of clinical practice guidelines for acute procedural pain on neonates. Clin J Pain. (2020) 36:390–8. doi: 10.1097/AJP.0000000000000808
42. Merritt TA, Gold M. Holland J. A critical evaluation of clinical practice guidelines in neonatal medicine: does their use improve quality and lower costs? J Eval Clin Pract. (1999) 5:169–77. doi: 10.1046/j.1365-2753.1999.00185.x
43. Lapillonne A, Carnielli VP, Embleton ND, Mihatsch W. Quality of newborn care: adherence to guidelines for parenteral nutrition in preterm infants in four European countries. BMJ open. (2013) 3:e003478. doi: 10.1136/bmjopen-2013-003478
44. Milton R, Gillespie D, Dyer C, Taiyari K, Carvalho MJ, Thomson K, et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Global Health. (2022) 10:e661–72. doi: 10.1016/S2214-109X(22)00043-2
45. Ou Z, Yu D, Liang Y, et al. Global trends in incidence and death of neonatal disorders and its specific causes in 204 countries/territories during 1990–2019. BMC Public Health. (2022) 22:360. doi: 10.1186/s12889-022-12765-1
46. Florez ID, Brouwers MC, Kerkvliet K, Spithoff K, Alonso-Coello P, et al. Assessment of the quality of recommendations from 161 clinical practice guidelines using the Appraisal of Guidelines for Research and Evaluation-Recommendations Excellence (AGREE-REX) instrument shows there is room for improvement. Implement Sci. (2020) 15:79. doi: 10.1186/s13012-020-01036-5
47. Brouwers MC, Florez ID, McNair SA, Vella ET, Yao X. Clinical practice guidelines: tools to support high quality patient care. Semin Nucl Med. (2019) 49:145–52. doi: 10.1053/j.semnuclmed.2018.11.001
48. Lotfi T, Stevens A, Akl EA, Falavigna M, Kredo T, Mathew JL, et al. eCOVID Collaborators. Getting trustworthy guidelines into the hands of decision-makers and supporting their consideration of contextual factors for implementation globally: recommendation mapping of COVID-19 guidelines. J Clin Epidemiol. (2021) 135:182–6. doi: 10.1016/j.jclinepi.2021.03.034
Keywords: neonatal sepsis, pediatrics, clinical practice guidelines, systematic review, AGREE II instrument, quality assessment
Citation: Amer YS, Shaiba LA, Hadid A, Anabrees J, Almehery A, AAssiri M, Alnemri A, Darwish ARA, Baqawi B, Aboshaiqah A, Hneiny L, Almaghrabi RH, El-Malky AM and Al-Dajani NM (2022) Quality assessment of clinical practice guidelines for neonatal sepsis using the Appraisal of Guidelines for Research and Evaluation (AGREE) II Instrument: A systematic review of neonatal guidelines. Front. Pediatr. 10:891572. doi: 10.3389/fped.2022.891572
Received: 07 March 2022; Accepted: 14 July 2022;
Published: 16 August 2022.
Edited by:
Venkataseshan Sundaram, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Tao Xiong, Sichuan University, ChinaDeepak Chawla, Government Medical College and Hospital, India
Copyright © 2022 Amer, Shaiba, Hadid, Anabrees, Almehery, AAssiri, Alnemri, Darwish, Baqawi, Aboshaiqah, Hneiny, Almaghrabi, El-Malky and Al-Dajani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasser S. Amer, eWFtZXJAa3N1LmVkdS5zYQ==; eWFzc2Vyc2FtaWFtZXJAZ21haWwuY29t
 Yasser S. Amer
Yasser S. Amer Lana A. Shaiba
Lana A. Shaiba Adnan Hadid
Adnan Hadid Jasim Anabrees
Jasim Anabrees Abdulrahman Almehery9
Abdulrahman Almehery9 Manal AAssiri
Manal AAssiri Abdulrahman Alnemri
Abdulrahman Alnemri Amira R. Al Darwish
Amira R. Al Darwish Badi Baqawi
Badi Baqawi Ahmad Aboshaiqah
Ahmad Aboshaiqah Layal Hneiny
Layal Hneiny Rana H. Almaghrabi
Rana H. Almaghrabi Ahmed M. El-Malky
Ahmed M. El-Malky Nawaf M. Al-Dajani
Nawaf M. Al-Dajani