- Neonatal Department, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
In recent years, it has been verified that placental transfusion can replenish blood volume of neonates, improve organ perfusion in the early postnatal stage, and facilitate the transition from fetal circulation to adult circulation. Meanwhile, placental transfusion can reduce the need for blood transfusion and the onset of intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, and other complications. Furthermore, it can improve the iron store and the long-term prognosis of central nervous system, and reduce infant mortality. Different methods have been used, including delayed cord clamping, intact umbilical cord milking, and cut umbilical cord milking. The World Health Organization (WHO) and other academic organizations recommend the routine use of delayed cord clamping at birth for the most vigorous term and preterm neonates. However, details of placental transfusion should be clarified, and the short/long-term impacts of this technology on some infants with special conditions still require further study.
Introduction
The concept of placental transfusion has evolved over a long period of time. Following delivery, the two umbilical arteries gradually contract and the bloodstream from the fetus to the placenta rapidly decreases. Meanwhile, the umbilical venous flow can be maintained for up to a few minutes (1, 2). Therefore, keeping the umbilical cord intact over a period of time, rather than clamping it immediately after birth, can increase blood volume for the neonate and facilitate the transition from fetal circulation to adult circulation. It also helps the perfusion of important organs. Perspectives on placental transfusion have rapidly expanded with the development of maternal-fetal medicine and neonatology, and now it has been accepted as a routine procedure at most deliveries. The World Health Organization (WHO), the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics all recommend delayed umbilical cord clamping (DCC) in most vigorous term and preterm infants at birth. However, there are still problems in special cases with regard to the benefits and risks of this technique, and further study is still needed (3, 4). This article seeks to review and summarize most current literature on placental transfusion. A table of studies evaluating the topic is presented (Table 1).
Physiological basis of placental transfusion
The placenta is an indispensable organ during pregnancy, which plays a variety of essential roles, such as providing a means of maternal-fetal oxygen and carbon dioxide exchange, supplying nutrients to the fetus, transporting various substances, secreting significant factors and hormones, and also acting as an immune organ and barrier to the fetus (5).
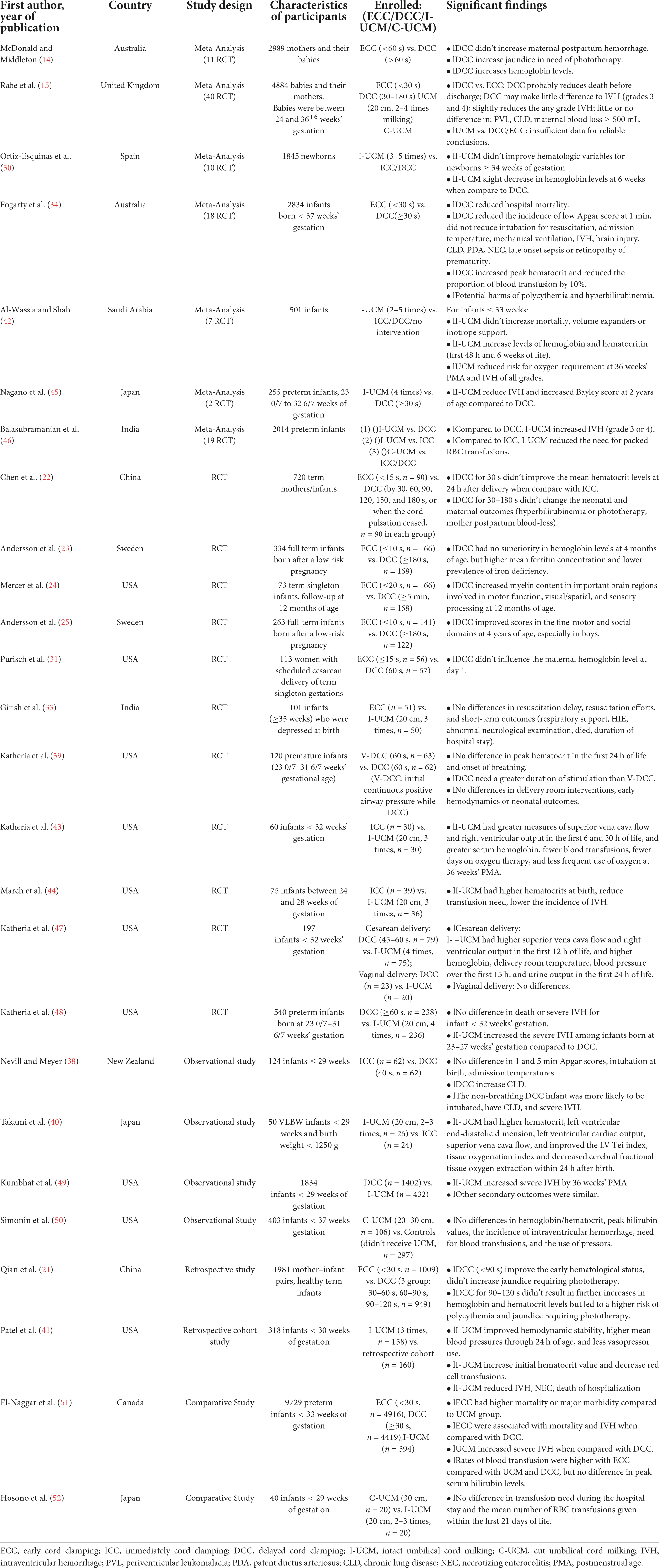
Placental function relies on maternal and fetal circulation (Figure 1). The umbilical arteries originate from the bilateral internal iliac arteries, which pass through the umbilical ring in the fetal abdominal wall and enter the umbilical cord. Here, the two umbilical arteries and one umbilical vein form a rope-like structure that links the fetus and the placenta. Usually, the two umbilical arteries form an anastomotic connection at the site where the umbilical cord approaches the placenta, and then branch out into the placental villi. The umbilical vein passes through the umbilical ring and into the abdomen of the fetus. Some of the blood in the umbilical vein imports into the ductus venosus and enters the inferior vena cava, thereafter, passes from the right to the left atrium through the foramen ovale. Blood flowing into the left branch of the portal vein nourishes the left side of liver, while the remaining blood nourishes the right side of the liver, after passing through the ductus venosus (6).
The combined ventricular output (CVO) of the fetus was about 450 ml/min/kg over the latter two-thirds of gestation, the right ventricular output became the dominant at second half of second trimester (7). In the near-term fetus, around 15% of the CVO reaches the lungs, account for only a quarter of right ventricular output; the remaining three quarters blood flows through the ductus arteriosus to descending aortic. Around 75% relatively well-oxygenated left ventricular output perfuses the head, neck, and arms; the remaining 25% flows to the descending aorta. The less-oxygenated descending aorta blood flows to the abdomen, lower body and the placenta, of which placenta hold about 50% (30% of CVO) (8).
Umbilical cord blood flow increases steadily to about 105 ml/kg/min before 20 weeks of gestation, and then gradually decreases to about 65 ml/kg/min near term. Between 20 and 30 weeks of gestation, the placental circulation accounts for 30% of the fetus’s total cardiac output, and this drops to 20% near term. For fetuses with severe growth retardation, the blood flow to the placenta is only 10% or less. Most of the blood circulates within the fetus, however, this type of “self-circulation” without exchange with maternal substances would eventually lead to an exacerbation of fetal growth retardation due to lack of support and regulation from maternal sources via the placenta (1).
The placenta is the foremost blood storage site for the fetus during pregnancy. Compared with the fetus, it is relatively large, and during the second trimester it holds a similar blood volume to that of the fetal body, while near term it still holds about 1/3 of the fetal blood volume. The liver and other visceral organs have a limited buffering capacity for blood reserve, but they are inferior in this regard to the placenta (1, 9).
Placental blood delivery to the fetus is not only important during the prenatal period, but also during delivery. Here it can improve the blood volume of the newborn, which leads to a better coordination with the initiation of pulmonary circulation and the dramatic change of the systemic circulation load in the early postnatal period; these all help the neonate to make a smooth transition from fetal to adult circulation. With the support of placental transfusion, the perfusion of important organs is guaranteed in the immediate postnatal period, which contributes to reducing intraventricular hemorrhage (IVH) and necrotizing enterocolitis (NEC).
The development of placental transfusion
Prior to the advancement of modern medicine, the common practice was to keep the umbilical cord intact until placental expulsion. There were even some reports of maintaining the umbilical cord attached to the placenta until natural separation, a practice termed “Lotus Birth”; however, this procedure increases the risk of neonatal sepsis (10–12).
From the 1940s–1950s, immediate cord clamping (ICC) after birth quickly became a common practice due to concerns on increasing maternal postpartum hemorrhage, although this practice was not supported by evidence (13). Actually, systematic retrospective analyses of DCC and ICC show that DCC doesn‘t increase the duration of the third stage of labor, maternal mortality, postpartum hemorrhage, nor the need for postpartum transfusion (14–16). Subsequently, two optional methods of umbilical cord milking (UCM) were developed: intact umbilical cord milking (I-UCM) and cut umbilical cord milking (C-UCM).
Research and guidelines for placental transfusion in term infants
The effect of placental transfusion on neonates varies on conditions. Factors such as delayed ligation, lung inflation, mode of delivery, gravity, and the use of oxytocin to stimulate uterine contraction, can all impact the effect of placental transfusion (17–19).
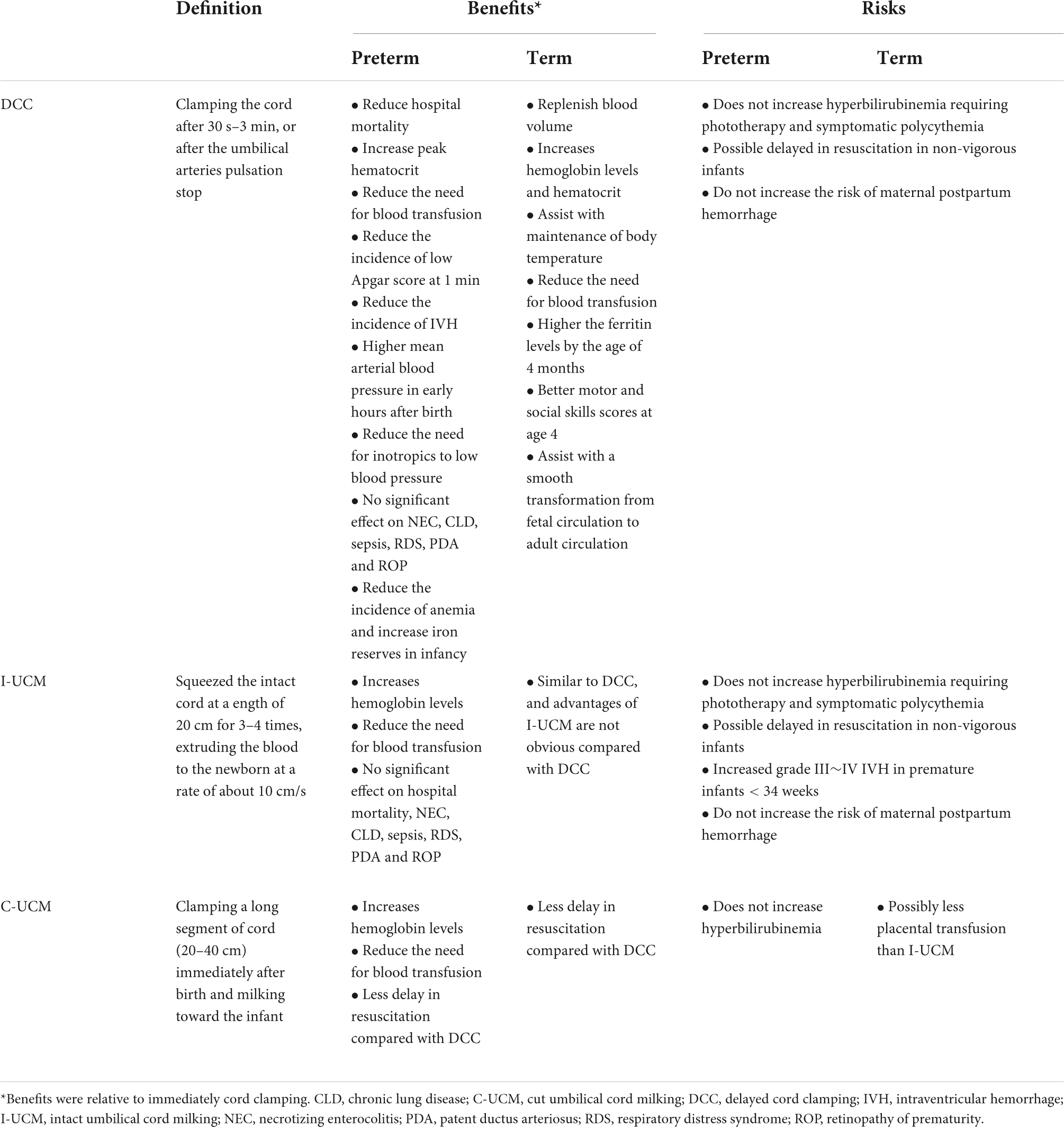
Delayed umbilical cord clamping is the most important factor affecting placental transfusion. ICC is defined as clamping the umbilical cord within 10–15 s after birth. According to the 7th edition of the Textbook of Neonatal Resuscitation, DCC is recommended as the standard procedure at birth (2). The umbilical cord bloodstream should be maintained at least 30–60 s after birth for most vigorous term and preterm infants. Ideally, it should be maintained until umbilical cord pulsation stops. The Italian Guidelines for Placental Transfusion in 2018 made the same recommendation (14).
Delayed umbilical cord clamping plays a role in many ways, the continuous blood flow from the placenta to fetus replenishes blood volume in neonates, increases hemoglobin levels and hematocrit, and can also assist with maintenance of body temperature. Multiple studies have shown that increased hemoglobin level could effectively reduce the need for blood transfusion, without increasing the incidence of polycythemia and hyperbilirubinemia that require phototherapy (20–22). The benefits of DCC were even sustained into childhood. As described by Andersson et al. (23), infant performed DCC at least 3 min didn’t observed higher hemoglobin levels at 4 months of age compared with ICC (<10 s), but ferritin levels were superior than ICC group. Mercer et al. (24) studied a longer time of DCC, they compared more than 5-min delay in umbilical cord to ICC (≤20 s), found the similar results by Andersson et al. (23) moreover, greater myelin content in important brain regions were observed in DCC group when followed up to 12 months, and the brain regions involved in motor function, visual or spatial, and sensory processing. Another study by Andersson et al. (25) also reported the neurodevelopmental benefits from DCC in term infants, they found that infants receiving DCC (≥3 min) had better motor and social skills scores at age 4 than ICC (<10 s), especially in boys.
Neonates face enormous circulatory challenges after birth. Those who treated with ICC usually experience a sudden reduction in preload and a significant increase in afterload. Before birth, the unexpanded lung induces high pulmonary circulation resistance, with very little blood moving through the pulmonary circulation. Blood rich in oxygen and nutrients from the umbilical vein passes through the foramen ovale and systemic circulation, to mainly supply the brain and upper trunk. Since the resistance of placental circulation is low, blood flow in the umbilical arteries accounts for a large part of the systemic blood flow. A sharp increase of systemic circulatory resistance could be expected after birth due to immediate ligation of the umbilical cord and interruption of placental transfusion, which brings quick increase of left ventricular afterload. Thereafter, pulmonary circulation resistance decreases, and blood flow increases significantly when the lungs dilate; at this point blood flow through the foramen ovale into the systemic circulation falls quickly, and the preload of the left heart is presented with a transient and dramatic decrease. DCC can precisely supplement left ventricular blood volume and slowly improve systemic circulation resistance based on contraction of the umbilical arteries. Therefore, DCC can assist neonates to make a smoother transition from fetal circulation to adult circulation and maintain a more stable blood pressure (2, 13, 20, 26).
Due to the advantages far outweighing the disadvantages, DCC is now recommended for most vigorous term or preterm infants. Some researchers suggest that DCC should be extended to more neonates with special conditions. In congenital diaphragmatic hernia patients, organs such as the gastrointestinal tract herniate into the chest and cause a high incidence of persistent pulmonary hypertension, which has a great influence on the cardiorespiratory system (27, 28). These patients usually need advanced therapies. DCC is suggested to optimize the condition of infants with congenital diaphragmatic hernia during the early stage of birth, and to help them transit from fetal circulation to adult circulation. The Children’s Hospital of Philadelphia has begun an ongoing study of DCC in neonates with congenital diaphragmatic hernia. Similarly, infants with congenital heart disease often need respiratory support after birth, and sometimes even early surgical therapy. In recent years, researchers have noted that DCC did not increase the risk of asphyxia, or intubation in term infants with prenatally diagnosed congenital heart disease; it also reduced blood transfusion during hospitalization (29).
Many studies have shown that DCC has multiple advantages and it has been widely recognized, but the therapeutic effects are affected by many factors. In the third stage of labor, uterine contraction contributes to 25–30% of placental transfusion. The intrauterine umbilical venous pressure is about 40–50 mmHg, but it can reach 100 mmHg during a contraction. For neonates delivered by cesarean section, owing to the lack of increased umbilical venous pressure caused by uterine contraction, the effect of the opening of the pulmonary circulation and right ventricular afterload decreasing–that is caused by the newly established respiration–will play a greater role (9). Some scholars worry that the position of mother and baby will influence the effect of placental transfusion in DCC. A multi-center randomized controlled study published showed that the position of the neonate before cord clamping did not impact the effect of placental transfusion. When vigorous neonates born vaginally were held for 2 min before cord clamping at the level of the vagina or on the mother’s abdomen or chest, the volume of placental transfusion didn’t vary significantly (19). Based on the data, gravity seems to have little influence on DCC following a vaginal delivery. Current guidelines or specifications point out that putting those infants born vaginally on the mother’s abdomen after birth is also beneficial owing to the skin-to-skin contact that helps keep them warm. While neonates delivered by cesarean section do not experience effective uterine contraction, it is wise to put these babies below the mother’s position to promote the placental transfusion; at the same time, other measures should be taken to keep the baby warm. DCC and initial resuscitation should be performed simultaneously (2).
As a natural process, DCC does not increase the incidence of adverse reactions such as hyperbilirubinemia and polycythemia. However, DCC cannot be used in some term infants. Due to the need for emergent resuscitation, 14–22% of neonates undergo ICC. This is unfortunate because these infants may benefit the most from DCC (20). According to International Resuscitation Course, ventilation support is the most critical step during resuscitation (2). The 2012 WHO Guidelines for neonatal resuscitation suggested that DCC could be performed during positive pressure ventilation in hospitals where clinicians have the relevant experience, but this practice has not been recommended (23). It is also mentioned in the 2016 International Resuscitation course that the resuscitator should communicate with the obstetrician before delivery on the presence of placental abruption, placenta previa with bleeding, vasa previa with bleeding, or umbilical cord tearing, etc. It is believed that ICC should be adopted if the integrity of placental circulation is disturbed. Neonatologists need to engage in a lively discussion with obstetricians on some conditions, such as fetal retardation, ultrasound monitoring for abnormal umbilical blood flow, abnormal placenta, and other conditions that affect umbilical cord or placenta blood flow; DCC may be beneficial for neonates under such situations (2). Since most DCC studies excluded multiple pregnancies, there are no clear recommendation for newborns with multiple births. The 2018 Italian Guidelines for Placental Transfusion do not suggest DCC for single chorionic twins to avoid acute fetal transfusion syndrome; and the recommendation of DCC for dichorionic twins is also weak due to low level evidences. Shoulder dystocia, amniotic fluid embolism, feto-fetal transfusion syndrome, and mothers with HIV are all contraindications for DCC (13).
Given the above-mentioned issues with DCC, Umbilical cord milking (UCM) is another method of placental transfusion. For term infants, UCM has similar effects compared with DCC, and even superior to DCC in some respects (30, 31). The cord is milked only once in C-UCM, and the blood volume obtained by C-UCM may be less than that obtained by I-UCM (32). Generally, UCM takes less time than DCC, so I-UCM may shows advantages in asphyxiated newborns who needs immediate resuscitation. Small randomized controlled trial showed that, I-UCM used as a placental transfusion strategy in late preterm and term neonates requiring resuscitation with no significant adverse short-term outcomes compared to ICC. I-UCM maybe a feasible strategy for depressed infants, but still need more discussion, including long-term outcome study (33). UCM technology is mainly used in premature infants, whereas DCC is still preferred in term infant (10, 13, 26). UCM will be discussed more below.
Advances in placental transfusion of premature infants
Premature infants are more likely to be admitted into NICU than term infants, some of them even need respiratory and circulatory support. Potentially they could benefit most from placental transfusion.
Delayed umbilical cord clamping plays an important role in premature infants. It can reduce in-hospital mortality, increase hematocrit, reduce the need for blood transfusion and inotropic, improve the mean arterial blood pressure in early hours after birth (14, 34). In recent years, a number of studies have found that intracranial hemorrhage in premature infants is closely related to insufficient blood flow in the superior vena cava. Compared with ICC, DCC can improve hemodynamic stability in early life and reduce the occurrence of intracranial hemorrhage (15, 35–37).
Although premature infants can also benefit from DCC, some studies show that DCC may be inadequate under some circumstances. Preterm deliveries often require more resuscitation, let alone this may happen with multiple pregnancy. DCC is also unfairly treated due to misunderstandings of the relationship between placental transfusion and maintenance of body temperature by some perinatal practitioners.
Aladangady et al. (17) found that the blood volume was significantly increased in vaginally born preterm infants (24–32 gestational weeks) receiving DCC procedure, but the same changes did not appear in premature infants delivered by cesarean section. Meanwhile, Nevill et al. (38) found that 70% of preterm infants (<29 gestational weeks) in the 40 s‘ DCC group established spontaneous breathing within 1 min after birth, and there was no significant difference in Apgar score, the number requiring intubation at birth, and admission temperatures compared with ICC group, Instead, the apneic infants in DCC group were more likely to be intubated, have chronic lung disease, and severe IVH. Characteristics of this subgroup led to a higher incidence of chronic lung disease in DCC group. Katheria et al. (39) found no significant difference on establishment of spontaneous breathing, cerebral oxygenation, cardiac output, and other neonatal outcomes in the two groups of preterm infants (<32 gestational weeks) initially stimulated or ventilated during DCC. This is the first successful exploration of feasibility of ventilatory support for preterm infants during DCC procedure. However, tactile stimulation didn’t show any advantages over positive pressure ventilation during DCC procedure, including neonatal mortality.
Meanwhile, the interest on UCM technique is also growing. For UCM, the cord remains intact after delivery. 20 cm‘ s umbilical cord is held within the thumb and forefinger and gently squeezed 2–4 times, the blood will be forced to flow from the cord to the neonate at a rate about 10 cm/s (9, 13).
The benefits of UCM over ICC have been widely verified. UCM can significantly improve the clinical status of preterm babies, such as blood volume, hemoglobin level, arterial pressure, cerebral oxygenation, urine output during early life, and effectively reduce blood transfusion (40–43). A meta-analysis showed that UCM had no significant effect on hospital mortality, NEC, chronic lung disease (CLD), sepsis, respiratory distress syndrome (RDS), patent ductus arteriosus (PDA) and retinopathy of prematurity (ROP) compared with ICC (15). Hemodynamics evaluation by ultrasound also shows that premature infants treated by UCM were superior to ICC in left ventricular end-diastolic volume, left ventricular cardiac output, and superior vena cava blood flow (41). Regarding the nervous system, several studies have suggested that UCM can effectively reduce the occurrence of preterm IVH compared with ICC (42–44).
Previous studies have suggested that UCM may be superior to DCC in preterm infants. Meta-analysis by Nagano et al. (45) demonstrated low quality evidence for reduced IVH and improved Bayley score at 2 years of age with UCM compared to DCC in preterm infants; UCM (4 stripping) provided more blood volume than DCC (30–60 s) in this study, but longer duration of DCC haven’t been investigated. Recent studies warning that UCM may be harmful to premature babies. Systematic reviews by Balasubramanian et al. (46) comparing UCM with DCC in preterm infants have reported that UCM significantly increased the risk of severe intraventricular hemorrhage in preterm infants < 34 weeks of gestation; this result were mainly influenced by the large multi-center trial conducted by Katheria et al. (48). Retrospectively data from Canadian Neonatal Network showed that both DCC and UCM were associated with better short-term outcomes than ICC in preterm infants; however, the odds of severe intraventricular hemorrhage were higher with UCM compared with DCC both in gestational age < 33 weeks and < 28 weeks (51). Result from another multicenter retrospective study also reported that extremely preterm infant exposed to UCM had higher odds of severe IVH than DCC (49).
Besides, another feasible method is C-UCM, which has been initially explored in some Asian countries. The cord was cutted after delivery, remained a 30–40 cm‘ s segment attach to newborn. The cutted cord was gently squeezed once at a rate about 10 cm/s. Hosono et al. (52) verified the safety and effectiveness of the C-UCM technique, and found that there was no significant difference in blood transfusion requirements between C-UCM and I-UCM group. Blood volume transported to the baby was limited by squeezed the sheared cord. An observational cohort of preterm infant < 35 weeks of gestation suggested that C-UCM neither improved hemoglobin levels nor reduced neonatal morbidities when compared with ICC (50). If C-UCM can be an effective method of placental transfusion of premature infants remains to be further explored.
Complications of placental transfusion
Although there is a high demand for placental blood transfusion in preterm infants, who are expected to benefit most from this technique, some researchers still have concerns about placental transfusion for premature babies in some clinical settings. Researchers worry that the placental transfusion can lead to hyperviscosity syndrome, or polycythemia. Case-controlled trial shows that blood viscosity is related to gestational age, and all subjects’ viscosity was lower than 12P which was the diagnostic threshold for hyperviscosity (53). Other studies have not found higher rates of polycythemia or significantly increased phototherapy needs in DCC or UCM treated newborns.
In 2019, a multi-center trial (NCT 03019367) (48) regarding placental blood transfusion was terminated early. This study was to investigate the effects of different methods of placental transfusion on intracranial hemorrhage in preterm infants less than 32 weeks of gestational age. In the midterm analysis, it was found that the risk of severe IVH was significantly higher in the subgroup of vaginal delivered extremely preterm infants (23–27 gestational weeks) receiving UCM procedure, who also had higher hemoglobin levels than DCC cases. In the UCM group, the umbilical cord was squeezed four times. Similar results were not found in other gestational age subgroups. The possible reasons are as follows. First, more than 50% of extremely preterm infants face pressure associated cerebral perfusion fluctuation, which happens in only more than 20% of very preterm infants (54). Repeated UCM in preterm lambs, equivalent to human fetuses of 26 gestational weeks, resulted in significant fluctuations in carotid arterial blood flow (55). Therefore, repeated UCM in extremely preterm infants may directly cause fluctuation of cerebral blood flow, and consequently increase the risk of IVH. Second, inflammatory mediators of fetal inflammation can pass through the blood-brain barrier, and cause an inflammatory cascade reaction of central nervous system. In this case, the germinal matrix and cerebrovascular system become vulnerable to blood flow fluctuations. Lastly, for premature infants, UCM can effectively improve hemoglobin level, blood volume, and organ perfusion, but it may have an opposite effect on the immature brain. Several large cohort studies have found that the incidence of IVH in naturally delivered premature infants is significantly higher than those of cesarean section (56, 57) and UCM increases the risk of IVH. Although this study differs from other studies in many aspects, such as experimental methods, subject selection, and details of umbilical cord and placenta management, it still suggests that future studies should be more careful in assessing benefits and risks, with the ultimate goal of facilitating the survival of preterm infants and improving long-term neurological outcomes. A summary of three strategies for placental transfusion is showed in Table 2.
Conclusion
Based on recent research, the mechanisms of placental transfusion have become relatively clear, and the benefits to neonates have been widely demonstrated. ICC is a practice that disrupts the normal physiologic transition at birth, it used to be widely practiced without the support by high quality evidence. DCC is widely recommended as the most physiological method of placental transfusion, meanwhile, a growing number of studies has cast doubt on the practice of UCM in premature infants. Although there are still some difficulties in performing placental transfusion for every newborn, especially for asphyxia and infants at high risk for maternal or fetal appendages. However, neonatologists should be confident in placental transfusion technology. Developing of a protocol to standardize the selection of appropriate placental transfusion strategies for different neonatal populations is also a vital task. A series of well-designed studies are warranted to assess the effects of placental transfusion in neonates with special conditions.
Author contributions
JL wrote the manuscript. JL, GY, QW, and XZ collected and analyzed the data. RJ revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 8217060614), the Sichuan Science and Technology Program (Grant Nos. 2018JY0355 and 2021YJ0190), and Medical Technology Program of Health Commission of Sichuan Province (Grant No. 21PJ129).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Richard AP, Steven HA, David HR, William EB, William WF. Fetal and Neonatal Physiology. 5th ed. Philadelphia, PA: Elsevier Inc (2017). p. 600–10.
2. Gary MW, Jeanette Z. Textbook of Neonatal Resuscitation. 7th ed. Elk Grove Village, IL: American Academy of Pediatrics and American Heart Association (2016). p. 36–7.
3. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Delayed umbilical cord clamping after birth: ACOG committee opinion, number 814. Obstet Gynecol. (2020) 136:e100–6. doi: 10.1097/AOG.0000000000004167
4. World Health Organization. Guideline: Delayed Umbilical Cord Clamping for Improved Maternal and Infant Health and Nutrition Outcomes. Geneva: World Health Organization (2014).
5. Sibley CP, Brownbill P, Glazier JD, Greenwood SL. Knowledge needed about the exchange physiology of the placenta. Placenta. (2018) 64(Suppl. 1):S9–15. doi: 10.1016/j.placenta.2018.01.006
6. Blackburn S. Placental, fetal, and transitional circulation revisited. J Perinat Neonatal Nurs. (2006) 20:290–4. doi: 10.1097/00005237-200610000-00006
7. Rudolph AM. Circulatory changes during gestational development of the sheep and human fetus. Pediatr Res. (2018) 84:348–51. doi: 10.1038/s41390-018-0094-9
8. Finnemore A, Groves A. Physiology of the fetal and transitional circulation. Semin Fetal Neonatal Med. (2015) 20:210–6. doi: 10.1016/j.siny.2015.04.003
9. Dunn PM. The placental venous pressure during and after the third stage of labour following early cord ligation. J Obstet Gynaecol Br Commonwealth. (1966) 73:747–56. doi: 10.1111/j.1471-0528.1966.tb06078.x
10. Royal College of Obstetricians and Gynaecologists.RCOG Statement on Umbilical Non-Severance or “Lotus Birth. (2017). Available online at: https://www.rcog.org.uk/en/news/rcog-statement-on-umbilical-nonseverance-or-lotus-birth/ (Accessed April 12)
11. Burns E. More than clinical waste? Placenta rituals among Australian home-birthing women. J Perinat Educ. (2014) 23:41–9. doi: 10.1891/1058-1243.23.1.41
12. Baker AN, Rao LM, Yeganeh N. Case 3: seizures in a 2-day-old Infant. NeoReviews. (2017) 18:e445–7. doi: 10.1542/neo.18-7-e445
13. Ghirardello S, Di Tommaso M, Fiocchi S, Locatelli A, Perrone B, Pratesi S, et al. Italian recommendations for placental transfusion strategies. Front Pediatr. (2018) 6:372. doi: 10.3389/fped.2018.00372
14. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes (review). Cochrane Database Syst Rev. (2008) 2:CD004074. doi: 10.1002/14651858.CD004074.pub3
15. Rabe H, Gyte GM, Díaz-Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. (2019) 9:CD003248. doi: 10.1002/14651858.CD003248.pub4
16. Eichenbaum-Pikser G, Zasloff JS. Delayed clamping of the umbilical cord: a review with implications for practice. J Midwifery Womens Health. (2009) 54:321–6. doi: 10.1016/j.jmwh.2008.12.012
17. Aladangady N, McHugh S, Aitchison TC, Wardrop CA, Holland BM. Infants’ blood volume in a controlled trial of placental transfusion at preterm delivery. Pediatrics. (2006) 117:93–8. doi: 10.1542/peds.2004-1773
18. Airey RJ, Farrar D, Duley L. Alternative positions for the baby at birth before clamping the umbilical cord. Cochrane Database Syst Rev. (2010) 6:CD007555. doi: 10.1002/14651858.CD007555.pub2
19. Vain NE, Satragno DS, Gorenstein AN, Gordillo JE, Berazategui JP, Alda MG, et al. Effect of gravity on volume of placental transfusion: a multicentre, randomised, non-inferiority trial. Lancet. (2014) 384:235–40. doi: 10.1016/S0140-6736(14)60197-5
20. DuPont TL, Ohls RK. Placental transfusion: current practices and future directions. NeoReviews. (2018) 19:e1–10. doi: 10.1080/14767058.2016.1269319
21. Qian Y, Lu Q, Shao H, Ying X, Huang W, Hua Y. Timing of umbilical cord clamping and neonatal jaundice in singleton term pregnancy. Early Hum Dev. (2020) 142:104948. doi: 10.1016/j.earlhumdev.2019.104948
22. Chen X, Li X, Chang Y, Li W, Cui H. Effect and safety of timing of cord clamping on neonatal hematocrit values and clinical outcomes in term infants: a randomized controlled trial. J Perinatol. (2018) 38:251–7. doi: 10.1038/s41372-017-0001-y
23. Andersson O, Hellström-Westas L, Andersson D, Domellöf M. Effect of delayed versus early umbilical cord clamping on neonatal outcomes and iron status at 4 months: a randomised controlled trial. BMJ. (2011) 343:d7157. doi: 10.1136/bmj.d7157
24. Mercer JS, Erickson-Owens DA, Deoni SCL, Dean Iii DC, Tucker R, Parker AB, et al. The effects of delayed cord clamping on 12-month brain myelin content and neurodevelopment: a randomized controlled trial. Am J Perinatol. (2022) 39:37–44. doi: 10.1055/s-0040-1714258
25. Andersson O, Lindquist B, Lindgren M, Stjernqvist K, Domellöf M, Hellström-Westas L. Effect of delayed cord clamping on neurodevelopment at 4 years of age: a randomized clinical trial. JAMA Pediatr. (2015) 169:631–8. doi: 10.1001/jamapediatrics.2015.0358
26. Who Guidelines Approved by the Guidelines Review Committee.Guidelines on Basic Newborn Resuscitation. Geneva: World Health Organization (2012).
27. Weems MF, Jancelewicz T, Sandhu HS. Congenital diaphragmatic hernia: maximizing survival. NeoReviews. (2016) 17:e705–18. doi: 10.1542/neo.17-12-e705
28. Canadian Congenital Diaphragmatic Hernia Collaborative Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, et al. Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ. (2018) 190:E103–12. doi: 10.1503/cmaj.170206
29. Backes CH, Huang H, Cua CL, Garg V, Smith CV, Yin H, et al. Early versus delayed umbilical cord clamping in infants with congenital heart disease: a pilot, randomized, controlled trial. J Perinatol. (2015) 35:826–31. doi: 10.1038/jp.2015.89
30. Ortiz-Esquinas I, Rodríguez-Almagro J, Gómez-Salgado J, Arias-Arias Á, Ballesta-Castillejos A, Hernández-Martínez A. Effects of cord milking in late preterm infants and full-term infants: a systematic review and meta-analysis. Birth. (2020) 47:259–69. doi: 10.1111/birt.12500
31. Panburana P, Odthon T, Pongmee P, Hansahiranwadee W. The effect of umbilical cord milking compared with delayed cord clamping in term neonates: a randomized controlled trial. Int J Womens Health. (2020) 12:301–6. doi: 10.1001/jama.2019.15995
32. McAdams RM, Fay E, Delaney S. Whole blood volumes associated with milking intact and cut umbilical cords in term newborns. J Perinatol. (2018) 38:245–50. doi: 10.1038/s41372-017-0002-x
33. Girish M, Jain V, Dhokane R, Gondhali SB, Vaidya A, Aghai ZH. Umbilical cord milking for neonates who are depressed at birth: a randomized trial of feasibility. J Perinatol. (2018) 38:1190–6. doi: 10.1038/s41372-018-0161-4
34. Fogarty M, Osborn DA, Askie L, Seidler AL, Hunter K, Lui K, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. (2018) 218:1–18. doi: 10.1016/j.ajog.2017.10.231
35. Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2000) 82:F188–94. doi: 10.1136/fn.82.3.f188
36. Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, et al. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics. (2012) 129:e667–72. doi: 10.1542/peds.2011-2550
37. Meyer MP, Mildenhall L. Delayed cord clamping and blood flow in the superior vena cava in preterm infants: an observational study. Arch Dis Child Fetal Neonatal Ed. (2012) 97:F484–6. doi: 10.1136/adc.2010.199703
38. Nevill E, Meyer MP. Effect of delayed cord clamping (DCC) on breathing and transition at birth in very preterm infants. Early Hum Dev. (2015) 91:407–11. doi: 10.1016/j.earlhumdev.2015.04.013
39. Katheria A, Poeltler D, Durham J, Steen J, Rich W, Arnell K, et al. Neonatal resuscitation with an intact cord: a randomized clinical trial. J Pediatr. (2016) 178:75.e–80.e. doi: 10.1016/j.jpeds.2016.07.053
40. Takami T, Suganami Y, Sunohara D, Kondo A, Mizukaki N, Fujioka T, et al. Umbilical cord milking stabilizes cerebral oxygenation and perfusion in infants born before 29 weeks of gestation. J Pediatr. (2012) 161:742–7. doi: 10.1016/j.jpeds.2012.03.053
41. Patel S, Clark EA, Rodriguez CE, Metz TD, Abbaszadeh M, Yoder BA. Effect of umbilical cord milking on morbidity and survival in extremely low gestational age neonates. Am J Obstet Gynecol. (2014) 21:.e1–519. doi: 10.1016/j.ajog.2014.05.037
42. Al-Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta-analysis. JAMA Pediatr. (2015) 169:18–25. doi: 10.1001/jamapediatrics.2014.1906
43. Katheria AC, Leone TA, Woelkers D, Garey DM, Rich W, Finer NN. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. J Pediatr. (2014) 164:1045.e–50.e. doi: 10.1016/j.jpeds.2014.01.024
44. March MI, Hacker MR, Parson AW, Modest AM, de Veciana M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J Perinatol. (2013) 33:763–7. doi: 10.1038/jp.2013.70
45. Nagano N, Saito M, Sugiura T, Miyahara F, Namba F, Ota E. Benefits of umbilical cord milking versus delayed cord clamping on neonatal outcomes in preterm infants: a systematic review and meta-analysis. PLoS One. (2018) 13:e0201528. doi: 10.1371/journal.pone.0201528
46. Balasubramanian H, Ananthan A, Jain V, Rao SC, Kabra N. Umbilical cord milking in preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2020) 105:572–80. doi: 10.1136/archdischild-2019-318627
47. Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics. (2015) 136:61–9. doi: 10.1542/peds.2015-0368
48. Katheria A, Reister F, Essers J, Mendler M, Hummler H, Subramaniam A, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. (2019) 322:1877–86. doi: 10.1001/jama.2019.16004
49. Kumbhat N, Eggleston B, Davis AS, DeMauro SB, Van Meurs KP, Foglia EE, et al. Umbilical cord milking vs delayed cord clamping and associations with in-hospital outcomes among extremely premature infants. J Pediatr. (2021) 232:87.e–94.e. doi: 10.1016/j.jpeds.2020.12.072
50. Simonin A, Safarulla A, Farmer Z, Coleman J, Sutton D, Wheeler K, et al. Cut umbilical cord milking: an ineffective method of placental transfusion in preterm infants? J Matern Fetal Neonatal Med. (2020) 33:3132–5. doi: 10.1080/14767058.2019.1569616
51. El-Naggar W, Afifi J, Dorling J, Bodani J, Cieslak Z, Canning R, et al. Canadian neonatal network and the Canadian preterm birth network investigators. A comparison of strategies for managing the umbilical cord at birth in preterm infants. J Pediatr. (2020) 225:58.e–64.e. doi: 10.1016/j.jpeds.2020.05.018
52. Hosono S, Mugishima H, Takahashi S, Takahashi S, Masaoka N, Yamamoto T, et al. One-time umbilical cord milking after cord cutting has same effectiveness as multiple-time umbilical cord milking in infants born at <29 weeks of gestation: a retrospective study. J Perinatol. (2015) 35:590–4. doi: 10.1038/jp.2015.15
53. Christensen RD, Baer VL, Gerday E, Sheffield MJ, Richards DS, Shepherd JG, et al. Whole-blood viscosity in the neonate: effects of gestational age, hematocrit, mean corpuscular volume and umbilical cord milking. J Perinatol. (2014) 34:16–21. doi: 10.1038/jp.2013.112
54. Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. (2007) 61:467–73. doi: 10.1203/pdr.0b013e31803237f6
55. Blank DA, Polglase GR, Kluckow M, Gill AW, Crossley KJ, Moxham A, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed. (2018) 103:F539–46. doi: 10.1136/archdischild-2017-314005
56. Humberg A, Härtel C, Paul P, Hanke K, Bossung V, Hartz A, et al. German neonatal network (GNN). Delivery mode and intraventricular hemorrhage risk in very-low-birth-weight infants: observational data of the German neonatal network. Eur J Obstet Gynecol Reprod Biol. (2017) 212:144–9. doi: 10.1016/j.ejogrb.2017.03.032
Keywords: neonates, placental transfusion, delayed cord clamping, intact-umbilical cord milking, cut-umbilical cord milking
Citation: Lu J, Yue G, Wang Q, Zhou X and Ju R (2022) A review on development of placental transfusion in term and preterm infants. Front. Pediatr. 10:890988. doi: 10.3389/fped.2022.890988
Received: 07 March 2022; Accepted: 30 August 2022;
Published: 15 September 2022.
Edited by:
Suksham Jain, Government Medical College and Hospital, IndiaCopyright © 2022 Lu, Yue, Wang, Zhou and Ju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Ju, anVyb25nQHVlc3RjLmVkdS5jbg==
 Jiangyi Lu
Jiangyi Lu Guang Yue
Guang Yue Rong Ju
Rong Ju