
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 31 May 2022
Sec. Pediatric Immunology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.890755
This article is part of the Research Topic Case Reports in Pediatric Immunology 2022 View all 11 articles
 Takayuki Suzuki1*
Takayuki Suzuki1* Tomohiro Suenaga1
Tomohiro Suenaga1 Aiko Sakai2
Aiko Sakai2 Masaya Sugiyama2
Masaya Sugiyama2 Masashi Mizokami2
Masashi Mizokami2 Ayumi Mizukami3
Ayumi Mizukami3 Satoshi Takasago3
Satoshi Takasago3 Hiromichi Hamada4
Hiromichi Hamada4 Nobuyuki Kakimoto1
Nobuyuki Kakimoto1 Takashi Takeuchi1
Takashi Takeuchi1 Mina Ueda5
Mina Ueda5 Yuki Komori5
Yuki Komori5 Daisuke Tokuhara1
Daisuke Tokuhara1 Hiroyuki Suzuki1,6
Hiroyuki Suzuki1,6Multisystem inflammatory syndrome in children (MIS-C) is a new syndrome involving the development of severe dysfunction in multiple organs after severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection. Because the pathophysiology of MIS-C remains unclear, a treatment strategy has not yet been established. We experienced a 12-year-old boy who developed MIS-C at 56 days after SARS-CoV-2 infection and for whom ciclosporin A (CsA) was effective as a third-line treatment. He had a high fever on day 1, and developed a rash on the trunk, swelling in the cervical region, and palmar erythema on day 2. On days 3, he developed conjunctivitis and lip redness, and fulfilled the criteria for classical Kawasaki disease (KD). Although intravenous immunoglobulin infusion (IVIG) was started on day 4, fever persisted and respiratory distress and severe abdominal pain developed. On day 5, because he fulfilled the criteria for MIS-C, methylprednisolone pulse was started for 3 days as a second-line treatment. However, he did not exhibit defervescence and the symptoms continued. Therefore, we selected CsA as a third-line treatment. CsA was so effective that he became defervescent and his symptoms disappeared. In order to clarify the relationship with treatment and the change of clinical conditions, we examined the kinetics of 71 serum cytokines to determine their relationships with his clinical course during the three successive treatments. We found that CsA suppressed macrophage-activating cytokines such as, IL-12(p40), and IL-18 with improvement of his clinical symptoms. CsA may be a useful option for additional treatment of patients with MIS-C refractory to IVIG + methylprednisolone pulse.
Multisystem inflammatory syndrome in children (MIS-C) is a new syndrome that was first reported in Europe and the United States in 2020. Although the mechanism for onset of MIS-C remains unclear, recent manuscripts have suggested potential mechanisms from the viewpoint of various immunological angles (1–6). Based on the cytokine kinetics in MIS-C, a wide variety of cells related to both innate immunity and adaptive immunity, such as macrophages, neutrophils, T cells, and B cells, may be activated (4–6).
Regarding treatment of MIS-C, no definite treatment has been established to date. However, based on the similarity with KD vasculitis in many clinical aspects, intravenous immunoglobulin infusion (IVIG) alone or IVIG plus steroid are recommended as the first line treatment (7). If patients with MIS-C are refractory to the first-line treatment, biological agents such as infliximab (IFX; anti-tumor necrosis factor-α antagonist) (8), anakinra (interleukin [IL]-1 blocker) (9, 10), and tocilizumab (IL-6 receptor inhibitor) (11) are recommended as a second-line treatment. However, these options are also not definitively established.
Here, we report a patient with MIS-C in whom ciclosporin A (CsA) was effective as a third-line treatment. We examined the kinetics of 71 serum cytokines and gave discussion to determine their changes in accordance with the treatments and clinical symptoms during the patient’s clinical course.
A 12-year-old boy with no particular history of medical problems was diagnosed with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection by polymerase chain reaction (PCR) analysis of a nasopharyngeal swab sample at 56 days before onset of MIS-C. He was admitted to his previous hospital because of low-grade fever and sore throat. His father was Caucasian, and his mother was Japanese.
At 56 days after the diagnosis of SARS-CoV-2 infection, he developed a fever (39.8°C) on day 1. On the next day (day 2), he had a rash on his trunk, swelling and pain in both sides of the cervical region, and palmar erythema. He was therefore re-admitted to the previous hospital, and ceftriaxone (50 mg/kg/day) was initiated. On day 3, he developed conjunctivitis and lip redness. On day 4, his fever persisted, and because he fulfilled the criteria for classical KD. Therefore, IVIG (2 g/kg) and aspirin (30 mg/kg) were started. However, the fever persisted, and abdominal pain and chest discomfort developed. On day 5, he was transferred to our hospital.
On admission, he had a fever of 41.0°C, severe pain throughout the abdomen, and bilateral cervical lymph node swelling with pain. Physical examination revealed multilobular cervical lymphadenopathy with a maximum diameter of 20 mm, and obvious redness of the lips and oral cavity. There were significant bilateral bulbar conjunctival congestions, and rashes on the face, trunk, and distal extremities. Although his blood pressure was 99/37 mmHg and within the reference range, he showed respiratory distress, including heart rate of 142 beats/min, respiratory rate of 50 breaths/min, and SpO2 of 99% (nasal O2: 2 L/min). Furthermore, cardiomegaly (cardiothoracic ratio: CTR; 54.6%) and pulmonary congestion were seen on both chest X-ray and computed tomography examinations, and intestinal gas showed prominent expansion on an abdominal X-ray (Supplementary Figures 1A,B). Electrocardiogram showed sinus tachycardia (heart rate: 142 beats/min), with flat and negative T waves on the V5 and V6 leads, suggesting myocardial damage (Supplementary Figure 1C). Although he had a negative test result for SARS-CoV-2, based on a TRC Ready (Tosoh Bioscience, Tokyo, Japan) analysis of a nasopharyngeal swab, SARS-CoV-2 Antibody Detection Kit (IgG/IgM) (Kurabo Industries Ltd., Osaka, Japan) was positive and negative, respectively.
Laboratory data on admission are shown in Table 1. Elevated neutrophil count (7.137 × 103/μL) and percentage of leukocytes (90%), reduced lymphocyte count (469/μL) and platelet count (116 × 103/μL), and elevated C-reactive protein level (22.04 mg/dL) were noted. In addition, elevated soluble IL-2 receptor level (7,044 U/mL, reference range 122–496 U/mL), normal ferritin level (173 ng/mL, reference range 13–277 ng/mL), elevated fibrin degradation product level (36.2 μg/mL, reference range 0–5 μg/mL), elevated brain natriuretic hormone level (461.9 pg/mL, reference range < 18.4 pg/mL), and elevated troponin I (708.6 pg/mL, reference range < 26.2 pg/mL) were found. The urinalysis did not show pyuria. Based on the clinical symptoms and laboratory data, he was diagnosed with MIS-C because he fulfilled the World Health Organization (WHO), Centers for Disease Control and Prevention (CDC) and Royal College of Paediatrics and Child Health (RCPCH) criteria (12–14). His clinical disease course following hospitalization is shown in Figure 1. We selected a methylprednisolone pulse (mPSL; 25 mg/kg/day) as a second-line treatment according to the guideline (7–9). Anticoagulation therapy (heparin: 10 IU/kg/h) was administered during the methylprednisolone pulse administration. Dobutamine was started simultaneously for the signs of cardiac failure such as cardiomegaly and pulmonary edema. Although his fever increased and decreased with the three consecutive mPSL pulses, he did not exhibit defervescence. In addition, his KD-like symptoms, decreased cardiac function, and abdominal pain persisted. Most guidelines recommend a biological agent as a second-line and/or third-line treatment after IVIG and steroid treatment. However, we discontinued glucocorticoid therapy and selected CsA as the third-line treatment because his clinical symptoms also fulfilled the six principal diagnostic criteria for classical KD (15–17). Initially, CsA was started by continuous intravenous injection (3 mg/kg/day) because he had severe abdominal pain at that time (day 8). The route of CsA treatment was changed from continuous intravenous injection to oral administration (3.75 mg/kg/day, divided by 2) on day 13 when his main symptoms, decreased cardiac function, and abdominal pain disappeared. He became defervescent and his symptoms, such as severe pain, disappeared within several days starting CsA treatment. We confirmed that no CAAs developed by both repeated transthoracic echocardiography and 3D computed tomography during his clinical course (Supplementary Figure 2). He was discharged after improvement of symptoms on day 28, and he has been doing well since discharge without any complications. Therefore, we judged CsA to be clearly effective for this patient with MIS-C refractory to IVIG + mPSL. Although various symptoms and signs disappeared after CsA treatment, his peripheral limbs (both hands and feet) turned purple and severe pain occurred when they hung below the heart, such as in the standing and/or sitting position, on day 11. There were no significant findings such as thrombus on vascular echography and magnetic resonance imaging or right heart failure. We judged these phenomena to be venous stasis, because the pain and color changes to the skin rapidly improved on elevation of the peripheral limbs. Elastic bandage use improved these symptoms, and thus exercise therapy was performed with an elastic bandage. Under exercise rehabilitation therapy, the symptoms improved in 10 days (18).
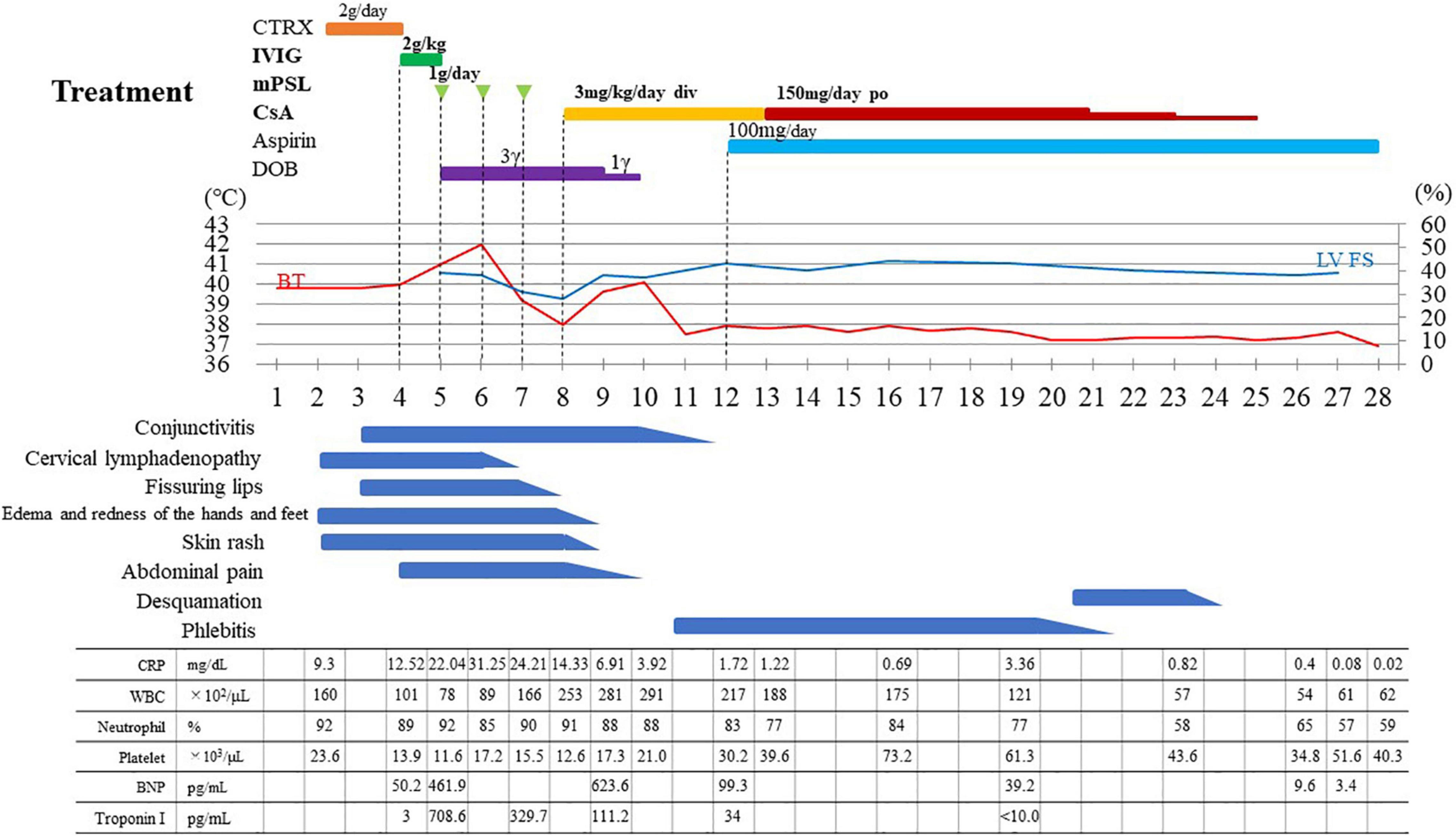
Figure 1. Treatment and clinical course of the patient. CTRX, ceftriaxone; IVIG, intravenous immunoglobulin infusion; mPSL, methylprednisolone pulse; CsA, ciclosporin A; DOB, dobutamine; LV FS, left ventricular fractional shortening; BT, body temperature; CRP, C-reactive protein (reference range < 0.14); WBC, white blood cell (reference range 4,000–10,700); BNP, B-type natriuretic peptide (reference range < 18.4); Toroponin I (reference range < 26.2); CTR, cardiothoracic ratio.
We evaluated his cytokine kinetics using archived serum samples collected intermittently from day 2 to day 166 of illness, in order to clarify the relationship with treatment and the change of clinical conditions. We performed comprehensive assays for cytokines using a BioPlex 3D system (Bio-Rad Laboratories Inc., Hercules, CA, United States) and BioPlex Human cytokine48/chemokine40 screening panel (Bio-Rad Laboratories Inc.). We also performed chemiluminescence enzyme immunoassay analyses using an HISCL-5000 system (Sysmex Asia Pacific Pte Ltd., Singapore) and ELISA assay (R&D Systems Inc., Minneapolis, MN, United States). Using these methods, we investigated the changes in 71 cytokines during the patient’s disease course. We classified a treatment response when a cytokine decreased by 50% from its peak level, referenced by the day 166 level for that cytokine. IL-2 and CXCL2 decreased before the initiation of treatment. Although no cytokines were completely suppressed by IVIG alone, proinflammatory cytokines (IL-6, IL-1β), interferon (IFN)-γ and IFN-γ-related chemokines (CXCL9, CXCL10, and CXCL11), and many chemokines (CCL1, CCL2, CCL8, CCL19, CCL20, CCL22, and CCL27) were strongly suppressed by mPSL (Table 2 and Figure 2). Although IL-6 was completely suppressed by mPSL, the patient did not become defervescent. As the third-line treatment, CsA mainly suppressed macrophage-activating cytokines such as IL-12(p40), and IL-18, and he became defervescent with disappearance of abdominal pain (Supplementary Figures 3-1, 3-2).
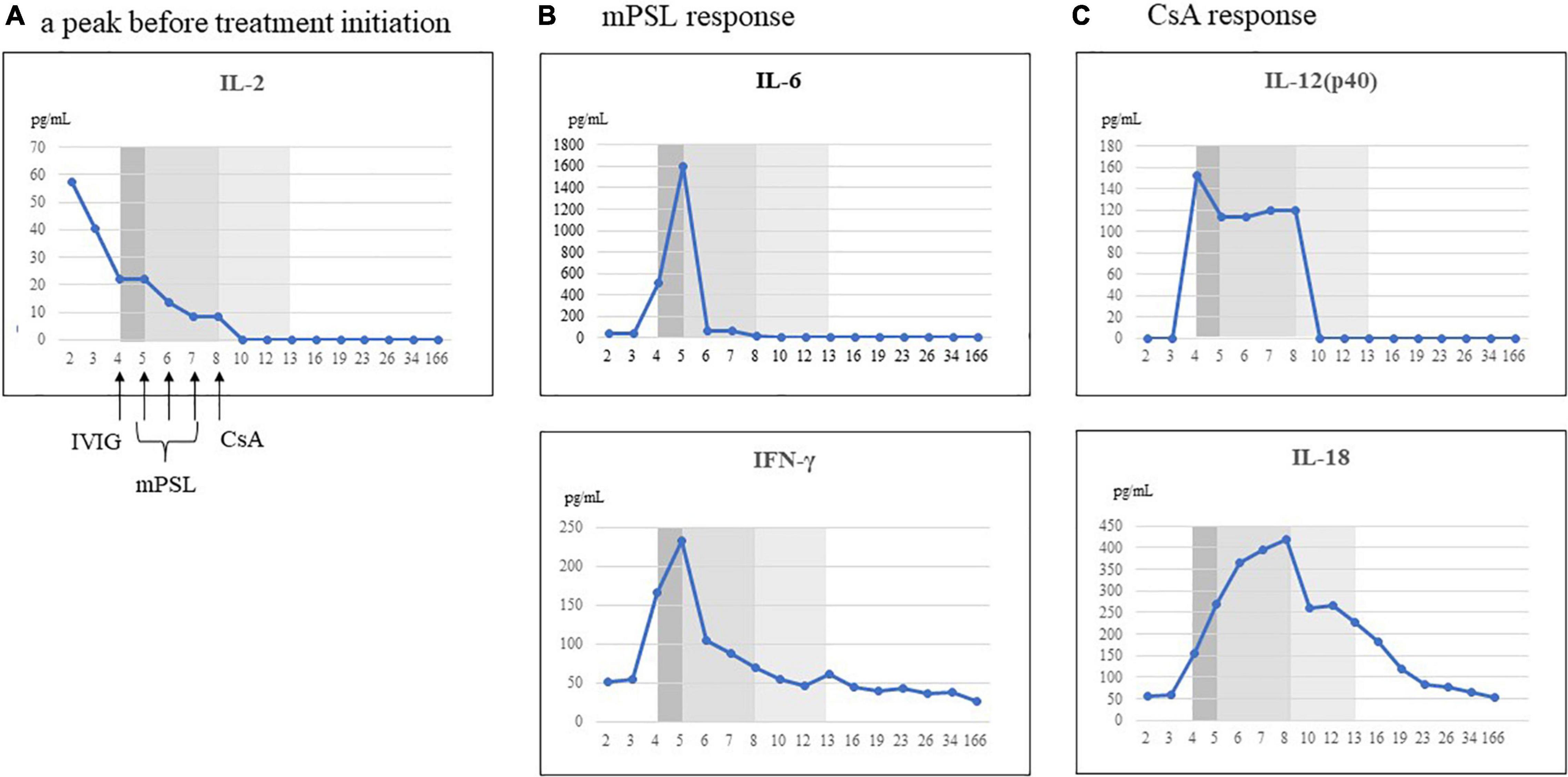
Figure 2. Representative cytokines that responded to each treatment. We classified the treatment response when the cytokine decreased by 50% from the peak levels, referenced to the day 166 levels for each cytokine. (A) Cytokine decreased prior to initiation of treatment. (B) Cytokines that responded to methylprednisolone (mPSL) treatment. (C) Cytokines that responded to ciclosporin A (CsA) treatment. IL, interleukin; IFN, interferon; IVIG, intravenous immunoglobulin infusion.
We reported a patient with MIS-C in whom CsA was effective as a third-line treatment. We examined the kinetics of 71 serum cytokines during the patient’s clinical course. In the present case, three successive treatments (IVIG, mPSL, and CsA) were performed during the time course, and thus we could distinguish the relationship between each treatment and the cytokine changes. Few cytokines were suppressed by IVIG alone. However, IL-6, IFN-γ, and IFN-γ-induced chemokines (CXCL9, CXCL10, and CXCL11) (19) and chemokines (CCL1, CCL2, CCL8, CCL19, and CCL20) were strongly suppressed by mPSL. Although IL-6 was completely suppressed by mPSL, the patient did not become defervescent. Meanwhile, CsA suppressed cytokines related to innate immune responses such as IL-12(p40), IL-18, and growth factors such as vascular endothelial growth factor and hepatocyte growth factor. Thereafter, the patient became defervescent. In our previous study, we reported the kinetics of 71 cytokines in a 9-year-old girl with MIS-C during her entire clinical course (6). However, both IVIG and prednisolone were started simultaneously in that case, and we could not distinguish the relationship between each treatment and the cytokine changes. Nevertheless, after IVIG and prednisolone administration as the first-line treatment, IL-6, IL-10, IL-17, IL-8, and CCL20 rapidly decreased, and the patient became defervescent. Meanwhile, IFN-γ is an indicator of Th1 type immune responses, and IFN-γ-induced cytokines such as CXCL9, CXCL10 decreased gradually, rather than rapidly. There is a report that IFN-γ plays a central role in the pathophysiology of MIS-C, indicating that IFN-γ may be a notable cytokine for understanding the state of MIS-C patients (19, 20). Furthermore, the present case was considered to be a more severe case of MIS-C, with higher levels of IL-12(p40), IL-18, IFN-γ, and IFN-γ-induced chemokines such as CXCL9 and CXCL10 than in our previous report.
Because these cytokine kinetics were assessed for three separate treatments during the time course, the relationships between each of the treatments and the changes in cytokines seemed to be clear in this report. However, it is not definitive whether the cytokines kinetics tightly correspond to each treatment, and it remains possible that there may be synergetic effects with the prior treatment. Among the data, it is particularly interesting that the patient did not become defervescent after complete suppression of IL-6 by mPSL, and required a third-line treatment. Thus, it is possible that tocilizumab as an additional treatment may be an inappropriate choice, especially those who are refractory to previous steroid treatment.
MIS-C and KD are similar in many clinical presentations, and 25–65.5% of MIS-C patients also have symptoms that resemble classical KD (21). Because our case was not only definite MIS-C but also fulfilled the criteria for classical KD, we selected CsA as the third-line treatment when he showed resistance to both IVIG and mPSL (16, 17). We usually select oral administration for CsA, but chose continuous infusion (3 mg/kg/day) in the present patient between day 8 and day 13, because he had remarkable gastrointestinal symptoms such as severe pain. After his abdominal pain disappeared, we changed the administration route of CsA from infusion to per oral (150 mg/day, divided by 2). His plasma level of CsA was 321–372 ng/ml at infusion and his trough level was 87-97 ng/ml at oral administration. All symptoms and signs of MIS-C in our patient improved after CsA treatment and no CAAs developed.
Regarding the effect of CsA in the present case, it is possible that the suppressive effects of CsA on macrophages and dendritic cells may have contributed to the clinical improvements, because the levels of cytokines related to innate immune responses such as IL-12(p40) and IL-18 were decreased at defervescence.
Ciclosporin A was originally considered to suppress the immune system through inactivation of the nuclear factor of activated T-cells pathway. However, according to pathophysiology of MIS-C, both innate immunity and adaptive immunity are highly activated. If this hypothesis is true, the mechanism of action for CsA may not be sufficient to control the total immune activation in MIS-C. Therefore, CsA may not have been recommended as an additional option for treatment of MIS-C in the treatment guideline (7–9). However, recent studies have clarified that CsA may exert direct suppressive effects on not only T cells but also innate immune cells such as dendritic cells, macrophages, and neutrophils (22–24). Indeed, cytokines related to innate immunity were suppressed by CsA in the present patient with MIS-C. These findings suggest that CsA may be a useful option for MIS-C refractory to IVIG + mPSL, which is known to involve activation of both innate immunity and adaptive immunity.
Although we have no evidence to explain why our patient developed venous stasis, we propose one possibility. The changes in skin color of his limbs were not chilblain-like skin lesions associated with SARS-CoV-2 infection (25), because the changes developed depending on the vertical position of the limbs and disappeared immediately in the horizontal position. Thus, we judged these phenomena to be venous stasis. Therefore, vasculitis caused by MIS-C may be not only arteritis but also phlebitis. Several reports have described that autoantibodies to autoantigens expressed in endothelial and cardiac tissues may trigger onset of MIS-C (1, 4, 26). Thus, it is possible that vasculitis may develop regardless of involvement of an artery or a vein. Therefore, the function of venous valves may be insufficient.
There are several limitations in the present case report. This is the first report to discuss the possibility of CsA treatment for MIS-C refractory to IVIG + mPSL. In addition, it is possible that the effect of CsA as a third-line treatment may have involved the summed results for IVIG and mPSL. Further studies are warranted to evaluate CsA treatment in refractory MIS-C patients.
The findings in the present case regarding the relationship between cytokine kinetics and treatment response suggest that CsA may be a useful option for patients with MIS-C who are refractory to IVIG + methylprednisolone pulse treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Wakayama Medical University (No: 3282) and National Center for Global Health and Medicine (NCGM-S-004245). Written informed consent was obtained from the patient and his parents for the publication of this case report. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
TaS performed the literature review and wrote the first draft of the manuscript. MU, YK, ToS, NK, TT, and HS assisted in the treatment of the patient. AS, MS, MM, AM, and ST contributed to the analysis and interpretation of cytokines and assisted in the preparation of the manuscript. HH and DT contributed to critically review of the manuscript. All authors have approved the final manuscript and have agreed to be accountable for all aspects of the work.
This work was supported in part by the Japan Agency for Medical Research and Development (AMED) (Grant Numbers: JP20fk0108104 and JP20fk0108416), and NCGM intramural research fund (Grant Number: 20A2009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Alison Sherwin, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.890755/full#supplementary-material
Supplementary Figure 1 | (A) Chest and abdominal X-ray image. Cardiothoracic ratio is 54.6%, pulmonary congestion is present, and intestinal gas shows prominent expansion. (B) Computed tomography image. Hair line is present. (C) Electrocardiogram. Heart rate is 142 beats/min, sinus rhythm is present, and T waves on the V5 and V6 leads are flat and negative, suggesting myocardial damage.
Supplementary Figure 2 | 3D-Computed Tomography (day 45).
Supplementary Figure 3-1 | Cytokines which responded to ciclosporin A (CsA). IL, interleukin; ra, receptor antagonist; Rα, receptor α; CCL, C–C motif ligand; CX3CL, C-X3-C motif ligand; IFN, interferon.
Supplementary Figure 3-2 | Cytokines which responded to ciclosporin A (CsA). TNF, tumor necrosis factor; LIF, leukemia inhibitory factor; HGF, hepatocyte growth factor; MIF, macrophage migration inhibitory factor; GM-CSF, granulocyte macrophage colony-stimulating factor; SCF, stem cell factor; VEGF, vascular endothelial growth factor; NGF, nerve growth factor; IFN, interferon.
1. Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell. (2020) 183:982–95.e14. doi: 10.1016/j.cell.2020.09.034
2. Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. (2020) 183:968–81.e7. doi: 10.1016/j.cell.2020.09.016
3. Sancho-Shimizu V, Brodin P, Cobat A, Biggs CM, Toubiana J, Lucas CL, et al. SARS-CoV-2–related MIS-C: a key to the viral and genetic causes of Kawasaki disease? J Exp Med. (2021) 218:e20210446. doi: 10.1084/jem.20210446
4. Ramaswamy A, Brodsky NN, Sumida TS, Comi M, Asashima H, Hoehn KB, et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity. (2021) 54:1083–95.e7. doi: 10.1016/j.immuni.2021.04.003
5. Yang C-A, Huang Y-L, Chiang B-L. Innate immune response analysis in COVID-19 and kawasaki disease reveals MIS-C predictors. J Formos Med Assoc. (2022) 121:623–32. doi: 10.1016/j.jfma.2021.06.009
6. Takasago S, Sakai A, Sugiyama M, Mizokami M, Hamada H, Ishizaka Y, et al. Case report: changes in cytokine kinetics during the course of disease in a Japanese patient with multisystem inflammatory syndrome in children. Front Pediatr. (2021) 9:702318. doi: 10.3389/fped.2021.702318
7. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated With SARS–CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol. (2022) 74:e1–20. doi: 10.1002/art.42062
8. Cattalini M, Taddio A, Bracaglia C, Cimaz R, Paolera SD, Filocamo G, et al. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): a diagnostic and treatment guidance from the Rheumatology Study Group of the Italian Society of Pediatrics. Ital J Pediatr. (2021) 47:24. doi: 10.1186/s13052-021-00980-2
9. Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. (2021) 5:133–41. doi: 10.1016/S2352-4642(20)30304-7
10. Abdel-Haq N, Asmar BI, Deza Leon MP, McGrath EJ, Arora HS, Cashen K, et al. SARS-CoV-2-associated multisystem inflammatory syndrome in children: clinical manifestations and the role of infliximab treatment. Eur J Pediatr. (2021) 180:1581–91. doi: 10.1007/s00431-021-03935-1
11. Çelikel E, Tekin ZE, Aydin F, Emeksiz S, Uyar E, Özcan S, et al. Role of Biological Agents in the Treatment of SARS-CoV-2–Associated Multisystem Inflammatory Syndrome in Children. JCR J Clin Rheumatol. (2022) 28:e381–7. doi: 10.1097/RHU.0000000000001734
12. World Health Organization [WHO].Multisystem inflammatory syndrome in children and adolescents with COVID-19. (2020). Available online at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (Accessed March 1, 2022).
13. Centers for Disease Control and Prevention [CDC].Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). (2020). Available online at: https://emergency.cdc.gov/han/2020/han00432.asp (Accessed March 1, 2022).
14. Royal College of Paediatrics and Child Health [RCPCH].Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. (2020). Available online at: https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance (Accessed March 1, 2022).
15. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
16. Suzuki H, Terai M, Hamada H, Honda T, Suenaga T, Takeuchi T, et al. Cyclosporin A Treatment for Kawasaki Disease Refractory to Initial and Additional Intravenous Immunoglobulin. Pediatr Infect Dis J. (2011) 30:871–6. doi: 10.1097/INF.0b013e318220c3cf
17. Hamada H, Suzuki H, Onouchi Y, Ebata R, Terai M, Fuse S, et al. Efficacy of primary treatment with immunoglobulin plus ciclosporin for prevention of coronary artery abnormalities in patients with Kawasaki disease predicted to be at increased risk of non-response to intravenous immunoglobulin (KAICA): a randomised cont. Lancet. (2019) 393:1128–37. doi: 10.1016/S0140-6736(18)32003-8
18. Kinoshita T, Nishimura Y, Umemoto Y, Koike Y, Kouda K, Ogawa T, et al. Case report: rehabilitation for lower extremity pain due to venous stasis in a patient with multisystem inflammatory syndrome in children. Front Pediatr. (2022) 9:810811. doi: 10.3389/fped.2021.810811
19. Hoste L, Roels L, Naesens L, Bosteels V, Vanhee S, Dupont S, et al. TIM3+ TRBV11-2 T cells and IFNγ signature in patrolling monocytes and CD16+ NK cells delineate MIS-C. J Exp Med. (2022) 219:e20211381. doi: 10.1084/jem.20211381
20. Caldarale F, Giacomelli M, Garrafa E, Tamassia N, Morreale A, Poli P, et al. Plasmacytoid dendritic cells depletion and elevation of IFN-γ dependent chemokines CXCL9 and CXCL10 in children with multisystem inflammatory syndrome. Front Immunol. (2021) 12:654587. doi: 10.3389/fimmu.2021.654587
21. Zhang Q-Y, Xu B-W, Du J-B. Similarities and differences between multiple inflammatory syndrome in children associated with COVID-19 and Kawasaki disease: clinical presentations, diagnosis, and treatment. World J Pediatr. (2021) 17:335–40. doi: 10.1007/s12519-021-00435-y
22. Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol. (2019) 163:472–80. doi: 10.1016/j.bcp.2019.03.022
23. Fric J, Zelante T, Wong AYW, Mertes A, Yu H-B, Ricciardi-Castagnoli P. NFAT control of innate immunity. Blood. (2012) 120:1380–9. doi: 10.1182/blood-2012-02-404475
24. Zanoni I, Granucci F. Regulation and dysregulation of innate immunity by NFAT signaling downstream of pattern recognition receptors (PRRs). Eur J Immunol. (2012) 42:1924–31. doi: 10.1002/eji.201242580
25. Piccolo V, Neri I, Filippeschi C, Oranges T, Argenziano G, Battarra VC, et al. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatology Venereol. (2020) 34:e291–3. doi: 10.1111/jdv.16526
Keywords: MIS-C, cytokine, ciclosporin A, Kawasaki disease, treatment option, phlebitis
Citation: Suzuki T, Suenaga T, Sakai A, Sugiyama M, Mizokami M, Mizukami A, Takasago S, Hamada H, Kakimoto N, Takeuchi T, Ueda M, Komori Y, Tokuhara D and Suzuki H (2022) Case Report: Ciclosporin A for Refractory Multisystem Inflammatory Syndrome in Children. Front. Pediatr. 10:890755. doi: 10.3389/fped.2022.890755
Received: 06 March 2022; Accepted: 26 April 2022;
Published: 31 May 2022.
Edited by:
Ankur Kumar Jindal, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Angelo Ravelli, University of Genoa, ItalyCopyright © 2022 Suzuki, Suenaga, Sakai, Sugiyama, Mizokami, Mizukami, Takasago, Hamada, Kakimoto, Takeuchi, Ueda, Komori, Tokuhara and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takayuki Suzuki, dGFzdXp1a2lAd2FrYXlhbWEtbWVkLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.