- 1State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Cardiopulmonary Bypass, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Department of Anesthesiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Background: The association of high-sensitivity C-reactive protein (hsCRP) and anemia with postoperative acute kidney injury (AKI) in neonates with congenital heart disease (CHD) is still unclear. The purpose of this study was to examine whether anemia-associated AKI is modulated by hsCRP in neonates.
Methods: This study included 253 consecutive neonatal patients who underwent CHD surgery in a national tertiary hospital. We investigated the association between postoperative AKI with baseline hsCRP, anemia, and their interaction by multivariable logistic regression analyses.
Results: The incidence of AKI was 24.1% in the entire cohort. After being adjusted for covariates, hsCRP level was negatively correlated with AKI (P < 0.01 for 1 mg/L threshold), whereas anemia emerged as an independent risk factor of AKI (P = 0.02). In addition, there was a significant interaction between anemia and hsCRP level (P = 0.01). In neonates with hsCRP < 1 mg/L, anemia was positively associated with AKI (P = 0.03). However, no significant association was found between anemia and AKI in the context of hsCRP ≥ 1 mg/L. Combination of anemia and hsCRP < 1 mg/L was independently correlated with the risk of AKI (P < 0.01), while concomitant anemia and hsCRP ≥ 1 mg/L or hsCRP < 1 mg/L combined with non-anemia was not.
Conclusions: In neonates with CHD, the risk of anemia-associated AKI may be modulated by hsCRP level. Attention should be paid to neonates with preoperative anemia and baseline hsCRP < 1 mg/L to reduce the risk of postoperative AKI.
Introduction
Early repair in the neonatal period or young infancy has become the treatment target of most patients with biventricular congenital heart disease (CHD) (1). Despite advances in neonatal CHD surgery, postoperative acute kidney injury (AKI) remains one of the leading complications that is independently correlated with morbidity and mortality (2, 3). Considerable risks despite symptomatic therapies have generated efforts to identify modifiable indicators and therapeutic measures for neonatal AKI (4, 5). Anemia is one putative target for mitigating the burden of AKI, according to authoritative data in adult cardiac surgery (6–8). However, the correlation between preoperative anemia and postoperative AKI has not been investigated in neonates, and it remains unclear which group of neonates would benefit the most from the elimination of anemia.
Systemic inflammation, which is caused by cardiovascular malformations and hypoperfusion of vital organs, has emerged as another potential pathogenesis exacerbating renal impairment (9–13). The interrelationship between inflammatory response and anemia may have a sophisticated effect on postoperative AKI. Among all inflammatory mediators, high-sensitivity C-reactive protein (hsCRP) has been demonstrated incontrovertibly as the most extensive predictive value for postoperative AKI in the context of adult cardiac surgery or cardiovascular intervention therapy (9–11, 14). Yet the consistency of its predictive role in adults and neonates is disputable (15). This study aimed to explore the hypothesis that a synergism between anemia and hsCRP on AKI in neonates undergoing CHD surgery, and whether anemia-associated AKI would be modulated by the degree of hsCRP, relying on the large-scale national tertiary hospital.
Materials and methods
Study population and data collection
We conducted a single-center retrospective study on neonatal patients (≤28 days) who underwent pump CHD primary repair from 1 November 2012 to 28 February 2021 at Fuwai Hospital, Chinese Academy of Medical Sciences. Preterm infants and neonates with preoperative extracorporeal membrane oxygenation support or emergency operation were excluded. This study was approved by the Institutional Review Board of Fuwai Hospital and performed with a waiver of informed consent from guardians in view of the retrospective nature.
Demographic characteristics, echocardiographic examination, predominant diagnosis, laboratory tests, intraoperative management, and postoperative information were collected. Based on the definition that concentration of hemoglobin (Hb) >2 standard deviations (SD) below the average of postnatal age (16) and the threshold of consideration for blood transfusion for neonates in our center, neonatal clinically relevant anemia was defined as Hb < 120 g/L. Serum hsCRP was routinely examined 1 day before operation by the clinical laboratory on Cobas c501 (Roche Diagnostics GmbH, Mannheim, Germany).
Intraoperative management
The standard cardiopulmonary bypass (CPB) was established following systemic heparinization and median sternotomy. Red blood cell was generally not transfused before surgery. CPB circuit was primed with Plasmalyte A 40 ml, albumin 30 ml, packed red blood cell 80 ml, and sodium bicarbonate 10 ml. Cold histidine-tryptophan-ketoglutarate cardioplegia was perfused as body temperature cooled down to 27–30 °C. Hematocrit (Hct) was targeted at 0.24–0.27 during bypass. After lesions are repaired, neonates would be weaned from CPB only when hemodynamic state and body temperature reached satisfactory levels as sinus rhythm returned to normal. α-stat management was adopted during CPB. Hct was maintained at 0.35–0.40 by modified ultrafiltration. All neonates were transferred to the pediatric intensive care unit (PICU) after the procedure.
AKI assessment
The primary outcome of this study was AKI. Neonates developing AKI at POD1 and still diagnosed with AKI 1 week after the operation were defined as having AKI within 1 week of surgery (17). AKI was determined by neonatal modified-Kidney Disease: Improving Global Outcomes (nKDIGO) criteria regardless of perioperative urine volume (18). AKI was detailed as follows: serum creatinine (SCr) increases of ≥26.5 μmol/L within 48 h or ≥1.5 times the baseline value within 1 week or need for renal replacement therapy. Preoperative SCr rather than estimated glomerular filtration rate was employed in AKI diagnosis because the reliability of its formula has not been proven in neonates. Secondary outcomes included prolonged postoperative hospital stay (≥21 days), prolonged PICU stay (≥7 days), and delayed extubation (≥72 h). We also investigated the relationship between hepatic dysfunction and hsCRP level to explore the underlying cause of neonatal stress disorder. Hepatic dysfunction was defined as a total bilirubin level >2.5 mg/dl or >2-fold increase of alanine aminotransferase or aspartate aminotransferase from the baseline level (19).
Statistical analysis
Continuous variables were presented as mean ± SD for normal distribution and median [interquartile range (IQR)] for non-normal distribution and were compared using independent-sample Student's t-test or Mann–Whitney U test, respectively. Categorical variables were reported as frequency (percentage) and were compared by Pearson χ2 test or Fisher's exact test when appropriate. Logistic regression models were generated to evaluate the association between preoperative hsCRP, anemia, and AKI, as well as interaction test, by adjusting for demographic characteristics, baseline laboratory tests, and intraoperative variables. The risk of AKI was compared between different combinations of hsCRP level and anemia. Two-tailed P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 25.0 software (SPSS Inc., Chicago, IL, United States), and the figure was processed by GraphPad prism 8.0 (GraphPad Software Inc., San Diego, CA, United States).
Results
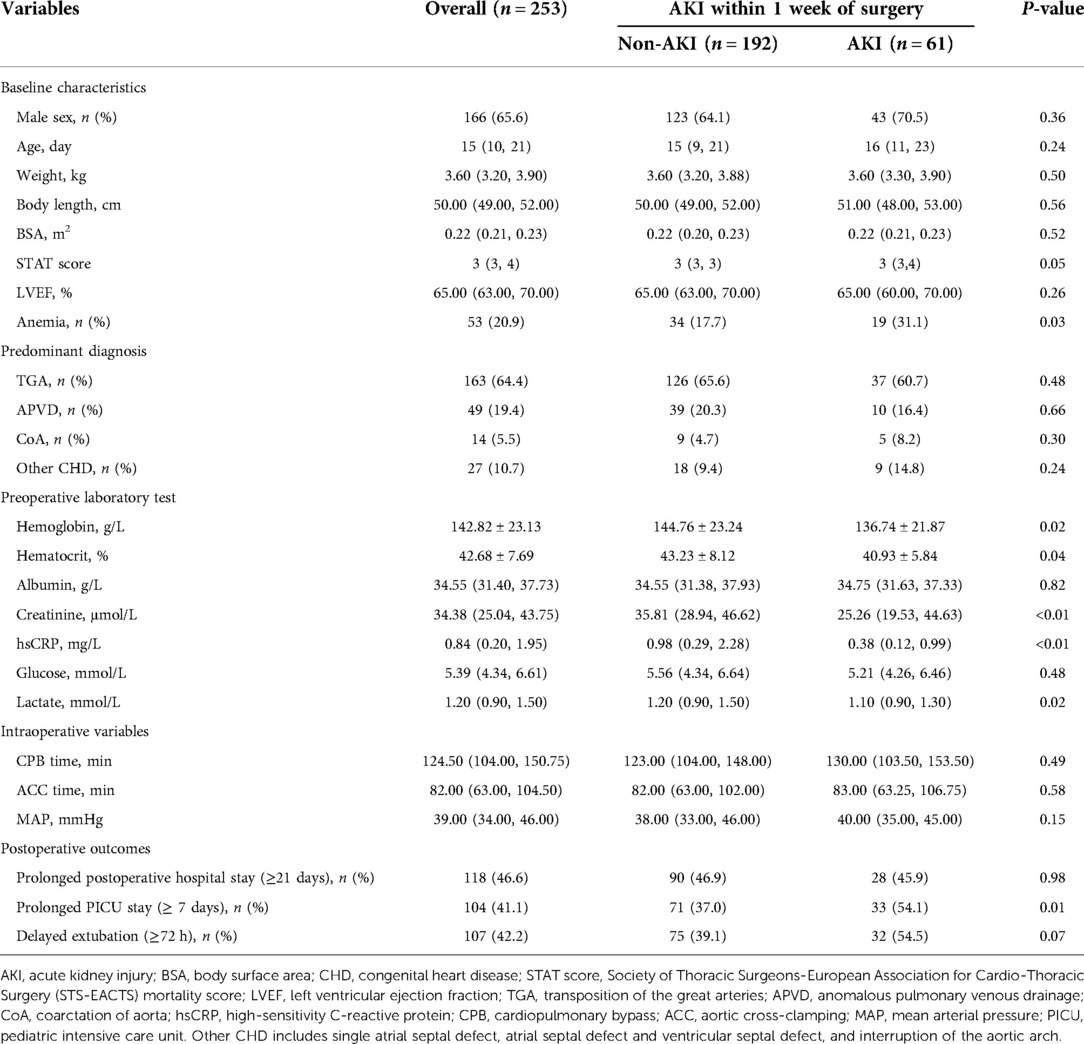
Baseline characteristics and perioperative information
A total of 262 consecutive neonates were identified, and 253 patients with complete medical records were finally included in this analysis. Baseline characteristics of the study population were classified by the presence (n = 61) or absence (n = 192) of AKI within 1 week of surgery (Table 1). Of the 253 neonates, 65.6% were male. The mean value of Hb and median of hsCRP were 142.82 g/L and 0.84 mg/L respectively. Neonates with AKI had a higher prevalence of anemia, compared with those without AKI (P = 0.03). Baseline hsCRP concentration was significantly lower in patients with AKI than those without (P < 0.01). For postoperative outcomes, there was a remarkably higher prevalence of prolonged PICU stay (≥7 days) among neonates with AKI (P = 0.01).
As Figure 1 illustrates, neonates with postoperative AKI had significantly lower Hb and Hct before the operation and during bypass hypothermia compared with those without AKI (P < 0.05).

Figure 1. Perioperative hemoglobin and hematocrit of the two groups at different time points. T0, before surgery; T1, bypass hypothermia; T2, bypass rewarming; T3, weaning of bypass. *P < 0.05.
Association of hsCRP and anemia with AKI
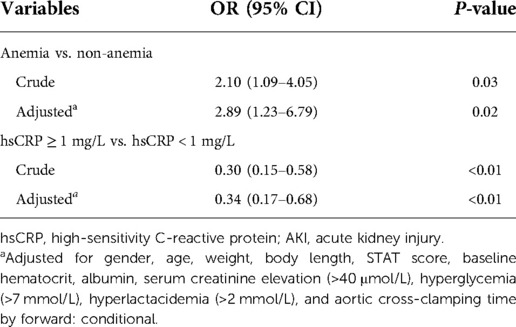
Table 2 depicts the crude and multivariable-adjusted association between AKI and hsCRP or anemia. Baseline and intraoperative variables in Table 1 were submitted into multivariable analysis (forward stepwise regression). HsCRP elevation was defined as ≥1 mg/L in line with the cut-off value (hsCRP = 1.09 mg/L) of the receiver operating characteristic curve for AKI in this population (Supplementary Figure S1). As Table 2 presents, AKI was negatively associated with hsCRP assessed by threshold of 1 mg/L (odds ratio (OR): 0.34, 95% CI: 0.17–0.68, P < 0.01), and anemia was an independent risk factor for AKI after adjustment for cofounders (OR: 2.89, 95% CI: 1.23–6.79, P = 0.02).
Association between AKI and anemia according to hsCRP threshold
As is revealed in Table 3, we stratified the entire cohort according to hsCRP threshold of 1 mg/L and found that anemia was correlated with a remarkably greater risk of AKI when hsCRP < 1 mg/L (OR: 2.67, 95% CI: 1.11–6.38, P = 0.03). Whereas in the setting of hsCRP ≥ 1 mg/L, anemia was not significantly associated with AKI. In addition, there was a significant interaction for AKI between anemia and hsCRP divided by the 1 mg/L threshold (P = 0.01).
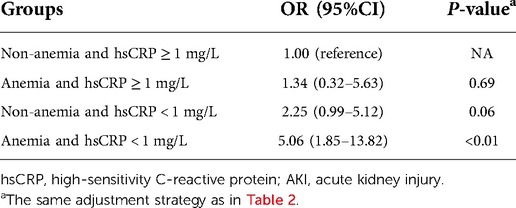
Risk of AKI in different combination groups of anemia and hsCRP
Table 4 indicates the results of the fully adjusted association between the risk of AKI and neonates classified by different combinations of anemia and hsCRP. Neither anemia combined with hsCRP ≥ 1 nor hsCRP < 1 mg/L without anemia was associated with a higher risk of AKI, compared with the reference group (non-anemia and hsCRP ≥ 1 mg/L). However, the combination of anemia and hsCRP < 1 mg/L significantly raised the risk of AKI (OR: 5.06, 95% CI: 1.85–13.82, P < 0.01).
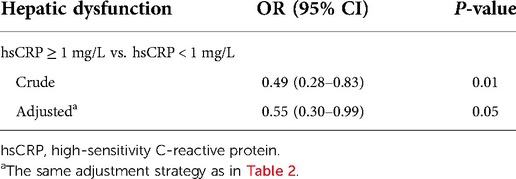
Association between hsCRP and hepatic dysfunction
Table 5 shows that hsCRP < 1 mg/L was positively associated with postoperative hepatic dysfunction after being adjusted for cofounders (OR: 0.55, 95% CI: 0.30–0.99, P = 0.05).
Discussion
The core finding of this study was that the risk of anemia-associated AKI could be affected by the level of hsCRP in neonates undergoing CHD surgery. This is the first study, to our knowledge, that investigated the correlation of hsCRP level and anemia with adverse outcomes among neonates. Based on a relatively large neonatal cohort, we demonstrated that anemia was positively associated with AKI. Interestingly, neonates with concomitant existence of anemia and lower hsCRP level (<1 mg/L) had a higher risk of AKI, whereas neonates with concomitant anemia and hsCRP ≥ 1 or hsCRP < 1 mg/L combined with non-anemia did not, compared with those who had elevated hsCRP without anemia.
Given the prevalence of transient AKI in pediatric CHD surgery, AKI within 1 week of surgery was adopted as the primary endpoint of observation to avoid overdiagnosis. A recent study found that persistent AKI was strongly correlated with poorer in-hospital prognosis after CHD surgery (17). The remarkable risk of postoperative AKI remains despite advancement in the perioperative management of neonates (2, 4), constituting an impetus to the efforts for exploring modifiable risk factors. In the present study, we identified a 24.1% incidence of AKI consistent with previous studies (20, 21). As was reported in several infant studies (15, 22–25), lower preoperative SCr was the independent risk factor of cardiovascular surgery-associated AKI, especially for neonates. It corresponds with the results of this study. One possible explanation is that lower SCr in neonates may indicate less muscle mass and inferior nutritional status (15, 22–24), which may add to the risk of AKI. In addition, preoperative fluid overload may render a decrease in preoperative SCr level, which is also related to the higher risk of AKI (15). By adjusting for the factor of baseline SCr > 40 μmol/L in multivariate logistic regression analyses, we eliminated the interference of latent renal insufficiency.
Compelling evidence in adults suggested that preoperative anemia was an independent predictor of AKI (6–8, 26). The major mechanisms underlying anemia-associated AKI involve renal oxygen supply impairment (16), exacerbation of oxidative stress (6), and hemostasis imbalance (7). This study provided consistent results that anemia was an independent risk factor for AKI in neonates undergoing cardiovascular surgery. However, we have noticed in clinical practice that postoperative AKI remained common in neonates even after anemia was corrected. Therefore, with the promotion of blood-saving strategy in infant CHD surgery (27) and the recognition of intraoperative blood transfusion-related risks (7), identifying the high-risk population of anemia-related AKI according to novel indicators is of vital importance to improve the prognosis of neonates as well as optimize the rational use of blood products.
The predictive value of baseline hsCRP for AKI has also been widely proposed in adult populations (9–12, 28). Previous adult studies identified hsCRP elevation as an independent biomarker of AKI in settings of percutaneous coronary intervention (10, 11) and coronary artery bypass grafting (9). Interestingly, the present study indicated a contrary to these results in adult studies, that in neonates undergoing CHD surgery, hsCRP elevation was negatively correlated with postoperative AKI.
In the context of hypoxic stress, CRP, which is also known as acute phase protein, was secreted by hepatocytes. CRP plays a critical role in preventing the generation of superoxide by neutrophils and stimulating the synthesis of interleukin-1 receptor antagonists, thus exerting a net anti-inflammatory effect (29). However, in vivo study indicated that CRP acts as a mediator in the pathogenesis of AKI and that overexpression of CRP inhibits the regeneration of G1/S-dependent tubular epithelial cells (30). Therefore, excessively high or low expression of CRP in stress response may both have a detrimental impact.
Generally, neonates with CHD bear extensive stress response due to hypoxia and hypoperfusion of vital organs. Immature organ development is the most distinguished characteristic of neonates that differs from patients of other ages. Our results showed that hsCRP < 1 mg/L was significantly correlated with hepatic dysfunction. An underdeveloped hepatic function may weaken the adaptive acute phase response of neonates to hypoxic stress. Also, it was demonstrated that younger patients may have a more pronounced systemic inflammatory response to critical diseases (31), and those preoperative CRP concentrations were inversely associated with the age of infants (1). Accordingly, we assumed that in the acute phase response, neonates with stress disorder characterized by limited elevation of hsCRP may have a higher risk of adverse events. Additionally, children suffering from CHD may commonly have chronic sympathetic activation and increased release of angiotensin (32). These mechanisms synergistically prompt the redistribution of blood flow from the kidneys to the brain and/or heart, which may lead to simulated preoperative renal ischemia preconditioning. The elevation of hsCRP (≥1 mg/L) may be a reflection of the activation of the inflammatory pathway induced by renal ischemic preconditioning (33). A period of nonfatal ischemic preconditioning has been tentatively conceptualized as protection against ischemic AKI (34). Furthermore, CRP has been reported to exert some anti-inflammatory effects by stimulating the release of anti-inflammatory factors including IL-10 and IL-1ra (35). CRP could also recruit several complement inhibitors when activating the complement system (36). Consequently, the net effect of CRP in vivo tends to be weak anti-inflammation (37), which may be another underlying explanation for the negative association between hsCRP elevation and AKI in this study.
The interaction between inflammation and anemia has not been investigated in neonates. In adult cohorts with chronic kidney disease, scholars have found that CRP exacerbated anemia by inhibiting endogenous/exogenous erythropoietin (38, 39), which may contribute to a synergic effect between inflammation and anemia on unfavorable events (39). However, data in this study indicated a different pattern between the interaction of hsCRP and anemia in neonates, that anemia was correlated with AKI only when hsCRP < 1 mg/L while this correlation was not valid in neonates with hsCRP ≥ 1 mg/L. Briefly, the risk of anemia-associated AKI in neonates could be mediated by hsCRP threshold. Therefore, what this study offered was the potential for refining the risk of AKI by identifying neonates with concomitant existence of hsCRP < 1 mg/L (may represent underlying stress disorder) and anemia.
Limitations
There were certain limitations in this study. First, this study had all the inherent limitations of a retrospective study. Second, the single-center design limited the intensity of evidence though the sample size was relatively large as a neonate cohort. Third, the existing data could not confirm the causal relationship between hsCRP or anemia and adverse outcomes. Next, there was a lack of other inflammatory mediators in this study, which may otherwise help determine the degree of inflammation indicated by hsCRP. Furthermore, the incidence of AKI may be underestimated because we did not define AKI in terms of urine output. In addition, although preterm infants were excluded from the population in this study, gestational age may affect SCr and Hb. However, because our hospital is a specialized cardiovascular center and the neonatal patients were transported from other hospitals after birth, the data on gestational age in our database is incomplete to determine the effect of gestational age on the results. As there was a lack of SCr examination records more than 1 week after surgery for many neonates, we were unable to explore the AKI recovery since then retrospectively, which may exaggerate the correlation between hsCRP, anemia and AKI in this study. Therefore, corroboration of our conclusion needs to be verified in well-designed randomized controlled trials.
Conclusions
Anemia-related AKI may be mediated by hsCRP level in the context of neonatal CHD surgery. This study offered a potential perspective that rectification of anemia in neonates, especially those combined with hsCRP < 1 mg/L may be beneficial for the improvement of clinical prognosis. Although the results of this study are interesting, it is only a preliminary study. The value of this study lies mainly in the use of limited data from a single center to emphasize the differences in the pathophysiology between neonates and older patients with CHD. We hope that this study could function as a starting point for multicenter studies to explore AKI prevention strategies or targets for the neonatal population specifically.
Data availability statement
The datasets presented in this article are not readily available because of data protection policies. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Fuwai Hospital. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin. This study was approved by the Institutional Review Board of Fuwai Hospital and performed with a waiver of informed consent from guardians in view of the retrospective nature.
Author contributions
PZ conceived the primary research question. PZ, JL, and DY designed the study. PZ drafted the manuscript. YT, YL, and PZ collected data. YT, DY, YJ, LB, PG, WW, JH, XD, and JL provided critical revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China Grant Awards (grant number 81670375), CAMS Innovation Fund for Medical Sciences (CIFMS) (grant numbers 2020-LC23 and 2019XK320051), and High-level Hospital Clinical Research Funds of Fuwai Hospital, Chinese Academy of Medical Sciences (zero balance 2022-GSP-GG-22).
Acknowledgments
We appreciate Dr. Yue Yuan for her great assistance in language polishing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.882739/full#supplementary-material.
References
1. Allan CK, Newburger JW, McGrath E, Elder J, Psoinos C, Laussen PC, et al. The relationship between inflammatory activation and clinical outcome after infant cardiopulmonary bypass. Anesth Analg. (2010) 111(5):1244–51. doi: 10.1213/ANE.0b013e3181f333aa
2. Ueno K, Shiokawa N, Takahashi Y, Nakae K, Kawamura J, Imoto Y, et al. Kidney disease: improving global outcomes in neonates with acute kidney injury after cardiac surgery. Clin Exp Nephrol. (2020) 24(2):167–73. doi: 10.1007/s10157-019-01805-7
3. Taylor ML, Carmona F, Thiagarajan RR, Westgate L, Ferguson MA, del Nido PJ, et al. Mild postoperative acute kidney injury and outcomes after surgery for congenital heart disease. J Thorac Cardiovasc Surg. (2013) 146(1):146–52. doi: 10.1016/j.jtcvs.2012.09.008
4. Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics. (2015) 136(2):e463–73. doi: 10.1542/peds.2014-3819
5. Alten JA, Cooper DS, Blinder JJ, Selewski DT, Tabbutt S, Sasaki J, et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med. (2021) 49(10):e941–51. doi: 10.1097/ccm.0000000000005165
6. Karkouti K, Yip P, Chan C, Chawla L, Rao V. Pre-operative anaemia, intra-operative hepcidin concentration and acute kidney injury after cardiac surgery: a retrospective observational study. Anaesthesia (2018) 73(9):1097–102. doi: 10.1111/anae.14274
7. Karkouti K, Grocott HP, Hall R, Jessen ME, Kruger C, Lerner AB, et al. Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth. (2015) 62(4):377–84. doi: 10.1007/s12630-014-0302-y
8. Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M, et al. Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation. (2009) 119(4):495–502. doi: 10.1161/CIRCULATIONAHA.108.786913
9. Han SS, Kim DK, Kim S, Chin HJ, Chae DW, Na KY. C-reactive protein predicts acute kidney injury and death after coronary artery bypass grafting. Ann Thorac Surg. (2017) 104(3):804–10. doi: 10.1016/j.athoracsur.2017.01.075
10. Gao F, Zhou YJ, Zhu X, Wang ZJ, Yang SW, Shen H. C-reactive protein and the risk of contrast-induced acute kidney injury in patients undergoing percutaneous coronary intervention. Am J Nephrol. (2011) 34(3):203–10. doi: 10.1159/000329534
11. Shacham Y, Leshem-Rubinow E, Steinvil A, Keren G, Roth A, Arbel Y. High sensitive C-reactive protein and the risk of acute kidney injury among ST elevation myocardial infarction patients undergoing primary percutaneous intervention. Clin Exp Nephrol. (2015) 19(5):838–43. doi: 10.1007/s10157-014-1071-1
12. Fu EL, Franko MA, Obergfell A, Dekker FW, Gabrielsen A, Jernberg T, et al. High-sensitivity C-reactive protein and the risk of chronic kidney disease progression or acute kidney injury in post-myocardial infarction patients. Am Heart J. (2019) 216:20–9. doi: 10.1016/j.ahj.2019.06.019
13. Smilowitz NR, Kunichoff D, Garshick M, Shah B, Pillinger M, Hochman JS, et al. C-reactive protein and clinical outcomes in patients with COVID-19. Eur Heart J. (2021) 42(23):2270–9. doi: 10.1093/eurheartj/ehaa1103
14. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. (2016) 118(1):145–56. doi: 10.1161/CIRCRESAHA.115.306656
15. Morgan CJ, Zappitelli M, Robertson CM, Alton GY, Sauve RS, Joffe AR, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. (2013) 162(1):120–7 e121. doi: 10.1016/j.jpeds.2012.06.054
16. Colombatti R, Sainati L, Trevisanuto D. Anemia and transfusion in the neonate. Semin Fetal Neonatal Med. (2016) 21(1):2–9. doi: 10.1016/j.siny.2015.12.001
17. Jia Y, Luo Q, Su Z, Xiong C, Wang H, Li Y, et al. The incidence and risk factors for persistent acute kidney injury following total cavopulmonary connection surgery: a single-center retrospective analysis of 465 children. Front Pediatr. (2021) 9:566195. doi: 10.3389/fped.2021.566195
18. Shalaby MA, Sawan ZA, Nawawi E, Alsaedi S, Al-Wassia H, Kari JA. Incidence, risk factors, and outcome of neonatal acute kidney injury: a prospective cohort study. Pediatr Nephrol. (2018) 33(9):1617–24. doi: 10.1007/s00467-018-3966-7
19. Tong Y, Liu J, Zou L, Feng Z, Zhou C, Lv R, et al. Perioperative outcomes of using different temperature management strategies on pediatric patients undergoing aortic arch surgery: a single-center, 8-year study. Front Pediatr. (2018) 6:356. doi: 10.3389/fped.2018.00356
20. Piggott KD, Liu A, Monczka J, Fakioglu H, Narasimhulu SS, Pourmoghadam K, et al. Inadequate preoperative nutrition might be associated with acute kidney injury and greater illness severity postoperatively. J Thorac Cardiovasc Surg. (2018) 155(5):2104–9. doi: 10.1016/j.jtcvs.2017.12.080
21. Che R, Quadri MM, Zhang A. The epidemiology and management of pediatric AKI in Asia. Semin Nephrol. (2020) 40(5):516–32. doi: 10.1016/j.semnephrol.2020.08.008
22. Li S, Krawczeski CD, Zappitelli M, Devarajan P, Thiessen-Philbrook H, Coca SG, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. (2011) 39(6):1493–9. doi: 10.1097/CCM.0b013e31821201d3
23. Morgan CJ, Gill PJ, Lamand S, Joffe AR. Peri-operative interventions, but not inflammatory mediators, increase risk of acute kidney injury after cardiac surgery: a prospective cohort study. Intensive Care Med. (2013) 39(5):934–41. doi: 10.1007/s00134-013-2849-4
24. Zappitelli M, Moffett BS, Hyderand A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. (2011) 26(1):144–50. doi: 10.1093/ndt/gfq375
25. Zappitelli M, Krawczeski CD, Devarajan P, Wang Z, Sint K, Thiessen-Philbrook H, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. (2011) 80(6):655–62. doi: 10.1038/ki.2011.123
26. Padmanabhan H, Siau K, Curtis J, Ng A, Menon S, Luckraz H, et al. Preoperative anemia and outcomes in cardiovascular surgery: systematic review and meta-analysis. Ann Thorac Surg. (2019) 108(6):1840–8. doi: 10.1016/j.athoracsur.2019.04.108
27. Boettcher W, Dehmel F, Redlin M, Sinzobahamvya N, Photiadis J. Cardiopulmonary bypass strategy to facilitate transfusion-free congenital heart surgery in neonates and infants. Thorac Cardiovasc Surg. (2020) 68(1):2–14. doi: 10.1055/s-0039-1700529
28. Cosentino N, Genovese S, Campodonico J, Bonomi A, Lucci C, Milazzo V, et al. High-sensitivity C-reactive protein and acute kidney injury in patients with acute myocardial infarction: a prospective observational study. J Clin Med. (2019) 8(12):2192. doi: 10.3390/jcm8122192
29. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. (1999) 340(6):448–54. doi: 10.1056/nejm199902113400607
30. Tang Y, Huang XR, Lv J, Chung AC, Zhang Y, Chen JZ, et al. C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin Sci (Lond). (2014) 126(9):645–59. doi: 10.1042/CS20130471
31. Butler J, Pathi VL, Paton RD, Logan RW, MacArthur KJ, Jamieson MP, et al. Acute-phase responses to cardiopulmonary bypass in children weighing less than 10 kilograms. Ann Thorac Surg. (1996) 62(2):538–42. doi: 10.1016/0003-4975(96)00325-6
32. Hirano D, Miwa S, Kakegawa D, Umeda C, Takemasa Y, Tokunaga A, et al. Impact of acute kidney injury in patients prescribed angiotensin-converting enzyme inhibitors over the first two years of life. Pediatr Nephrol. (2021) 36(7):1907–14. doi: 10.1007/s00467-021-04920-4
33. Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. (2011) 121(11):4210–21. doi: 10.1172/JCI45161
34. Thiele RH, Isbelland JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. (2015) 10(3):500–14. doi: 10.2215/CJN.07830814
35. Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. (2018) 70:61–75. doi: 10.1016/j.bbi.2018.02.013
36. Mihlan M, Blom AM, Kupreishvili K, Lauer N, Stelzner K, Bergström F, et al. Monomeric C-reactive protein modulates classic complement activation on necrotic cells. FASEB J. (2011) 25(12):4198–210. doi: 10.1096/fj.11-186460
37. Eisenhardt SU, Thiele JR, Bannasch H, Starkand GB, Peter K. C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle. (2009) 8(23):3885–92. doi: 10.4161/cc.8.23.10068
38. Gunnell J, Yeun JY, Depner TA, Kaysen GA. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis. (1999) 33(1):63–72. doi: 10.1016/s0272-6386(99)70259-3
Keywords: neonates, acute kidney injury, high-sensitivity C-reactive protein, anemia, congenital heart disease
Citation: Zhang P, Tong Y, Yuan D, Li Y, Jin Y, Bai L, Gao P, Wang W, Hu J, Duan X and Liu J (2022) Association of high-sensitivity C-reactive protein and anemia with acute kidney injury in neonates. Front. Pediatr. 10:882739. doi: 10.3389/fped.2022.882739
Received: 24 February 2022; Accepted: 7 October 2022;
Published: 2 November 2022.
Edited by:
Oswin Grollmuss, Université Paris-Sud, FranceReviewed by:
Bineeta Kashyap, University of Delhi, IndiaMarissa DeFreitas, University of Miami, United States
Rachel Victoria Jimenez, Moffitt Cancer Center, United States
© 2022 Zhang, Tong, Yuan, Li, Jin, Bai, Gao, Wang, Hu, Duan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinping Liu bGl1amlucGluZ0BmdXdhaS5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Peiyao Zhang1,2,†
Peiyao Zhang1,2,† Deshan Yuan
Deshan Yuan Liting Bai
Liting Bai Peng Gao
Peng Gao Wenting Wang
Wenting Wang Jinping Liu
Jinping Liu