
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 09 June 2022
Sec. Pediatric Pulmonology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.881908
This article is part of the Research Topic Hot Topics in Pediatrics View all 50 articles
Background: Post-infectious bronchiolitis obliterans (PIBO) is a long-term sequela after an initial insult to the lower respiratory tract. A comprehensive understanding of the factors that contribute to a high risk of developing PIBO is important to help define therapeutic strategies and improve prognosis.
Methods: We performed a systematic review of published literature available in the online databases including PubMed, Embase, Web of Science, CNKI, Wan Fang, and VIP, with the last search updated on 27 January 2022. Observational studies and case-control studies that provide sufficient data to examine associations between potential risk factors and PIBO were included. Pooled odds ratio (OR) or mean difference (MD) with 95% confidence interval (CI) and heterogeneity were calculated.
Results: A total of 14 risk factors were selected from 9 studies included in the analysis. The strongest risk factors were hypoxemia, mechanical ventilation, tachypnea, and wheezing. Hypoxemia conferred the greatest risk with pooled OR of 21.54 (95% CI: 10–46.36, p < 0.001). Mechanical ventilation ranked second (pooled OR 14.61, 95% CI: 7.53–28.35, p < 0.001). Use of γ-globulin, use of glucocorticoids, co-infection of bacteria, a history of wheezing, and being male were other prominent risk factors. The effects of premature birth, allergic rhinitis, and imaging finding (pulmonary consolidation, atelectasis, pleural effusion) are less clear and require further confirmation. Cases that developing PIBO had a lower age compared with controls (MD, −8.76 months, 95% CI: −16.50 to −1.02, p = 0.03). No significant differences were observed in the duration of fever (MD, 1.74 days, 95% CI: −0.07 to 3.54, p = 0.06). Children diagnosed with PIBO had higher LDH levels (MD, 264.69 U/L, 95% CI: 67.43 to 461.74, p = 0.008) and duration of hospitalization (MD, 4.50 days, 95% CI: 2.63 to 6.37, p < 0.001).
Conclusion: In this study, we found that the strongest risk factors for PIBO were hypoxemia, mechanical ventilation, tachypnea, and wheezing. Use of glucocorticoids, γ-globulin, co-infection of bacteria, a history of wheezing, and being male may also play a role. The factors discussed above can inform the generation of a clinical prediction model for the developing PIBO in children.
There are three forms of bronchiolitis obliterans (BO) seen by pediatricians: post-infectious bronchiolitis obliterans (PIBO); BO post lung transplantation; and BO after bone marrow transplantation (BMT) or hematopoietic stem cell transplantation (HSCT) (1, 2). Of these, PIBO is, by far, the most common in children. PIBO is a chronic small-airway disease caused by initial insult to the lower airways and subsequent epithelial and subepithelial inflammation and fibrotic narrowing of the bronchioles (3–5). The injury to the lower respiratory tract can be caused by various pathogens, such as adenovirus, influenza, parainfluenza, respiratory syncytial virus, mycoplasma pneumonia, or measles (6–10).
The prognosis of PIBO is overall poor, which is related to the late diagnosis, irreversible pulmonary fibrosis, and airway obstruction (1, 11). To date, there were several attempts to investigate an association between various risk factors and PIBO in children, but no systematic reviews of published literature assessed the strength of association between the suspected risk factors and PIBO.
Therefore, it is extremely necessary to identify the risk factors predicting the occurrence of PIBO following lower respiratory tract infections. A better understanding and knowledge of the topic can alert the professionals to the need of designing effective strategic measures to reduce the incidence, take early intervention, and improve the prognosis of PIBO in children. This study aimed to assess the quality of available evidence and present summary estimates of the strength of association between the risk factors and PIBO in children using meta-analysis.
This meta-analysis was conducted and reported according to MOOSE guidelines.
The PUBMED, EMBASE, Web of Science, and Chinese databases (Wan fang, CNKI, and VIP) were systematically searched with the restriction of language only in English and Chinese (last search updated on 27 January 2022). We used the Medical Subject Headings (MeSH): “bronchiolitis Obliterans” as the core search term in case of some unexpected missing of any potentially eligible studies. The references list of each of the initially included studies was also checked for further articles of interest. The final search strategies of PubMed, Web of Science, and Embase are presented in online Supplementary Material.
Criteria for inclusion: (1) case-control study, retrospective or prospective observational study, cross-sectional study, and cohort study; (2) all subjects were children (aged 0–18 years) diagnosed with or without PIBO after lower respiratory tract infections; (3) the definition of PIBO is clear; (4) the effect estimates of potential risk factors for PIBO are reported; and (5) sufficient data were provided to calculate odds ratio (OR). Exclusion criteria were as follows: (1) case reports, reviews, or unpublished literature; (2)BO caused by lung transplantation, heart-lung transplantation, HSCT, BMT, and Stevens–Johnson Syndrome; (3)Irrelevant reporting topics: such as organizing pneumonia, interstitial lung disease, bronchiectasis, and cystic fibrosis; and (4) adequate information could be obtained for categorical variables and continuous variables.
Data were independently extracted by JL and LZ; any inconsistency was resolved by QZ after referring to the original article. The following variables were collected and recorded in an Excel table: the first author, publication year, region, sample size, age, and risk factors were included. When full data cannot be obtained from the study, we tried to contact the corresponding author to obtain all the data.
The Newcastle–Ottawa Scale (NOS) for case-control studies was used to assess study quality concerning cases and controls selection (four items), comparability (one item), and exposure (three items). The star system ranges from 0 to 9, with higher scores representing better study quality. JL and DL accomplished this process independently, and disagreements were resolved by QZ.
Statistical analysis was performed using the Review Manager 5.4 software (The Nordic Cochrane Collaboration, Copenhagen). Raw data of continuous variables were converted into mean and standardized difference (SD) when suitable (12). Heterogeneity was tested by the I2 statistic, and I2 > 50% was considered significant. If there was significant heterogeneity, a random-effects model was used, or else, a fixed-effects model. The pooled effect estimates were reported as an OR with 95% confidence intervals (CI) for dichotomous data and mean difference (MD) with 95% CI for continuous outcomes. The significance of the pooled effects was determined by the Z-test, two-tailed p < 0.05 was considered statistically significant.
The flowchart of the study selection process is shown in Figure 1. Of 814 articles initially searched, 207 duplicates were firstly removed. After screening the titles and abstracts, 551 studies that were irrelevant to the risk factors of PIBO were excluded, and another 47 studies that did not provide detailed origin data or were not in accordance with our inclusion criteria were excluded after assessing the full text. Finally, only nine studies satisfied our inclusion criteria and were enrolled in our meta-analysis, including three case-control studies, five retrospective studies, and one prospective study.
The nine studies included in the qualitative analysis comprised 377 cases and 1,342 controls. The general characteristics were summarized in Table 1. Except for two South America (Chile, Argentina) studies, all the others were on the Asian population (China and Korea). A total of 14 risk factors were eligible for meta-analysis: male, premature birth, a history of wheezing, allergic rhinitis, wheezing, tachypnea, hypoxemia, pulmonary consolidation, atelectasis, pleural effusion, co-infection of bacteria, use of glucocorticoids, use of γ-globulin, and mechanical ventilation. Forest plots for individual risk factors for PIBO are available in Supplementary Figures 1–14. A summary of the pooled OR for all risk factors is given in Table 2. Based on the NOS quality assessment, all the included studies had a seven-plus score (Table 1), guaranteeing the eligibility for meta-analysis.
Eight studies (five studies from China and one each from Argentina, Korea, and Chile), were included, comprising 343 cases and 1,237 controls, to study the association between age and PIBO from 1991 to 2020 (13–20). Cases that developing PIBO had a lower age compared with controls as shown in Figure 2A (MD, −8.76 months, 95% CI: −16.50 to −1.02, p = 0.003).
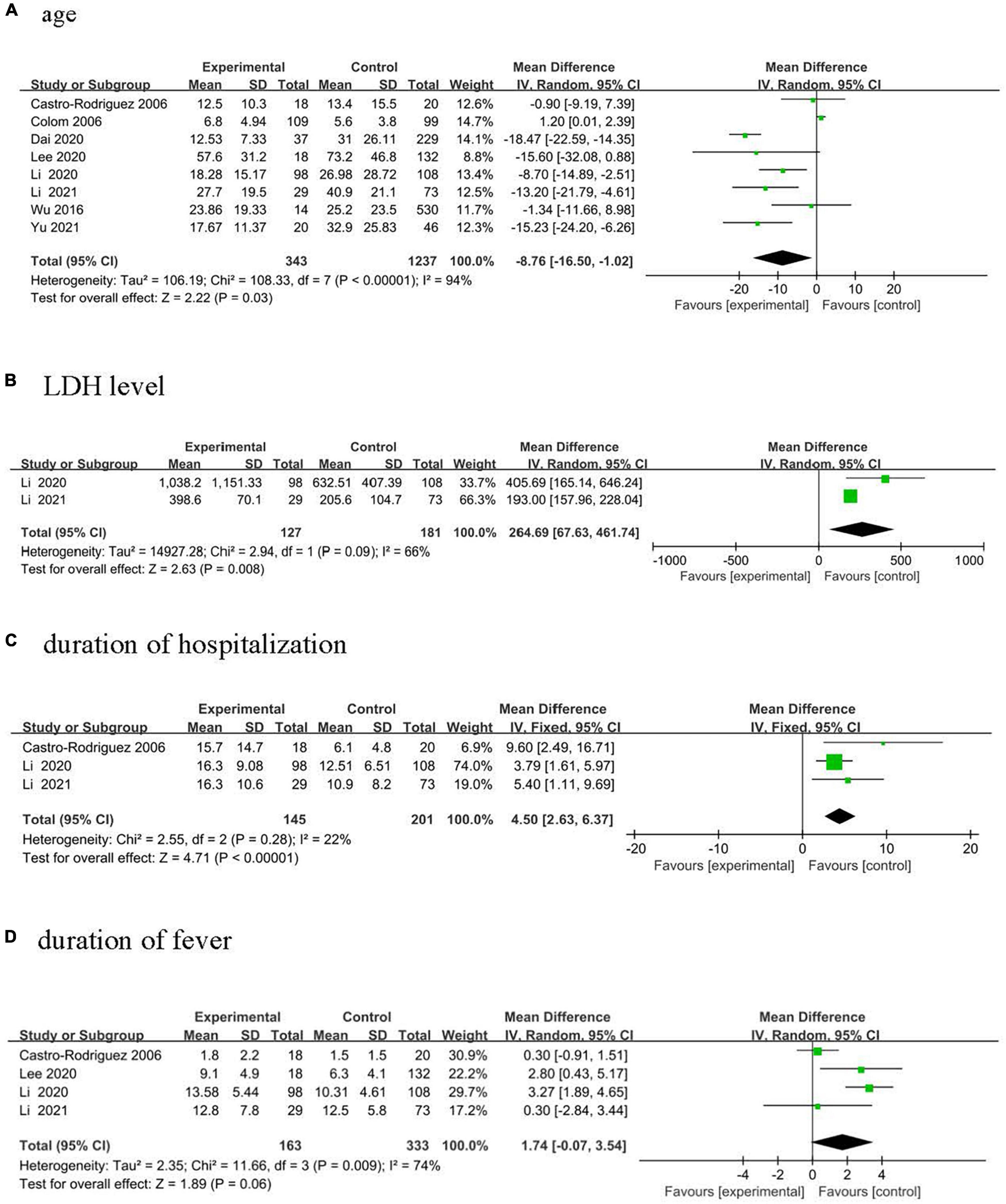
Figure 2. Forest plots of MD estimates for the following risk factors: (A) age; (B) LDH level; (C) duration of hospitalization; and (D) duration of fever.
Nine studies (three studies from China and one each from Argentina, Korea, and Chile) were included, consisting of 377 cases and 1,342 controls, to study the association between being male and PIBO from 1991 to 2020 (13–21). Meta-analysis calculated a pooled OR of 1.52 (95% CI: 1.14–2.01).
Two studies from China were included, comprising 57 cases and 275 controls, to analyze the association between hypoxemia and PIBO from 2011 to 2020 (13, 18). Meta-analysis calculated a pooled OR of 21.54 (95% CI: 10–46.36).
Five studies (three studies from China and one each from Argentina and Chile), were included, comprising 195 cases and 800 controls, to study the association between mechanical ventilation and PIBO from 1991 to 2020 (13, 15, 16, 20, 21). Meta-analysis calculated a pooled OR of 14.61 (95% CI: 7.53–28.35).
Three studies (two studies from China and one from Chile) were included, comprising 84 cases and 322 controls, to study the association between tachypnea and PIBO from 1998 to 2020 (16–18). Meta-analysis calculated a pooled OR of 10.14 (95% CI: 2.66–38.74).
Four studies were included (three studies from China and one from Chile), consisting of 104 cases and 368 controls, to study the association between wheezing and PIBO from 1998 to 2020 (13, 16–18). Meta-analysis calculated a pooled OR of 7.73 (95% CI: 2.73–21.93).
Three studies from China were included, consisting of 58 cases and 681 controls, to study the association between the use of γ-globulin and PIBO from 2011 to 2020 (13, 20, 21). Meta-analysis calculated a pooled OR of 4.77 (95% CI: 2.34–9.73).
Four studies (three studies from China and one from Chile) were included, consisting of 86 cases and 701 controls, to study the association between the use of glucocorticoids and PIBO from 1998 to 2020 (13, 16, 20, 21). Meta-analysis calculated a pooled OR of 4.46 (95% CI: 1.26–15.79).
Four studies from China were included, consisting of 120 cases and 453 controls, to study the association between co-infection of bacteria and PIBO from 2011 to 2020 (13, 17, 20, 21). Meta-analysis calculated a pooled OR of 2.23 (95% CI: 1.40–3.53).
Four studies (six studies from China and one from Chile) were included, consisting of 173 cases and 403 controls, to study the association between a history of wheezing and PIBO from 1998 to 2020 (13, 16, 18, 19). Meta-analysis calculated a pooled OR of 2.22 (95% CI: 1.36–3.63).
Two studies from China were included, comprising 127 cases and 181 controls, to analyze the association between LDH levels and PIBO from 2012 to 2020 (17, 19). Children diagnosed with PIBO had higher LDH levels (Figure 2B, MD, 264.69 U/L, 95% CI: 67.43–461.74, p = 0.008).
Three studies from China were included, comprising 145 cases and 201 controls, to analyze the association between duration of hospitalization and PIBO from 1998 to 2020 (16, 17, 19). Children diagnosed with PIBO had a higher duration of hospitalization (Figure 2C, MD, 4.5 days, 95% CI: 2.63–6.37, p < 0.001).
Three studies evaluated lung imaging evidence (13, 17, 21). Meta-analysis calculated a pooled OR respectively, pulmonary consolidation of 1.20 (95% CI: 0.66–2.19, p = 0.55), atelectasis of 1.25 (95% CI: 0.46–3.38, p = 0.67), pleural effusion of 0.65 (95% CI: 0.34–1.25, p = 0.20). No significant differences were observed in Premature birth (pooled OR 1.50, 95% CI: 066–3.43, p = 0.33) (13, 18, 20, 21), Allergic rhinitis (pooled OR 1.60, 95% CI: 0.38–6.70, p = 0.52) (14, 16, 17), and duration of fever (Figure 2D, MD = 1.74 days, 95% CI: −0.07 to 3.54, p = 0.06) (14, 16, 17, 19).
This is the first comprehensive systematic review and meta-analysis to date, which attempt to systematically assess the effect of a multitude of possible risk factors on PIBO in children. We identified 14 risk factors in total. This meta-analysis was the first to show that hypoxemia was most strongly associated with PIBO (OR 21.54). Mechanical ventilation was found to confer a significant risk (OR 14.61). Clinical manifestations, such as tachypnea and wheezing, were also observed to be prominent risk factors (OR 10.14 and 7.73, respectively). Use of γ-globulin (OR 4.77), use of glucocorticoids (OR 4.46), co-infection of bacteria (OR 2.23), a history of wheezing (OR 2.22) and being male (OR 1.52) were other prominent risk factors. The effects of premature birth, allergic rhinitis, duration of fever, and imaging finding (pulmonary consolidation, atelectasis, and pleural effusion) are less clear and require further confirmation. Cases that developing PIBO had a lower age compared with controls (MD, −8.76 months, 95% CI: −16.50 to −1.02). Both LDH levels and duration of hospitalization had a statistically significant association with PIBO (MD, 264.69 U/L, 95% CI: 67.43 to 461.74 and MD, 4.50 days, 95% CI: 2.63 to 6.37, respectively). This is the first meta-analysis to examine the association between hypoxemia, mechanical ventilation, tachypnea, and wheezing with PIBO.
In this meta-analysis, we found that hypoxemia was the strongest predictor of PIBO, which was a novel finding. Hypoxemia usually occurs earlier than mechanical ventilation, which made it a more sensitive and good early warning indicator than other predictors. No mechanisms have been proposed to explain the adverse effects of hypoxia on the development of PIBO. Based on the limited data shown in animal studies, Matthew and colleagues showed the development of BO and pulmonary fibrosis 1 month after sulfur mustard (SM) inhalation exposure in rats (22). They found that respiratory distress developed over time after SM inhalation, with progressive hypoxemia, respiratory distress, and weight loss. TGF-b1 and PDGF, two profibrotic cytokines and pathways, remained chronically elevated in rat lungs. The role of hypoxemia needs to be further investigated in animal models in the future.
Although our meta-analysis found that mechanical ventilation was a significant risk factor for PIBO, our results do not indicate that mechanical ventilation causes injury to the lung that increases the risk of developing PIBO, or as an indicator of disease severity. Further studies are needed to elucidate the relationship between mechanical ventilation and PIBO and to investigate the need for lung-protective strategies in this susceptible population (15).
Hypoxemia and mechanical ventilation were associated with severe lung injury. Based on the results of the meta-analysis, we speculated that predictors of PIBO showed that factors representing acute pulmonary insult severity were correlated with PIBO development, and it seemed that the more severe the episode of initial pneumonia, the higher the incidence of PIBO.
Most patients with PIBO are younger than 3 years of age at onset. Huang calculated that the median age of the 56 children with PIBO was 17.71 (7–91) months (23). Hong Kong’s 20-year single-center research data show that the median age at diagnosis was 1.39 years (IQR: 0.84–4.99 years), 26 patients were included with a male predominance (72.2%) (24). Our meta-analysis showed that younger children and males were at higher risk of PIBO than older children. Therefore, careful attention should be paid especially to younger male children.
In our study, specific clinical manifestations such as tachypnea and wheezing were significant risk factors of PIBO. The patient usually presents wheezing, tachypnea, dyspnea, and persistent cough for weeks or months after the initial infection (25–27). Yazan et al. evaluated 114 children with PIBO and found that persistent wheezing was the most common complaint (28). The typical ventilatory pattern of the PIBO disease is a severe ventilatory obstruction, which often does not respond to the treatments administered (29). After the initial attack, the disease can persist for years. Jerkic et al. demonstrated that in patients with PIBO, pulmonary function decreased significantly showing persistent obstruction over an average follow-up period of 8 years (30).
Our analysis also confirms that the association between several initial imaging factors and PIBO has a very small effect. These include pulmonary consolidation, atelectasis, and pleural effusion. The most common HRCT finding is the mosaic pattern in children with PIBO (31–33). It is not clear if CT changes significantly progress to deterioration as children grow up (34).
Our study also found that a history of wheezing was significantly associated with developing PIBO compared with allergic rhinitis. The number of studies included was small, and we did not assess the relationship between atopic dermatitis, history of eczema, allergic sensitization early in life, allergic sensitization early in life, tobacco, and PIBO. It appears that more well-designed clinical studies are needed to identify risk factors of PIBO.
Although this study has summarized the major risk factors for the development of PIBO, our systematic review has also limitations. The timing of assessment of the clinical manifestations and treatment of disease was generally not available. The definition and method of summarizing risk factors, the method of analysis, and the method of reporting results were heterogeneous. Given the limited number of studies included in the analysis, the findings from our meta-analysis should be confirmed in future research. One aim of the study was to precisely quantify the risk associated with these factors by combining center estimates in a meta-analysis. To pursue this aim, an agreement to supply and combine common data from many centers is required. This might be achieved through a special working group in the provision of the relevant data to central registries for analysis. Fortunately, since 2016, an international consortium of experts consisting of pediatric pulmonologists, radiologists, pathologists, physical therapists, psychologists, basic scientists, and statisticians has gathered regularly for a workshop on PIBO in Geisenheim, Germany (35). Further multicenter prospective trials are required to understand the complexity of the disease and to define risk factors.
In future studies, additional analyses are required to test other factors that may also be predictive of PIBO in children. Furthermore, studies should investigate the role of hypoxemia and another possible case mechanism by studying larger samples and by more careful measurement and analysis of possible confounding factors. Further multicenter prospective trials are required to understand the complexity of the disease and to define risk factors.
In conclusion, this systematic review and meta-analysis have found hypoxemia to be the most significant risk factor for PIBO, followed by mechanical ventilation, tachypnea, and wheezing. Use of glucocorticoids, use of γ-globulin, co-infection of bacteria, a history of wheezing, and being male may also play a role. The factors identified by our meta-analysis can inform the generation of a clinical prediction model for the developing PIBO in children. Further investigation is required to verify this relationship and explore other aspects of the aetiopathogenesis of PIBO.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
DL, JL, and QZ designed the study. JL contributed to the literature search, data collection, statistical analysis, and drafting of the manuscript. DL, LZ, and YC contributed to the literature search and data collection. QZ performed the manuscript review. All authors have read and approved the content of the final manuscript.
This work was financially supported by the Scientific Research Foundation of China-Japan Friendship Hospital (2019-RC-3). The funder had no role in any other part of the study (including study design, data collection, data interpretation, et al.) or in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.881908/full#supplementary-material
1. Kavaliunaite E, Aurora P. Diagnosing and managing bronchiolitis obliterans in children. Expert Rev Respir Med. (2019) 13:481–8. doi: 10.1080/17476348.2019.1586537
2. Aguilar PR, Michelson AP, Isakow W. Obliterative Bronchiolitis. Transplantation. (2016) 100:272–83. doi: 10.1097/TP.0000000000000892
3. Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. (2014) 370:1820–8. doi: 10.1056/NEJMra1204664
4. Fischer GB, Sarria EE, Mattiello R, Mocelin HT, Castro-Rodriguez JA. Post infectious bronchiolitis obliterans in children. Paediatr Respir Rev. (2010) 11:233–9. doi: 10.1016/j.prrv.2010.07.005
5. Champs NS, Lasmar LM, Camargos PA, Marguet C, Fischer GB, Mocelin HT. Post-infectious bronchiolitis obliterans in children. J Pediatr (Rio J). (2011) 87:187–98. doi: 10.2223/JPED.2083
6. Marinopoulos GC, Huddle KR, Wainwright H. Obliterative bronchiolitis: virus induced? Chest. (1991) 99:243–5. doi: 10.1378/chest.99.1.243
7. Casas Maldonado F, Gallardo Medina M, Franco Campos MA, Conde Valero A, érez Chica GP, Cruz Molina JM. [Bronchiolitis obliterans with organized pneumonia associated with measles virus]. Arch Bronconeumol. (1997) 33:541–4.
8. Massie R, Armstrong D. Bronchiectasis and bronchiolitis obliterans post respiratory syncytial virus infection: think again. J Paediatr Child Health. (1999) 35:497–8. doi: 10.1046/j.1440-1754.1999.355369.x
9. Becroft DM. Bronchiolitis obliterans, bronchiectasis, and other sequelae of adenovirus type 21 infection in young children. J Clin Pathol (1971) 24:72–82. doi: 10.1136/jcp.24.1.72
10. Coultas DB, Samet JM, Butler C. Bronchiolitis obliterans due to Mycoplasma pneumoniae. West J Med. (1986) 144:471–4.
11. Yu J. Postinfectious bronchiolitis obliterans in children: lessons from bronchiolitis obliterans after lung transplantation and hematopoietic stem cell transplantation. Korean J Pediatr. (2015) 58:459–65. doi: 10.3345/kjp.2015.58.12.459
12. Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. (2020) 11641–54. doi: 10.1002/jrsm.1429
13. Yu X, Ma Y, Gao Y, You H. Epidemiology of adenovirus pneumonia and risk factors for bronchiolitis obliterans in children during an outbreak in Jilin, China. Front Pediatr. (2021) 9:722885. doi: 10.3389/fped.2021.722885
14. Lee E, Young Lee Y. Risk factors for the development of post-infectious bronchiolitis obliterans after Mycoplasma pneumoniae pneumonia in the era of increasing macrolide resistance. Respir Med. (2020) 175:106209. doi: 10.1016/j.rmed.2020.106209
15. Colom AJ, Teper AM, Vollmer WM, Diette GB. Risk factors for the development of bronchiolitis obliterans in children with bronchiolitis. Thorax. (2006) 61:503–6. doi: 10.1136/thx.2005.044909
16. Castro-Rodriguez JA, Daszenies C, Garcia M, Meyer R, Gonzales R. Adenovirus pneumonia in infants and factors for developing bronchiolitis obliterans: a 5-year follow-up. Pediatr Pulmonol. (2006) 41:947–53. doi: 10.1002/ppul.20472
17. Li XL, He W, Shi P, Wang LB, Zheng HM, Wen YJ, et al. Risk factors of bronchiolitis obliterans after adenovirus pneumonia: a nested case-control study. Chin J Evid Based Pediatr. (2021) 16:233–6. doi: 10.3969/j.issn.1673-5501.2021.03.012
18. Dai G, Wang T, Jing WJ, Sun H, Wang M, Chen Z, et al. Clinical analysis of 37 cases of bronchiolitis obliterans after adenovirus pneumonia. Chin J Appl Clin Pediatr. (2020) 35:1235–8. doi: 10.3760/cma.j.cn101070-20190830-00826
19. Liu J, Liu X, Xu B, Li J, Yin J, Zhao Z Risk factors for bronchiolitis obliterans in children with adenovirus pneumonia. Chin J Med. (2020) 55:283–7. doi: 10.3969/j.issn.1008-1070.2020.03.015
20. Wu PQ, Li X, Jiang WH, Yin GQ, Lei AH, Xiao Q, et al. Hypoxemia is an independent predictor of bronchiolitis obliterans following respiratory adenoviral infection in children. Springerplus. (2016) 5:1622. doi: 10.1186/s40064-016-3237-7
21. Zhong L, Lin J, Dai J. Risk factors for the development of bronchiolitis obliterans in children with severe adenovirus pneumonia: a retrospective study with dose-response analysis. J Med Virol. (2020) 92:3093–9. doi: 10.1002/jmv.25703
22. McGraw MD, Dysart MM, Hendry-Hofer TB, Houin PR, Rioux JS, Garlick RB, et al. Bronchiolitis obliterans and pulmonary fibrosis after sulfur mustard inhalation in rats. Am J Respir Cell Mol Biol. (2018) 58:696–705. doi: 10.1165/rcmb.2017-0168OC
23. Huang F, Ma YC, Wang F, Li YN. Clinical analysis of adenovirus postinfectious bronchiolitis obliterans and nonadenovirus postinfectious bronchiolitis obliterans in children. Lung India. (2021) 38:117–21. doi: 10.4103/lungindia.lungindia_374_20
24. Chan KC, Yu MW, Cheung TWY, Lam DSY, Leung TNH, Tsui TK, et al. Childhood bronchiolitis obliterans in Hong Kong-case series over a 20-year period. Pediatr Pulmonol. (2021) 56:153–61. doi: 10.1002/ppul.25166
25. Colom AJ, Maffey A, Garcia Bournissen F, Teper A. Pulmonary function of a paediatric cohort of patients with postinfectious bronchiolitis obliterans. A long term follow-up. Thorax. (2015) 70:169–74. doi: 10.1136/thoraxjnl-2014-205328
26. Wang X, Liu C, Wang M, Zhang YI, Li H, Liu G. Clinical features of post-infectious bronchiolitis obliterans in children undergoing long-term azithromycin treatment. Exp Ther Med. (2015) 9:2379–83. doi: 10.3892/etm.2015.2418
27. Zhang XM, Lu AZ, Yang HW, Qian LL, Wang LB, Zhang XB. Clinical features of postinfectious bronchiolitis obliterans in children undergoing long-term nebulization treatment. World J Pediatr. (2018) 14:498–503. doi: 10.1007/s12519-018-0193-z
28. Yazan H, Khalif F, Shadfaan LA, Bilgin S, Nursoy M, Cakir FB, et al. Post-infectious bronchiolitis obliterans in children: clinical and radiological evaluation and long-term results. Heart Lung. (2021) 50:660–6. doi: 10.1016/j.hrtlng.2021.05.001
29. Cazzato S, Poletti V, Bernardi F, Loroni L, Bertelli L, Colonna S, et al. Airway inflammation and lung function decline in childhood post-infectious bronchiolitis obliterans. Pediatr Pulmonol. (2008) 43:381–90. doi: 10.1002/ppul.20784
30. Jerkic SP, Koc-Gunel S, Herrmann E, Kriszeleit L, Eckrich J, Schubert R, et al. Long-term course of bronchial inflammation and pulmonary function testing in children with postinfectious bronchiolitis obliterans. Pediatr Pulmonol. (2021) 56:2966–72. doi: 10.1002/ppul.25547
31. Chen IC, Hsu JS, Chen YW, Liu YC, Wu YH, Hsu JH, et al. Post-infectious bronchiolitis obliterans: HRCT, DECT, pulmonary scintigraphy images, and clinical follow-up in eight children. Front Pediatr. (2020) 8:622065. doi: 10.3389/fped.2020.622065
32. Bandeira T, Negreiro F, Ferreira R, Salgueiro M, Lobo L, Aguiar P, et al. Clinical, radiological, and physiological differences between obliterative bronchiolitis and problematic severe asthma in adolescents and young adults: the early origins of the overlap syndrome? Pediatr Pulmonol. (2011) 46:573–80. doi: 10.1002/ppul.21405
33. Yalcin E, Dogru D, Haliloglu M, Ozcelik U, Kiper N, Gocmen A. Postinfectious bronchiolitis obliterans in children: clinical and radiological profile and prognostic factors. Respiration. (2003) 70:371–5. doi: 10.1159/000072900
34. Mattiello R, Sarria EE, Mallol J, Fischer GB, Mocelin H, Bello R, et al. Post-infectious bronchiolitis obliterans: can CT scan findings at early age anticipate lung function? Pediatr Pulmonol (2010) 45:315–9. doi: 10.1002/ppul.21115
Keywords: meta-analysis, post-infectious bronchiolitis obliterans, bronchiolitis obliterans, risk factors, children
Citation: Liu D, Liu J, Zhang L, Chen Y and Zhang Q (2022) Risk Factors for Post-infectious Bronchiolitis Obliterans in Children: A Systematic Review and Meta-Analysis. Front. Pediatr. 10:881908. doi: 10.3389/fped.2022.881908
Received: 23 February 2022; Accepted: 03 May 2022;
Published: 09 June 2022.
Edited by:
Lian-Ping He, Taizhou University, ChinaReviewed by:
Amrita Dosanjh, University of California, San Diego, United StatesCopyright © 2022 Liu, Liu, Zhang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Zhang, emhhbmdxaWtleWFuQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.