
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 13 April 2022
Sec. Pediatric Rheumatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.872313
This article is part of the Research TopicInsights in Pediatric Rheumatology: 2021View all 13 articles
Takayasu Arteritis (TAK) is a rare large vessel vasculitis affecting the aorta and its major branches. The heterogeneous and often severe clinical manifestations result from systemic and local inflammation as well as end-organ ischemia. Disease flares are common and contribute to accrued damage over time with significant morbidity and mortality. Newer understanding of the pathogenesis in TAK has paved the way for the use of pathway targeting agents such as tumor necrosis factor (TNF)α- or interleuking (IL)-6-inhibitors with improved disease control. Nevertheless, long-term data are lacking, particularly in children; prognosis often remains guarded and the disease burden high. This article aims at providing a comprehensive review of childhood-onset TAK with a focus on recent publications.
Takayasu Arteritis (TAK) is the most common large vessel vasculitis in children. It is characterized by granulomatous inflammation of the aorta, its major branches and the pulmonary arteries that may result in segmental stenosis, occlusion, dilatation and/or aneurysms. Systemic inflammation, local inflammatory processes and organ dysfunction secondary to ischemia lead to a highly variable clinical presentation and significant morbidity if untreated. Therapy is often extrapolated from adult studies given the very low prevalence of the disease in children. Better understanding of pathophysiologic mechanisms has resulted in the use of cytokine targeting therapies. Although early recognition and therapy seem to improve outcome in childhood-onset TAK, long-term follow-up data are lacking and prognosis remains guarded. This article reviews recent publications on epidemiology, pathogenesis, clinical presentation, laboratory biomarkers, imaging, treatment, and outcomes with a focus on the pediatric literature.
The incidence and prevalence of TAK are much higher in adults compared to children. A recent systematic review and meta-analysis found an incidence rate of 1.11 per million person-years (95% CI 0.70, 1.76) with considerable variation across different populations (1). Indeed, the described incidence rates of TAK range from 0.3 to 3.3 per million per year and the prevalence from 0.9 to 360 cases per million depending on the population and geographic region studied, with higher rates observed particularly in Asia (2).
Epidemiological data in childhood-onset TAK are scarce. The annual incidence rate reported in a Swedish study was 0.4 (95% CI 0, 1.1) per million for childhood-onset TAK (3). The prevalence estimated from a National Health Insurance database in South Korea varied between 0.04 (95% CI 0.00, 0.08) for children 0 to 4 years old and 0.63 (95% CI 0.36, 0.91) per 100,000 for those 15–19 years old, with an increase of the age-standardized prevalence of TAK over the years (4).
Takayasu Arteritis most commonly affects young women with a peak incidence between 20 and 40 years of age. It is unknown why TAK occurs predominantly in women. In children, female preponderance is lower than in adult-onset TAK and estimated around 2.5:1–3:1 (5). Mean age at disease onset in the pediatric population is 12 years, but TAK has been described across all ages and even in infants (6–9).
While there are still major gaps in our understanding, the etiology of TAK has begun to be elucidated. Current knowledge of pathophysiology is mostly extrapolated from adult studies and animal models of large vessel vasculitis (10). Both, the innate and adaptive immune systems have been implicated in the pathophysiology of TAK (11). Histologically, the inflammatory process usually predominates in the adventitia and the outer part of the media, but may affect all three blood vessel layers. The consequences are vessel wall damage with laminar necrosis and elastic fiber fragmentation, and eventually fibrosis and arterial remodeling (12). Inflammatory cell infiltrates of the arterial wall consist of macrophages and lymphoid cells (αß CD4+ and CD8+ cells, γδ T-cells, natural killer cells, and B cells) (13).
Multiple proinflammatory cytokines have been implicated in the pathogenesis of TAK (14). Increased levels of tumor necrosis factor (TNF)α, interferon (IFN)α, IFNγ, interleukin (IL)-6, IL-8, IL-12, IL-17, and IL-18 have been observed in the peripheral blood of patients with TAK compared to healthy controls. Serum IL-6 and IL-18 levels have been shown to correlate with disease activity (15–18). Novel insights in disease pathways and identification of key pro-inflammatory cytokines have led to the use of cytokine targeting agents such as TNFα- or IL- 6-, and more recently janus kinase (JAK)-inhibitors.
T cells, and particularly Th1 and Th17 responses seem to play an important role in driving the systemic and vascular manifestations in TAK, as demonstrated by increased expression of Th1 and Th17-related cytokines in patients with TAK that correlate with disease activity (17).
Furthermore, recent data indicate the implication of the mammalian target of rapamycin (mTOR) pathway in T cell activation and the development of vascular lesions in TAK. mTOR is a kinase that drives different signaling pathways to regulate cell differentiation, proliferation and metabolism (19), as well as vascular remodeling (20, 21). In addition, mTOR Complex 1 (mTORC1) has been involved in the differentiation of Th1 and Th17 cells (22). In patients with TAK, mTORC1 pathway is hyperactivated in CD4+ T cells and correlates with disease progression (23). This hyperactivity of mTORC1 has been identified as a critical mechanism underlying the altered differentiation of Th1 and Th17 cells (23). In vitro and mouse model studies show, that blockade of mTORC1 by sirolimus, a specific mTOR inhibitor, or by genetic knockdown, successfully suppresses the hyperactivation of the mTOR pathway, altered differentiation of CD4+ T cells and arterial inflammation (23, 24). Thus, targeting the mTORC1 pathway may represent an interesting novel therapeutic strategy in patients with TAK (25).
Growing evidence supports the crucial role of the Janus Kinase/Signal Transducers and Activators of Transcription (JAK-STAT) signaling pathway in the pathophysiology of TAK. This was first shown in a large vessel vasculitis mouse-model, in which the JAK1/3 inhibitor tofacitinib significantly reduced vessel wall infiltrates and expression of IFNγ, IL-17, and IL-21, and further diminished angiogenesis and hyperplasia of the intima (26). Using transcriptome analysis, Régnier et al. found an important enrichment for pathways linked to IFN, and especially type I IFN in patients with TAK (27). The upregulation of type I specific IFN gene signature was confirmed in patients with TAK compared to healthy controls, and treatment with either ruxolitinib, a JAK1/2 inhibitor, or tofacitinib reduced T cell activation and restored T cell homeostasis in vitro (27). The same group has recently established a specific follicular helper T cell signature that characterizes TAK and highlighted the cooperation of follicular helper T cells and B cells through the JAK/STAT pathway in patients with TAK, further supporting the use of JAK-inhibitors as promising treatment strategy (28). In summary, cytokine signaling dependent on JAK/STAT is critically important in TAK and opens new therapeutic avenues, and studies are currently underway.
The involvement of humoral immune mechanisms has previously been demonstrated by the presence of circulating anti-endothelial cell antibodies (29), autoantibody-producing B cells in inflammatory vascular lesions (30), and increased numbers of plasmablasts in patients with TAK, which correlate with disease activity (31). More recently, the identification of two major endothelial autoantigens, both negative regulators of endothelial activation, and their corresponding autoantibodies further highlighted the involvement of the adaptive immune system in the pathophysiology of TAK (32). These findings supported the use of anti B cell agents in TAK (33).
Furthermore, a complex genetic predisposition may contribute, at least in parts, to the pathogenesis of TAK. Multiple susceptibility loci, including both HLA class I and II, have been identified in various studies, but only HLA-B*52 has been associated with TAK beyond ethnicity (11, 34). Genome-wide association studies established non-HLA susceptibility loci such as IL12B, IL6, RPS9/LILRB3, and FCGR2A/FCGR3A, which represent potential targets in pathophysiology and treatment of TAK (35).
More recently, monogenic causes including mutations in NOD2, STAT1 gain-of-function, and XIAP have been described in association with TAK (36–39). Clinical features associated with monogenic causes of TAK are early-onset of symptoms, associated other inflammatory and autoimmune diseases such as inflammatory bowel disease or thyroid disorder, and/or features reminding of primary immunodeficiency.
Finally, triggers of the immune response, such as the involvement of Mycobacterium tuberculosis has been suggested for a long time. Although high prevalence of tuberculosis has been described in patients with TAK (i.e., detection of active tuberculosis in up to 18% of patients with TAK, presence of mycobacterium tuberculosis DNA in aortic tissue in up to 70% of cases), particularly in regions with a high prevalence of tuberculosis infection, there is not enough evidence to establish a causal relationship (40). A possible cross-reaction between mycobacterial and arterial antigens is matter of ongoing discussion.
To summarize, almost all arms of the immune response, from cellular components of both the innate and adaptive immune systems to downstream humoral mediators and their signaling components, have been implicated in the pathogenesis of TAK, pointing to the complexity of the immune reaction. It remains to be elucidated, what is causation and what association.
The diagnosis of childhood-onset TAK is based on clinical criteria and angiographic abnormalities, and is supported by laboratory findings. It requires a high index of suspicion, especially in children, because (1) onset is often insidious with non-specific symptoms mimicking many inflammatory conditions, (2) the clinical presentation greatly varies due to variable localization and extent of the vessels involved, and (3) the occurrence is rare.
Different sets of classification criteria have been proposed for childhood-onset TAK. The initial 1990 American College of Rheumatology (ACR) classification criteria for TAK were based on data from adult patients with TAK (41). In 2005, the vasculitis working group of the European League against Rheumatism/Pediatric Rheumatology European Society (EULAR/PReS) proposed new classification criteria for childhood-onset TAK (42). These were subsequently endorsed by EULAR, the Pediatric Rheumatology International Trial Organization (PRINTO), and PReS (EULAR/PRINTO/PReS) (43). They optimized the 1990 ACR classification criteria by including the mandatory criterion of angiographic abnormalities (not only conventional angiography, but also more recent imaging modalities such as CT or MRI), as well as the extra criteria arterial hypertension and elevated acute phase reactants. The latter was added to help differentiate TAK from non-inflammatory conditions [e.g., fibromuscular dysplasia (FMD)]. The EULAR/PRINTO/PReS classification criteria for childhood-onset TAK, presented in Table 1, have a sensitivity and specificity of 100 and 99.9%, respectively (43). Of note, these are classification criteria, that are not required to be met to make a diagnosis of TAK and initiate treatment.
Disease often presents with non-specific constitutional symptoms, including fever and systemic inflammation (pre-pulseless stage), and evolves to vascular symptoms attributable to occlusive arteritis. Up to 25% of children are diagnosed during the late inactive, “burnt-out” phase of the disease, where symptoms result from irreversible vascular damage rather than active vasculitis lesions (44–46). TAK may be associated with other inflammatory diseases, such as inflammatory bowel disease, spondyloarthritis or sarcoidosis (47–50). A child with TAK, pyoderma gangrenosum and chronic recurrent multifocal osteomyelitis has also been described (51).
In children, the aorta (arch, thoracic or abdominal) is most commonly affected, followed by the renal, subclavian, carotid, and splanchnic arteries (5, 52; Figure 1). Stenotic lesions predominate, but occlusion, concentric vessel wall thickening and aneurysms may also be observed. Lesions are characteristically located close to the origin of the aortic branches, with an often segmental and patchy distribution (53).
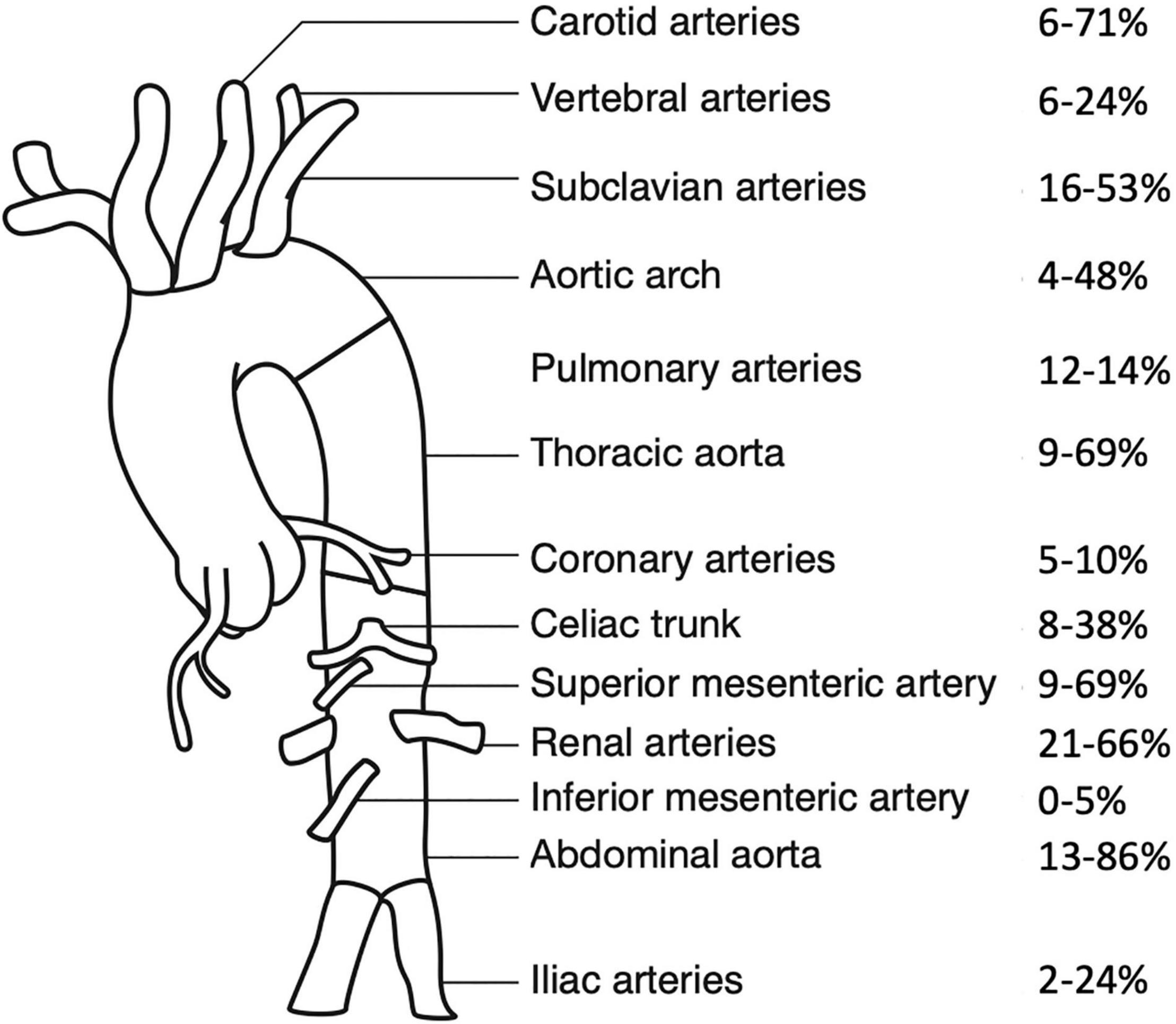
Figure 1. Frequency of arterial involvement at presentation, modified according to Aeschlimann et al. (44). Frequencies of arterial involvement in pediatric Takayasu Arteritis are reported as percentages, paired vessels are presented as one combined value. Data are extracted from Refs. (6, 7, 44, 46, 52, 54, 55, 62).
Table 2 summarizes childhood-onset TAK cohorts published during the last decade (6, 7, 44–46, 52, 54–62). Children may present with non-specific symptoms such as fever, dyspnea, headaches, weight loss or abdominal pain. Unlike adults, musculoskeletal symptoms including arthritis are infrequent (5), although they are more commonly described in pediatric TAK cohorts from South America (54, 56, 63). Cutaneous involvement (erythema nodosum, pyoderma gangrenosum) (51, 64) and ocular disease such as retinal vasculitis (47, 65) are rare in children. Presentation may be dramatic and life-threatening when acute hypertensive crisis, heart failure or arterial dissection develop (66–69).
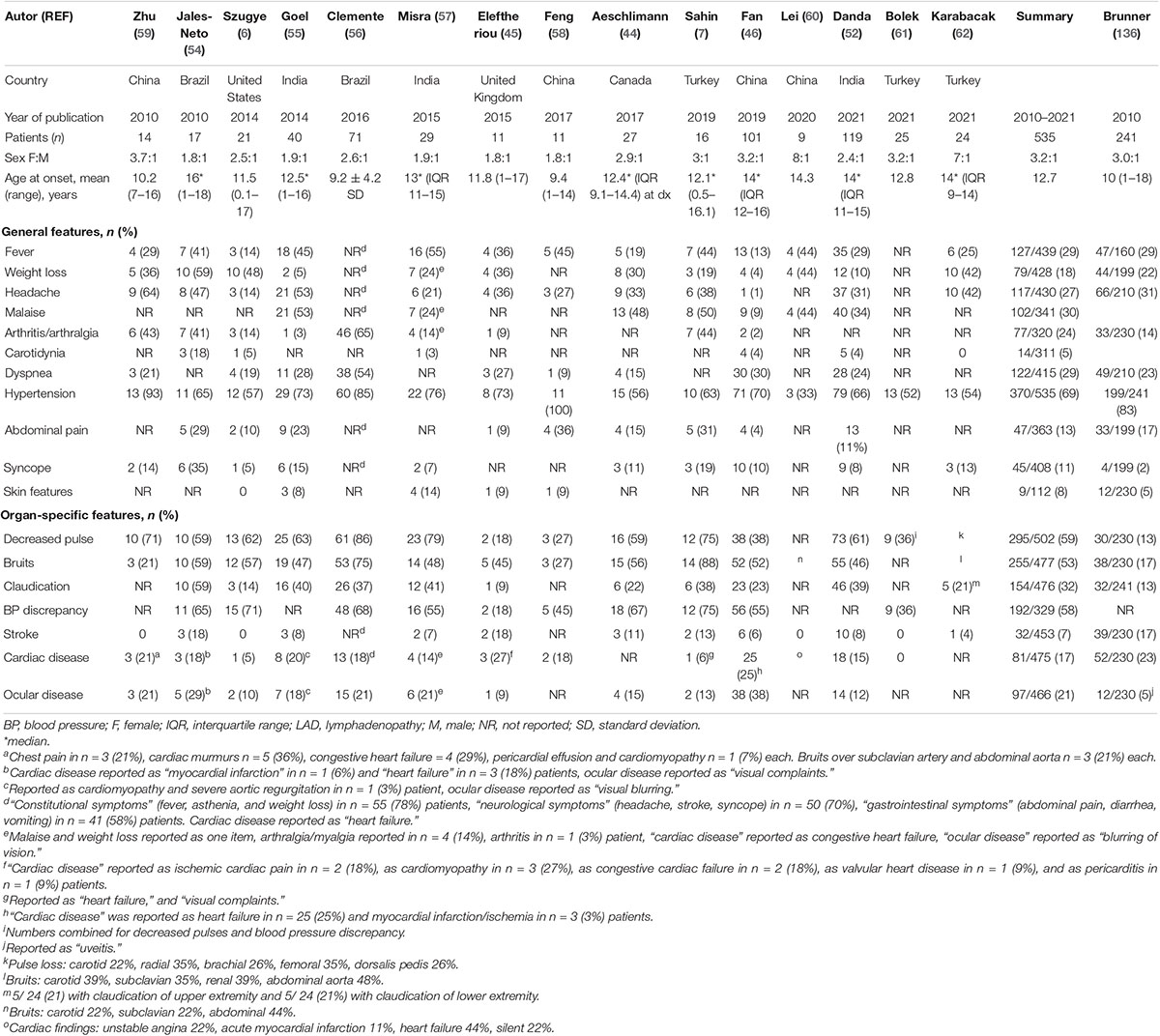
Table 2. Demographic and clinical characteristics of pediatric TAK cohorts, modified according to Aeschlimann et al. (135).
Organ-specific manifestations result from ischemia secondary to vascular stenosis. Arterial hypertension is the main presenting feature (56–100% of children depending on ethnicity, with higher prevalence in Asians) and is primarily related to renal artery stenosis and subsequent renovascular hypertension (44, 58). Arterial hypertension and other clinical signs of hypoperfusion such as blood pressure discrepancy between limbs, decreased peripheral pulses and bruits over large arteries are found in over 60% of children at diagnosis and highlight the necessity of a rigorous physical exam, especially in a child with unexplained systemic inflammation. Claudication of extremities, secondary to decreased blood supply, is reported in a third of children at presentation. Abdominal pain, often related to vasculitis of the abdominal aorta or the mesenteric arteries may occur. Prevalence of cardiovascular complications such as cardiomyopathy, ischemic heart disease, heart failure and valvular disease is estimated between 5 and 27% (6, 45), but coronary artery involvement is reported in only about 11% of children with TAK (44). Neurologic involvement including headache, dizziness, seizures, transitory ischemic attacks, and stroke may be observed. In adults, stroke is more often ischemic than hemorrhagic. While ischemic strokes are more commonly a consequence of steno-occlusive lesions in the carotid and vertebrobasilar vessels, hemorrhagic strokes usually occur as a result of steno-occlusive lesions in the abdominal aorta and renal arteries (70).
More recently, several groups have been directly comparing pediatric and adult TAK cohorts (5, 52, 54, 60–62). Childhood-onset TAK has a lower female predominance (5, 52, 54) and a shorter diagnostic delay, possibly due to a more pro-inflammatory presentation (5, 62). Children have more commonly arterial hypertension, less claudication of the upper extremities, and less carotidynia. This reflects the vascular disease pattern, which more often affects the aorta and the infra-diaphragmatic renal and mesenteric arteries in children, but more frequently the aortic arch in adults (5, 52, 61, 62). Higher disease activity scores such as the Indian TAK Clinical Activity Score (ITAS 2010) at diagnosis have been reported in the pediatric compared to the adult population, but this does not seem to be associated with accrued damage over time (52, 54, 62).
Duration of symptoms before treatment initiation has been positively associated with disease extent and damage (52). Disease course is most commonly relapsing-remitting, especially when untreated, but monophasic evolution may be observed.
Although various biomarkers have been explored for monitoring disease activity in TAK, none has yet been identified for reliable use in clinical practice. In children, elevation of acute phase reactants such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) is commonly observed (45, 55), but their sensitivity to reflect disease activity is uncertain, and they also lack specificity (71). In addition, they rapidly normalize and are masked under IL-6 blockade, adding further challenges to disease activity assessment in tocilizumab-treated patients (72). Anemia and thrombocytosis may be associated with chronic inflammation, particularly in children.
Among inflammatory molecules, IL-6, a pro-inflammatory cytokine that drives CRP synthesis and thrombocytosis and increases ESR seems promising as a biomarker and a treatment target. In tocilizumab-treated patients with TAK, longitudinal IL-6 monitoring might be useful for assessing disease activity and for detecting infections. While infections seem to trigger a significant, but short-term peak in serum IL-6, persistent IL-6 elevation may indicate subclinical disease activity or relapse (73, 74).
Further, IL-6 independent inflammatory biomarkers, such as S100 proteins or pentraxin-3 are being investigated (75). Pentraxin-3, for example, an acute phase protein synthetized locally at sites of inflammation, has been reported to correlate with disease activity in adults with TAK and may help to predict subclinical vascular inflammation (76). As mentioned previously, anti-endothelial antibodies have recently been identified in patients with TAK (32), but like many other serological biomarkers, they have limited availability and are not used in clinical practice; as a result, their clinical value yet remains to be defined. Studies searching for novel laboratory biomarkers are ongoing.
Vascular imaging aims at depicting morphologic anomalies of the aorta and its branches suggestive of arteritis, and at distinguishing active inflammation from chronic inactive disease. Thus, imaging is crucial for diagnosis, assessment of disease extent and follow-up management of TAK. Imaging modalities include conventional angiography, magnetic resonance (MR) angiography, computer tomography (CT) angiography, Doppler ultrasound (US), and fluorodeoxyglucose positron emission tomography (PET) (18F-FDG-PET). Although listed in the EULAR/PRINTO/PReS classification criteria for childhood-onset TAK, conventional angiography is nowadays rarely used and restricted to very few, specific indications such as angiographic imaging prior to revascularization procedures (77).
In childhood-onset TAK, contrast-enhanced MR angiography is the most popular imaging modality (Figure 2). It is proposed as the first imaging for suspected TAK by the EULAR recommendations on imaging of large vessel vasculitis (77), and is particularly appealing for repeated evaluations in the pediatric population due to lack of invasiveness and of radiation (78). However, recent research evidenced accumulation of gadolinium in the brain and other human tissues with repeated exposure. This raises concerns about potential toxicity particularly in the pediatric population and underlines the importance to carefully consider the indications for each MRI (79). Similar to MR imaging, CT angiography provides information on anatomical changes of the vascular lumen and wall, and the extent of arterial lesions with good spatial resolution. However, radiation exposure remains an important concern, especially in children and with repeated exposure. Doppler US is non-invasive and non-radiating, it visualizes the arterial wall and lumen, as well as altered blood flow characteristics. Challenges with its use include the lack of pediatric radiologists with expertise in vasculitis imaging, as well as acoustic technical limits, restricting the accessibility of certain vessels such as the descending aorta, especially in children (80).
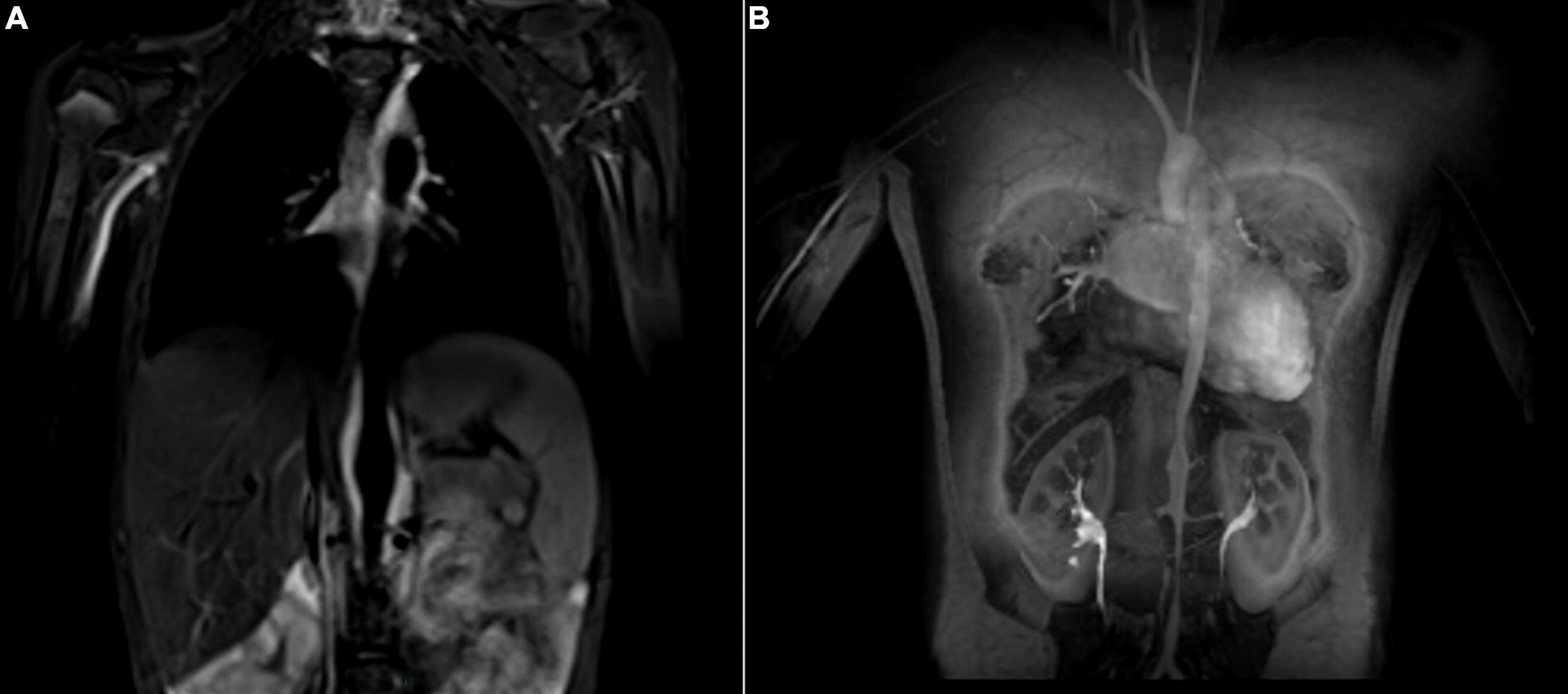
Figure 2. Takayasu Arteritis in a 13-year old girl who presented with fatigue, dyspnea, anemia, and claudication of the lower extremities. The MR angiogram (T2 black blood sequence, coronal view) shows hypersignal (inflammation) of the abdominal aorta (A). Post contrast angiography (coronal view) demonstrates multifocal narrowing of the aorta (B).
More recently, 18F-FDG-PET has been suggested to support radiologic assessment of disease activity (81). In children though, 18F-FDG-PET plays a minor role for routine imaging monitoring of disease activity, mainly due to the high radiation dose.
While these imaging modalities (MR angiography, CT angiography, Doppler ultrasound, and 18F-FDG-PET) reliably detect signs suggestive of vascular inflammation and allow diagnosis of TAK even in the early pre-stenotic phase, their role for follow-up monitoring is less evident. As an example, vascular changes such as vessel wall thickening or 18F-FDG uptake are not specific to active TAK, but may also be induced by healing processes or fibrotic remodeling. This causes difficulties for interpretation of imaging as there is no clear correlation of imaging results with disease activity during the course of disease. Hence, it is crucial to combine clinical, laboratory and imaging evaluation for disease management.
The interested reader is referred to a recent extensive review on imaging in adult and childhood-onset TAK for more information (82).
Efforts to describe angiographic patterns of vascular involvement in TAK have resulted in the identification of different patterns with distinct clinical presentation in various ethnicities (53, 83). Most recently, three distinct clusters with distinct clinical symptoms and outcomes were identified in a large cohort of 806 adults with TAK of Indian and North American origins (84). Yet, these angiography-based disease classifications require validation regarding prognostic prediction.
Given the variable clinical presentation with often non-specific symptoms, differential diagnosis of childhood-onset TAK is broad and also depends on disease presentation. For example, differential diagnoses of fever of unknown origin have to be considered during the early disease phase, when non-specific systemic inflammatory symptoms predominate. Infections including tuberculosis and syphilis may cause aortitis, bacterial infections such as staphylococcus aureus, streptococcus, salmonella, or brucella should be considered in children with more acute clinical presentations (85, 86).
Differential diagnosis also includes other primary vasculitides (Behçet disease, Kawasaki disease, polyarteritis nodosa) and vasculitides secondary to systemic lupus erythematosus, spondylarthritis or sarcoidosis. Non-inflammatory diseases such as aortic coarctation, Williams syndrome, Marfan or Ehlers-Danlos syndrome, and fibromuscular dysplasia (FMD) may mimic childhood-onset TAK. In contrast to TAK, FMD is not an inflammatory disease; but it may be difficult to differentiate from TAK during the “burned-out” disease phase as–unlike in adults–the characteristic angiographic “string of beads” pattern is rarely observed in childhood-onset FMD (87).
Because of progressive or relapsing disease most children with TAK require immunosuppressive therapy to control systemic and vascular inflammation. Challenges in clinical management include the timely treatment initiation and assessment of disease activity to guide therapeutic decision-making. Diagnostic delay can result in irreversible vascular damage even prior to diagnosis and assessment of treatment response is difficult, because reliable laboratory and radiological biomarkers for disease activity are lacking.
Treatment recommendations are extrapolated from adult TAK studies, as high-level evidence including randomized controlled trials is not available to guide treatment in childhood-onset TAK. To date, high-dose corticosteroids remain the mainstay for induction of remission (78, 88), although recent treatment approaches in adults have used protocols without corticosteroids (89). Relapses are common in patients on corticosteroid monotherapy (90), and side effects of long-term high dose corticosteroids may be devastating, especially in children. Therefore, early initiation of second-line, corticosteroid sparing agents has been recommended (78, 88, 91). Traditionally, cyclophosphamide has been used in children with extensive or life-threatening disease, while conventional disease-modifying antirheumatic drugs (DMARDs) such as methotrexate, azathioprine, and mycophenolate mofetil have been favored in less severe cases (45, 91).
Better understanding of disease pathophysiology has resulted in the use of cytokine- and pathway-targeting agents such as TNFα-, IL-6, and more recently JAK-inhibitors (15, 16, 27). Beneficial effects of TNFα- and IL-6-inhibitors have been reported for more than a decade in mainly retrospective case series and case reports. Based on these data, the use of biologic agents on a case-to-case basis was proposed in the recent European consensus-based recommendations for the treatment of childhood vasculitis (78). More recently, and particularly in children, biologic agents have been preferred over cyclophosphamide when they can be accessed and afforded as they present a more favorable toxicity profile.
For TNFα-inhibitors, evidence is mainly extrapolated from adults. The largest cohort study retrospectively included 209 adults with TAK and compared efficacy of TNFα-inhibitors (63% of patients) and the IL-6 inhibitor tocilizumab (37% of patients) (92). Efficacy was equivalent between biologic agents: complete response was observed in 66% of patients on TNFα-inhibitors and 70% of patients on tocilizumab. A total of 103 relapses (median of 36 months follow-up) were reported with similar rates between TNFα-inhibitors and tocilizumab (92). These results are supported by previous studies evaluating the efficacy of TNFα-inhibitors (93–96) and comparing rates of treatment response to TNFα-inhibitors and tocilizumab in patients with TAK (97, 98).
In children, data on TNFα-inhibitors are limited to small retrospective cohorts and case reports. In a retrospective cohort of childhood-onset TAK from Canada, eleven mostly treatment refractory children were treated with TNFα-inhibitors (n = 10 treatment episodes) or tocilizumab (n = 2 treatment episodes). Biologic agents were associated with significantly better 2-year flare-free survival rates and greater likelihood to reach inactive disease at last follow-up compared with conventional DMARDs or corticosteroids alone (44). Filocamo retrospectively reported four children with mainly refractory TAK treated with TNFα-inhibitors: two achieved remission and two partially responded (99). In addition, several other small case series and case reports have described the beneficial use of TNFα-inhibitors in children with treatment refractory TAK (100–102). TNFα-inhibitors with different mechanisms of action (monoclonal anti-TNFα antibodies and TNFα receptor) have been used, but most commonly the monoclonal anti-TNFα antibody infliximab, followed by adalimumab.
Beneficial effects and safety profiles of the IL-6 inhibitor tocilizumab have been reported in several adult and pediatric TAK cohorts (103–109). Two large retrospective cohort studies of mostly DMARD-refractory adults with TAK demonstrated significantly better event-free survival with tocilizumab compared to conventional DMARDs, and complete response rates in up to 70% of tocilizumab-treated patients (92, 109). However, a randomized, placebo-controlled trial of patients with TAK who had recently relapsed did not find a statistically significant difference between patients receiving tocilizumab and those in the placebo group, although patients receiving tocilizumab trended toward fewer relapses. Among the 36 enrolled patients, six children over the age of 12 years were included (four receiving TCZ, two placebo) and there were no new safety concerns (110). The long-term extension study confirmed a corticosteroid-sparing effect and stable/improved disease on imaging evaluation for up to 96 weeks (111). More recently, a prospective multicenter open-label trial showed high efficacy of tocilizumab in combination with corticosteroids in treatment-naïve patients with TAK, with high remission rates of 85% and corticosteroid discontinuation rates of 54% after 6 months of therapy. However, relapse rates were of 45% after tocilizumab discontinuation, highlighting the necessity of maintenance therapy (112).
In children with TAK, data on tocilizumab are scarce. Apart from the few patients included in the randomized controlled trial (110) there are only a few retrospective case series published, reporting children with TAK with mostly DMARD-refractory disease and good response to tocilizumab with no adverse events (44, 104, 105, 113).
The discovery of the critical role of the JAK/STAT pathway in the pathophysiology of TAK paved the way for the use of JAK-inhibitors. Forty-two mainly refractory patients with TAK treated with a JAK-inhibitor (9/42 treatment-naïve patients, 3/42 with childhood-onset TAK) have been published to date with promising results (27, 114–121). Most patients were treated with the JAK 1/3 inhibitor tofacitinib (39/42) in combination with prednisone ± MTX or other conventional DMARDs. The largest cohort prospectively compared the efficacy and safety of corticosteroids and tofacitinib (n = 27 adults with TAK) with corticosteroids and methotrexate (n = 26 adults with TAK) (121). Patients receiving tofacitinib had significantly higher complete remission rates at 12 months (88.6 vs. 56.5%, p = 0.02), lower relapse rates (11.5 vs. 34.8%, p = 0.052) and significantly lower average corticosteroid doses during the study period compared to those on methotrexate. Treatments were well tolerated with a good safety profile (121).
Various other biologic agents have been used with partial success in adults with TAK. Rituximab has been proposed as a therapeutic option following evidence of an implication of B cells in the pathophysiology of TAK (31) and its potential benefits have been reported in retrospective case reports of treatment-refractory adult patients with TAK (31, 33). The use of rituximab in childhood-onset TAK has been described, but sound data are lacking (45). Ustekinumab, a monoclonal antibody against IL-12/IL-23 has been used following detection of IL12B as a susceptibility gene for TAK in genome-wide association studies (122). Clinical and laboratory response has been reported to be good, although improvement was not observed on imaging (123). Finally, a randomized, placebo-controlled trial in adult patients with TAK did not find better flare-free survival in patients treated with the T cell co-stimulation inhibitor abatacept compared with controls (124).
However, although biologic agents, and in particular TNFα-, IL- 6-, and JAK-inhibitors seem promising, not all patients respond to these treatments. For anti-TNFα agents, for example, some controversy emerges from reports of patients who developed TAK while they were being treated with a TNFα-inhibitor for another disease and primary non-response or even disease progression has been described under TNFα-, IL-6, and JAK-inhibitors (44, 114, 115, 125–129). Of note, assessment of disease activity is even more challenging in tocilizumab-treated patients, as biologic inflammation may be suppressed and disease activity scores that include acute phase reactants may not be sensitive enough (72, 129).
More data are required to better understand, when to start a biologic agent, which biologic therapy to choose for an individual patient and how long to continue treatment. While biologic agents are currently considered mainly in case of relapsing or refractory disease despite conventional DMARDs, recent treatment approaches have used them at treatment initiation and independently from disease severity in treatment-naïve adults (89, 112) and children (44), but the long-term outcome has yet to be determined. Further studies will be needed to provide more data to guide therapeutic management.
Antiplatelet therapy is often prescribed in the management of TAK, although there is no evidence to support its usefulness. Its benefits need to be weighed against potential side effects, principally gastrointestinal hemorrhage.
Endovascular interventions or reconstructive surgery may be required to treat major vascular complications. Ideally, they should be performed in phases of stable remission, but urgent interventions may be necessary in case of arterial dissection or critical vascular ischemia (130). In children with TAK, endovascular interventions are performed mainly for treatment-resistant reno-vascular hypertension, restenosis is observed in about half of the patients within one year (131, 132).
Assessment of disease activity is often difficult in clinical practice because an outcome measure that reliably reflects vascular inflammation does not exist. The current tools insufficiently reflect disease activity in pediatric large vessel vasculitis, although the Pediatric Vasculitis Activity Score (PVAS) has been validated in children. Other disease activity measurement tools such as the ITAS 2010 and ITAS-A, which additionally includes acute phase reactants, have been developed specifically for TAK, but are only validated in adults.
The US National Institute of Health (NIH) criteria, commonly used to assess disease activity in TAK, define active disease as the presence of constitutional symptoms, new bruits, increased acute phase reactants or new angiographic findings (133).
Although tools for assessment of disease damage exist for adults with TAK, none has been validated in children. These scores may help to assess accumulated damage over time, but discrimination between disease and treatment-related damage may be difficult (134).
Children with TAK present with more systemic inflammation and more widespread vascular disease than adults. However, relapses and accrued damage are high and equally frequent in both groups and seem to be associated with longer duration of symptoms (44, 52, 61).
Recent advances in disease recognition and therapeutic strategies have decreased morbidity and mortality in TAK. Several recent studies have reported the benefit of biologic therapies such as TNFα-inhibitors or Tocilizumab in adults, with up to 80% of patients achieving complete remission at 6 months (92, 112). In children, data are scarce. A retrospective cohort study demonstrated significantly higher 2-year flare-free survival rates and higher rates of inactive disease at last follow-up in children treated with biologic agents, compared with those on non-biologic therapies (44).
Young age and high CRP at disease onset, stroke, lower BMI, longer duration of symptoms and high accrued damage scores have been associated with poor outcomes including increased mortality in childhood-onset TAK (45, 46, 52). In the recent pediatric cohorts, mortality rate varied between 0 and 27% (6, 7, 44–46, 54–59). Despite growing literature, more data are needed, particularly in children, to better describe the long-term outcome of new therapeutic strategies and to identify factors predicting treatment response and relapses.
Timely diagnosis of childhood-onset TAK is important because the disease is associated with a significant morbidity and mortality. Despite persisting gaps in the knowledge of the pathophysiology of TAK, critical inflammatory pathways contributing to the disease begin to be elucidated and provide avenues for new treatment approaches. Treatment recommendations are mostly based on adult TAK studies with level of evidence two or three, although few randomized controlled trials have been published with biologic agents. Corticosteroids remain the mainstay for induction of remission, but biologic agents such as TNFα- or IL-6- and JAK-inhibitors are increasingly used, particularly in severe, relapsing or DMARD-refractory cases. Whether upfront use of biologic agents improve long-term outcome, yet needs to be investigated.
Large international collaborative efforts are required to conduct well-designed studies to determine efficacy of current therapeutic regimens, to identify reliable biomarkers that help to assess disease activity and guide treatment choice, and to better define the long-term outcome of pediatric TAK using validated pediatric outcome measures. One of the main goals is thereby finding therapeutic strategies to reduce cumulative corticosteroid use and to achieve the best possible efficiency-tolerance balance with minimal cumulative damage.
FA reviewed the literature and drafted the manuscript. RY and RL reviewed the literature and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
RY was supported by the Hak-Ming and Deborah Chiu Chair in Paediatric Translational Research at the Hospital for Sick Children, University of Toronto.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Francesca Raimondi and Simon W. M. Eng for technical help with the images.
1. Rutter M, Bowley J, Lanyon PC, Grainge MJ, Pearce FA. A systematic review and meta-analysis of the incidence rate of Takayasu arteritis. Rheumatology (Oxford). (2021) 60:4982–90. doi: 10.1093/rheumatology/keab406
3. Mossberg M, Segelmark M, Kahn R, Englund M, Mohammad AJ. Epidemiology of primary systemic vasculitis in children: a population-based study from southern Sweden. Scand J Rheumatol. (2018) 47:295–302. doi: 10.1080/03009742.2017.1412497
4. Jang SY, Seo SR, Park SW, Kim DK. Prevalence of Takayasu’s arteritis in Korea. Clin Exp Rheumatol. (2018) 36(Suppl. 111): 163–4.
5. Aeschlimann FA, Barra L, Alsolaimani R, Benseler SM, Hebert D, Khalidi N, et al. Presentation and disease course of childhood-onset versus adult-onset Takayasu arteritis. Arthritis Rheumatol. (2019) 71:315–23. doi: 10.1002/art.40690
6. Szugye HS, Zeft AS, Spalding SJ. Takayasu arteritis in the pediatric population: a contemporary United States-based single center cohort. Pediatr Rheumatol Online J. (2014) 12:21. doi: 10.1186/1546-0096-12-21
7. Sahin S, Hopurcuoglu D, Bektas S, Belhan E, Adrovic A, Barut K, et al. Childhood-onset Takayasu arteritis: a 15-year experience from a tertiary referral center. Int J Rheum Dis. (2019) 22:132–9. doi: 10.1111/1756-185X.13425
8. Sandeep S, Unni VN, Sreekumar KP, Mathew A, Nair RR, Kurian G. Takayasu arteritis in an infant. Indian J Nephrol. (2014) 24:257–9.
9. Liu H, Sun L, Upadhyaya RS, Chen Y, Ajoje OO. Case report: Takayasu arteritis in a 3-month-old Chinese girl. Medicine (Baltimore). (2018) 97:e12637. doi: 10.1097/MD.0000000000012637
10. Tombetti E, Mason JC. Takayasu arteritis: advanced understanding is leading to new horizons. Rheumatology (Oxford). (2019) 58:206–19. doi: 10.1093/rheumatology/key040
11. Terao C. Revisited HLA and non-HLA genetics of Takayasu arteritis–where are we? J Hum Genet. (2016) 61:27–32. doi: 10.1038/jhg.2015.87
12. Mason JC. Surgical intervention and its role in Takayasu arteritis. Best Pract Res Clin Rheumatol. (2018) 32:112–24. doi: 10.1016/j.berh.2018.07.008
13. Inder SJ, Bobryshev YV, Cherian SM, Lord RS, Masuda K, Yutani C. Accumulation of lymphocytes, dendritic cells, and granulocytes in the aortic wall affected by Takayasu’s disease. Angiology. (2000) 51:565–79. doi: 10.1177/000331970005100705
14. Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev. (2011) 11:61–7. doi: 10.1016/j.autrev.2011.08.001
15. Park MC, Lee SW, Park YB, Lee SK. Serum cytokine profiles and their correlations with disease activity in Takayasu’s arteritis. Rheumatology (Oxford). (2006) 45:545–8. doi: 10.1093/rheumatology/kei266
16. Noris M, Daina E, Gamba S, Bonazzola S, Remuzzi G. Interleukin-6 and RANTES in Takayasu arteritis: a guide for therapeutic decisions? Circulation. (1999) 100:55–60. doi: 10.1161/01.cir.100.1.55
17. Saadoun D, Garrido M, Comarmond C, Desbois AC, Domont F, Savey L, et al. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol. (2015) 67:1353–60. doi: 10.1002/art.39037
18. Nakajima T, Yoshifuji H, Shimizu M, Kitagori K, Murakami K, Nakashima R, et al. A novel susceptibility locus in the IL12B region is associated with the pathophysiology of Takayasu arteritis through IL-12p40 and IL-12p70 production. Arthritis Res Ther. (2017) 19:197. doi: 10.1186/s13075-017-1408-8
19. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. (2012) 149:274–93. doi: 10.1016/j.cell.2012.03.017
20. Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, et al. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. (2014) 129:864–74. doi: 10.1161/CIRCULATIONAHA.113.004581
21. Canaud G, Terzi F. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. (2014) 371:1554–5.
22. Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. (2011) 12:295–303. doi: 10.1038/ni.2005
23. Zhang J, Zhao L, Wang J, Cheng Z, Sun M, Zhao J, et al. Targeting mechanistic target of rapamycin complex 1 restricts proinflammatory T Cell differentiation and ameliorates Takayasu arteritis. Arthritis Rheumatol. (2020) 72:303–15. doi: 10.1002/art.41084
24. Hadjadj J, Canaud G, Mirault T, Samson M, Bruneval P, Régent A, et al. mTOR pathway is activated in endothelial cells from patients with Takayasu arteritis and is modulated by serum immunoglobulin G. Rheumatology (Oxford). (2018) 57:1011–20. doi: 10.1093/rheumatology/key017
25. Sakai H, Oyama N, Kishimoto N, Takahashi M, Urasawa K, Tsutsui H. Revascularization of malignant coronary instent restenosis resulting from Takayasu’s arteritis using sirolimus-eluting stents. Int Heart J. (2006) 47:795–801. doi: 10.1536/ihj.47.795
26. Zhang H, Watanabe R, Berry GJ, Tian L, Goronzy JJ, Weyand CM. Inhibition of JAK-STAT signaling suppresses pathogenic immune responses in medium and large vessel vasculitis. Circulation. (2018) 137:1934–48. doi: 10.1161/CIRCULATIONAHA.117.030423
27. Régnier P, Le Joncour A, Maciejewski-Duval A, Desbois AC, Comarmond C, Rosenzwajg M, et al. Targeting JAK/STAT pathway in Takayasu’s arteritis. Ann Rheum Dis. (2020) 79:951–9. doi: 10.1136/annrheumdis-2019-216900
28. Desbois AC, Régnier P, Quiniou V, Lejoncour A, Maciejewski-Duval A, Comarmond C, et al. Specific follicular helper T cell signature in Takayasu arteritis. Arthritis Rheumatol. (2021) 73:1233–43. doi: 10.1002/art.41672
29. Chauhan SK, Tripathy NK, Nityanand S. Antigenic targets and pathogenicity of anti-aortic endothelial cell antibodies in Takayasu arteritis. Arthritis Rheum. (2006) 54:2326–33. doi: 10.1002/art.21921
30. Wang H, Ma J, Wu Q, Luo X, Chen Z, Kou L. Circulating B lymphocytes producing autoantibodies to endothelial cells play a role in the pathogenesis of Takayasu arteritis. J Vasc Surg. (2011) 53:174–80. doi: 10.1016/j.jvs.2010.06.173
31. Hoyer BF, Mumtaz IM, Loddenkemper K, Bruns A, Sengler C, Hermann KG, et al. Takayasu arteritis is characterised by disturbances of B cell homeostasis and responds to B cell depletion therapy with rituximab. Ann Rheum Dis. (2012) 71:75–9. doi: 10.1136/ard.2011.153007
32. Mutoh T, Shirai T, Ishii T, Shirota Y, Fujishima F, Takahashi F, et al. Identification of two major autoantigens negatively regulating endothelial activation in Takayasu arteritis. Nat Commun. (2020) 11:1253. doi: 10.1038/s41467-020-15088-0
33. Pazzola G, Muratore F, Pipitone N, Crescentini F, Cacoub P, Boiardi L, et al. Rituximab therapy for Takayasu arteritis: a seven patients experience and a review of the literature. Rheumatology (Oxford). (2018) 57:1151–5. doi: 10.1093/rheumatology/kex249
34. Sahin Z, Bicakcigil M, Aksu K, Kamali S, Akar S, Onen F, et al. Takayasu’s arteritis is associated with HLA-B*52, but not with HLA-B*51, in Turkey. Arthritis Res Ther. (2012) 14:R27. doi: 10.1186/ar3730
35. Renauer PA, Saruhan-Direskeneli G, Coit P, Adler A, Aksu K, Keser G, et al.. Identification of susceptibility Loci in IL6, RPS9/LILRB3, and an intergenic locus on chromosome 21q22 in Takayasu arteritis in a genome-wide association study. Arthritis Rheumatol. (2015) 67:1361–8. doi: 10.1002/art.39035
36. Rose CD, Eichenfield AH, Goldsmith DP, Athreya BH. Early onset sarcoidosis with aortitis–“juvenile systemic granulomatosis?”. J Rheumatol. (1990) 17:102–6.
37. Khubchandani RP, Hasija R, Touitou I, Khemani C, Wouters CH, Rose CD. Blau arteritis resembling Takayasu disease with a novel NOD2 mutation. J Rheumatol. (2012) 39:1888–92. doi: 10.3899/jrheum.120156
38. Takeuchi I, Kawai T, Nambu M, Migita O, Yoshimura S, Nishimura K, et al. X-linked inhibitor of apoptosis protein deficiency complicated with Crohn’s disease-like enterocolitis and Takayasu arteritis: a case report. Clin Immunol. (2020) 217:108495. doi: 10.1016/j.clim.2020.108495
39. Maeshima K, Ishii K, Shibata H. An adult fatal case with a STAT1 Gain-of-function mutation associated with multiple autoimmune diseases. J Rheumatol. (2019) 46:325–7. doi: 10.3899/jrheum.180210
40. Pedreira ALS, Santiago MB. Association between Takayasu arteritis and latent or active mycobacterium tuberculosis infection: a systematic review. Clin Rheumatol. (2020) 39:1019–26. doi: 10.1007/s10067-019-04818-5
41. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American college of rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. (1990) 33:1129–34. doi: 10.1002/art.1780330811
42. Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K, Davin JC, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. (2006) 65:936–41. doi: 10.1136/ard.2005.046300
43. Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, et al. EULAR/PRINTO/PRES criteria for henoch-schönlein purpura, childhood polyarteritis nodosa, childhood wegener granulomatosis and childhood Takayasu arteritis: ankara 2008. part II: final classification criteria. Ann Rheum Dis. (2010) 69:798–806. doi: 10.1136/ard.2009.116657
44. Aeschlimann FA, Eng SWM, Sheikh S, Laxer RM, Hebert D, Noone D, et al. Childhood Takayasu arteritis: disease course and response to therapy. Arthritis Res Ther. (2017) 19:255. doi: 10.1186/s13075-017-1452-4
45. Eleftheriou D, Varnier G, Dolezalova P, McMahon AM, Al-Obaidi M, Brogan PA. Takayasu arteritis in childhood: retrospective experience from a tertiary referral centre in the United Kingdom. Arthritis Res Ther. (2015) 17:36. doi: 10.1186/s13075-015-0545-1
46. Fan L, Zhang H, Cai J, Yang L, Liu B, Wei D, et al. Clinical course and prognostic factors of childhood Takayasu’s arteritis: over 15-year comprehensive analysis of 101 patients. Arthritis Res Ther. (2019) 21:31.
47. Clemente G, Silva CA, Sacchetti SB, Ferriani VPL, Oliveira SK, Sztajnbok F, et al. Takayasu arteritis in childhood: misdiagnoses at disease onset and associated diseases. Rheumatol Int. (2018) 38:1089–94. doi: 10.1007/s00296-018-4030-4
48. Betancourt BY, Ahlman MA, Grayson PC. Clinical images: sarcoidosis concomitant with Takayasu arteritis, identified by advanced molecular imaging. Arthritis Rheumatol. (2019) 71:990. doi: 10.1002/art.40847
49. Guzel Esen S, Armagan B, Atas N, Ucar M, Varan O, Erden A, et al. Increased incidence of spondyloarthropathies in patients with Takayasu arteritis: a systematic clinical survey. Joint Bone Spine. (2019) 86:497–501. doi: 10.1016/j.jbspin.2019.01.020
50. Sy A, Khalidi N, Dehghan N, Barra L, Carette S, Cuthbertson D, et al. Vasculitis in patients with inflammatory bowel diseases: a study of 32 patients and systematic review of the literature. Semin Arthritis Rheum. (2016) 45:475–82. doi: 10.1016/j.semarthrit.2015.07.006
51. Vettiyil G, Punnen A, Kumar S. An Unusual Association of Chronic Recurrent Multifocal osteomyelitis, pyoderma gangrenosum, and Takayasu arteritis. J Rheumatol. (2017) 44:127–8. doi: 10.3899/jrheum.160491
52. Danda D, Goel R, Joseph G, Kumar ST, Nair A, Ravindran R, et al. Clinical course of 602 patients with Takayasu’s arteritis: comparison between Childhood-onset versus adult onset disease. Rheumatology (Oxford). (2021) 60:2246–55. doi: 10.1093/rheumatology/keaa569
53. Moriwaki R, Noda M, Yajima M, Sharma BK, Numano F. Clinical manifestations of Takayasu arteritis in India and Japan–new classification of angiographic findings. Angiology. (1997) 48:369–79. doi: 10.1177/000331979704800501
54. Jales-Neto LH, Levy-Neto M, Bonfa E, de Carvalho JF, Pereira RM. Juvenile-onset Takayasu arteritis: peculiar vascular involvement and more refractory disease. Scand J Rheumatol. (2010) 39:506–10. doi: 10.3109/03009741003742730
55. Goel R, Kumar TS, Danda D, Joseph G, Jeyaseelan V, Surin AK, et al. Childhood-onset Takayasu arteritis – experience from a tertiary care center in South India. J Rheumatol. (2014) 41:1183–9. doi: 10.3899/jrheum.131117
56. Clemente G, Hilario MO, Len C, Silva CA, Sallum AM, Campos LM, et al. Brazilian multicenter study of 71 patients with juvenile-onset Takayasu’s arteritis: clinical and angiographic features. Rev Bras Reumatol Engl Ed. (2016) 56:145–51. doi: 10.1016/j.rbre.2016.01.004
57. Misra DP, Aggarwal A, Lawrence A, Agarwal V, Misra R. Pediatric-onset Takayasu’s arteritis: clinical features and short-term outcome. Rheumatol Int. (2015) 35:1701–6. doi: 10.1007/s00296-015-3272-7
58. Feng Y, Tang X, Liu M, Zhou J, Zhao X, Li Q. Clinical study of children with Takayasu arteritis: a retrospective study from a single center in China. Pediatr Rheumatol Online J. (2017) 15:29. doi: 10.1186/s12969-017-0164-2
59. Zhu WH, Shen LG, Neubauer H. Clinical characteristics, interdisciplinary treatment and follow-up of 14 children with Takayasu arteritis. World J Pediatr. (2010) 6:342–7. doi: 10.1007/s12519-010-0234-8
60. Lei C, Huang Y, Yuan S, Chen W, Liu H, Yang M, et al. Takayasu arteritis with coronary artery involvement: differences between pediatric and adult patients. Can J Cardiol. (2020) 36:535–42. doi: 10.1016/j.cjca.2019.08.039
61. Bolek EC, Kaya Akca U, Sari A, Sag E, Demir S, Kilic L, et al. Is Takayasu’s arteritis more severe in children? Clin Exp Rheumatol. (2021) 39(Suppl. 129)::32–8.
62. Karabacak M, Kaymaz-Tahra S, Şahin S, Yıldız M, Adroviç A, Barut K, et al. Childhood-onset versus adult-onset Takayasu arteritis: a study of 141 patients from Turkey. Semin Arthritis Rheum. (2021) 51:192–7. doi: 10.1016/j.semarthrit.2020.10.013
63. Morales E, Pineda C, Martinez-Lavin M. Takayasu’s arteritis in children. J Rheumatol. (1991) 18:1081–4.
64. Barrera-Vargas A, Granados J, Garcia-Hidalgo L, Hinojosa-Azaola A. An unusual presentation of Takayasu’s arteritis in two Mexican siblings. Mod Rheumatol. (2015) 25:802–5. doi: 10.3109/14397595.2013.844384
65. Wu SY, Chen CH, Cheng CC, Fan HC. Takayasu’s arteritis presenting as monocular visual loss. Pediatr Neonatol. (2015) 56:435–8. doi: 10.1016/j.pedneo.2015.04.007
66. Aeschlimann FA, Grosse-Wortmann L, Benseler SM, Laxer RM, Hebert D, Yeung RS. Arterial dissection in childhood Takayasu Arteritis: not as rare as thought. Pediatr Rheumatol Online J. (2016) 14:56. doi: 10.1186/s12969-016-0115-3
67. An X, Han Y, Zhang B, Qiao L, Zhao Y, Guo X, et al. Takayasu arteritis presented with acute heart failure: case report and review of literature. ESC Heart Fail. (2017) 4:649–54. doi: 10.1002/ehf2.12174
68. Fan L, Zhang H, Cai J, Ma W, Song L, Lou Y. Middle aortic syndrome because of pediatric Takayasu arteritis admitted as acute heart failure: clinical course and therapeutic strategies. J Hypertens. (2018) 36:2118–9. doi: 10.1097/HJH.0000000000001847
69. Yang MC, Yang CC, Chen CA, Wang JK. Takayasu arteritis presenting with acute heart failure. J Am Coll Cardiol. (2013) 61:1302. doi: 10.1016/j.jacc.2012.09.070
70. Yang L, Zhang H, Jiang X, Song L, Qin F, Zou Y, et al. Clinical features and outcomes of Takayasu arteritis with neurological symptoms in China: a retrospective study. J Rheumatol. (2015) 42:1846–52. doi: 10.3899/jrheum.150097
71. Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with Takayasu arteritis. a preliminary report from The International network for the study of the systemic vasculitides (INSSYS). Int J Cardiol. (1998) 66(Suppl. 1):S191–4. doi: 10.1016/s0167-5273(98)00181-8
72. Dikkes A, Aschwanden M, Imfeld S, Glatz K, Messerli J, Staub D, et al. Takayasu arteritis: active or not, that’s the question. Rheumatology (Oxford). (2017) 56:1818–9. doi: 10.1093/rheumatology/kex213
73. Berger CT, Rebholz-Chaves B, Recher M, Manigold T, Daikeler T. Serial IL-6 measurements in patients with tocilizumab-treated large-vessel vasculitis detect infections and may predict early relapses. Ann Rheum Dis. (2019) 78:1012–4. doi: 10.1136/annrheumdis-2018-214704
74. Sakumura N, Irabu H, Inoue N, Mizuta M, Shimizu M. Clinical usefulness of longitudinal IL-6 monitoring in a patient with Takayasu aortitis receiving tocilizumab. Rheumatology (Oxford). (2020) 59:252–4. doi: 10.1093/rheumatology/kez245
75. Tombetti E, Hysa E, Mason JC, Cimmino MA, Camellino D. Blood biomarkers for monitoring and prognosis of large vessel vasculitides. Curr Rheumatol Rep. (2021) 23:17. doi: 10.1007/s11926-021-00980-5
76. Tombetti E, Di Chio MC, Sartorelli S, Papa M, Salerno A, Bottazzi B, et al. Systemic pentraxin-3 levels reflect vascular enhancement and progression in Takayasu arteritis. Arthritis Res Ther. (2014) 16:479. doi: 10.1186/s13075-014-0479-z
77. Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. (2018) 77:636–43. doi: 10.1136/annrheumdis-2017-212649
78. de Graeff N, Groot N, Brogan P, Ozen S, Avcin T, Bader-Meunier B, et al. European consensus-based recommendations for the diagnosis and treatment of rare paediatric vasculitides - the SHARE initiative. Rheumatology (Oxford). (2019) 58:656–71. doi: 10.1093/rheumatology/key322
79. Shah R, D’Arco F, Soares B, Cooper J, Brierley J. Use of gadolinium contrast agents in paediatric population: Donald Rumsfeld meets hippocrates! Br J Radiol (2019) 92:20180746. doi: 10.1259/bjr.20180746
80. Löffler C, Hoffend J, Benck U, Krämer BK, Bergner R. The value of ultrasound in diagnosing extracranial large-vessel vasculitis compared to FDG-PET/CT: a retrospective study. Clin Rheumatol. (2017) 36:2079–86. doi: 10.1007/s10067-017-3669-7
81. Grayson PC, Alehashemi S, Bagheri AA, Civelek AC, Cupps TR, Kaplan MJ, et al. F-Fluorodeoxyglucose-positron emission tomography as an imaging biomarker in a prospective. longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol. (2018) 70:439–49. doi: 10.1002/art.40379
82. Aeschlimann FA, Raimondi F, Leiner T, Aquaro GD, Saadoun D, Grotenhuis HB. Overview of imaging in adult- and childhood-onset Takayasu arteritis. J Rheumatol. (2021) jrheum.210368. doi: 10.3899/jrheum.210368 [Epub ahead of print].
83. Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. (1996) 54(Suppl):S155–63.
84. Goel R, Gribbons KB, Carette S, Cuthbertson D, Hoffman GS, Joseph G, et al. Derivation of an angiographically based classification system in Takayasu’s arteritis: an observational study from India and North America. Rheumatology (Oxford). (2020) 59:1118–27. doi: 10.1093/rheumatology/kez421
85. Lopes RJ, Almeida J, Dias PJ, Pinho P, Maciel MJ. Infectious thoracic aortitis: a literature review. Clin Cardiol. (2009) 32:488–90. doi: 10.1002/clc.20578
86. Schwartz SB, Fisher D, Reinus C, Shahroor S. Infectious aortitis: a rare cause of chest pain in a child. Pediatr Emerg Care. (2011) 27:654–6. doi: 10.1097/PEC.0b013e318222561f
87. Tullus K. Renovascular hypertension–is it fibromuscular dysplasia or Takayasu arteritis. Pediatr Nephrol. (2013) 28:191–6. doi: 10.1007/s00467-012-2151-7
88. Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. (2009) 68:318–23.
89. Saito S, Okuyama A, Okada Y, Shibata A, Sakai R, Kurasawa T, et al. Tocilizumab monotherapy for large vessel vasculitis: results of 104-week treatment of a prospective, single-centre, open study. Rheumatology (Oxford). (2020) 59:1617–21. doi: 10.1093/rheumatology/kez511
90. Kotter I, Henes JC, Wagner AD, Loock J, Gross WL. Does glucocorticosteroid-resistant large-vessel vasculitis (giant cell arteritis and Takayasu arteritis) exist and how can remission be achieved? A critical review of the literature. Clin Exp Rheumatol. (2012) 30:S114–29.
91. Ozen S, Duzova A, Bakkaloglu A, Bilginer Y, Cil BE, Demircin M, et al. Takayasu arteritis in children: preliminary experience with cyclophosphamide induction and corticosteroids followed by methotrexate. J Pediatr. (2007) 150:72–6. doi: 10.1016/j.jpeds.2006.10.059
92. Mekinian A, Biard L, Dagna L, Novikov P, Salvarani C, Espita O, et al. Efficacy and safety of TNF-α antagonists and tocilizumab in Takayasu arteritis: multicenter retrospective study of 209 patients. Rheumatology. (2021) keab635. doi: 10.1093/rheumatology/keab635 [Epub ahead of print].
93. Comarmond C, Plaisier E, Dahan K, Mirault T, Emmerich J, Amoura Z, et al. Anti TNF-α in refractory Takayasu’s arteritis: cases series and review of the literature. Autoimmun Rev. (2012) 11:678–84. doi: 10.1016/j.autrev.2011.11.025
94. Molloy ES, Langford CA, Clark TM, Gota CE, Hoffman GS. Anti-tumour necrosis factor therapy in patients with refractory Takayasu arteritis: long-term follow-up. Ann Rheum Dis. (2008) 67:1567–9. doi: 10.1136/ard.2008.093260
95. Gudbrandsson B, Molberg Ø, Palm Ø. TNF inhibitors appear to inhibit disease progression and improve outcome in Takayasu arteritis; an observational, population-based time trend study. Arthritis Res Ther. (2017) 19:99. doi: 10.1186/s13075-017-1316-y
96. Mertz P, Kleinmann JF, Lambert M, Puéchal X, Bonnin A, Boulon C, et al. Infliximab is an effective glucocorticoid-sparing treatment for Takayasu arteritis: results of a multicenter open-label prospective study. Autoimmun Rev. (2020) 19:102634. doi: 10.1016/j.autrev.2020.102634
97. Mekinian A, Comarmond C, Resche-Rigon M, Mirault T, Kahn JE, Lambert M, et al. Efficacy of biological-targeted treatments in Takayasu arteritis: multicenter, retrospective study of 49 patients. Circulation. (2015) 132:1693–700. doi: 10.1161/CIRCULATIONAHA.114.014321
98. Alibaz-Oner F, Kaymaz-Tahra S, Bayındır Ö, Yazici A, Ince B, Kalkan K, et al. Biologic treatments in Takayasu’s Arteritis: a comparative study of tumor necrosis factor inhibitors and tocilizumab. Semin Arthritis Rheum. (2021) 51:1224–9. doi: 10.1016/j.semarthrit.2021.09.010
99. Filocamo G, Buoncompagni A, Viola S, Loy A, Malattia C, Ravelli A, et al. Treatment of Takayasu’s arteritis with tumor necrosis factor antagonists. J Pediatr. (2008) 153:432–4. doi: 10.1016/j.jpeds.2008.04.049
100. Eleftheriou D, Melo M, Marks SD, Tullus K, Sills J, Cleary G, et al. Biologic therapy in primary systemic vasculitis of the young. Rheumatology (Oxford). (2009) 48:978–86. doi: 10.1093/rheumatology/kep148
101. Stern S, Clemente G, Reiff A, Ramos MP, Marzan KA, Terreri MT. Treatment of pediatric Takayasu arteritis with infliximab and cyclophosphamide: experience from an American-Brazilian cohort study. J Clin Rheumatol. (2014) 20:183–8. doi: 10.1097/RHU.0000000000000106
102. Buonuomo PS, Bracaglia C, Campana A, Insalaco A, Pardeo M, Cortis E, et al. Infliximab therapy in pediatric Takayasu’s arteritis: report of two cases. Rheumatol Int. (2011) 31:93–5. doi: 10.1007/s00296-009-1147-5
103. Abisror N, Mekinian A, Lavigne C, Vandenhende MA, Soussan M, Fain O, et al. Tocilizumab in refractory Takayasu arteritis: a case series and updated literature review. Autoimmun Rev. (2013) 12:1143–9. doi: 10.1016/j.autrev.2013.06.019
104. Batu ED, Sonmez HE, Hazirolan T, Ozaltin F, Bilginer Y, Ozen S. Tocilizumab treatment in childhood Takayasu arteritis: case series of four patients and systematic review of the literature. Semin Arthritis Rheum. (2017) 46:529–35. doi: 10.1016/j.semarthrit.2016.07.012
105. Bravo Mancheno B, Perin F, Guez Vazquez Del Rey Mdel M, Garcia Sanchez A, Alcazar Romero PP. Successful tocilizumab treatment in a child with refractory Takayasu arteritis. Pediatrics. (2012) 130:e1720–4. doi: 10.1542/peds.2012-1384
106. Goel R, Danda D, Kumar S, Joseph G. Rapid control of disease activity by tocilizumab in 10 ‘difficult-to-treat’ cases of Takayasu arteritis. Int J Rheum Dis. (2013) 16:754–61. doi: 10.1111/1756-185X.12220
107. Nishimoto N, Nakahara H, Yoshio-Hoshino N, Mima T. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. (2008) 58:1197–200. doi: 10.1002/art.23373
108. Yamazaki K, Kikuchi M, Nozawa T, Kanetaka T, Hara R, Imagawa T, et al. Tocilizumab for patients with takayasu arteritis in childhood refractory to conventional therapy. Pediatric Rheumatology. (2013) 11:O24.
109. Mekinian A, Resche-Rigon M, Comarmond C, Soriano A, Constans J, Alric L, et al. Efficacy of tocilizumab in Takayasu arteritis: multicenter retrospective study of 46 patients. J Autoimmun. (2018) 91:55–60. doi: 10.1016/j.jaut.2018.04.002
110. Nakaoka Y, Isobe M, Takei S, Tanaka Y, Ishii T, Yokota S, et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis. (2018) 77:348–54. doi: 10.1136/annrheumdis-2017-211878
111. Nakaoka Y, Isobe M, Tanaka Y, Ishii T, Ooka S, Niiro H, et al. Long-term efficacy and safety of tocilizumab in refractory Takayasu arteritis: final results of the randomized controlled phase 3 TAKT study. Rheumatology (Oxford). (2020) 59:2427–34. doi: 10.1093/rheumatology/kez630
112. Mekinian A, Saadoun D, Vicaut E, Thietart S, Lioger B, Jego P, et al. Tocilizumab in treatment-naïve patients with Takayasu arteritis: TOCITAKA French prospective multicenter open-labeled trial. Arthritis Res Ther. (2020) 22:218. doi: 10.1186/s13075-020-02311-y
113. Asano T, Sato S, Temmoku J, Fujita Y, Furuya MY, Matsuoka N, et al. Effectiveness of Tocilizumab in juvenile patients with refractory Takayasu arteritis: two case reports. Medicine (Baltimore). (2020) 99:e18890. doi: 10.1097/MD.0000000000018890
114. Palermo A, Marvisi C, Casali M, Pipitone N, Muratore F, Salvarani C. Tofacitinib for the treatment of refractory Takayasu’s arteritis: description of 2 cases. Clin Exp Rheumatol. (2020) 38(Suppl. 124):234–5.
115. Li J, Li M, Tian X, Zeng X. Tofacitinib in patients with refractory Takayasu’s arteritis. Rheumatology (Oxford). (2020) 59:e95–8. doi: 10.1093/rheumatology/keaa281
116. Sato S, Matsumoto H, Temmoku J, Fujita Y, Matsuoka N, Furuya M, et al. A case of Takayasu arteritis complicated by refractory ulcerative colitis successfully treated with tofacitinib. Rheumatology (Oxford). (2020) 59:1773–5. doi: 10.1093/rheumatology/kez580
117. Yamamura Y, Matsumoto Y, Asano Y, Katayama Y, Hayashi K, Ohashi K, et al. Refractory Takayasu arteritis responding to the oral Janus kinase inhibitor, tofacitinib. Rheumatol Adv Pract. (2020) 4:rkz050. doi: 10.1093/rap/rkz050
118. Kuwabara S, Tanimura S, Matsumoto S, Nakamura H, Horita T. Successful remission with tofacitinib in a patient with refractory Takayasu arteritis complicated by ulcerative colitis. Ann Rheum Dis. (2020) 79:1125–6. doi: 10.1136/annrheumdis-2019-216606
119. Rios Rodriguez V, Rademacher J, Protopopov M, Torgutalp M, Haibel H, Vahldiek JL, et al. Comment on: ‘successful remission with tofacitinib in a patient with refractory Takayasu arteritis complicated by ulcerative colitis’ by Kuwabara. Ann Rheum Dis. (2020) annrheumdis-2020-217894. doi: 10.1136/annrheumdis-2020-217894 [Epub ahead of print].
120. Wang CR, Tsai YS, Liu YW, Li YH. Extended-release tofacitinib improves refractory Takayasu’s arteritis. Scand J Rheumatol. (2022) 51:72–5. doi: 10.1080/03009742.2021.1911054
121. Kong X, Sun Y, Dai X, Wang L, Ji Z, Chen H, et al. Treatment efficacy and safety of tofacitinib versus methotrexate in Takayasu arteritis: a prospective observational study. Ann Rheum Dis. (2022) 81:117–23. doi: 10.1136/annrheumdis-2021-220832
122. Terao C, Yoshifuji H, Kimura A, Matsumura T, Ohmura K, Takahashi M, et al. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. Am J Hum Genet. (2013) 93:289–97. doi: 10.1016/j.ajhg.2013.05.024
123. Terao C, Yoshifuji H, Nakajima T, Yukawa N, Matsuda F, Mimori T. Ustekinumab as a therapeutic option for Takayasu arteritis: from genetic findings to clinical application. Scand J Rheumatol. (2016) 45:80–2. doi: 10.3109/03009742.2015.1060521
124. Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol. (2017) 69:837–45. doi: 10.1002/art.40044
125. Liebling EJ, Peterson R, Victoria T, Burnham JM. Aortic ulceration in a tocilizumab-treated patient with Takayasu arteritis. Ann Rheum Dis. (2019) 78:e116. doi: 10.1136/annrheumdis-2018-214191
126. Sanchez-Alvarez C, Koster M, Duarte-García A, Warrington KJ. Disease progression of Takayasu arteritis in two patients treated with tocilizumab. Ann Rheum Dis. (2020) 79:e21. doi: 10.1136/annrheumdis-2018-214642
127. Muratore F, Salvarani C. Aortic dilatation in a patient with Takayasu arteritis treated with tocilizumab. Ann Rheum Dis. (2021) 80:e121. doi: 10.1136/annrheumdis-2019-215459
128. Bonilla-Abadía F, Cañas CA, Echeverri AF. Outcomes of patients with takayasu arteritis treated with infliximab. J Rheumatol. (2013) 40:1930–1. doi: 10.3899/jrheum.130154
129. Xenitidis T, Horger M, Zeh G, Kanz L, Henes JC. Sustained inflammation of the aortic wall despite tocilizumab treatment in two cases of Takayasu arteritis. Rheumatology (Oxford). (2013) 52:1729–31. doi: 10.1093/rheumatology/ket107
130. Saadoun D, Lambert M, Mirault T, Resche-Rigon M, Koskas F, Cluzel P, et al. Retrospective analysis of surgery versus endovascular intervention in Takayasu arteritis: a multicenter experience. Circulation. (2012) 125:813–9. doi: 10.1161/CIRCULATIONAHA.111.058032
131. Zhu G, He F, Gu Y, Yu H, Chen B, Hu Z, et al. Angioplasty for pediatric renovascular hypertension: a 13-year experience. Diagn Interv Radiol. (2014) 20:285–92. doi: 10.5152/dir.2014.13208
132. Ladapo TA, Gajjar P, McCulloch M, Scott C, Numanoglu A, Nourse P. Impact of revascularization on hypertension in children with Takayasu’s arteritis-induced renal artery stenosis: a 21-year review. Pediatr Nephrol. (2015) 30:1289–95. doi: 10.1007/s00467-015-3049-y
133. Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. (1994) 120: 919–29.
134. Aydin SZ, Merkel PA, Direskeneli H. Outcome measures for Takayasu’s arteritis. Curr Opin Rheumatol. (2015) 27:32–7. doi: 10.1097/BOR.0000000000000129
135. Aeschlimann FA, Twilt M, Yeung RSM. Childhood-onset Takayasu Arteritis. Eur J Rheumatol. (2020) 7:S58–66.
Keywords: Takayasu Arteritis, large vessel vasculitis, childhood vasculitis, pediatrics, review
Citation: Aeschlimann FA, Yeung RSM and Laxer RM (2022) An Update on Childhood-Onset Takayasu Arteritis. Front. Pediatr. 10:872313. doi: 10.3389/fped.2022.872313
Received: 09 February 2022; Accepted: 03 March 2022;
Published: 13 April 2022.
Edited by:
Natasa Toplak, Univerzitetnega Kliniènega Centra Ljubljana, SloveniaReviewed by:
Sezgin Sahin, Istanbul University-Cerrahpaşa, TurkeyCopyright © 2022 Aeschlimann, Yeung and Laxer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florence A. Aeschlimann, ZmxvcmVuY2UuYWVzY2hsaW1hbm5AYXBocC5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.