
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 17 May 2022
Sec. Pediatric Surgery
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.869518
Parts of this article's content have been modified or rectified in:
Erratum: Neuroblastic tumors of the adrenal gland in elderly patients: A case report and review of the literature
 Philip Deslarzes1
Philip Deslarzes1 Reza Djafarrian1
Reza Djafarrian1 Maurice Matter1
Maurice Matter1 Stefano La Rosa2
Stefano La Rosa2 Carole Gengler2
Carole Gengler2 Maja Beck-Popovic3
Maja Beck-Popovic3 Tobias Zingg1*
Tobias Zingg1*Background: Neuroblastic neoplasms (NN) include ganglioneuromas (GN), ganglioneuroblastomas (GNB), and neuroblastomas (NB). They generally arise in childhood from primitive sympathetic ganglion cells. Their incidence in adults, especially among elderly, is extremely low.
Case Presentation: This is the case of a 74-year-old woman with history of abdominal pain, weakness and night sweating since several months. Blood pressure was normal. CT-scan showed a 10 cm left adrenal mass, without other pathologic findings. An open left-sided adrenalectomy was performed. Recovery was uneventful with hospital length of stay of 8 days. Based on morphological, immunohistochemical, and molecular features the diagnosis was a nodular GNB. A positron emission tomography (PET) performed 6 weeks after the resection did not show any residual tumor or distant metastases. The patient was followed-up with annual clinical and radiological exams.
Conclusion: This case presentation, associated with a review of the literature, illustrates the importance to include NN in the preoperative differential diagnosis of adrenal tumors in adults and highlights the need for multidisciplinary patient work-up and management.
Neuroblastic neoplasms (NN) are a heterogeneous group of tumors deriving from the sympatho-adrenal lineage of the neural crest forming the peripheral sympathetic nervous system. They include a wide spectrum of neoplasms with various degrees of cellular maturation and biological aggressiveness (1–4). Ganglioneuromas (GN) show an indolent behavior with benign clinical course, while, on the other hand, neuroblastomas (NB) are malignant tumors with a much worse prognosis. Between GN and NB, ganglioneuroblastomas (GNB) exhibit some intermediate malignant potential depending on the tumor subtype (intermixed or nodular) and on other specific criteria (3).
NB are the most frequent extra cranial solid tumors in early childhood, (8–10% of childhood cancers), while GNB are very rare, including in the pediatric population (0.5 cases/100,000 children) (4–7). In adults and elderly (>65 years) population, the incidence of NB and GNB is even rarer (0.01 cases /100,000) (8, 9). More than 97% of cases occur in patients younger than 10 years-old and the median age at diagnosis is 2 years (10). The most common sites of origin of NB and GNB are the adrenal glands (40%) and extra-adrenal retroperitoneum (25%), although they can arise anywhere throughout the sympathetic nervous system (neck, chest, pelvis) (11, 12). One of the most important prognostic factors is age at diagnosis. Of note, prognosis of NB in elderly is generally poor (13). Elderly people with NB have a significantly worse outcome than children younger than 5 years (14–16).
Because of the rarity of NN in adults, the natural history in this population is unknown and there are no established treatment guidelines. To the best of our knowledge, no case of adrenal GNB in elderly patients has been reported in literature until now (Table 1). In the present paper, we discuss the clinico-pathological features, work-up and management of an adrenal GNB diagnosed in a 74-year-old patient.
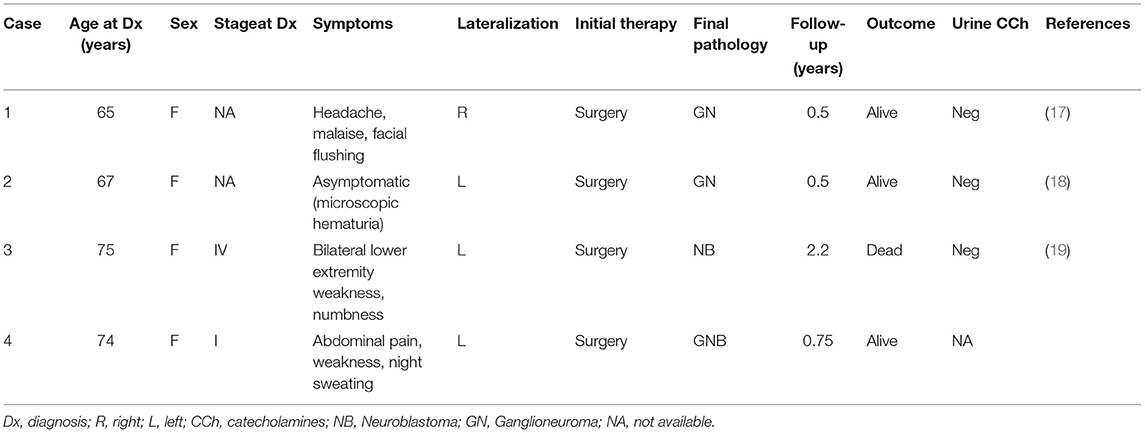
Table 1. Adrenal neuroblastic neoplasms in elderly patients (>65 years) in the English literature from 1976 to 2021.
A 74-year-old woman consulted her GP with history of abdominal pain, weakness and night sweating for months. She was on prednisone and pregabalin for fibromyalgia. The patient had no history of hypertension. The physical examination showed a palpable mass in the left upper quadrant, blood pressure was normal. A thoraco-abdominal contrast-enhanced CT-scan, showed a 10 × 10 × 8 cm mass at the upper pole of the left kidney (Figures 1, 2) and no other suspicious lesions. A CT-guided core needle biopsy of the mass was performed with histology findings compatible with a possible adrenocortical tumor (immunohistochemistry was positive for vimentine, racemase and synaptophysin, but was negative for S-100 and chromogranin).
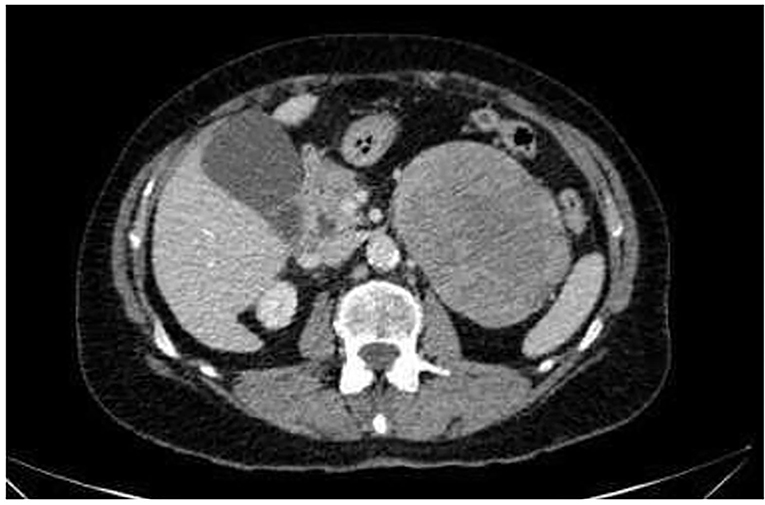
Figure 1. CT-scan of the abdomen. Axial CT scan of the abdomen reveals a large abdominal mass measuring 10 × 8 × 10 cm without lymphadenopathy.
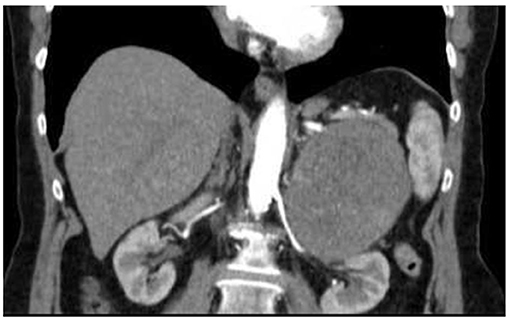
Figure 2. CT-scan of the abdomen. Coronal-view CT scan of the abdomen reveals a large abdominal mass measuring 10 × 8 × 10 cm without lymphadenopathy.
Serum catecholamines showed elevated nor-metanephrine (9.72 nmol/l, N: 0.04–1.39) and methoxytyramine (0.62 nmol/l, N: <0.06), but normal metanephrine levels (high negative predictive value for excluding pheochromocytoma) (17). Urine catecholamines were not evaluated. The other laboratory work-up showed normal cortisol, androstenedione, DHEA and testosterone levels.
An open left adrenalectomy was performed through a left subcostal laparotomy. The definitive diagnosis was nodular GNB (Figure 3).
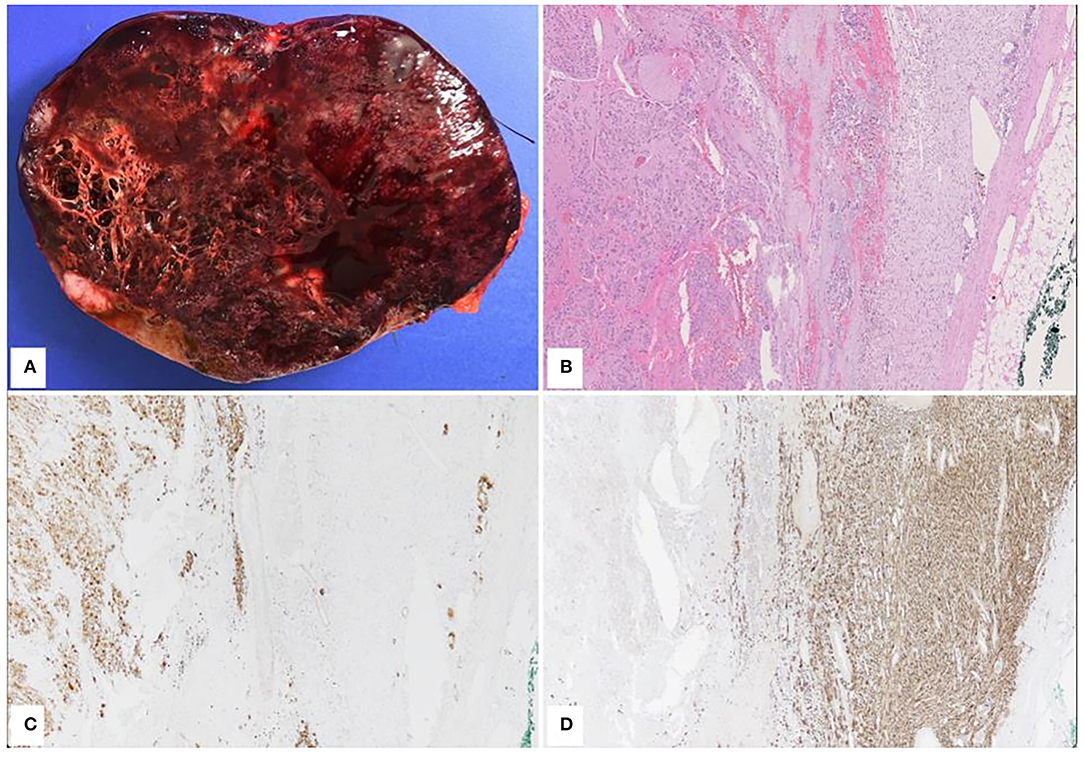
Figure 3. Pathological features. Macroscopically, The cut surface was heterogeneous with solid and microcystic features, hemorrhagic and necrotic areas, and some white nodules. The tumor was well-delimitated showing solid and microcystic features with hemorrhagic and necrotic areas. (A) Histologically, the tumor showed two main components. The central portion of the tumor was composed of ganglion cells at different stage of maturation, embedded in a fibrillary network (B) left portion of the image. The ganglion cells were positive for synaptophysin, chromogranin and MAP2 while they were negative for SF1, inhibin, MYC, CD99, cytokeratin, GFAP, and ATRX. SDHB was expressed. (C) The second component of the tumor was located at the periphery and included spindle Schwann cells positive for S100 (D).
Recovery was uneventful with hospital stay of 8 days. Following our multidisciplinary endocrinology board, the staging was completed with PET-CT, which showed no distant lesions. It was programmed a clinical follow up at 1 month after the surgery with a favorable clinical course and a resolution of all symptoms. The patient was followed with annual clinical exams and CT-scans.
The clinical presentation of NN is very heterogeneous and depends on the site of origin and the presence of metastatic disease (18). Approximately two thirds of NN are localized in the abdomen and two thirds of the abdominal NN originate from the adrenal glands (11, 12). However, it is worth noting that they can arise anywhere throughout the sympathetic nervous system, such as the thorax or the neck (11).
NB and GNB can secrete catecholamines, and their derivates such as vanillylmandelic acid and homovanillic acid. Determination of catecholamine levels and their serum metabolites is important for the initial screening, diagnosis, differential diagnosis (i.e., pheochromocytoma vs. adrenocortical carcinoma), and for the evaluation of treatment efficacy (19). Neuroblastoma cells may release ferritin and patients with high ferritin level or increased levels of lactate dehydrogenase tend to have a worse prognosis (20).
According to a recent retrospective study, 48% of patients with abdominal NB and 29% of patients with abdominal GNB had metastatic disease (18). Thus, functional imaging like I123-MIBG-scan or FDG PET-CT, are essential in the assessment of NB and GNB to define the extent of disease at diagnosis and to follow the treatment response (21, 22). Notably, it was shown that FDG can be useful when interpretation of MIBG is unclear (23). Some studies have shown that Ga68-DOTATATE PET-CT has a better sensitivity, quicker clearance, limited toxicity and less radiation exposure than 123I-MIBG and could be used for staging of NN (24–26). Due to the rarity of intracranial metastases (0.7% in stage IV disease in children), brain CT or MRI should be only performed if intracranial involvement is clinically suspected (27, 28). The final diagnosis has to be confirmed by histology.
GNB, NB and GN are three separate diseases with different outcomes: malignant for NB, intermediate for GNB and benign for GN. NB and GNB have different clinical behaviors with some tumors showing spontaneous regression and others progressing to metastatic dissemination (27).
There are currently two classification systems. The International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Risk Group Staging System (INRGSS), are summarized in Tables 2, 3 (30). The risk stratification is based on age at diagnosis, INRG tumor stage, grade of tumor differentiation, histologic category, DNA ploidy, and number of copies at the MYCN oncogene locus and at chromosome 11q (29).

Table 2. INRG Tumor staging system (29).

Table 3. INSS Tumor staging system (29).
According to the INRGSS and INSS, our patient qualifies as a stage L1 and a stage 1, respectively. Adults with L1 disease experience an OS of 94, 90, and 69% at 3, 5, and 10 years, respectively (19). The most important prognostic factor is age, but in adults and elderly population data are limited. According to few case reports, the prognosis in adults is worse than among children, because of unfavorable histological characteristics (8, 9, 12, 19). In a retrospective study, Conter et al. observed an OS of adult patients of 18.1 years, 9.8 years and 1.6 years for stage L1, L2 and M, respectively (19).
NN patients with a high quantity of neurotrophin receptors, found on the surface of normal nerve cells and some neuroblastoma cells, especially the nerve growth factor receptor TrkA, may have a better prognosis (20).
In older children, but not in infants and adults, MYCN amplification represents a negative prognostic factor (31). The amplification for MYCN was negative in our patient, strengthening our multidisciplinary board decision for a clinical/radiological follow-up. MYCN amplification occurs in about 40% of high-risk tumors (32).
Outcomes of NN in adults are generally poor, because half of the patients have a stage IV NB at the time of diagnosis (11, 13). 45% of NB are classified as high risk (33).
In children, treatment of NB and GNB is well-defined but remains demanding. Surgical resection is the mainstay for low-risk pediatric cases, whereas high-dose chemotherapy with stem cell rescue, radiation therapy and immunotherapy are indicated in high-risk patients (20). In children with INSS stage 4 disease, intensive cytotoxic chemotherapy offered a better progression-free survival than standard chemotherapy (31). In high-risk tumors (50% of pediatric NB and GNB), multiagent chemotherapy, surgical resection, radiotherapy, myeloablative chemotherapy and autologous stem cell rescue, as well as immunotherapy (i.e., anti-GD2 immunoglobulins, ALK-inhibition molecules) can be used (34).
Regarding treatment, the presented patient had surgery without chemotherapy or radiation therapy. In adults, due to rarity, there are no standardized guidelines or treatment protocols for NN (22). According to the three case reports mentioned above, the treatment of the GN and NB was surgical without adjuvant treatment. Treatment guidelines usually follow those existing for children or those from rare retrospective studies (4, 14, 20). For adult patients with INRG stage L1 NB, surgery combined with adjuvant radiotherapy offer a better OS than surgical resection alone (20). In the same study, chemotherapy did not show any additional benefit for stages L1 and L2. In high-risk patients, high-dose chemotherapy with hematopoietic stem cell rescue did not show the same benefit when compared to pediatric patients (20). According to pediatric guidelines, surgery alone should be performed for low-risk tumors, while multiagent chemotherapy is effective in patients with relapsing low-risk tumors or in those with intermediate-risk tumors (33). The treatment of choice for metastatic patients is chemotherapy (22).
Follow-up with urinary catecholamine levels and imaging is necessary for all pediatric patients treated for NB and GNB (35). As functional tumors are rare in adults, it was decided not to follow-up the urinary catecholamine level in our patient (12).
There are no clear guidelines concerning imaging-based follow-up, but the frequency of visits and duration of observation period depend on age, stage and localization of the tumor. In some children, ultrasound may be used for surveillance, while CT, MRI or even MIBG-scan can be necessary for adults or advanced-stage metastatic disease (34, 36). FDG PET-CT is not needed when a MIBG-scan has been performed, however some NN cells do not absorb MIBG (36). Due to paucity of NB, each case should be discussed in the setting of a multidisciplinary board.
GNB should be included in the preoperative differential diagnosis of adrenal tumors in adults. In the absence of specific guidelines, management of adrenal NN in elderly is surgical following pediatric protocols and retrospective observational studies.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
PD wrote the manuscript and the abstract. RD was the first corrector. MM and TZ were the second correctors. TZ was the coordinator. SL and CG added the pathology section and the pictures. MB-P added some informations concerning her medical specialties. All authors read and approved the final manuscript.
Open access funding was provided by the University of Lausanne.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ATRX, Alpha-Thalassemia Mental Retardation-X; DHEA, Dehydroepiandrosterone; CD99, Cluster of Differentiation–99; FDG, Fluorodeoxyglucose; GN, Ganglioneuromas; GNB, Ganglioneuroblastomas; GP, General Practician; GFAP, Glial Fibrillary Acidic Protein; MIBG, Metaiodobenzylguanidine; INSS, International Neuroblastoma Staging System; INRGSS, International Neuroblastoma Risk Group Staging System; NN, Neuroblastic Neoplasms; Neuroblastomas, NB; MAP-2, Microtubule Associated Protein 2; MYC, Myelocytomatosis oncogene, OS, Overall Survival, PET, Positron Emission Tomography; SF1, Splicing Factor 1; SDHB, Succinate Dehydrogenase.
1. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. The Lancet. (2007) 369. doi: 10.1016/S0140-6736(07)60983-0
2. Lonergan GJ, Schwab CM, Suarez ES, Carlson C. From the archives of the AFIP: neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. RadioGraphics. (2001) 22: 911–34. doi: 10.1148/radiographics.22.4.g02jl15911
3. Joshi VV. Peripheral neuroblastic tumors: pathologic classification based on recommendations of international neuroblastoma pathology committee (modification of Shimada classification). Pediatr. Dev Pathol. (2002) 3:184–99. doi: 10.1007/s100240050024
4. Maris JM. “Recent advances in neuroblastoma.” N Engl J Med. (2010) 362:2202–11. doi: 10.1056/NEJMra0804577
5. Morris JA, Shochat SJ, Smith E, Look AT, Brodeur GM, Cantor AB, et al. Biological variables in thoracic neuroblastoma: a pediatric oncology group study. J Pediatr Surg. (1995) 30:296–303. doi: 10.1016/0022-3468(95)90577-4
6. Qiu W, Ting L, Xiao Dong S, Yue Lv G. Onset of adrenal ganglioneuroblastoma in an adult after delivery. Ann Surg Treat Res. (2015) 89:220. doi: 10.4174/astr.2015.89.4.220
7. Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, et al. The International Neuroblastoma Pathology Classification (the Shimada System). Cancer. (1999) 86:364–72. doi: 10.1002/(SICI)1097-0142(19990715)86:2<364::AID-CNCR21>3.0.CO;2-7
8. Franks LM, Bollen A, Seeger RC, Stram DO, Matthay KK. Neuroblastoma in adults and adolescents: an indolent course with poor survival.” Cancer. (1997) 79:10. doi: 10.1002/(SICI)1097-0142(19970515)79:10<2028::AID-CNCR26>3.0.CO;2-V
9. Kushner BH, Kramer K, LaQuaglia MP, Modak S, Cheung NK. Neuroblastoma in adolescents and adults: the memorial sloan-kettering experience. Med Pediatr Oncol. (2003) 6:508–15. doi: 10.1002/mpo.10273
11. Jrebi NY, Iqbal CW, Joliat GR, Sebo TJ, Farley DR. Review of our experience with neuroblastoma and ganglioneuroblastoma in adults. World J Surg. (2014) 38: 2871–74. doi: 10.1007/s00268-014-2682-0
12. Podda MG, Luksch R, Polastri D, Gandola L, Piva L, Collini P, et al. Neuroblastoma in patients over 12 years old: a 20-year experience at the Istitu to nazionale tumori of milan. Tumori J. (2010) 5:684–89. doi: 10.1177/030089161009600507
13. Esiashvili N, Goodman M, Ward K, Marcus RB Jr, Johnstone P. Neuroblastoma in adults: incidence and survival analysis based on SEER Data. Pediat Blood Cancer. (2007) 49:41–46. doi: 10.1002/pbc.20859
14. Umehara S, Nakagawa A, Matthay KK, Lukens JN, Seeger RC, Stram DO, et al. Histopathology defines prognostic subsets of ganglioneuroblastoma, Nodular. Cancer. (2000) 89:1150–61. doi: 10.1002/1097-0142(20000901)89:5<1150::AID-CNCR25>3.0.CO;2-7
15. Rogowitz E, Babiker HM, Kanaan M, Millius RA, Ringenberg QS, Bishop M. Neuroblastoma of the elderly, an oncologist's nightmare: case presentation, literature review and SEER database analysis. Exp Hematol Oncol. (2014) 3:20. doi: 10.1186/2162-3619-3-20
16. Wu XL, Dai YJ, Sun GY Wank LK, Han L, Qu M, et al. Adult neuroblastoma in the retroperitoneum: a case report. Medicine. (2018) 97: 13750. doi: 10.1097/MD.0000000000013750
17. Joana S, do Carmo Cachulo M, Leiteum Qu MRA. Secondary hypertension to rare adrenal gland tumor. Arq Bras Cardiol. (2010) 6:144–47. doi: 10.1590/s0066-782x2010001600020
18. He WG, Yan Y, Tang W, Cai R, Ren G. Clinical and biological features of neuroblastic tumors: a comparison of neuroblastoma and ganglioneuroblastoma. Oncotarget. (2017) 8: 37730–39. doi: 10.18632/oncotarget.17146
19. Conter HJ, Gopalakrishnan V, Ravi V, Ater JL, Patel S, Araujo DM. Adult vs. pediatric neuroblastoma: the MD Anderson cancer center experience. Sarcoma. (2014) 2014:1–6. doi: 10.1155/2014/375151
20. Smith L, Minter S, O'Brien P, Kraveka JM, Medina AM, Lazarchick J. Neuroblastoma early detection, diagnosis, and staging. ACS.
21. Leavitt JR, Harold DL, Robinson RB. Adrenal ganglioneuroma: a familial case. Urology. (2000) 56:508. doi: 10.1016/S0090-4295(00)00695-6
22. Vassallo L, Fasciano M, Baralis I, Pellegrino L, Fortunato M, Fraternali Orcioni G, et al. A Rare case of adrenal ganglioneuroblastoma-intermixed in an adult and a review of literature. Radiol Case Rep. (2021) 16:2351–56. doi: 10.1016/j.radcr.2021.06.005
23. Sharp SE, Gelfand MJ, Shulkin BL. PET/CT in the evaluation of neuroblastoma PET Clinics (2008) 3:551. doi: 10.1016/j.cpet.2009.03.006
24. Alexander N, Vali R, Ahmadzadehfar H, Shammas A, Baruchel S. Review: the role of radiolabeled DOTA-conjugated peptides for imaging and treatment of childhood neuroblastoma. Curr Radiopharm. (2018) 11:14–21. doi: 10.2174/1874471011666171215093112
25. Ata URM, O'Doherty J, Djekidel M. 68anGa-DOTATATE PET/CT for neuroblastoma staging. Utility for clinical use. J Nucl Med Technol. (2021) 49:265–68. doi: 10.2967/jnmt.120.258939
26. Tugçe T, Ergün EL, Salanci B, Ozgen Kiralti P. The complementary role of 68Ga-DOTATATE PET/CT in neuroblastoma. Clin Nucl Med. (2020) 45: 326–29. doi: 10.1097/RLU.0000000000002961
27. Matthay KK, Brisse H, Couanet D, Couturier J, Bf childhood neurob, et al. Central nervous system metastases in neuroblastoma: radiologic, clinical, and biologic features in 23 patients. Cancer. (2003) 1:15503. doi: 10.1002/cncr.11448
28. D'Ambrosio N, Lyo JK, Young RJ, Haque SS, Karimi S. Imaging of metastatic CNS neuroblastoma. Am J Roentgenol. (2010) 194:1223–29. doi: 10.2214/AJR.09.3203
29. Zhang Haibin Ziwei F. Adrenal neuroblastoma in an elderly adult: a case report and review of the literature. Open j Clin Med Case Rep. (2019) 13:284. doi: 10.1186/s13256-019-2204-7
30. Brisse H, Beth McCarville JM, Granata C, Barbara Krug K, Wottom-Gorges SL, Kanegawa K, et al. Guidelines for imaging and staging of neuroblastic tumors: consensus report from the international neuroblastoma risk group project Radiology (2011) 261:243–57. doi: 10.1148/radiol.11101352
31. Whittle SB, Smith V, Doherty E, Zhao S, McCarty S, Zage PE. Overview and recent advances in the treatment of neuroblastoma. Expert Rev Anticancer Ther. (2017) 4:369–86. doi: 10.1080/14737140.2017.1285230
32. Bordow SB, Norris MD, Haber PS, Marshall GM, Haber M. Prognostic significance of MYCN oncogene expression in childhood neuroblastoma” J Clin Oncol (1998) 16:3286–94. doi: 10.1200/JCO.1998.16.10.3286
33. Smith L Minter S Once Once of MYCN oncogene expression Lazarchick J. Neuroblastoma in an adult: case presentation and literature review. Ann Clin Lab Sci. (2013) 43:81–4.
34. Papaioannou G, McHugh K. Neuroblastoma in childhood: review and radiological findings. Cancer Imag. (2005) 5:116–27. doi: 10.1102/1470-7330.2005.0104
35. Conte M, Parodi S, De Bernardi B, Milanaccio C, Mazzocco K, Angelini P, et al. Neuroblastoma in adolescents: the Italian EXPERIENCE. Cancer. (2006) 106:1409–17. doi: 10.1002/cncr.21751
Keywords: neuroblastic tumors, ganglioneuroblastoma, elderly patient, preoperative diagnosis, surgical and medical therapy, staging, prognosis, follow up
Citation: Deslarzes P, Djafarrian R, Matter M, La Rosa S, Gengler C, Beck-Popovic M and Zingg T (2022) Neuroblastic Tumors of the Adrenal Gland in Elderly Patients: A Case Report and Review of the Literature. Front. Pediatr. 10:869518. doi: 10.3389/fped.2022.869518
Received: 08 February 2022; Accepted: 20 April 2022;
Published: 17 May 2022.
Edited by:
Jürgen Schleef, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyReviewed by:
Alice Helena Dutra Violante, Federal University of Rio de Janeiro, BrazilCopyright © 2022 Deslarzes, Djafarrian, Matter, La Rosa, Gengler, Beck-Popovic and Zingg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tobias Zingg, dG9iaWFzLnppbmdnQGNodXYuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.