- 1Sydney Children's Hospital, Department of Sleep Medicine, Sydney, NSW, Australia
- 2University of New South Wales, School of Women's and Children's Health, Kensington, NSW, Australia
Average volume assured pressure support (AVAPS) is a modality of non-invasive ventilation that enables the machine to deliver a pre-set tidal volume by adjusting the inspiratory pressure support within a set range. Data on its use in the pediatric population are limited to case reports and single centre case series. This article reviews paediatric data on use of AVAPS and highlights the need for validation to help develop specific guidelines on use of AVAPS in children.
Introduction
The use of home non-invasive ventilation (NIV) has increased substantially in children over the last few decades, at least in part due to enhanced survival of children with chronic medical conditions along with improvements in home ventilator technology and provision of suitably sized pediatric masks (1, 2). When NIV is initiated, parameters are generally determined based on clinical assessment followed by an in-laboratory polysomnographic titration study where parameters are adjusted throughout the recording to determine optimal ventilatory settings for adequate gas exchange andupper airway patency (3). Although conventional fixed pressure NIV has been the mainstay therapy for children with neuromuscular and hypoventilation syndromes requiring respiratory support, several pediatric centres are reporting favorable outcomes with the use of average volume assured pressure support (AVAPS) for home ventilation (4–7). AVAPS enables the machine to deliver a pre-set tidal volume by automatically adjusting the inspiratory pressure support within a set range. It offers several advantages over fixed pressure NIV support such as its ability to compensate for the changes in tidal volume which occur with changes in lung compliance and sleep stages. It intuitively varies the inspiratory pressure, using higher pressure during REM sleep compared to NREM sleep resulting in a more stable ventilation and potentially improving adherence (5, 8). Despite its advantages, data on its use in children are sparse and recommendations on initiation and settings are extrapolated from adult experience.
AVAPS Function and Settings
Rather than having a fixed IPAP setting, AVAPS has the capability to set a range of values for IPAP, a maximum and a minimum IPAP, to target delivery of a set tidal volume. Pressure support is no longer fixed and changes within the set parameters. The ventilator uses an inbuilt algorithm to either increase or decrease the inspiratory pressure from breath to breath to ensure delivery of the pre-set tidal volume1. The IPAP maximum also serves as a safety parameter to prevent barotrauma from excessive pressure. For the minimum IPAP delivery, the machine makes a selection from three pre-set algorithms (IPAP min; VT 60 ml/cmH2O + EPAP or 8 cmH2O + EPAP), choosing the highest value (9). Several additional parameters such as AVAPS rate of change ensure that patient-machine desynchronization is minimized and the IPAP pressure changes swiftly and efficiently to ensure patient comfort and optimal ventilation1. Tidal volume varies with each breath and it may take several breaths for AVAPS to attain the targeted tidal volume (10). EPAP is fixed similar to conventional BPAP, although there is an AVAPS auto-titrating EPAP (AVAPS AE) feature to regulate the EPAP as well in some devices (11).
The suggested settings are based on the information provided by the manufacturer and early adult studies1 (12, 13). In adults, a target tidal volume of 8 ml/kg of ideal body weight is recommended. The use of ideal body weight is to ensure optimal calculation of tidal volume for obese patients. The manufacturer provides pre-calculated ideal weights for a range of adult heights1. The maximum IPAP is 25 cmH2O and depending on the patient pathology can be set to a lower level. The minimum IPAP is set at EPAP +4 cmH2O, generally no <8 cmH2O. The set rate is generally 2 to 3 breaths below resting respiratory rate. The AVAPS rate of change determines the rate of change of IPAP and is kept shorter (range 1–5 cmH2O) for more unstable patients. The rise time is adjusted for patient comfort. The use of modem enabled devices help monitor pressures, air leaks and objective adherence to patient's healthcare provider. Recommendations for pediatric settings are lacking.
Patient Selection
Although AVAPS debuted in 2003, a study published in 2006 by Storre et al. provided evidence of improvement in ventilation in a randomized crossover trial in adult patients with obesity hypoventilation syndrome. Adult studies have reported successful use in conditions such as chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), hypercapnia, encephalopathy, kyphoscoliosis and neuromuscular disorders (14–17). Pediatric studies have been limited to case series and case reports. A function similar to AVAPS, called iVAPS (intelligent volume assured pressure support), targets alveolar ventilation by adjusting pressure support (7). For purposes of this review, we have presented pediatric studies on volume assured pressure support ventilation below.
Search Methods
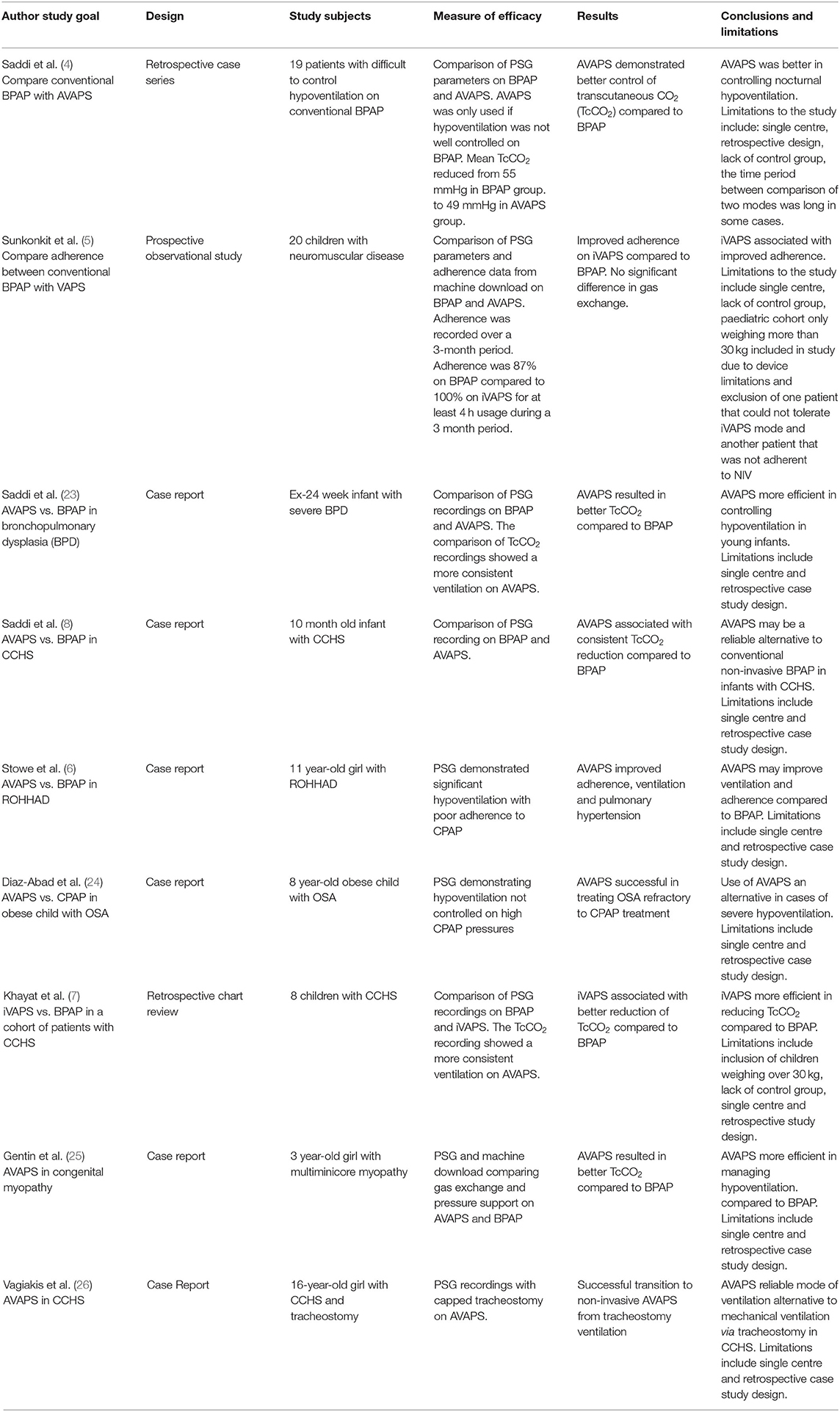
A literature search of MEDLINE, Embase and PubMed was undertaken. No time limits were placed. The key terms included pediatrics, children, bilevel ventilation (BiPAP/BPAP), non-invasive ventilation and AVAPS. Inclusion criteria were: (1) AVAPS has been initiated either in an acute/subacute setting (pediatric intensive care unit) or electively (in stable setting, during or after a sleep study) and (2) English language articles and (3) Pediatric population (0–18 years). None of the studies were excluded from the review based on quality assessment. A total of 9 articles meeting the inclusion criteria were identified. These included 2 retrospective chart reviews, 1 prospective observational study and 6 case reports (Table 1).
Device Considerations
The ability to reliably deliver small tidal volumes is critical to the use of AVAPS in pediatric populations. The minimum tidal volume threshold is 50 ml for the more advanced AVAPS-capable devices. Table 2 provides minimum tidal volume delivery for devices currently available with the AVAPS feature.
Pediatric Settings
There are no pediatric specific guidelines for initiation of AVAPS. As discussed above, data on safety and reliability are based on single center studies with relatively small numbers. There are subtle differences between pediatric and adult AVAPS initiation that must be highlighted. The recommendation from the manufacturer to use ideal body weight (IBW) for calculation of tidal volume was presumably based on experience with obese adult patients. Although pediatric obesity is a growing problem, our centre has used AVAPS for a number of non-obese children. There is no universally accepted formula for calculation of IBW in pediatrics. A recent study published in JAMA Pediatrics comparing five different methods of calculating IBW in children revealed significant differences and variability in calculations using different methods (18). Furthermore, calculation of height is problematic for children with neuromuscular problems. Arm span is often used as a surrogate for height in children but upper limb contractures can make accurate measurements difficult. The data on arm span as a surrogate for height are based on small studies, mainly in children under 10 years of age (19, 20). One large study found ulnar length to be more reproducible and representative of height in healthy school age children, supporting its use in children with neuromuscular weakness (21). Furthermore, use of IBW for very young non-obese children may be unnecessary for calculation of tidal volumes. At our centre, we adopt the following approach:
1. For children with weight between 3rd and 95th centile we use actual body weight;
2. For children above healthy weight range (above 95th centile) or underweight (below 3rd centile) we use IBW for calculation of tidal volume as recommended;
3. For children shorter than 5 ft, the IBW in kg is calculated as ([height in centimeters]2 × 1.65)/1000. For boys taller than 5 ft, the IBW in kg is calculated as 39 + (2.27 × [height in inches – 60]). For girls taller than 5 ft, the IBW in kg is calculated as 42 + (2.27 × [height in inches – 60]) (22).
Other popular methods to calculate IBW include those proposed by McLaren, Moore, the American dietary association and the BMI 50 method (18). For children with disability where height cannot be measured, we use arm span as a surrogate for height measurement. It is important that height or arm span is measured meticulously to reduce errors in tidal volume calculation.
For the purposes of tidal volume calculation, a tidal volume of 6to 10 ml/kg is targeted (27). The IPAP minimum is calculated by adding 4 cmH2O to EPAP, similar to adults. The IPAP maximum depends on the patient's underlying condition with higher values for restrictive lung conditions.
In summary, the overall evidence on use of AVAPS in pediatric population remains confined to single centre and case studies without control groups. Prospective randomized control trials with long term follow up are needed. The recommendations and guidelines on initiation and titration of AVAPS in children are lacking. Further validations with data from other centres will help guide the optimal approach to use of AVAPS in pediatric settings.
Author Contributions
VS drafted the initial manuscript. GT, BM, GB, and AT equally contributed to its development and approved the final version. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors have published several articles included in this review. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CCHS, congenital central hypoventilation syndrome; ROHHAD, rapid-onset obesity with hypothalamic dysregulation, hypoventilation, and autonomic dysregulation.
Footnotes
References
1. Chatwin M, Tan H-L, Bush A, Rosenthal M, Simonds AK. Long term non-invasive ventilation in children: impact on survival and transition to adult care. PLOS ONE. (2015) 10:E0125839. doi: 10.1371/journal.pone.0125839
2. Edwards EA, Hsiao K, Nixon GM. Paediatric home ventilatory support: the auckland experience. J Paediatr Child Health. (2005) 41:652–8. doi: 10.1111/j.1440-1754.2005.00753.x
3. Kushida CA, Chediak A, Berry RB, Brown LK, Gozal D, Iber C, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. (2008) 4:157–71. doi: 10.5664/jcsm.27133
4. Saddi V, Thambipillay G, Pithers S, Moody M, Martin B, Blecher G, et al. Average volume-assured pressure support vs. conventional bilevel pressure support in pediatric nocturnal hypoventilation: a case series. J Clin Sleep Med. (2021) 17:925–30. doi: 10.5664/jcsm.9084
5. Sunkonkit K, Al-Saleh S, Chiang J, Hamilton A, Medin D, Syed F, et al. Volume-assured pressure support mode for noninvasive ventilation: can it improve overnight adherence in children with neuromuscular disease? Sleep Breath. (2021) 21:1. doi: 10.1007/s11325-021-02288-1
6. Stowe RC, Afolabi-Brown O. Pulmonary hypertension and chronic hypoventilation in Rohhad syndrome treated with average-volume assured pressure support. Pediatr Investig. (2019) 3:253–6. doi: 10.1002/ped4.12168
7. Khayat A, Medin D, Syed F, Moraes TJ, Bin-Hasan S, Narang I, et al. Intelligent volume-assured pressured support (ivaps) for the treatment of congenital central hypoventilation syndrome. Sleep Breath. (2017) 21:513–9. doi: 10.1007/s11325-017-1478-5
8. Saddi V, Teng A, Thambipillay G, Allen H, Pithers S, Sullivan C. Nasal mask average volume-assured pressure support in an infant with congenital central hypoventilation syndrome. Respirol Case Rep. (2019) 7:E00448–E. doi: 10.1002/rcr2.448
9. Yarrarapu SNS SH, Sanghavi D. Average Volume-Assured Pressure Support. (2021). in: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing (2022). Available online at: https://www.Ncbi.nlm.nih.gov/Books/NBK560600/ (accessed August 14, 2021).
10. Hess DR. Patient-ventilator interaction during noninvasive ventilation. Respir Care. (2011) 56:153–65. doi: 10.4187/respcare.01049
11. Patout M, Gagnadoux F, Rabec C, Trzepizur W, Georges M, Perrin C, et al. AVAPS-AE Versus ST Mode: a randomized controlled trial in patients with obesity hypoventilation syndrome. Respirology. (2020) 25:1073–81. doi: 10.1111/resp.13784
12. Storre JH, Seuthe B, Fiechter R, Milioglou S, Dreher M, Sorichter S, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest. (2006) 130:815–21. doi: 10.1378/chest.130.3.815
13. Heiat A. Impact of age on definition of standards for ideal weight. Prev Cardiol. (2003) 6:104–7. doi: 10.1111/j.1520-037X.2003.01046.x
14. Magdy DM, Metwally A. Effect of average volume-assured pressure support treatment on health-related quality of life in COPD patients with chronic hypercapnic respiratory failure: a randomized trial. Respir Res. (2020) 21:64. doi: 10.1186/s12931-020-1320-7
15. Mittal A, Forte M, Leonard R, Sangani R, Sharma S. Refractory acute respiratory distress syndrome secondary to COVID-19 successfully extubated to average volume-assured pressure support non-invasive ventilator. Cureus. (2020) 12:E7849–E. doi: 10.7759/cureus.7849
16. Piesiak P, Brzecka A, Kosacka M, Jankowska R. Efficacy of noninvasive volume targeted ventilation in patients with chronic respiratory failure due to kyphoscoliosis. Adv Exp Med Biol. (2015) 838:53–8. doi: 10.1007/5584_2014_68
17. Patel SI, Gay P, Morgenthaler TI, Olson EJ, Shamoun FE, Kashyap R, et al. Practical implementation of a single-night split-titration protocol with bpap-st and avaps in patients with neuromuscular disease. J Clinic Sleep Med. (2018) 14:2031–5. doi: 10.5664/jcsm.7530
18. Moylan A, Appelbaum N, Clarke J, Feather C, Tairraz AF, Maconochie I, et al. Assessing the agreement of 5 ideal body weight calculations for selecting medication dosages for children with obesity. JAMA Pediatrics. (2019) 173:597–8. doi: 10.1001/jamapediatrics.2019.0379
19. Yousafzai AK, Filteau SM, Wirz SL, Cole TJ. Comparison of armspan, arm length and tibia length as predictors of actual height of disabled and nondisabled children in Dharavi, Mumbai, India. Eur J Clin Nutr. (2003) 57:1230–4. doi: 10.1038/sj.ejcn.1601705
20. Socrates C, Grantham-McGregor SM, Harknett SG, Seal AJ. poor nutrition is a serious problem in children with cerebral palsy in Palawan, the Philippines. Int J Rehabil Res. (2000) 23:177–84. doi: 10.1097/00004356-200023030-00007
21. Gauld LM, Kappers J, Carlin JB, Robertson CF. Height prediction from ulna length. Development Med Child Neurol. (2004) 46:475–80. doi: 10.1111/j.1469-8749.2004.tb00508.x
22. Traub SL, Johnson CE. Comparison of methods of estimating creatinine clearance in children. Am J Hosp Pharm. (1980) 37:195–201. doi: 10.1093/ajhp/37.2.195
23. Saddi V, Thambipillay G, Teng A. Non-invasive home ventilation using the average volume assured pressure support feature in an infant with severe bronchopulmonary dysplasia and chronic respiratory failure. Pediatr Investig. (2020) 4:222–4. doi: 10.1002/ped4.12221
24. Diaz-Abad M, Isaiah A, Rogers VE, Pereira KD, Lasso-Pirot A. Use of noninvasive ventilation with volume-assured pressure support to avoid tracheostomy in severe obstructive sleep apnea. Case Rep Pediatr. (2018) 2018:4701736. doi: 10.1155/2018/4701736
25. Gentin N, Williamson B, Thambipillay G, Teng A. Nocturnal respiratory failure in a child with congenital myopathy - management using average volume-assured pressure support (AVAPS). Respirol Case Rep. (2015) 3:115–7. doi: 10.1002/rcr2.117
26. Vagiakis E, Koutsourelakis I, Perraki E, Roussos C, Mastora Z, Zakynthinos S, et al. Average volume-assured pressure support in a 16-year-old girl with congenital central hypoventilation syndrome. J Clinic Sleep Med: JCSM : Offic Publicat Am Acad Sleep Med. (2010) 6:609–12. doi: 10.5664/jcsm.27997
Keywords: pediatrics, ventilation, sleep, AVAPS, infant, hypoventilation
Citation: Saddi V, Thambipillay G, Martin B, Blecher G and Teng A (2022) Pediatric Average Volume Assured Pressure Support. Front. Pediatr. 10:868625. doi: 10.3389/fped.2022.868625
Received: 03 February 2022; Accepted: 07 March 2022;
Published: 04 May 2022.
Edited by:
Bülent Taner Karadag, Marmara University, TurkeyReviewed by:
Brenda M. Morrow, University of Cape Town, South AfricaGül Gürsel, Gazi University, Turkey
Copyright © 2022 Saddi, Thambipillay, Martin, Blecher and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vishal Saddi, dmlzaGFsLnNhZGRpQGhlYWx0aC5OU1cuZ292LmF1
 Vishal Saddi
Vishal Saddi Ganesh Thambipillay1,2
Ganesh Thambipillay1,2