- 1Department of Women's and Children's Health, University Hospital of Padova, Padova, Italy
- 2Paediatric Neurology and Neurophysiology Unit, Department of Women's and Children's Health, University Hospital of Padova, Padova, Italy
- 3Neuroimmunology Group, Paediatric Research Institute “Città della Speranza”, Padova, Italy
Neuronal surface antibody syndromes (NSAS) are an expanding group of autoimmune neurological diseases, whose most frequent clinical manifestation is autoimmune encephalitis (AE). Anti-NMDAR, anti-LGI1, and anti-CASPR2 autoimmunity represent the most described forms, while other NSAS are rarer and less well-characterized, especially in children. We carried out a systematic literature review of children with rare NSAS (with antibodies targeting D2R, GABAAR, GlyR, GABABR, AMPAR, amphiphysin, mGluR5, mGluR1, DPPX, IgLON5, and neurexin-3alpha) and available individual data, to contribute to improve their clinical characterization and identification of age-specific features. Ninety-four children were included in the review (47/94 female, age range 0.2–18 years). The most frequent NSAS were anti-D2R (28/94, 30%), anti-GABAAR (23/94, 24%), and anti-GlyR (22/94, 23%) autoimmunity. The most frequent clinical syndromes were AE, including limbic and basal ganglia encephalitis (57/94, 61%; GABAAR, D2R, GABABR, AMPAR, amphiphysin, and mGluR5), and isolated epileptic syndromes (15/94, 16%; GlyR, GABAAR). With the limitations imposed by the low number of cases, the main distinctive features of our pediatric literature cohort compared to the respective NSAS in adults included: absent/lower tumor association (exception made for anti-mGluR5 autoimmunity, and most evident in anti-amphiphysin autoimmunity); loss of female preponderance (AMPAR); relatively frequent association with preceding viral encephalitis (GABAAR, D2R). Moreover, while SPS and PERM are the most frequent syndromes in adult anti-GlyR and anti-amphiphysin autoimmunity, in children isolated epileptic syndromes and limbic encephalitis appear predominant, respectively. To our knowledge, this is the first systematic review on rare pediatric NSAS. An improved characterization may aid their recognition in children.
Introduction
Neurological syndromes with neuronal surface antibodies (NSAbs) are an expanding group of conditions whose most frequent clinical manifestation is autoimmune encephalitis (AE). They are characterized by more recent identification, rarer tumor association, higher frequency in children, direct pathogenic role of autoantibodies, and therefore better immunotherapy response compared with syndromes with antibodies against intracellular neuronal antigens (1–9).
The most frequent and best characterized NSAS are anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis (10–12), and autoimmunity with NSAbs targeting the leucine-rich, glioma-inactivated protein-1 (LGI1), and the contactin-associated protein-2 (CASPR2) (13–15). In children, myelin oligodendrocyte glycoprotein (MOG)-associated disorders (MOGAD) represent another major class of autoimmune disorders where MOG, a minor component of the central nervous system myelin sheath, is the target antigen; pediatric MOGAD most frequently manifest with acute demyelinating syndromes such as acute disseminated encephalomyelitis or optic neuritis, but rarer manifestations, including cortical encephalitis, have been described (16).
Rarer and less characterized NSAS, especially in children, include those with antibodies targeting the glycine receptor (GlyR) (17), the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) (18), the γ-aminobutyric acid-A and B receptor (GABAAR, GABABR) (19, 20), the metabotropic glutamate receptor 1 and 5 (mGluR1, mGluR5) (9, 21, 22), the dopamine-2 receptor (D2R) (23), the dipeptidyl-peptidase-like protein-6 (DPPX) (24), the immunoglobulin-like cell adhesion molecule 5 (IgLON5) (25), neurexin-3alpha (26), and amphiphysin (27).
We hereby carry out a systematic literature review of these latter rarer and less characterized NSAS in children, with the aim of contributing to their clinical description.
Methods
We conducted a systematic literature review on rare NSAS in children (D2R, GABAAR, GlyR, GABABR, AMPAR, amphiphysin, mGluR5, mGluR1, DPPX, IgLON5, and neurexin-3alpha).
We searched Pubmed from inception up to 10 September 2021, with the following search keys: “((complete name of the receptor) or (abbreviation of the name)) and (autoimmune or antibod* or abs) and (encephalit* or syndrome).” We included published details of the patients with onset of neurological symptoms in pediatric age (0–18 years) with serum and/or cerebrospinal fluid (CSF) positivity for the searched antibodies, and available individual data (minimum data: age and clinical syndrome); large cohorts providing only pooled data were excluded. In the results section and in Table 1, the denominators may differ due to heterogeneous data availability.
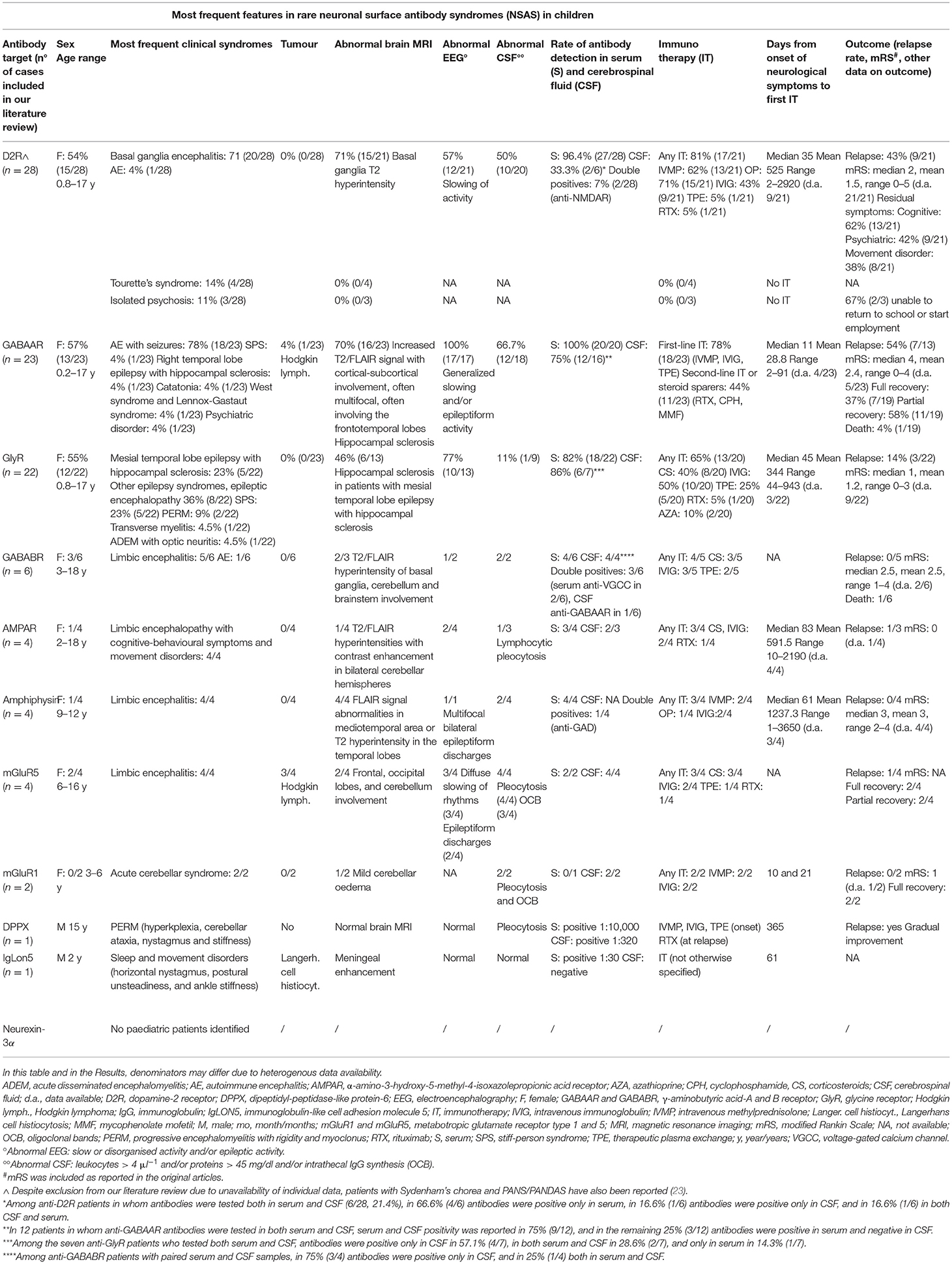
Table 1. Most frequent features associated with rare NSAS in paediatric age according to our systematic literature review (antibodies targeting D2R, GABAAR, GlyR, GABABR, AMPAR, amphiphysin, mGluR5, mGluR1, DPPX, IgLON5, and neurexin-3alpha).
Results
Our literature review disclosed 94 published cases meeting the inclusion criteria (Table 1, Supplementary Table 1).
Anti-D2R Autoimmunity
Demographics
Twenty-eight children with anti-D2R autoimmunity were identified (23, 28–33) (15/28, 53.6% female). The median age at onset was 5.5 years (mean 5.4, range 0.8–17).
Clinical Syndromes and Symptoms
Four main clinical syndromes were identified, namely, basal ganglia encephalitis (20/28, 71.4%) (BGE), Tourette's syndrome (4/28, 14.3%), isolated psychosis (3/28, 10.7%), and AE (1/28, 3.6%). It should also be noted that despite exclusion from our literature review due to unavailability of individual data, patients with Sydenham's chorea and PANS/PANDAS have also been reported (23).
In BGE-AE patients (21/28), the most frequent subgroups of symptoms were movement disorders (20/21, 95.2%): dystonia, chorea, parkinsonism, ocular symptoms; psychiatric symptoms (15/21, 71.4%): agitation, personality change and psychosis; cognitive symptoms (15/21, 62.5%): cognitive decline, impaired executive functions, attention, fine motor coordination, memory; speech disorder (9/21, 42.9%); sleep disorders (5/21, 23.8%); cerebellar signs (5/21, 23.8%); seizures (4/21, 19%). Among patients with Tourette's syndrome, 4/4 had tics, and 2/4 had psychiatric disorders. Psychiatric disturbances were the only symptom in 3/3 in the isolated psychosis subgroup.
None of the patients had associated malignancies (0/28, 0%). Infections preceding the neurological onset were reported in 42.9% (9/21) of cases, including previous herpes simplex virus 1 (HSV1) encephalitis, or post-vaccine (2/21, 9.5%). In 1/4 patients with Tourette's syndrome a previous streptococcal infection was diagnosed and in 2/4 elevated antistreptolysin-O titer was detected. All 3/3 patients with isolated psychosis had past neuropsychiatric comorbidities and positive psychiatric family history.
Anti-D2R Antibodies
Antibodies were tested in serum in 100% (28/28) patients (positive in 27/28, 96.4%), and in CSF in 21.4% (6/28) (positive in 2/6, 33.3%). About 7.1% (2/28) of patients had coexistent anti-NMDAR antibodies. Antibody assay used was cell-based assay (CBA) in 26/28, and ELISA in 2/28 (31, 33).
Investigations
Among the BGE-AE subgroup, abnormal brain magnetic resonance imaging (MRI) was reported in 71.4% (15/21), mostly showing basal ganglia T2 hyperintensity, resolving during the recovery phase in 60% (9/15). Abnormal electroencephalography (EEG) was reported in 57.1% (12/21), showing non-specific slowing. CSF data was available in 95.2% (20/21) patients and disclosed abnormalities in 50% (10/20). No abnormalities on imaging (MRI or computerised tomography scan) were found among patients with Tourette's syndrome and with isolated psychosis. EEG and CSF were not performed in these latter two subgroups of patients.
Treatment
Immunotherapy was used in 89.9% (17/21) of BGE-AE patients: intravenous methylprednisolone (13/21, 61.9%) (IVMP), oral prednisone (15/21, 71.4%) (OP), intravenous immunoglobulin (9/21, 42.9%) (IVIG), therapeutic plasma exchange (1/21, 4.8%) (TPE), rituximab (1/21, 4.8%), and steroid sparing agents (azathioprine and mycophenolate mofetil) (3/21, 14.3%). About 28.6% (6/21) patients had a complete response, 61.9% (13/21) had a partial response, and 9.5% (2/21) had no clinical response. Patients with Tourette's syndrome and with isolated psychosis did not receive immunotherapies. Patients with psychotic symptoms received antipsychotics and mood stabilisers.
Median time from onset of neurological symptoms to first immunotherapy was 35 days (mean 525, range 2–2,920; data available in 9/21 patients with BGE-AE).
Outcome
In the BGE-AE subgroup, at a median follow-up of 5 years (mean 6.6, range 0.4–18, data available in 19/21) relapses occurred in 42.3% (9/21). At follow-up, 38.1% (8/21) had residual movement disorders (mostly mild dystonia), 42.3% (9/21) developed psychiatric disorders (particularly anxiety disorder), and 61.9% (13/21) had cognitive symptoms. Median mRS at last follow-up was 2 (mean 1.5, range 0–5, data available in 21/21). Data on outcome was not available in patients with Tourette's syndrome. In the psychosis subgroup, 2/3 were unable to return to school or start employment.
Anti-GABAAR Autoimmunity
Demographics
Twenty-three children with anti-GABAAR autoimmunity were identified (20, 34–42) (13/23, 56.5% female). Median age at onset was 11 years (mean 9.6, range 2 months – 17 years).
Clinical Syndromes and Symptoms
AE was the most frequent clinical syndrome (18/23, 78.2%), followed by stiff-person syndrome (SPS), right temporal lobe epilepsy with hippocampal sclerosis, catatonia of unknown origin, West syndrome and Lennox-Gastaut syndrome, psychiatric disorder (1/23, 4.3% each). The most frequently described symptoms were seizures (21/23, 91.3%) cognitive impairment (9/23, 39%), behavioural change (5/23, 21.7%), movement disorders (39.1%, 9/23), and dysautonomia (17.4%, 4/23).
Hodgkin lymphoma was diagnosed in one patient, 10 months before neurological onset (1/23, 4.3%). About 17.4% of children (4/23) had viral infections before the onset of neurological symptoms: herpes labialis, HSV1 encephalitis, human herpes virus 6 (HHV6) encephalitis, and a concomitant parvovirus B19 encephalitis. One additional patient had fever without confirmed infection in the 6 days preceding neurological onset.
Anti-GABAAR Antibodies
Antibodies were tested in serum in 87% (20/23) patients (positive in 20/20, 100%), and in CSF in 69.6% (16/23) (positive in 12/16, 75%). 30.5% (7/23) patients had other coexistent autoantibodies: 4% (1/23) anti-GABABR antibodies (see also the anti-GABABR section), 13% (3/23) anti-NMDAR antibodies, and 13% (3/23) anti-GAD65 antibodies. CBA was used in 23/23 patients.
Investigations
Abnormal MRI was reported in 69.5% (16/23) patients, most commonly with increased T2/FLAIR signal in frontotemporal lobes (10/23, 43.5%), often multifocal and with cortical-subcortical involvement. In all cases with available data (17/23), EEG showed generalised slowing and/or epileptiform activity (17/17, 100%). CSF was abnormal in 66.7% (12/18) patients with available data.
Treatment
Overall, 78.2% (18/23) patients received first-line immunotherapy (IVMP and/or OP, IVIG, TPE), and 43.5% (11/23) received second-line immunotherapy and/or steroid sparers: rituximab (8/11), cyclophosphamide (2/11), and mycophenolate mofetil (1/11). Anti-seizure medications (ASM) were used in 52.2% (12/23) patients.
Median time from onset of neurological symptoms to first immunotherapy was 11 days (mean 28.8, range 2–91; data available in 4/23).
Outcome
At last follow-up (median 8 months, mean 13.3, range 2–36; data available in 12/23), relapses occurred in 53.8% (7/13) of patients with available data on disease course. Data on outcome was available in 19/23 patients. Median mRS at last follow-up was 4 (mean 2.4, range 0–4; data available in 5/19). Complete recovery was reported in 36.8% (7/19) patients, all treated with immunotherapy. Partial recovery was reported in 57.9% (11/19), and residual dysfunctions were cognitive deficits, need for chronic ASM, persistence of SPS symptoms. The death occurred in a 3-year-old child (1/19, 4%) with coexistent anti-GABABR antibodies, for sepsis during refractory status epilepticus (20).
Anti-GlyR Autoimmunity
Demographic
Twenty-two children with anti-GlyR autoimmunity were identified (39, 43–53) (12/22, 54.5% female). The median age at onset was 9 years (mean 9.7, range 9 months −17 years).
Clinical Syndromes and Symptoms
Reported clinical syndromes were: mesial temporal lobe epilepsy with hippocampal sclerosis (MTLE-HS, 5/22, 22.7%), other epilepsy syndromes and epileptic encephalopathy (8/22, 36.4%), SPS (5/22, 22.7%), progressive encephalomyelitis with rigidity and myoclonus (PERM) (2/22, 9.1%), transverse myelitis (1/22, 4.5%), and acute disseminated encephalomyelitis associated with optic neuritis (1/22, 4.5%). Seizures (13/22, 59.1%), cognitive impairment (8/22, 36.4%), rigidity (7/22, 31.8%), myoclonus (7/22, 31.8%), and psychiatric disturbances (6/22, 27.3%) were the most frequent symptoms overall, followed by consciousness disturbances, dysautonomia, sleep disturbances, movement disorders, cranial nerve deficits, and brainstem signs. Seizures, cognitive impairment, and psychiatric symptoms were most represented in the MTLE-HS, epileptic encephalopathy, and epilepsy group, while rigidity and myoclonus were most frequent in patients with SPS and PERM.
No malignancies were reported. One patient “had a cold” 5 days before the neurological onset (47). About 13.6% (3/22) patients had other autoimmune diseases (one diabetes mellitus and two autoimmune thyroiditis).
Anti-GlyR Antibodies
Antibodies were tested in serum in 100% (22/22) of patients (positive in 18/22, 81.8%), and in CSF in 31.8% (7/22) (positive in 6/7, 85.7%). About 22/22 were tested with CBA.
Investigations
When available, abnormal brain MRI was reported in 46.2% (6/13, including all five cases of MTLE-HS reporting hippocampal sclerosis), abnormal EEG in 76.9% of patients (10/13, almost all cases in the MTLE-HS, epilepsy, and epileptic encephalopathy groups), and abnormal CSF in 11% (1/9).
Treatment
About 65% (13/20) of patients with available treatment data received first-line immunotherapy: IVIG (10/20, 50%), corticosteroids (8/20, 40%: 3/20 IVMP, 1/20 OP, and 4/20 corticosteroids not otherwise specified), and TPE (5/20, 25%). Rituximab was used in 5% (1/20), azathioprine in 10% (2/10). About 35% (7/20) patients did not receive immunotherapy (all in the MTLE-HS and epilepsy group) but received ASM and/or epilepsy surgery.
Median time from onset of neurological symptoms to first immunotherapy was 45 days (mean 344, range 44–943; data available in 3/22).
Outcome
At median follow-up of 24 months (mean 18.6, range 3–31; data available in 13/22), relapses occurred in 13.6% (3/22). Median mRS at last follow-up was 1 (mean 1.2, range 0–3; data available in 9/22).
Anti-GABABR Autoimmunity
Demographics
Six children with anti-GABABR autoimmunity were identified (20, 54–58) (3/6 female). The median age at onset was 16 years (mean 13.6, range 3–18; data available in 6/6).
Clinical Syndromes and Symptoms
About 5/6 patients had limbic encephalitis and 1/6 AE. Limbic symptoms were described in all (6/6): memory deficits, psychomotor agitation, declining mental status, and personality changes; 4/6 had seizures. No tumours were reported.
Anti-GABABR Antibodies
Antibodies were tested in serum in 6/6 patients (positive in 5/6), and in CSF in 4/6 (positive in 4/4). The antibody assay used was CBA in 5/6; information was not available in 1/6 (54).
Investigations
When available, abnormal brain MRI was reported in 2/3 cases (mostly T2/FLAIR hyperintensity of basal ganglia, but also with cerebellum and brainstem involvement), abnormal EEG in 1/2, and abnormal CSF in 2/2 cases.
Treatment
Overall 4/5 patients with available treatment data received first-line immunotherapy: corticosteroids (3/5), IVIG (3/5), and TPE (2/5).
Outcome
Median follow-up was 12 months (mean 11, range 5–18; data available in 5/6). No relapses occurred (0/5). Patients (3/4) treated with first-line immunotherapy had a good response, while as described in anti-GABAAR section a 3-year-old patient with concomitant CSF anti-GABABR and anti-GABAAR antibodies died. One patient (1/5) did not receive immunotherapy and had a spontaneous recovery. Median mRS at last follow-up was 2.5 (mean 2.5, range 1–4; data available in 2/6).
Anti-AMPAR Autoimmunity
Demographics
Four children with anti-AMPAR autoimmunity were identified (59–63) (1/4 female). Median age at onset was 12 years (mean 11, range 2–18; data available in 4/4).
Clinical Syndromes and Symptoms
Patients (4/4) had encephalopathy with cognitive-behavioural disfunction (i.e., memory impairment, apathy, psychotic symptoms, hallucinations, bipolar disorder) and movement disorders.
No tumours were reported. Patients (2/4) had prodromal fever before onset of neurological symptoms.
Anti-AMPAR Antibodies
Antibodies were detected in serum in 3/4, and in CSF in 2/3 of patients tested. CBA was used in 3/4 patients, while type of antibody assay was not available in 1/4 (62).
Investigations
Abnormal brain MRI was reported in 1/4 cases, presenting T2/FLAIR hyperintensities with contrast enhancement in bilateral cerebellar hemispheres. EEG was abnormal in 2/4. CSF was abnormal in 1/3 cases, showing lymphocytic pleocytosis.
Treatment
Patients (3/4) received immunotherapy (corticosteroids and IVIG in 2/4, rituximab in 1/4). One patient received antipsychotics. Median time from onset of neurological symptoms to first immunotherapy was 83 days (mean 591.5, range 10–2,190; data available in 4/4).
Outcome
At last follow-up (median 36 months, mean 36, range 24–48; data available in 2/4), 2/4 of patients had full recovery while the remaining had progressive improvement. Relapses occurred in 1/3 of patients with available data on disease course. mRS was available in one patient only (mRS 4 at symptoms onset, mRS 0 at 48-month follow-up).
Anti-Amphiphysin Autoimmunity
Demographics
Four children with anti-amphiphysin autoimmunity were identified (64, 65) (1/4 female). The median age at onset was 12 years (mean 11.5, range 9–12; data available in 4/4).
Clinical Syndromes and Symptoms
Patients (4/4) had limbic encephalitis with fever, encephalopathy, memory impairment, and refractory temporal seizures.
No associated tumours were described. Preceding upper respiratory infection was reported in 2/4.
Anti-Amphiphysin Antibodies
Antibodies were positive in serum in 4/4 (CSF not tested). One patient (1/4) was also positive to anti-GAD antibodies. Patients (3/4) were tested with ELISA, whereas type of antibody assay was not available in 1/4 (65).
Investigations
Abnormal brain MRI was reported in 4/4 patients, showing abnormal FLAIR signal in mediotemporal areas or T2 temporal lobes hyperintensity. EEG data was available in only one patient, showing multifocal bilateral epileptiform discharges. Abnormal CSF was reported in 2/4 of patients.
Treatment
Overall 3/4 received immunotherapy (IVMP in 2/4; OP in 1/4; IVIG in 2/4), and had a partial response. Median time from onset of neurological symptoms to first immunotherapy was 61 days (mean 1,237.3, range 1–3,650; data available in 3/4).
Outcome
At a median follow-up of 9 years (mean 4.5 years, range 6 weeks – 11 years; data available in 4/4), no relapses occurred. All patients showed a chronic course with refractory epilepsy (4/4), and neuropsychiatric or cognitive symptoms (3/4); 2/4 developed a severe disability, precluding the possibility to live independently, whereas the other 2/4 had a mild disability. Median mRS at last follow-up was 3 (mean 3, range 2–4; data available in 4/4).
Anti-mGluR5 Autoimmunity
Demographics
Four children with anti-mGluR5 autoimmunity were identified (66) (2/4 female). Median age at onset was 15 years (mean 13, range 6–16 years; data available in 4/4).
Clinical Syndromes and Symptoms
Patients (4/4) had AE, with a decreased level of consciousness (3/4), seizures (3/4), and psychiatric disturbances (3/4) as most described symptoms.
3/4 patients had Hodgkin lymphoma; neurologic symptoms preceded tumour diagnosis.
Anti-mGluR5 Antibodies
Antibodies were tested in serum in 2/4 patients (positive in 2/2). Antibodies were tested in CSF in 4/4 patients (positive in 4/4). Immunochemistry and CBA were used in 4/4 patients.
Investigations
Brain MRI was abnormal in 2/4 of patients, showing frontal and occipital lobes and cerebellum involvement. EEG showed diffuse slowing of rhythms (3/4) and epileptiform discharges (2/4). CSF analysis showed pleocytosis (median 38 white blood cells, range 21–114; data available in 4/4) and oligoclonal bands (3/4).
Treatment
Patients (3/4) received immunotherapy: corticosteroids in 3/4, IVIG in 2/4, TPE in 1/4, rituximab in 1/4. Time from onset of neurological symptoms to first immunotherapy was not available.
Outcome
At last follow-up (median 33.5 months, mean 37.7, range 12–79; data available in 4/4) 2/4 of patients showed partial response while the remaining recovered completely. Relapse was documented in one patient presenting neurological symptoms in association with tumour relapse.
Anti-mGluR1 Autoimmunity
Demographics
Two children with anti-mGluR1 autoimmunity were identified (67, 68) (2/2 males); age at onset was 3 and 6 years, respectively.
Clinical Syndromes and Symptoms
Both patients had acute cerebellar syndrome; one had unsteady gait and mild behavioural changes, the other unsteady gait, dysarthria, intentional tremor, and movement disorder (choreiform movements of the face and jerky movements of the fingers).
No associated tumours were identified. One patient had prodromal headache, fever, nausea, and vomit, and had a previous history of streptococcal pharyngitis.
Anti-mGluR1 Antibodies
Antibodies were detected in CSF (2/2). Only one patient was tested in serum (negative). CBA was used in 2/2 patients.
Investigations
In one patient mild cerebellar oedema at brain MRI was reported. Data on EEG was not available. The CSF analysis showed pleocytosis and oligoclonal bands in both patients.
Treatment
Both patients received IVMP and IVIG. The time from onset of neurological symptoms to first immunotherapy was 10 and 21 days.
Outcome
At the last follow-up (2.5 and 7 months, respectively), both patients had a rapid full recovery. mRS was available in one patient only (mRS 1).
Anti-DPPX Autoimmunity
A 15-year-old male with anti-DPPX autoimmunity was identified (69), tested with indirect immunofluorescence CBA (Table 1).
Anti-IgLON5 Autoimmunity
A 2-year-old boy with anti-IgLON5 autoimmunity was identified (70), tested with CBA (Table 1).
Anti-Neurexin-3alpha Autoimmunity
No paediatric cases were identified.
Discussion
We have carried out a systematic literature review on rare paediatric NSAS (D2R, GABAAR, GlyR, GABABR, AMPAR, amphiphysin, mGluR5, mGluR1, DPPX, IgLON 5, and neurexin-3alpha autoimmunity) (Tables 1, 2, Supplementary Table 1), excluding NSAS that are already well-characterised in children (NMDAR, LGI1, and CASPR2 autoimmunity) and MOGAD. Amphiphysin, which is actually an intracellular synaptic vesicle protein involved in vesicle recycling at presynaptic nerve endings, was included since this epitope could be exposed to antibodies during synaptic vesicle fusion and reuptake, enabling the antibody binding process and its subsequent entrance in the cell (71).
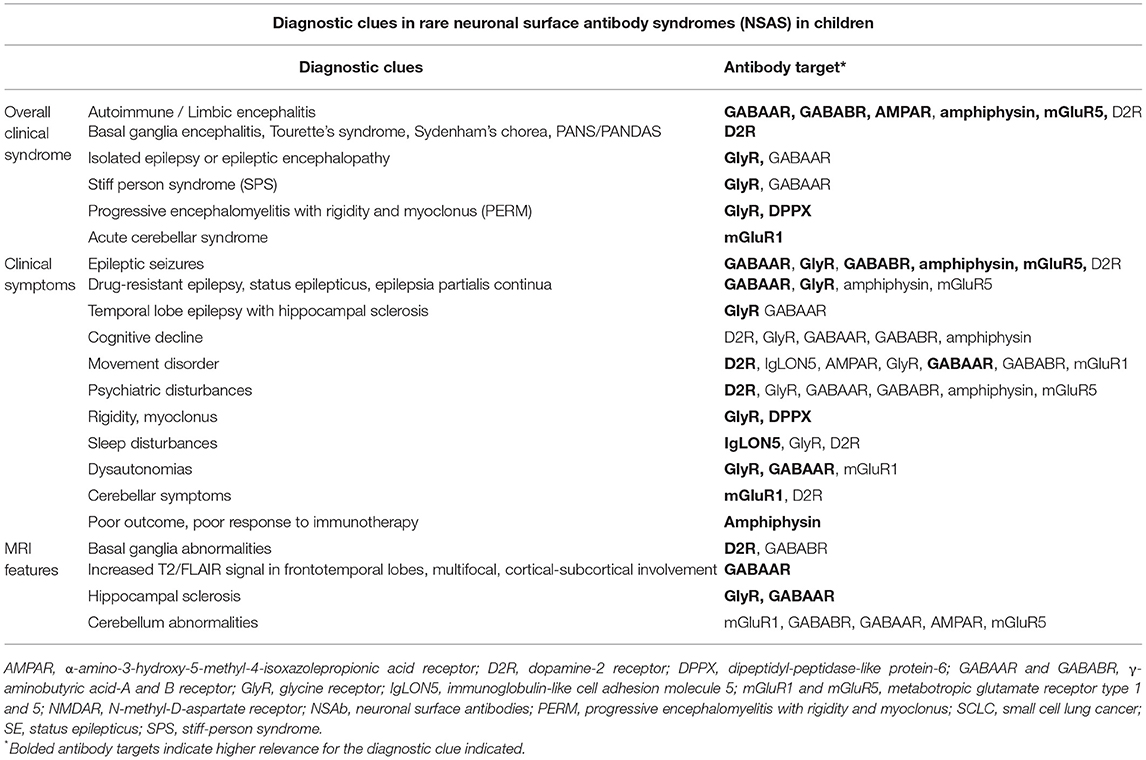
Table 2A. Diagnostic clues in rare NSAS in children, based on our literature review in paediatric age (antibodies targeting D2R, GABAAR, GlyR, GABABR, AMPAR, amphiphysin, mGluR5, mGluR1, DPPX, IgLON5, and neurexin-3alpha) and on previous literature data.
We have identified 94 published children with these rare NSAS and available individual patient data, the most frequent being anti-D2R (28/94), anti-GABAAR (23/94), and anti-GlyR (22/94) autoimmunity.
NSAb detection rate was generally higher in serum than in CSF, with rare exceptions (GABABR, GlyR, and mGluR1); whenever possible, both serum and CSF should be tested (72, 73). NSAbs bind to conformational extracellular epitopes of cell surface proteins, and their detection depends on methods that preserve the three-dimensional structure of the antigen. Therefore, CBAs with live or fixed eukaryotic cells to express the protein at the cell surface in its “physiological” state are the most used assays for NSAbs (72–74), and live CBAs are the gold standard in MOGAD (75). Commercial CBAs are generally used in clinical practice but antibody detection is improved by pairing with in-house diagnostics (immunohistochemistry, live CBAs, and neuronal cultures), although this may be challenging to implement in laboratories that lack the required expertise, and is therefore not always available (73, 76–79). Moreover, commercial CBAs do not cover all NSAbs (i.e., GABAR, GlyR, D2R, and neurexin-3alpha), posing an additional challenge in the identification of these rare NSAS.
Overall, AE was the most frequent clinical syndrome (57/94, 61%), including limbic encephalitis (GABABR, AMPAR, amphiphysin, mGluR5), BGE (D2R), and other AE (GABAAR, D2R). Although, other less common syndromes have variably been reported, such as isolated epilepsy/epileptic encephalopathy (15/94, 16%; GABAAR, GlyR), isolated psychiatric disorders (D2R, GABAAR), Tourette's syndrome, Sydenham's chorea, PANS/PANDAS (D2R), SPS (GlyR, GABAAR), PERM (GlyR, DPPX), acute cerebellar syndrome (mGluR1), and sleep and movement disorders (IgLON5) (Table 2A).
While several paediatric NSAS features are similar to the adult population, some important differences could be highlighted (Table 2B), the most striking being the rarer paraneoplastic aetiology in children (5/94, 5.3% of the whole cohort) (1–5), especially in anti-amphiphysin autoimmunity (where >80% of adult have an associated malignancy vs. 0/4 of our included pediatric patients), but except for anti-mGluR5 syndromes (associated with Hodgkin lymphoma in 3/4 children identified in our review). In our literature cohort, one additional patient with anti-GABAAR encephalitis had Hodgkin lymphoma and one with anti-IgLON5 autoimmunity was affected by Langerhans cell histiocytosis. The type of associated tumour may also differ from adults. Despite the overall rarer paraneoplastic aetiology in children, oncologic searches remain mandatory, also considering that neurological symptoms may precede tumour diagnosis (i.e., GABAAR, mGluR5) (66).
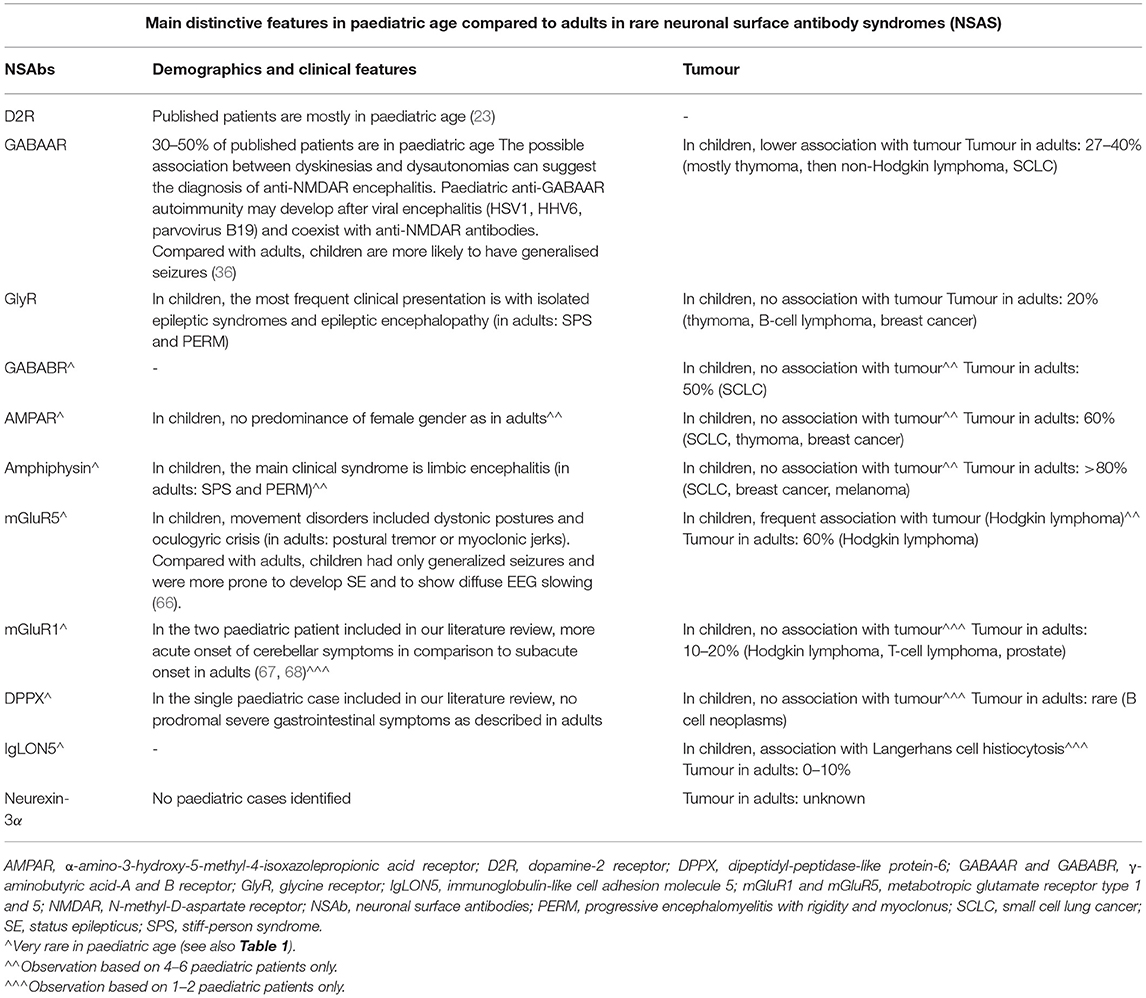
Table 2B. Rare NSAS (with antibodies targeting D2R, GABAAR, GlyR, GABABR, AMPAR, amphiphysin, mGluR5, mGluR1, DPPX, IgLON5, and neurexin-3alpha): main distinctive features in paediatric age compared to adults, as regards demographics, clinical features and association with tumour, based on our systematic literature review and on previous literature observations (3, 4, 23, 36, 66–68).
As previously observed in other NSAS (28, 80, 81), in our paediatric literature cohort a history of preceding viral encephalitis was relatively frequent (i.e., D2R, GABAAR) (28, 36), and overall there was less marked gender association than in adults (i.e., no female preponderance in AMPAR encephalitis).
Regarding clinical features, while SPS and PERM are the most frequent syndromes in adult anti-GlyR and anti-amphiphysin autoimmunity, in children isolated epileptic syndromes and limbic encephalitis appear predominant, respectively. In anti-GABAAR autoimmunity, children are more likely to have generalised seizures and a different type of movement disorder compared with adults; a subset of children has coexistent anti-NMDAR antibodies (36). Finally, in paediatric anti-mGluR1 autoimmunity, cerebellar ataxia appears to have a more acute onset of symptoms than in adults (67, 68).
The reason for the rarity of these NSAS overall and especially in children is not clear, possibly including their recent description, the general lower frequency of tumour in paediatric age (therefore decreasing one known potential trigger), and hypothetical age-dependent variations in the expression pattern of some neuronal surface antigens in childhood. Underdiagnosis is also possible, in view of their yet incomplete clinical characterisation and the above-mentioned diagnostic challenges, these latter also reflected by the considerable treatment delay observed in out literature cohort.
The effect of treatment timing and strategy on the final outcome is yet to be clarified in larger cohorts, as observed for anti-NMDAR encephalitis (82).
Limitations and Conclusion
Our literature review is strongly affected by its retrospective nature and the low number of patients, limiting the possibility of drawing definite conclusions. Moreover, we acknowledge that not all patients included in our literature review were diagnosed via CBA.
Despite these limitations, to our knowledge this is the first systematic review focusing on rare paediatric NSAS, and may therefore contribute to their characterisation. This review discloses antibody-specific features in children, helping clinicians suspect NSAS. We suggest examining both serum and CSF with CBA for a broad NSAbs panel in children presenting with new-onset focal or diffuse neurological deficits, cognitive difficulties, psychiatric symptoms, seizures, and/or movement disorder of unknown origin, even in the absence of definite MRI, EEG, or CSF abnormalities. Larger cohorts are warranted to elucidate the clinical features of these rare NSAS in children, their paraclinical findings and the most effective treatment strategies.
Author Contributions
CA, VM, MT, LT, AL, and CL: carried out the literature review and drafted the paper. MN, SS, and IT: supervised the literature review and contributed to the critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.866074/full#supplementary-material
Abbreviations
AE, autoimmune encephalitis; BGE, basal ganglia encephalitis; NSAbs, neuronal surface antibodies; NSAS, neuronal surface antibody syndromes.
References
1. Zuliani L, Graus F, Giometto B, Bien C, Vincent A. Central nervous system neuronal surface antibody associated syndromes: review and guidelines for recognition. J Neurol Neurosurg Psychiatry. (2012) 83:638–45. doi: 10.1136/jnnp-2011-301237
2. Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. (2017) 97:839–87. doi: 10.1152/physrev.00010.2016
3. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. (2018) 378:840–51. doi: 10.1056/NEJMra1708712
4. Dalmau J, Graus F. Antibody-mediated neuropsychiatric disorders. J Allergy Clin Immunol. (2022) 149:37–40. doi: 10.1016/j.jaci.2021.11.008
5. Nosadini M, Mohammad SS, Ramanathan S, Brilot F, Dale RC. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. (2015) 15:1391–419. doi: 10.1586/14737175.2015.1115720
6. Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. (2010) 30:5866–75. doi: 10.1523/JNEUROSCI.0167-10.2010
7. Peng X, Hughes EG, Moscato EH, Parsons TD, Dalmau J, Balice-Gordon RJ. Cellular plasticity induced by anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor encephalitis antibodies. Ann Neurol. (2015) 77:381–98. doi: 10.1002/ana.24293
8. Planaguma J, Leypoldt F, Mannara F, Gutierrez-Cuesta J, Martin-Garcia E, Aguilar E, et al. N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. (2015) 138:94–109. doi: 10.1093/brain/awu310
9. Sillevis Smitt P, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. (2000) 342:21–7. doi: 10.1056/NEJM200001063420104
10. Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. (2007) 61:25–36. doi: 10.1002/ana.21050
11. Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
12. Nosadini M, Granata T, Matricardi S, Freri E, Ragona F, Papetti L, et al. Anti-N-methyl-D-aspartate receptor encephalitis. Relapse risk factors in anti-N-methyl-D-aspartate receptor encephalitis. Dev Med Child Neurol. (2019) 61:1101–7. doi: 10.1111/dmcn.14267
13. Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. (2010) 9:776–85. doi: 10.1016/S1474-4422(10)70137-X
14. Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. (2010) 133:2734–48. doi: 10.1093/brain/awq213
15. Nosadini M, Toldo I, Tascini B, Bien CG, Parmeggiani L, De Gaspari P, et al. LGI1 and CASPR2 autoimmunity in children: systematic literature review and report of a young girl with Morvan syndrome. J Neuroimmunol. (2019) 335:577008. doi: 10.1016/j.jneuroim.2019.577008
16. Bruijstens AL, Lechner C, Flet-Berliac L, Deiva K, Neuteboom RF, Hemingway C, et al. EU paediatric MOG consortium consensus: part 1 - classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. (2020) 29:2–13. doi: 10.1016/j.ejpn.2020.10.006
17. Hutchinson M, Waters P, McHugh J, Gorman G, O'Riordan S, Connolly S, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. (2008) 71:1291–2. doi: 10.1212/01.wnl.0000327606.50322.f0
18. Lai M, Hughes EG, Peng X, Zhou L, Gleichman AJ, Shu H, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. (2009) 65:424–34. doi: 10.1002/ana.21589
19. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. (2010) 9:67–76. doi: 10.1016/S1474-4422(09)70324-2
20. Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. (2014) 13:276–86. doi: 10.1016/S1474-4422(13)70299-0
21. Coesmans M, Smitt PA, Linden DJ, Shigemoto R, Hirano T, Yamakawa Y, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol. (2003) 53:325–36. doi: 10.1002/ana.10451
22. Lancaster E, Martinez-Hernandez E, Titulaer MJ, Boulos M, Weaver S, Antoine JC, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. (2011) 77:1698–701. doi: 10.1212/WNL.0b013e3182364a44
23. Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. (2012) 135:3453–68. doi: 10.1093/brain/aws256
24. Boronat A, Gelfand JM, Gresa-Arribas N, Jeong HY, Walsh M, Roberts K, et al. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv42 potassium channels. Ann Neurol. (2013) 73:120–8. doi: 10.1002/ana.23756
25. Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. (2014) 13:575–86. doi: 10.1016/S1474-4422(14)70051-1
26. Gresa-Arribas N, Planagumà J, Petit-Pedrol M, Kawachi I, Katada S, Glaser CA, et al. Human neurexin-3α antibodies associate with encephalitis and alter synapse development. Neurology. (2016) 86:2235–42. doi: 10.1212/WNL.0000000000002775
27. De Camilli P, Thomas A, Cofiell R, Folli F, Lichte B, Piccolo G, et al. The synaptic vesicle-associated protein amphiphysin is the 128-kD autoantigen of Stiff-Man syndrome with breast cancer. J Exp Med. (1993) 178:2219–23. doi: 10.1084/jem.178.6.2219
28. Mohammad SS, Sinclair K, Pillai S, Merheb V, Aumann TD, Gill D, et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-Methyl-D-aspartate receptor or dopamine-2 receptor. Mov Disord. (2014) 29:117–22. doi: 10.1002/mds.25623
29. Pathmanandavel K, Starling J, Merheb V, Ramanathan S, Sinmaz N, Dale RC, et al. Antibodies to surface dopamine-2 receptor and N-methyl-D-aspartate receptor in the first episode of acute psychosis in children. Biol Psychiatry. (2015) 77:537–47. doi: 10.1016/j.biopsych.2014.07.014
30. Pawela C, Brunsdon RK, Williams TA, Porter M, Dale RC, Mohammad SS. The neuropsychological profile of children with basal ganglia encephalitis: a case series. Dev Med Child Neurol. (2017) 59:445–8. doi: 10.1111/dmcn.13351
31. Marques-Matos C, Melo C, Sampaio M, Rodrigues E, Sousa R, Alves D. Child Neurology: treatable bilateral striatal lesions related to anti-dopamine 2 receptor autoimmunity. Neurology. (2018) 91:98–101. doi: 10.1212/WNL.0000000000005774
32. Dai X, Kuang L, Feng L, Yi X, Tang W, Liao Q, et al. Anti-dopamine receptor 2 antibody-positive encephalitis in adolescent. Front Neurol. (2020) 11:471. doi: 10.3389/fneur.2020.00471
33. Salamatova Y, Malaty I, Ghosh S. Pediatric autoimmune Parkinsonism and response to deep brain stimulation. Childs Nerv Syst. (2022) 38:203–6. doi: 10.1007/s00381-021-05152-5
34. Pettingill P, Kramer HB, Coebergh JA, Pettingill R, Maxwell S, Nibber A, et al. Antibodies to GABAA receptor α1 and γ2 subunits: clinical and serologic characterization. Neurology. (2015) 84:1233–41. doi: 10.1212/WNL.0000000000001326
35. Baysal-Kirac L, Tuzun E, Erdag E, Ulusoy C, Vanli-Yavuz EN, Ekizoglu E, et al. Neuronal autoantibodies in epilepsy patients with peri-ictal autonomic findings. J Neurol. (2016) 263:455–66. doi: 10.1007/s00415-015-8002-2
36. Spatola M, Petit-Pedrol M, Simabukuro MM, Armangue T, Castro FJ, Barcelo Artigues MI, et al. Investigations in GABAA receptor antibody-associated encephalitis. Neurology. (2017) 88:1012–20. doi: 10.1212/WNL.0000000000003713
37. Caputo D, Iorio R, Vigevano F, Fusco L. Febrile infection-related epilepsy syndrome (FIRES) with super-refractory status epilepticus revealing autoimmune encephalitis due to GABAAR antibodies. Eur J Paediatr Neurol. (2018) 22:182–5. doi: 10.1016/j.ejpn.2017.11.005
38. Figlerowicz M, Kemnitz P, Mania A, Mazur-Melewska K, Tomczak E, Kuls K, et al. Autoimmune encephalitis with GABAA receptor antibodies in a 10-year-old girl. Clin Neurol Neurosurg. (2018) 164:160–3. doi: 10.1016/j.clineuro.2017.12.012
39. Tekturk P, Baykan B, Erdag E, Peach S, Sezgin M, Yapici Z, et al. Investigation of neuronal auto-antibodies in children diagnosed with epileptic encephalopathy of unknown cause. Brain Dev. (2018) 40:909–17. doi: 10.1016/j.braindev.2018.06.002
40. Nikolaus M, Knierim E, Meisel C, Kreye J, Prüss H, Schnabel D, et al. Severe GABAA receptor encephalitis without seizures: a paediatric case successfully treated with early immunomodulation. Eur J Paediatr Neurol. (2018) 22:558–62. doi: 10.1016/j.ejpn.2018.01.002
41. O'Connor K, Waters P, Komorowski L, Zekeridou A, Guo CY, Mgbachi VC, et al. GABAA receptor autoimmunity: a multicenter experience. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e552. doi: 10.1212/NXI.0000000000000552
42. Valle DAD, Santos MLSF, Spinosa MJ, Telles BA, Prando C, Cordeiro ML, et al. receptor encephalitis associated with human parvovirus B19 virus infection: case report. Medicine. (2021) 100:e26324. doi: 10.1097/MD.0000000000026324
43. Atmaca MM, Tuzun E, Erdag E, Bebek N, Baykan B, Gurses C. Investigation of anti-neuronal antibodies in status epilepticus of unknown etiology: a prospective study. Acta Neurol Belg. (2017) 117:841–8. doi: 10.1007/s13760-017-0796-5
44. Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, et al. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. (2014) 137:2178–92. doi: 10.1093/brain/awu142
45. Chan DWS, Thomas T, Lim M, Ling S, Woodhall M, Vincent A. Focal status epilepticus and progressive dyskinesia: a novel phenotype for glycine receptor antibody-mediated neurological disease in children. Eur J Paediatr Neurol. (2017) 21:414–7. doi: 10.1016/j.ejpn.2016.08.013
46. Clardy SL, Lennon VA, Dalmau J, Pittock SJ, Jones HR Jr, Renaud DL, et al. Childhood onset of stiff-man syndrome. J Am Med Assoc Neurol. (2013) 70:1531–6. doi: 10.1001/jamaneurol.2013.4442
47. Damásio J, Leite MI, Coutinho E, Waters P, Woodhall M, Santos MA, et al. Progressive encephalomyelitis with rigidity and myoclonus: the first pediatric case with glycine receptor antibodies. J Am Med Assoc Neurol. (2013) 70:498–501. doi: 10.1001/jamaneurol.2013.1872
48. Hacohen Y, Wright S, Waters P, Agrawal S, Carr L, Cross H, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. (2013) 84:748–55. doi: 10.1136/jnnp-2012-303807
49. Hacohen Y, Absoud M, Woodhall M, Cummins C, De Goede CG, Hemingway C, et al. Autoantibody biomarkers in childhood-acquired demyelinating syndromes: results from a national surveillance cohort. J Neurol Neurosurg Psychiatry. (2014) 85:456–61. doi: 10.1136/jnnp-2013-306411
50. McKeon A, Martinez-Hernandez E, Lancaster E, Matsumoto JY, Harvey RJ, McEvoy KM, et al. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. J Am Med Assoc Neurol. (2013) 70:44–50. doi: 10.1001/jamaneurol.2013.574
51. Piquet AL, Khan M, Warner JEA, Wicklund MP, Bennett JL, Leehey MA, et al. Novel clinical features of glycine receptor antibody syndrome: a series of 17 cases. Neurol Neuroimmunol Neuroinflamm. (2019) 6:e592. doi: 10.1212/NXI.0000000000000592
52. Vanli-Yavuz EN, Erdag E, Tuzun E, Ekizoglu E, Baysal-Kirac L, Ulusoy C, et al. Neuronal autoantibodies in mesial temporal lobe epilepsy with hippocampal sclerosis. J Neurol Neurosurg Psychiatry. (2016) 87:684–92. doi: 10.1136/jnnp-2016-313146
53. Wuerfel E, Bien CG, Vincent A, Woodhall M, Brockmann K. Glycine receptor antibodies in a boy with focal epilepsy and episodic behavioral disorder. J Neurol Sci. (2014) 343:180–2. doi: 10.1016/j.jns.2014.05.014
54. Chen X, Liu F, Li JM, Xie XQ, Wang Q, Zhou D, et al. Encephalitis with antibodies against the GABAB receptor: seizures as the most common presentation at admission. Neurol Res. (2017) 39:973–80. doi: 10.1080/01616412.2017.1351062
55. Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. (2013) 81:1500–6. doi: 10.1212/WNL.0b013e3182a9585f
56. Jeffery OJ, Lennon VA, Pittock SJ, Gregory JK, Britton JW, McKeon A, et al. receptor autoantibody frequency in service serologic evaluation. Neurology. (2013) 81:882–7. doi: 10.1212/WNL.0b013e3182a35271
57. Kruer MC, Hoeftberger R, Lim KY, Coryell JC, Svoboda MD, Woltjer RL, et al. Aggressive course in encephalitis with opsoclonus, ataxia, chorea, and seizures: the first pediatric case of γ-aminobutyric acid type B receptor autoimmunity. J Am Med Assoc Neurol. (2014) 71:620–3. doi: 10.1001/jamaneurol.2013.4786
58. Liu B, Liu J, Sun H, Xie M, Yang C, Pan Y, et al. Autoimmune encephalitis after Japanese encephalitis in children: a prospective study. J Neurol Sci. (2021) 424:117394. doi: 10.1016/j.jns.2021.117394
59. Qiao S, Wu HK, Wang KM, Zang KJ, Liu XW. Long-term follow-up of a child with anti-AMPA receptor encephalitis. Neurol Sci. (2021) 42:2107–9. doi: 10.1007/s10072-020-04900-w
60. Wang K, Shi Y, Du Q, Zhang RR, Wu H, Qiao S, et al. Clinical review and prognostic analysis of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor-associated encephalitis. Front Neurol. (2021) 12:665229. doi: 10.3389/fneur.2021.665229
61. Trung Hieu NL, Minh Duc N, Tra My TT, Hieu Anh B, Tan Lien Bang M, Minh Thong P. First reported case of anti-ampa receptor encephalitis in a Vietnamese adolescent. Clin Med Insights Case Rep. (2021) 14:11795476211037782. doi: 10.1177/11795476211037782
62. Quaranta G, Maremmani AG, Perugi G. Anti-AMPA-receptor encephalitis presenting as a rapid-cycling bipolar disorder in a young woman with turner syndrome. Case Rep Psychiatry. (2015) 2015:273192. doi: 10.1155/2015/273192
63. Laurido-Soto O, Brier MR, Simon LE, McCullough A, Bucelli RC, Day GS. Patient characteristics and outcome associations in AMPA receptor encephalitis. J Neurol. (2019) 266:450–60. doi: 10.1007/s00415-018-9153-8
64. Chou IJ, Wang HS, Lin JJ, Kuo CF, Lin KL, Chou ML, et al. Limbic encephalitis in Taiwanese children and adolescence: a single center study. Pediatr Neonatol. (2013) 54:246–53. doi: 10.1016/j.pedneo.2013.01.016
65. Lin JJ, Chou IJ, Lin KL, Wang HS. Childhood refractory focal epilepsy following acute febrile encephalopathy with anti-amphiphysin antibody. Eur J Neurol. (2011) 18:e70. doi: 10.1111/j.1468-1331.2010.03342.x
66. Spatola M, Sabater L, Planagumà J, Martínez-Hernandez E, Armangué T, Prüss H, et al. Encephalitis with mGluR5 antibodies: symptoms and antibody effects. Neurology. (2018) 90:e1964–72. doi: 10.1212/WNL.0000000000005614
67. Bien CG, Braig S, Bien CI. Antibodies against metabotropic glutamate receptor type 1 in a toddler with acute cerebellitis. J Neuroimmunol. (2020) 348:577366. doi: 10.1016/j.jneuroim.2020.577366
68. Spatola M, Petit Pedrol M, Maudes E, Simabukuro M, Muñiz-Castrillo S, Pinto AL et al. Clinical features, prognostic factors, and antibody effects in anti-mGluR1 encephalitis. Neurology. (2020) 95:e3012–25. doi: 10.1212/WNL.0000000000010854
69. Balint B, Jarius S, Nagel S, Haberkorn U, Probst C, Blöcker IM, et al. Progressive encephalomyelitis with rigidity and myoclonus: a new variant with DPPX antibodies. Neurology. (2014) 82:1521–8. doi: 10.1212/WNL.0000000000000372
70. Ye F, Fan C, Peng M, Liu S, Yu Y, Yang L. Anti-IgLON5 disease in a pediatric patient with Langerhans cell histiocytosis. Clin Chim Acta. (2021) 521:212–4. doi: 10.1016/j.cca.2021.07.008
71. Lancaster E, Dalmau J. Neuronal autoantigens–pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. (2012) 8:380–90. doi: 10.1038/nrneurol.2012.99
72. Dale RC, Gorman MP, Lim M. Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr Opin Neurol. (2017) 30:334–44. doi: 10.1097/WCO.0000000000000443
73. Budhram A, Dubey D, Sechi E, Flanagan EP, Yang L, Bhayana V, et al. Neural antibody testing in patients with suspected autoimmune encephalitis. Clin Chem. (2020) 66:1496–509. doi: 10.1093/clinchem/hvaa254
74. Prüss H. Autoantibodies in neurological disease. Nat Rev Immunol. (2021) 21:798–813. doi: 10.1038/s41577-021-00543-w
75. Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, et al. International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e674. doi: 10.1212/NXI.0000000000000674
76. Ruiz-García R, Muñoz-Sánchez G, Naranjo L, Guasp M, Sabater L, Saiz A, et al. Limitations of a commercial assay as diagnostic test of autoimmune encephalitis. Front Immunol. (2021) 12:691536. doi: 10.3389/fimmu.2021.691536
77. Gastaldi M, Nosadini M, Spatola M, Sartori S, Franciotta D. N-methyl-D-aspartate receptor encephalitis: laboratory diagnostics and comparative clinical features in adults and children. Expert Rev Mol Diagn. (2018) 18:181–93. doi: 10.1080/14737159.2018.1431124
78. Masi G, Spagni G, Campetella L, Monte G, Sabatelli E, Evoli A, et al. Assessing the role of a tissue-based assay in the diagnostic algorithm of autoimmune encephalitis. J Neuroimmunol. (2021) 356:577601. doi: 10.1016/j.jneuroim.2021.577601
79. Gadoth A, Segal Y, Paran Y, Aizenstein O, Alcalay Y. The importance of tissue-based assay in the diagnosis of autoimmune encephalitis. J Neurol. (2022) 2022:8. doi: 10.1007/s00415-022-10973-8
80. Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. (2018) 17:760–72. doi: 10.1016/S1474-4422(18)30244-8
81. Cavaliere E, Nosadini M, Pelizza MF, Ventura G, Toldo I, Sartori S. Anti-NMDAR encephalitis preceded by non-herpetic central nervous system infection: systematic literature review and first case of tick-borne encephalitis triggering anti-NMDAR encephalitis. J Neuroimmunol. (2019) 332:1–7. doi: 10.1016/j.jneuroim.2019.03.011
Keywords: neuronal surface antibody syndromes, autoimmune encephalitis, children, pediatrics, central nervous system
Citation: Ancona C, Masenello V, Tinnirello M, Toscano LM, Leo A, La Piana C, Toldo I, Nosadini M and Sartori S (2022) Autoimmune Encephalitis and Other Neurological Syndromes With Rare Neuronal Surface Antibodies in Children: A Systematic Literature Review. Front. Pediatr. 10:866074. doi: 10.3389/fped.2022.866074
Received: 30 January 2022; Accepted: 16 March 2022;
Published: 20 April 2022.
Edited by:
Erdem Tüzün, Istanbul University, TurkeyReviewed by:
Sudarshini Ramanathan, The University of Sydney, AustraliaSukhvir K. Wright, Aston University, United Kingdom
Copyright © 2022 Ancona, Masenello, Tinnirello, Toscano, Leo, La Piana, Toldo, Nosadini and Sartori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Ancona, Y2xhdWRpby5hbmNvbmFAYW9wZC52ZW5ldG8uaXQ=
†These authors have contributed equally to this work and share last authorship
 Claudio Ancona
Claudio Ancona Valentina Masenello1
Valentina Masenello1 Margherita Nosadini
Margherita Nosadini