
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 10 June 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.864115
This article is part of the Research TopicNeonatal Health in Low- and Middle-Income CountriesView all 41 articles
 Mubashir Hassan Shah1†
Mubashir Hassan Shah1† Samuel McAleese2*†
Samuel McAleese2*† Sandeep Kadam3
Sandeep Kadam3 Tushar Parikh3
Tushar Parikh3 Umesh Vaidya3
Umesh Vaidya3 Sonali Sanghavi4
Sonali Sanghavi4 Julia Johnson2,5
Julia Johnson2,5Objective: Treating neonatal bloodstream infections and meningitis in South Asia remains difficult given high rates of antimicrobial resistance (AMR). To evaluate changing epidemiology of neonatal infections, we assessed pathogen-specific and clinical features of culture-proven infections in neonates admitted to a neonatal intensive care unit (NICU) in Pune, India.
Materials and Methods: This retrospective cohort study was performed in the King Edward Memorial Hospital and Research Center NICU over 2 years between January 1, 2017 and December 31, 2018. We included all neonates admitted to the NICU with positive blood or cerebrospinal fluid cultures. Demographic, clinical, and microbiologic data were collected from the medical record. We reviewed antimicrobial susceptibility testing (AST) of all isolates.
Results: There were 93 culture-positive infections in 83 neonates, including 11 cases of meningitis. Fifteen (18%) neonates died. Gram-negative pathogens predominated (85%) and AST showed 74% resistance to aminoglycosides, 95% resistance to third/fourth generation cephalosporins, and 56% resistance to carbapenems. Resistance to colistin was present in 30% of Klebsiella pneumoniae isolates. Birth weight <1,000 g [odds ratio (OR) 6.0, p < 0.002], invasive respiratory support (OR 7.7, p = 0.001), and antibiotics at the time of culture (OR 4.2, p = 0.019) were associated with increased risk of mortality. Rates of AMR to all major antibiotic classes were similar between early onset and late onset infections. There was no association between carbapenem resistance and mortality.
Conclusion: In our NICU in India, there are high rates of AMR among Gram-negative pathogens that are predominantly responsible for infections. Given higher colistin resistance in this cohort than previously reported, hospitals should consider routinely testing for colistin resistance.
Neonatal sepsis causes a large proportion of all neonatal deaths worldwide (1). India reports higher rates of neonatal infections than other low- and middle-income countries (LMIC), and a recent review of neonatal infections in India reported a case fatality rate of 50% for culture-positive sepsis (2, 3). Compounding the difficulty and complexity of treating neonatal sepsis is antimicrobial resistance (AMR); as antibiotic use has increased and broader spectrum antibiotics are used more frequently, AMR has risen (4). Previously published longitudinal data from our neonatal intensive care unit (NICU) in western India from 2006 to 2008 already showed a worrying increase in multidrug-resistant (MDR) infection over the 2-year study period as well as persistently high rates of cephalosporin and carbapenem resistance (5). A more recent systematic review from 2019 on AMR in neonatal sepsis in South Asia (including 69 studies from India) shows that these challenges are ongoing and more widespread (6). This review reported rates of resistance between 67 and 86% to first-line drugs recommended by the World Health Organization (WHO), such as ampicillin, gentamicin, and third-generation cephalosporins, as well as high (50–70%) degree of MDR in isolates throughout India and South Asia.
AMR surveillance studies guide institutional infection prevention practices, help national efforts to standardize recommendations, and inform future implementation research. These studies can also inform practices outside the regions and countries being studied. Human travel and globalization have contributed to the global spread of select mutations that confer colistin resistance, reinforcing the notion that AMR is not only a local or regional concern (7). Given the worsening trends in AMR, ongoing surveillance is vital to better assess risk factors for infection and the appropriateness of commonly used empiric antibiotic regimens. The objective of this study was to evaluate pathogen-specific and clinical features of culture-proven infections in neonates admitted to the NICU at a tertiary care healthcare facility in western India.
This retrospective cohort study was conducted at King Edward Memorial Hospital & Research Center (KEMHRC), a 550-bed multispecialty tertiary care teaching hospital in urban Pune, India, with a 50-bed Level III NICU. As part of the largest non-governmental hospital in Pune and a major referral center for the state of Maharashtra, the Department of Obstetrics and Gynecology at KEMHRC performs over 3,000 deliveries annually including high-risk deliveries. Approximately 1,000 infants—predominantly born at KEMHRC—are admitted to the NICU annually, although the NICU also serves as a referral center for smaller facilities throughout Maharashtra. This two-year study included all neonates admitted to the NICU between January 1, 2017, and December 31, 2018 with a documented positive culture from blood or cerebrospinal fluid (CSF). Culture specimens were collected and processed according to routine laboratory standards in KEMHRC's nationally accredited microbiology laboratory. Data were manually extracted from the hospital's electronic medical record using a standardized case report form. Demographic, birth, and clinical data were collected, including gestational age, birth weight, presence of central line at time of positive culture, relevant clinical diagnoses, and disposition. Microbiologic data were collected, including organism isolated, results of antibiotic susceptibility testing (AST), and time to culture positivity.
We examined all culture-proven infections in neonates. Culture-proven infections were defined as positive blood or CSF culture. Blood and CSF samples of minimum 1 ml were collected using aseptic technique; samples were immediately transported to the in-hospital laboratory for automated processing using the BD FX™ system (BD, Franklin Lakes, NJ, USA). Cultures that tested positive were subcultured on blood agar, MacConkey agar, and chocolate agar (CSF cultures only). Positive cultures with the same organism from both blood and CSF were considered as part of the same culture-positive infection. Multiple positive cultures from the same neonate were considered part of the same culture-positive infection unless a distinct organism was isolated, or the culture was drawn more than 7 days after the prior culture. Early onset blood stream infection (BSI) was defined as positive blood culture in the first 72 h of life, and early onset meningitis was defined as positive CSF culture in the first 72 h of life. Late onset BSI and meningitis were defined as positive culture from blood or CSF, respectively, after 72 h of life. Necrotizing enterocolitis (NEC) was defined as stage II or greater on modified Bell's criteria (8). Retinopathy of prematurity (ROP) was defined as any stage per International Committee for Classification of Retinopathy of Prematurity (ICROP) criteria (9). Intraventricular hemorrhage was defined according to the Papile grading classification (10). AST for all antibiotics was performed on the Vitek 2® system (BioMérieux, Inc, Hazelwood, MO, USA). Susceptibility testing results were interpreted according to minimum inhibitory concentration breakpoints set by the Clinical and Laboratory Standards Institute (CLSI, Malvern, PA, USA). Antibiotic resistance was defined as resistance or intermediate susceptibility on AST; resistance to a class of antibiotics was defined as resistance or intermediate susceptibility to any antimicrobial in that class.
Descriptive analysis was performed on the study population. Statistical analysis was performed with Stata 15.1 (StataCorp, College Station, TX, USA). Univariate regression using a Pearson χ2-test was used to assess the associations between clinical risk factors, carbapenem resistance, and mortality.
This study was approved by the KEMHRC Ethics Committee (ID No. 2108).
There were 113 positive cultures during the study period, 101 from blood samples and 12 from CSF samples (see Figure 1). Three blood samples were excluded as contaminants [Brevundimonas spp. (n = 1), Granulicatella spp. (n = 1), Rhizobium spp. (n = 1)]. Six blood cultures and one CSF culture from four neonates were positive within 7 days of a prior culture from the same source with the same organism and were considered as part of the same culture-positive infection. Of 11 remaining positive CSF cultures, 10 resulted with the same pathogen as a positive blood culture drawn on the same day and were considered part of the same culture-positive infection. One infant had a positive CSF culture without concurrent positive blood culture. After exclusions, there were 93 culture-positive cases of BSI or meningitis in 83 neonates. Fifty-three percent (49/93) of all infections occurred in males and 84% (78/93) occurred in inborn neonates (Table 1). The median age at birth in completed gestational weeks was 31 [interquartile range (IQR) 29–33] and the median birth weight was 1,340 g (IQR 1,040–1,750). Twenty percent (17/83) of neonates were extremely low birth weight (ELBW, or <1,000 g at birth). Thirty-one percent (26/83) of neonates required invasive ventilation during their hospitalization. Median length of stay was 29 days (IQR 15–41). Fifteen (18%) infants died. Three of four neonates (75%) with documented fungal infections died. Neonates with repeat infections were smaller at birth (median birth weight 1,028 g, IQR 863–1,265) and had prolonged hospitalizations (median length of stay 49 days, IQR 32–62). Of positive cultures from the same neonates, 50% (5/10) were unique infections with the same organism, but minimal changes to susceptibility patterns were noted on AST. The median time between unique infections for these neonates was 14 days (IQR 9–19).
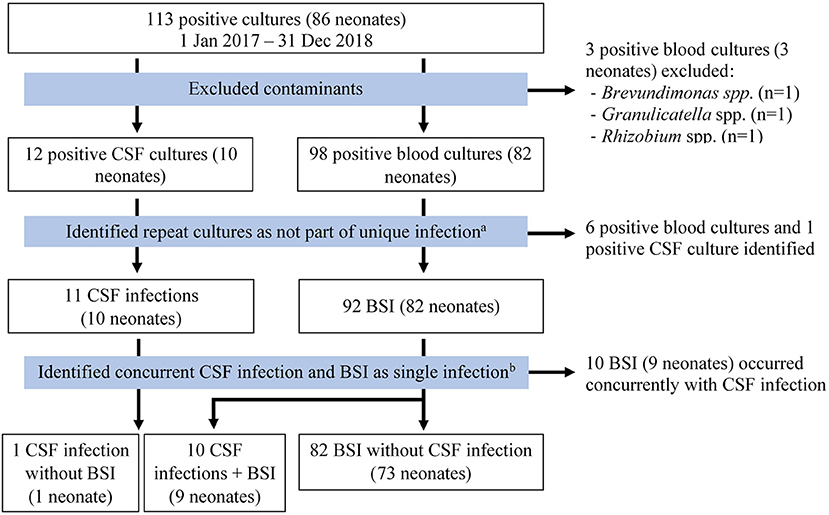
Figure 1. Flow diagram used to identify unique infections. Meningitis and CSF infection were used interchangeably. aMultiple positive cultures from the same neonate were considered part of the same culture-positive infection unless a distinct organism was isolated, or the culture was drawn more than 7 days after the most recent positive culture. bPositive cultures with the same organism from both blood and CSF were considered as part of the same culture-positive infection. BSI, bloodstream infection; CSF, cerebrospinal fluid.
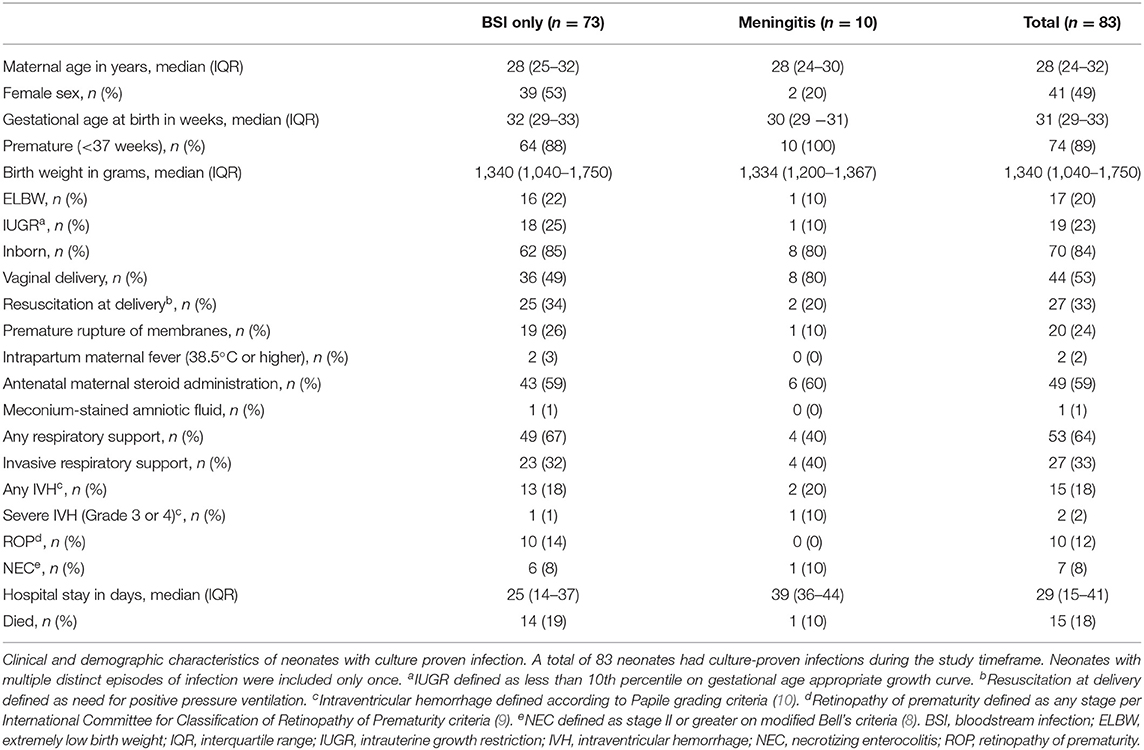
Table 1. Clinical and demographic characteristics of neonates with culture-positive bloodstream infections or meningitis.
The majority of infections were late onset (82%, 76/93) with a median age at time of positive culture of 7 days (IQR 4–11) (Table 2). Central lines were present at time of culture in 53% (49/93) of cases. Of infections with a central line present at time of positive culture, the median for total duration of central line use was 6 days (IQR 4–12). The median time to culture positivity (from incubation to growth) was 22 h (IQR 19–25).
Gram-negative infections were predominant (85%, 79/93) (Table 3). The most commonly isolated Gram-negative pathogens were Klebsiella pneumoniae (52% of all infections, 48/93), Acinetobacter spp. (15%, 14/93), and Escherichia coli (9%, 8/93). Enterococcus faecalis accounted for 60% of Gram-positive infections (6/10) and 6% of all infections (6/93). There were four fungal infections, all caused by Candida albicans.
Among the 10 neonates with meningitis, one neonate had two separate CSF infections (18 days apart). Neonates with meningitis were predominantly male (80%, 8/10); all 10 (100%) were premature. Of 11 cases of meningitis, 82% were late onset; the median day of life for a positive CSF culture was 10 days (IQR 4–20). Infants with meningitis had prolonged hospitalizations compared to the entire cohort (39 days, IQR 36–44). Only one (10%) neonate with meningitis died. Pathogens isolated from CSF cultures included E. faecalis (n = 3, 27%), K. pneumoniae (n = 3, 27%), Acinetobacter spp. (n = 2, 18%), Serratia marcescens (n = 1, 9%), Elizabethkingia spp. (n = 1, 9%), and Moraxella lacunata (n = 1, 9%). Only one infant, who developed S. marcescens meningitis on day of life three, did not have concurrent BSI with the same organism.
Among pathogens causing BSI or meningitis, AMR was common. For all Gram-negative isolates, resistance to commonly used empiric antibiotic classes such as aminoglycosides and third-generation cephalosporins was reported in 74% (57 of 77 isolates tested) and 95% (73/77), respectively (Table 4). Gentamicin was less effective than amikacin; 67% (58/87) of samples were resistant to gentamicin compared to 26% (17/66) that were resistant to amikacin. Carbapenem resistance was 56% (42/75); resistance to fluoroquinolones was 91% (70/77). Resistance to colistin was reported in six of 44 isolates tested (14%), all six of which were K. pneumoniae, corresponding to 30% colistin resistance for the most commonly isolated pathogen in our cohort.
Resistance to carbapenems was seen in 46% (6/13) of early onset infections and 58% (36/62) of late onset infections. Although rates of resistance to aminoglycosides were similar between early onset and late onset infections (67%, 11/16 vs. 74%, 53/72, respectively), this similarity was less notable for amikacin. Resistance to amikacin was seen in 8% (1/12) early onset infections and 29% (16/55) late onset infections. Rates of resistance to 3rd/4th generation cephalosporins (86%, 12/14 vs. 95%, 61/64), fluoroquinolones (93%, 13/14 vs. 91%, 58/64), and colistin (14%, 1/7 vs. 14%, 5/37) were similar in early onset infections and late onset infections.
Although Gram-negative infections were predominant, there were 10 Gram-positive infections and four blood cultures positive for C. albicans. All E. faecalis samples were susceptible to vancomycin and linezolid, but resistant to clindamycin. The four fungal isolates were susceptible to all antifungal therapies tested (amphotericin-B, caspofungin, fluconazole, flucytosine, and micafungin).
Among clinical characteristics of neonates, only ELBW [odds ratio (OR) 6.0, confidence interval (CI) 1.9–18.7, p = 0.002], invasive respiratory support (OR 7.7, CI 2.4–24.9, p = 0.001), and antibiotic use at time of culture (OR 4.2, CI 1.3–14.2, p = 0.019) were associated with an increased risk of mortality (Table 5). There were no deaths among neonates with colistin-resistant infections. Carbapenem resistance was not associated with mortality among all neonates. A sub-analysis of only preterm neonates (n = 74) was done. Clinical characteristics and AST testing of the 84 infections were similar compared to the entire cohort. In preterm infants, the presence of a central line was also associated with mortality (OR 5.4, CI 1.1–25.8, p = 0.037).
Gram-negative infections predominate in our study, with 52% of all infections caused by K. pneumoniae and 14% caused by Acinetobacter spp. This epidemiologic profile mirrors prior hospital data reported in India where Gram-negative infections (especially K. pneumoniae and Acinetobacter spp.) account for 60–70% of all culture proven infections in neonates admitted to NICUs (3, 6). The high rate of K. pneumoniae infections in our study aligns with prior data from our institution (41% in 2006–2008) (5).
This study includes detailed AST data from a large number of isolates. Unfortunately, we report a high incidence of AMR to commonly used and broad-spectrum antibiotics. The majority of isolates were resistant to third-generation cephalosporins, aminoglycosides, and carbapenems. Resistance to carbapenems varied widely depending on the organism isolated. Acinetobacter spp., a major health-care associated pathogen in India capable of acquiring resistance, was nearly universally resistant to carbapenems in our study (11). There were similar levels of resistance to all drugs within most antibiotic classes, with the exception of aminoglycosides; isolates showed more susceptibility to amikacin, compared to gentamicin. This discrepancy exists in other areas of India as well as in other LMIC countries (12–14). The high rate of resistance to gentamicin likely is influenced by widespread use driven by its inclusion as a first-line antibiotic for neonatal sepsis in the WHO's guidelines (15). Institutions such as our own, which are routinely using amikacin as the preferred aminoglycoside instead of gentamicin, may find themselves limited in the future as AMR to efficacious antibiotics increases with increased use. This highlights the need not only to select appropriate antibiotics, but to prioritize antimicrobial stewardship activities to reduce overall antibiotic use.
Rates of resistance to antibiotic classes or single agents (excepting amikacin) were similar among early onset infections and late onset infections. A higher rate of resistance to amikacin in late onset infections may suggest that nosocomial spread contributes to rising rates of AMR or that early exposure to amikacin confers resistance in later infections. Transmission of AMR infections may be driven by multiple reservoirs of transmission, including maternal colonization with community-acquired pathogens followed by vertical transmission or rapid colonization after birth and subsequent infection. Better understanding of the differences in epidemiology between early and late onset neonatal infections is required to direct infection prevention practices and antimicrobial stewardship initiatives. At KEMHRC, multidisciplinary infection prevention and control (IPC) efforts are supported by dedicated nursing staff and include a focus on staff education, hand-hygiene, environmental surveillance, and outbreak investigations.
Notably, a central line was present more often and used for longer durations in late-onset BSIs compared to early-onset BSIs. Our study was not designed to assess whether the reported BSIs were central-line associated blood stream infections, but highlights an opportunity for tailoring IPC practices. Central lines are a known risk factor for BSI and that risk changes over the lifetime of the central line. At KEMHRC, umbilical catheters are not used beyond 7 days of life, and infants with expected prolonged need for central access preferentially undergo peripherally inserted central catheter (PICC) placement. IPC practices should account for the different risks associated with different phases of central line use (insertion, compared to maintenance). Some universal practices which apply to all phases (and all patients) include effective hand hygiene before and after every patient encounter and aseptic technique when inserting or accessing a central line. Daily discussion of line necessity and prompt removal of central lines when appropriate is an integral component of central line associated BSI prevention (16).
Prior to the rise in difficult-to-treat, MDR infections, colistin, a polymyxin antibiotic with activity against Gram-negative infections, was routinely avoided given paucity of data in neonates and concerns for difficult pharmacokinetics, nephrotoxicity, and neurotoxicity (17, 18). In India, clinicians over the past 10 years are turning more frequently to colistin as part of routine practice (19). Worryingly, our study showed higher rates of colistin resistance (14% overall, 30% in K. pneumoniae isolates) than previously reported in the literature on neonates in India (0–10%) (3, 20–25). Polymyxins are classified as a “reserve” antibiotic class by the WHO (26). Unfortunately, there is mounting evidence that increased colistin use drives colistin resistance, especially in K. pneumoniae. Epidemiologic data from a 2017 report by the European Center for Disease Prevention and Control on polymyxin use in 30 European countries reported an association between total consumption of polymyxin antibiotics and resistance in K. pneumoniae (27). In studies of hospitalized adult patients, use of colistin was a risk factor for infection with colistin resistant K. pneumoniae and there are reports of patients developing colistin resistance during colistin therapy (28–30). More concerning, once resistance in K. pneumoniae isolates develops, resistant infections have been shown to spread rapidly via clonal spread and biofilm formation, including in neonates (31–34).
Limiting colistin use and effective IPC programs to prevent spread of resistance are necessary to prevent further increases in resistance. Even when colistin use is appropriately limited to MDR infections, how it is used can potentially create conditions that lead to increased resistance. While colistin monotherapy is traditionally used, some question whether a combination regimen that includes colistin with possible synergistic effects would be more effective or even protective against colistin resistance (35, 36). Adequate dosing in MDR infections remains key as sub-optimal dosing or prolonged therapy has been shown to increase the risk for acquired resistance, including in neonates (30). Addressing the rising trend in colistin resistance will require comprehensive approaches including stewardship programs, IPC programs, and improved education about colistin use. Important to all of these initiatives will be improved surveillance for colistin resistance. Colistin resistance was not universally tested for in our study. However, given the increased risk of emerging resistance, clinicians and microbiologists should consider routinely testing for colistin on AST.
Limitations for this study include its retrospective nature, focus on a single center in western India, and lack of confirmatory cultures for potential contaminant pathogens. In our study, Brevundimonas spp., Granulicatella spp., and Rhizobium spp. were excluded as likely contaminants. As prior case reports have reported true infections in neonates with these pathogens, we may have excluded potential real infections (37–39). Coagulase negative staphylococcus (CoNS) isolates were included as infections despite lack of confirmatory culture given the known role of CoNS as a neonatal pathogen, especially in late onset neonatal infections (40).
This study adds awareness to the growing concern about rising AMR in neonatal infections in India. The lack of narrow empiric regimens with adequate coverage for neonatal sepsis leads to clinicians using broader spectrum agents as empiric therapy, which in turn, leads to increasing resistance. As use of last-resort antibiotics increases, clinicians must remain vigilant in prescribing practices. Standardization of empiric antibiotic therapy, avoiding overlapping therapy, and timely reevaluation of the antibiotic regimen (including narrowing coverage when appropriate and stopping antibiotics in neonates with negative cultures) are practical areas of focus for neonatal practitioners. Strengthening IPC practices and antimicrobial stewardship programs will be paramount in reducing the burden of AMR neonatal sepsis in India and other LMIC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by King Edward Memorial Hospital Research Center Ethics Committee. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
MS: conceptualization, methodology, data collection, and data management. SM: literature review, data analysis, and writing—original draft, review, and editing. SK: conceptualization. TP: conceptualization and data collection. UV: project administration. SS: data collection. JJ: conceptualization, data analysis, and writing—review and editing. All authors have approved the submitted version.
JJ was supported by the National Institutes of Health (K23HD100594).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors wish to thank Shaoming Xiao for his technical expertise in preparation for data analysis in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.864115/full#supplementary-material
1. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. (2015) 385:430–40. doi: 10.1016/S0140-6736(14)61698-6
2. Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. (2018) 6:223–30. doi: 10.1016/S2213-2600(18)30063-8
3. Investigators of the Delhi Neonatal Infection Study (DeNIS) Collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health. (2016) 4:e752–60. doi: 10.1016/S2214-109X(16)30148-6
4. Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. (2013) 13:1057–98. doi: 10.1016/S1473-3099(13)70318-9
5. Tagare A, Kadam S, Vaidyaa U, Deodharb J, Panditb A. Multidrug resistant Klebsiella pneumoniae in NICU - what next? Trend of antibiotic resistance. J Pediatr Infect Dis. (2010) 5:119–24. doi: 10.3233/JPI-2010-0245
6. Chaurasia S, Sivanandan S, Agarwal R, Ellis S, Sharland M, Sankar MJ. Neonatal sepsis in South Asia: huge burden and spiralling antimicrobial resistance. BMJ. (2019) 364:k5314. doi: 10.1136/bmj.k5314
7. Wang R, van Dorp L, Shaw LP, Bradley P, Wang Q, Wang X, et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat Commun. (2018) 9:9. doi: 10.1038/s41467-018-03205-z
8. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/S0031-3955(16)34975-6
9. Members of an International Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. (2005) 123:991–9. doi: 10.1001/archopht.123.7.991
10. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
11. Hsu LY, Apisarnthanarak A, Khan E, Suwantarat N, Ghafur A, Tambyah PA. Carbapenem-resistant Acinetobacter baumannii and enterobacteriaceae in South and Southeast Asia. Clin Microbiol Rev. (2017) 30:1–22. doi: 10.1128/CMR.00042-16
12. Viswanathan R, Singh AK, Mukherjee S, Mukherjee R, Das P, Basu S. Aetiology and antimicrobial resistance of neonatal sepsis at a tertiary care Centre in Eastern India: a 3 year study. Indian J Pediatr. (2011) 78:409–12. doi: 10.1007/s12098-010-0272-1
13. Solomon S, Akeju O, Odumade OA, Ambachew R, Gebreyohannes Z, Van Wickle K, et al. Prevalence and risk factors for antimicrobial resistance among newborns with gram-negative sepsis. PLoS ONE. (2021) 16:e0255410. doi: 10.1371/journal.pone.0255410
14. Tewari VV, Jain N. Monotherapy with amikacin or piperacillin-tazobactum empirically in neonates at risk for early-onset sepsis: a randomized controlled trial. J Trop Pediatr. (2014) 60:297–302. doi: 10.1093/tropej/fmu017
15. Fuchs A, Bielicki J, Mathur S, Sharland M, Van Den Anker JN. Reviewing the WHO guidelines for antibiotic use for sepsis in neonates and children. Paediatr Int Child Health. (2018) 38:S3–15. doi: 10.1080/20469047.2017.1408738
16. Milstone AM, Reich NG, Advani S, Yuan G, Bryant K, Coffin SE, et al. Catheter dwell time and CLABSIs in neonates with PICCs: a multicenter cohort study. Pediatrics. (2013) 132:e1609–15. doi: 10.1542/peds.2013-1645
17. Nakwan N, Chokephaibulkit K, Imberti R. The use of colistin for the treatment of multidrug-resistant Gram-negative infections in neonates and infants: a review of the literature. Pediatr Infect Dis J. (2019) 38:1107–12. doi: 10.1097/INF.0000000000002448
18. Antachopoulos C, Iosifidis E. Colistin use in neonates and children with infections due to Carbapenem-resistant bacteria. Pediatr Infect Dis J. (2017) 36:905–7. doi: 10.1097/INF.0000000000001655
19. Dudeja S. Neonatal sepsis: treatment of neonatal sepsis in multidrug-resistant (MDR) infections: part 2. Indian J Pediatr. (2020) 87:122–4. doi: 10.1007/s12098-019-03152-7
20. Jajoo M, Manchanda V, Chaurasia S, Sankar MJ, Gautam H, Agarwal R, et al. Alarming rates of antimicrobial resistance and fungal sepsis in outborn neonates in North India. PLoS ONE. (2018) 13:e0180705. doi: 10.1371/journal.pone.0180705
21. Thakur S, Thakur K, Sood A, Chaudhary S. Bacteriological profile and antibiotic sensitivity pattern of neonatal septicaemia in a rural tertiary care hospital in North India. Indian J Med Microbiol. (2016) 34:67–71. doi: 10.4103/0255-0857.174108
22. Lamba M, Sharma R, Sharma D, Choudhary M, Maheshwari RK. Bacteriological spectrum and antimicrobial susceptibility pattern of neonatal septicaemia in a tertiary care hospital of North India. J Matern Fetal Neonatal Med. (2016) 29:3993–98. doi: 10.3109/14767058.2016.1152251
23. Dharmapalan D, Shet A, Yewale V, Sharland M. High reported rates of antimicrobial resistance in Indian neonatal and pediatric blood stream infections. JPIDS. (2017) 6:e62–8. doi: 10.1093/jpids/piw092
24. Mahich S, Angurana SK, Sundaram V, Gautam V. Epidemiology, microbiological profile, and outcome of culture positive sepsis among outborn neonates at a tertiary hospital in Northern India. J Matern Fetal Neonatal Med. (2021) 1–9. doi: 10.1080/14767058.2021.1939300. [Epub ahead of print].
25. Dhir SK, Sundaram V, Gautam V, Munda VS, Tiewsoh JB, Angurana SK, et al. Microorganisms profile and antimicrobial resistance pattern in outborn neonates in northern India: a hospital-based observational study. J Trop Pediatr. (2021) 67:fmab068. doi: 10.1093/tropej/fmab068
26. World Health Organization. The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. Geneva (2019). Available online at: https://list.essentialmeds.org/ (beta version 1.0) (accessed December 11, 2021).
27. European Centre for Disease Prevention and Control (ECDC), European Food Safety Authority (EFSA), European Medicines Agency (EMA). ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals. EFSA J. (2017) 15:e04872. doi: 10.2903/j.efsa.2017.4872
28. Matthaiou DK, Michalopoulos A, Rafailidis PI, Karageorgopoulos DE, Papaioannou V, Ntani G, et al. Risk factors associated with the isolation of colistin-resistant Gram-negative bacteria: a matched case-control study. Crit Care Med. (2008) 36:807–11. doi: 10.1097/CCM.0B013E3181652FAE
29. Bogdanovich T, Adams-Haduch JM, Tian GB, Nguyen MH, Kwak EJ, Muto CA, et al. Colistin-resistant Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin Infect Dis. (2011) 53:373–6. doi: 10.1093/cid/cir401
30. Ah YM, Kim AJ, Lee JY. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents. (2014) 44:8–15. doi: 10.1016/j.ijantimicag.2014.02.016
31. Singh SK, Gupta M. blaOXA-48 carrying clonal colistin resistant-carbapenem resistant Klebsiella pneumoniae in neonate intensive care unit, India. Microbial Pathog. (2016) 100:75–7. doi: 10.1016/j.micpath.2016.09.009
32. Rossi GI, Ferreira ML, Araujo BF, Campos PA, Royer S, Batistão DW, et al. Outbreaks of colistin-resistant and colistin-susceptible KPC-producing Klebsiella pneumoniae in a Brazilian intensive care unit. J Hosp Infect. (2016) 94:322–9. doi: 10.1016/j.jhin.2016.08.019
33. Battikh H, Harchay C, Dekhili A, Khazar K, Kechrid F, Zribi M, et al. Clonal spread of colistin-resistant Klebsiella pneumoniae coproducing KPC and VIM carbapenemases in neonates at a Tunisian university hospital. Microb Drug Resist. (2016) 23:468–72. doi: 10.1089/mdr.2016.0175
34. Goel G, Hmar L, Sarkar De M, Bhattacharya S, Chandy M. Colistin-resistant Klebsiella pneumoniae: report of a cluster of 24 cases from a new oncology center in eastern India. Infect Control Hosp Epidemiol. (2014) 35:1076–7. doi: 10.1086/677170
35. Alanezi G, Almulhem A, Aldriwesh M, Bawazeer M. A triple antimicrobial regimen for multidrug-resistant Klebsiella pneumonia in a neonatal intensive care unit outbreak: a case series. J Infect Public Health. (2021) 15:138–41. doi: 10.1016/j.jiph.2021.10.008
36. Vardakas KZ, Mavroudis AD, Georgiou M, Falagas ME. Intravenous colistin combination antimicrobial treatment vs. monotherapy: a systematic review and meta-analysis. Int J Antimicrob Agents. (2018) 51:535–47. doi: 10.1016/j.ijantimicag.2017.12.020
37. Ryan MP, Pembroke JT. Brevundimonas spp: emerging global opportunistic pathogens. Virulence. (2018) 9:480–93. doi: 10.1080/21505594.2017.1419116
38. Bizzarro MJ, Callan DA, Farrel PA, Dembry L-M, Gallagher PG. Granulicatella adiacens and early-onset sepsis in neonate. Emerg Infect Dis. (2011) 17:1971–3. doi: 10.3201/eid1710.101967
39. Khan S, Al-Sweih N, Othman AH, Dhar R. Bacteremia caused by Rhizobium radiobacter in a preterm neonate. Indian J Pediatr. (2014) 81:191–2. doi: 10.1007/s12098-013-1012-0
Keywords: neonatal sepsis, bloodstream infection, meningitis, antimicrobial resistance, colistin resistance
Citation: Shah MH, McAleese S, Kadam S, Parikh T, Vaidya U, Sanghavi S and Johnson J (2022) Emerging Antibiotic Resistance Patterns in a Neonatal Intensive Care Unit in Pune, India: A 2-Year Retrospective Study. Front. Pediatr. 10:864115. doi: 10.3389/fped.2022.864115
Received: 28 January 2022; Accepted: 11 May 2022;
Published: 10 June 2022.
Edited by:
K. C. Ashish, Uppsala University, SwedenCopyright © 2022 Shah, McAleese, Kadam, Parikh, Vaidya, Sanghavi and Johnson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel McAleese, c21jYWxlZTFAamguZWR1
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.