
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 12 May 2022
Sec. Pediatric Pulmonology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.856840
This article is part of the Research TopicInsights in Pediatric Pulmonology: 2021View all 15 articles
Background: Many patients with cystic fibrosis (CF) wheeze, and are dubbed as having CF-asthma. Understanding the determinants of such wheezing may avoid unnecessary treatments and open newer treatment avenues.
Objectives: Main: To evaluate the prevalence and characteristics of wheezing and a positive bronchodilatory response (BDR) in children with CF. Secondary: To identify the predictive markers and the impact of current wheezing a positive BDR.
Methods: A retrospective single-center study in children with CF. We determined the characteristics of physician-reported wheeze in patients <6 years, and a BDR in patients aged 6–17 years. Anthropometric, lung function, laboratory, genetic and microbiological data were recorded in all groups. Variables were compared using the Chi2 and Student t-tests, and ANOVA.
Results: 125 preschool and 69 school-aged children and adolescents with CF were included in the study. 71.2% of patients <6 years of age had had at least one episode of wheezing: 26.3% of patients were Transient Early Wheezers, 12.6% Late Onset Wheezers and 37.9% were Persistent Wheezers. The prevalence of a positive BDR was 73.5, 48.5, and 52.9% in the 6–8 years, 10–12 years, and 15–17 years age groups, respectively. Allergic factors were not predictive of wheezing in preschoolers. In the 6–8 years age group, the sum of wheal diameters of allergic skin prick tests (SPT, house dust mite + cat + dog dander) was greater in those with a BDR vs. no BDR (4 [2.0–8.8] vs. 1 [0–7.0] mm, p = 0.01). The presence of Pseudomonas aeruginosa in the bronchial secretions before 3 years of age was not significantly associated with either the presence of wheezing at the age of 6 years or a BDR in school-aged children and adolescents. The proportion of homozygous p.F508del patients was significantly lower in the group of patients who had wheezed by 6 years of age (60% vs. 72.7%, p = 0.009), but higher in the 6–8 years old group with a BDR vs. no BDR (64% vs. 36%, p = 0.04). Current wheezers at 6 years had a lower mean FEV1 vs. the non-current wheezers (91.5 ± 4.4% vs. 100.9 ± 2.4%; p = 0.047). Similarly, forced vital capacity (FVC) was significantly lower in the 6–8 years old group with BDR vs. no BDR (85 ± 19 vs. 101 ± 21%, p = 0.015).
Conclusion: Wheezing and BDR are very frequent findings in children with CF. Current wheeze at the age of 6 years was associated with worse lung function. Labeling wheezing in CF as “CF-Asthma” is misleading since the determinants are different, and may lead to inappropriate prescriptions of inhaled steroids.
Cystic fibrosis (CF) is one of the most frequent and severe genetic diseases affecting European populations. Clinical manifestations can vary from mild to severe forms, and prognosis depends to a large extent on the severity of respiratory impairment. This associates chronic bronchial inflammation and infection, and the presence of very thick mucus, a consequence of the absence or dysfunction of the CFTR channels present on the apical surface of bronchial epithelial cells. Bronchiectasis will ensue, followed by chronic respiratory failure.
Asthma and CF are both chronic inflammatory diseases of the lung, but their pathophysiology is quite different. Airway inflammation is mostly eosinophilic in allergic asthma, whereas it is predominantly neutrophilic in CF (1, 2). Wheezing is very common in asthma. A prevalence of 30–50% has been reported in preschool children, in whom less than half will undergo persistent symptoms (1). Wheezing, whether in patients with asthma or CF, is classically the consequence of airway obstruction due to inflammation, bronchospasm, non-discharged secretions and/or small airways.
Morphologically, the bronchi of patients with asthma and CF share common features, such as the development of bronchial remodeling, including hyperplasia of the bronchial smooth muscle (3, 4). Bronchial hyperresponsiveness (BHR) is also observed in both conditions. This is usually demonstrated by the onset of bronchoconstriction induced by a specific or non-specific stimulus, and/or reversibility after exposure to a bronchodilator (5). Up to half of all patients with CF have measurable BHR, which does not appear to be related to the patient’s atopic status (6–8). According to Weinberger BHR in CF shows characteristics which are different from asthma, e.g., bronchodilation on exertion (9).
Alterations in the signaling pathways regulating airway smooth muscle (ASM) contractility in CFTR-deficient patients have been described. As an example, persistent bacterial infection, especially by Pseudomonas aeruginosa, stimulates the release of interleukin-8 from the airway epithelium, resulting in neutrophilic inflammation. Increased neutrophilia and CFTR-deficient T-helper cells create an inflammatory environment characterized by high levels of Tumor Necrosis Factor (TNF). The presence of high levels of TNFα, Interleukin-8 and Interleukin-13, may contribute to increased ASM contractility which participates in wheezing and BHR (10).
Predictive indices and scores have been proposed to identify which of the early non-CF wheezing children are at risk of persistence (of wheezing) at school age. Two of these have been validated in different populations: the PIAMA score (11, 12) and the Asthma Predictive Index (API) (13). A positive API index indicates a 2.6–9.8 greater risk of current asthma during school age (6 and 13 years) (14). According to the PIAMA score the risk of asthma from age 6 to 8 years is as follows: <5% (score 0–7); 6–22% (score 8–15); 25–60% (score 16–23) (12).
There is a lack of such data in children with CF. Our primary objective was therefore to evaluate the prevalence and characteristics of wheezing and a bronchodilatory response (BDR) to short-acting beta-agonists as a marker of airway lability in a population of children with CF followed in a single large CF center. The secondary objectives were to identify the factors associated with wheezing and a positive BDR, and to determine the impact of current wheezing at the age of 6 years.
We conducted a retrospective, analytic, single-center study in children with CF, aged less than 18 years, followed at the Regional Pediatric CF-center in Bordeaux University Hospital, France. In this retrospective study French regulatory legislation requires that the protection of personal data is ensured, and this was applied in our study (15). The Bordeaux University Hospital Institutional Research Ethics Board authorized the conduct and publication of this research (Reference CERBDX-2022-03).
The first part of the study involved children aged less than 6 years, recruited from February 2016 to June 2016. The primary objective was to determine the overall frequency of wheezing in such children. The secondary objectives were to describe the characteristics of wheezing according to the TUCSON clinical phenotypes as described by Martinez et al. (16), to determine predictive markers for the persistence of wheezing at school age (6 years), and to describe the consequences of wheezing particularly with respect to lung function at 6 years. Children were assigned to four categories according to their history of wheezing: those who had no recorded lower respiratory tract illness with wheezing during the first 3 years of life and had no wheezing at 6 years of age (NW); those with at least one lower respiratory tract illness with wheezing during the first 3 years of life but no wheezing at 6 years of age (those with transient early wheezing, TW); those who had no lower respiratory tract illness with wheezing during the first 3 years of life but who had wheezing at 6 years of age (those with wheezing of late onset, LW); and those who had at least one lower respiratory tract illness with wheezing in the first 3 years of life and had wheezing at 6 years of age (those with persistent wheezing, PW) (16).
Data collected over the first 6 years of life were the following: Demographic (age, weight, and gestational age), family (asthma and atopy, in utero and postnatal cigarette smoke exposure), respiratory function (best lung function (LFT) results between age 6 and 7 years), immunological (allergic Skin Prick Tests and total IgE or specific IgE to the most common respiratory allergens: (house dust mite, cat or dog dander)), bacteriological (pathogens found in the sputum) and therapeutic (treatments prescribed for respiratory symptoms. From these data, we scored the Asthma Predictive Index, the PIAMA score and determined the “TUCSON” wheezing phenotypes (16).
The second part of the study involved children aged 6–17 years from 2002 to 2011 and focused on the presence of a BDR. The preschool children shown in the above section were not included in this >6 years age school age study group. Three age ranges were selected: ≥6 years to <8 years, ≥10 years to <12 years, and ≥15 years to <17 years. For each patient, anthropometric, clinical, allergic (total IgE, blood eosinophils, and allergic skin tests), bacteriological, genetic, and spirometric data were collected. LFT criteria regarding bronchial obstruction and the BDR were reported according to the ATS/ERS recommendations. The criteria for a positive BDR were: improvement in ppFEV1 by at least 12%, of ppFEF25–75 or ppFEV1 by at least 35%; decrease in ppRV (and/or ppRV/TLC) by at least 20%, decrease in Raw by at least 35%. The BDR was analyzed either based solely on ppFEV1 criteria, or on all the above-mentioned criteria (positive BDR if at least one of the functional reversibility parameter was present).
Current wheezing at 6 years of age was defined as children who had presented at least one episode of wheezing in the previous 12 months.
Regarding continuous variables, data are presented as medians and interquartile ranges, or means and standard deviations, according to their distribution. The statistical analysis was performed using NCSS software (Kaysville, Utah). The qualitative variables were compared using the Chi2 test. Quantitative variables were compared using a Student’s t-test if two groups were studied and by analysis of variance (ANOVA) in the presence of >2 groups (with a Bonferroni test to determine statistical differences between groups). A p-value < 0.05 was considered statistically significant.
One hundred and twenty-five patients aged less than 6 years of age and 69 patients above 6 years of age were included in the study.
Mean age of patients at the time of the retrospective data collection was 9.9 ± 4.9 years. The M:F sex ratio was 1:1.2 [girls: 69/125 (55.2%)]. These patients were full-term babies in 92% of cases, exposed to cigarette smoke in utero and postnatally in 25.6% and 59.5% of cases, respectively, and had at least one asthmatic parent in 20.3% (25/123) of cases. The p.Phe508del mutation was present in 87% (110/126) of cases (Table 1).
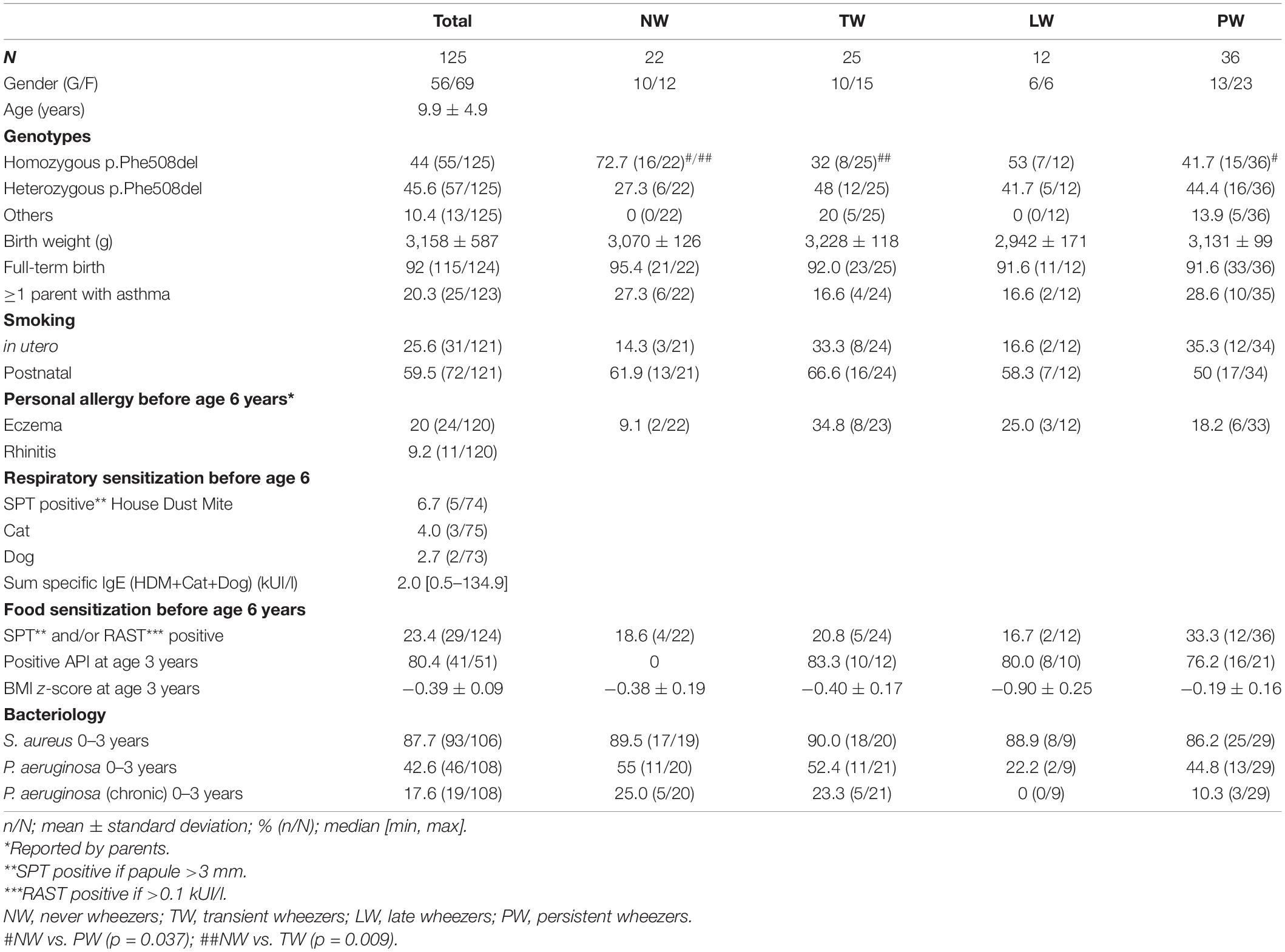
Table 1. Population characteristics according to the TUCSON phenotypes in preschool children aged less than 6 years.
The cumulative yearly frequency wheezing is shown in Figure 1. At least one episode of wheezing during the first 6 years of life was recorded in 71.2% (89/125) of cases. Regarding 115 children who had attained the age of 3 years, 66.1% (76/115) had had at least one episode of wheezing. In patients reaching the age of 6 years the cumulative prevalence of children having wheezed at least once was 76.8% (73/95) and 48.4% (46/95) had wheezed at least three times.
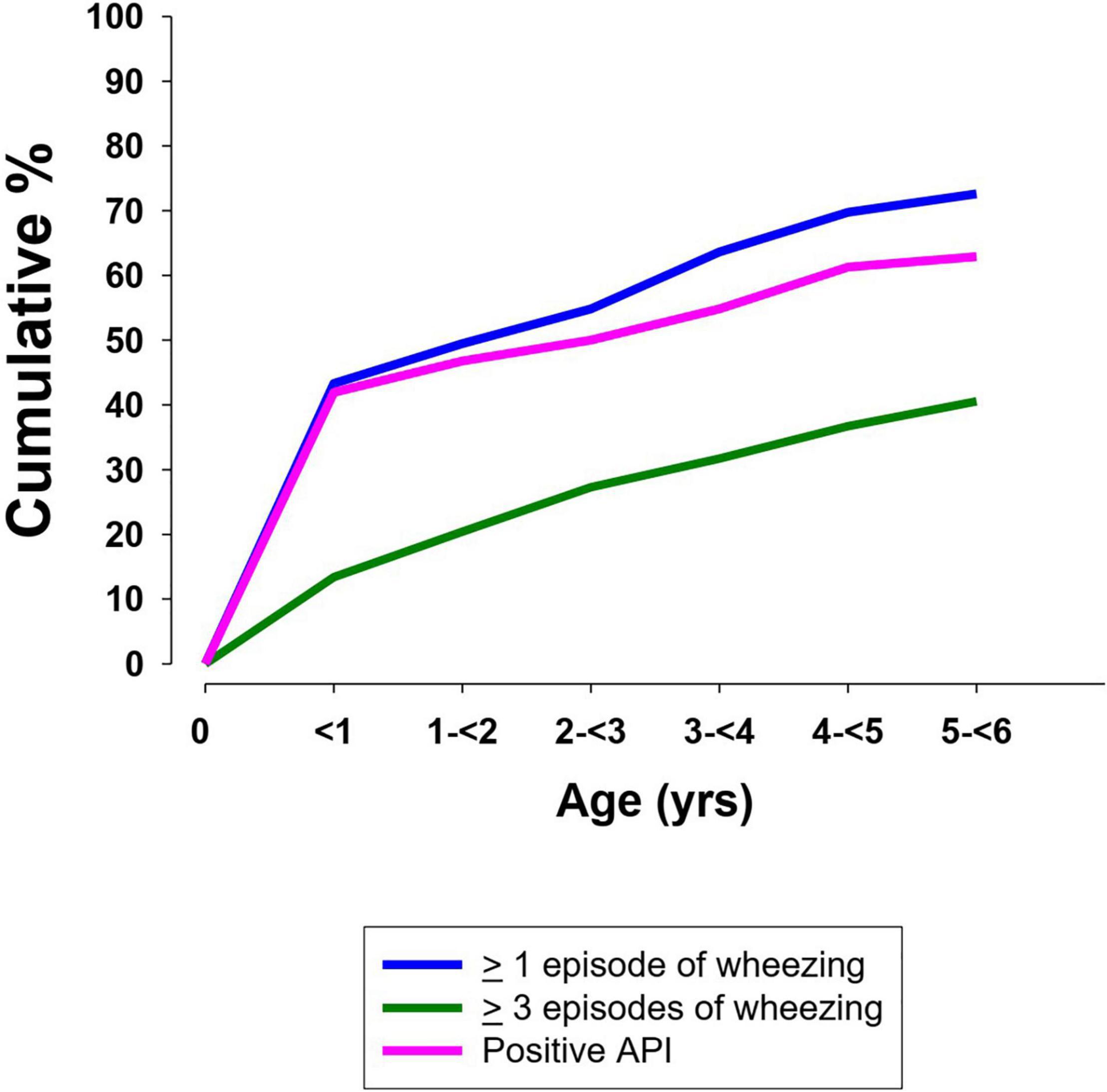
Figure 1. Cumulative frequency of wheezing and a positive Asthma Predictive Index in preschool children (<6 years) with CF (13).
The API index was available in 72 of 73 children who had wheezed at least once from birth to the age of 6 years. A positive API between 0 and 3 years of age was present in 77.3% (34/44) of cases. The yearly cumulative frequency of a positive API is shown in Figure 1. Figure 2 indicates the PIAMA risk score.

Figure 2. PIAMA* score in children with CF. Box and whisker indicating the median (horizontal line) and the upper and lower quartiles of the PIAMA* score. The black dots indicate the outliers. The blue horizontal lines indicate the risk of asthma in non-CF patients between 6 and 8 years of age according to the PIAMA* score: 0–7: low; 8–15: medium; 16–23: high. *PIAMA, prevention and incidence of asthma and mite allergy (12).
Twenty-two (23.2%) children had never wheezed by 6 years of age (NW). The distribution of 73 children with wheezing was as follows: 25 (26.3%) TW, 12 (12.6%) LW, and 36 (37.9%) PW (Figure 3).
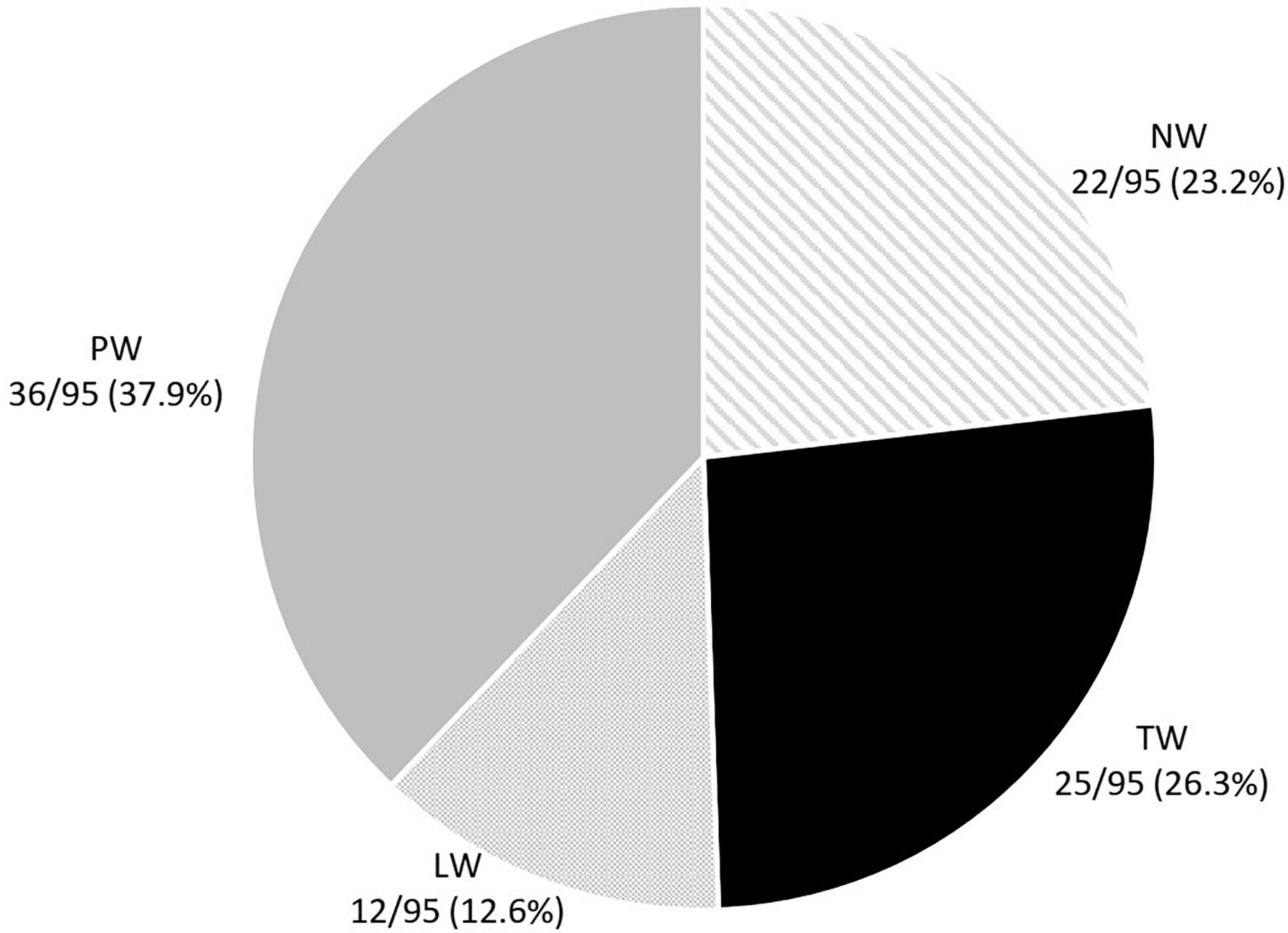
Figure 3. Distribution of the preschool wheezing phenotypes according to the TUCSON* phenotypes. *Tucson phenotypes: TW, transient earlier wheezers; NW, never wheezers; LW, late onset wheezers; PW, persistent wheezers (16).
These are shown in Table 2. Homozygous p.Phe508del mutations were present in 72.7% of the children in the NW group compared to 32% in the TW group (p = 0.009) and 41.7% in the PW group (p = 0.037).
The prevalence of “current” wheezers at the age of 6 years was 28.8% (21/73). The impact of wheezing in children according to their wheezing profiles or the existence of “current” wheezing at the age of 6 years is summarized in Tables 2, 3.
One of the main consequences of preschool wheezing was its impact on respiratory function. In the present cohort patients who had never wheezed had a mean ppFEV1 of 107.8 ± 3.4% vs. 98.0 ± 2.2% in those who had had at least one episode between birth and 6 years of age (p = 0.027, Figure 4). Figure 4 shows the ppFEV1 and ppFEF25–75 according to the types of wheezing profiles. FEV1 and forced vital capacity (FVC) values according to the different TUCSON phenotypes were as follows: NW > TW > PW > LW (NS).
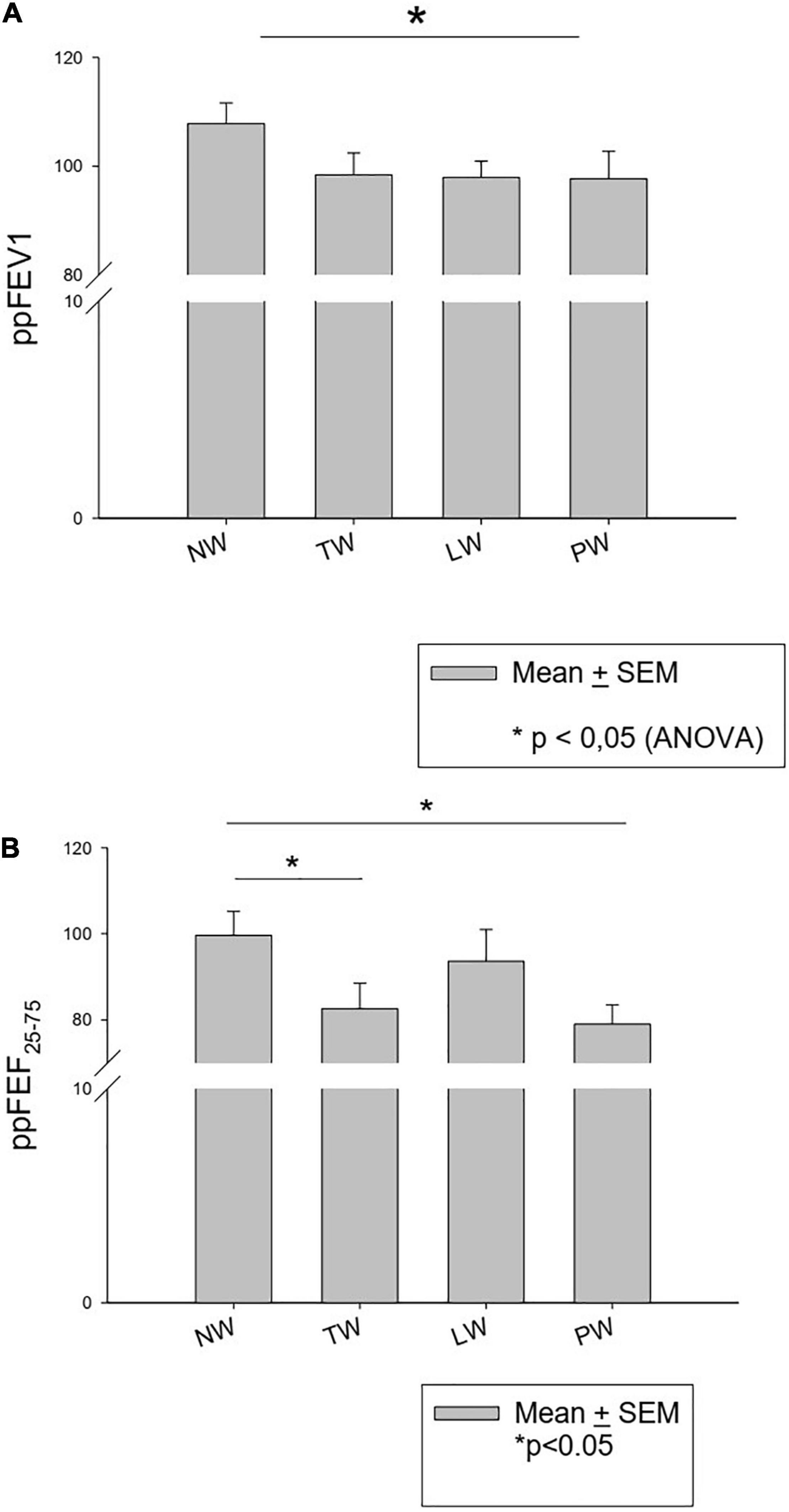
Figure 4. ppFEV1 (A) and ppFEF25–75 (B) at the age of 6 years according to the TUCSON wheezing phenotypes.
Prescriptions of short-acting bronchodilators and inhaled corticosteroids were significantly more frequent in symptomatic children (TW < LW < PW) compared to the NW groups (Table 3). At 6 years of age “current” wheezers had received more bronchodilator therapy (p = 0.039) and inhaled corticosteroids (NS) vs. non-current wheezers.
Sixty-nine patients were included in this group (Table 4). The M:F sex ratio was 1:1. A Phe508del homozygote genotype was present in 71% (49/69) of cases. Forty-nine children had had at least one annual checkup between ≥6 years and <8 years, 33 children between ≥10 years and <12 years, and 17 children between ≥15 years and <17 years.
A positive BDR was found in 73.5, 48.5, and 52.9% of children between 6 and 8 years, 10 and 12 years, and 15 and 17 years, respectively (Table 5). Longitudinally, among the 36 patients with early BDR (at 6–8 years), 8 out of 14 (57%) retained this BDR at 10–12 years, and 3/5 at 15–17 years (Table 5). Among the 13 patients without early BDR criteria, 3 of 6 (50%) progressed to a positive BDR response at the age of 10–12 years.
Using FEV1 as a sole criterion, a positive BDR was demonstrated in 34.7, 6.1, and 5.9% of the children between 6 and 8, 10 and 12, and 15 and 17 years of age, respectively (Table 5).
The homozygous Phe508del genotype was more frequent in the BDR + group compared to the BDR- group (64% vs. 36%, p = 0.041) in 6- to 8-year-olds (Table 6). The body mass index in the BDR + group was greater vs. the BDR- group in children aged 10–12 years (p = 0.01). The sum of the diameters of the prick test papules for 3 pre-defined common respiratory allergens was greater in the BDR+ patients at all ages, but this was significantly in the 6–8 years age group only (p = 0.005). The blood eosinophil counts were significantly lower in the BDR+ group of patients at the age of 10–12 years only (p = 0.013). The number of Aspergillus precipitation arcs was also lower in the BDR+ group at the age of 10–12 years (p = 0.05) (42).
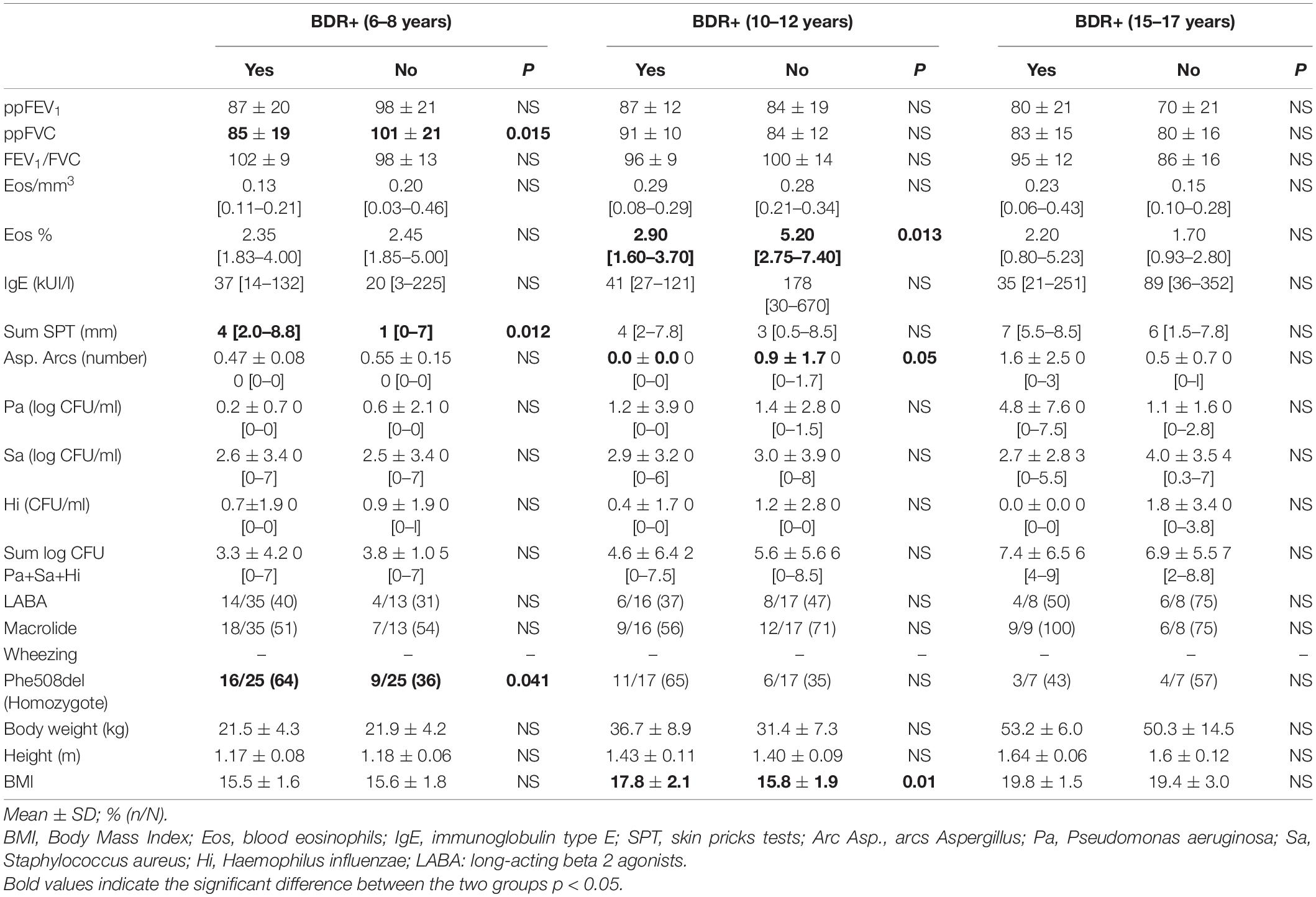
Table 6. Predictive markers and factors a positive bronchodilatory response (BDR) in patients aged >6 years.
FVC and FEV1 values of BDR + patients at the age of 6–8 years were lower than those of BDR- patients, with a significant difference regarding FVC only (85% vs. 101%, p = 0.015, Table 6).
The present study indicates that wheezing is a common phenomenon in preschool children with CF. Wheezing was not associated to p.Phe508del homozygosity, allergic factors or chronic Pseudomonas aeruginosa colonization. Persistent wheezing was associated with lower lung function at the age of 6 years. A positive BDR in older children with CF was also a common finding, and was associated with lower lung function between 6 and 8 years of age, increased BMI between 10 and 12 years of age, and a greater sum of the diameter of allergic prick test papules for three common respiratory allergens. In this study, we did not find identify allergic bronchopulmonary aspergillosis as a cause of wheezing.
The reported rate of wheezing in CF is variable. In a large population of patients with CF in the United States and Canada, the cumulative percentage of patients reaching onset of persistent wheezing was approximately 30% at the age of 6 years and 50% at the age of 15 years (17). In contrast, the European CF Epidemiologic Registry (ERCF) reported a rate of asthma of 14% in children with CF less than 6 years of age. Ren et al. showed that wheezing during the first 6 years of life was associated with lower lung function at the age of 6–8 years (18, 19). Based on the different TUCSON phenotypes (16), they reported that persistent wheezers (PW) had significantly lower FEV1 than never wheezers (NW) (19). Levine et al. showed that 39% of children aged 14.4 (4–76) years, median (range), with CF had reversible bronchial obstruction (20).
Physicians tend to extrapolate asthma treatment to the management of wheezing in CF. We and others (1, 17, 20) raise concerns regarding the abuse of inhaled steroids and bronchodilators which are too often prescribed in patients with CF. According to the French 2019 CF registry, 36.3 and 59.3% of patients received inhaled corticosteroids and bronchodilators, respectively (21). Our work confirmed the widespread use of these agents: short-acting bronchodilators were prescribed in 97.2% of PWs and 94.4% of such wheezers received inhaled corticosteroids. Ren et al. reported that 89.1% of PW received bronchodilators vs. 73.2% of NW (p < 0.05). Kent et al. (22) pointed out that there was little evidence for the efficacy of asthma therapy in CF. A Cochrane review concluded that no study has been able to demonstrate that the use of inhaled corticosteroids decreases lung inflammation in CF (23). In France, a Delphi study recommended restricting the use of inhaled corticosteroids in CF due to an insufficient level of evidence (24). According to Smith and Edwards neither long-acting beta-2 agonists nor long-acting muscarinic antagonist bronchodilators demonstrate improvement in FEV1 in CF (25). Moreover, the side effects of inhaled corticosteroids are not negligible. They can cause oral fungal infections and at high doses may negatively impact growth. Overall, under close supervision, teams caring for patients with CF may safely stop prescribing inhaled corticosteroids in these patients (26).
Wheezing in children with CF is more frequent compared to non-CF children [33.6% at 3 years of age and 48.6% at 6 years of age in the TUCSON Cohort (16)]. McColley et al. (17) found that children diagnosed by neonatal screening had a reduced risk of early onset of wheezing. The early diagnosis of the disease (associated with appropriate overall management by pediatric expert centers) is of vital importance to preserve the respiratory status in CF (14).
In our study, a positive API between 0 and 3 years of age was not significantly associated with any TUCSON wheezing phenotype, or with “current” wheezing at the age of 6 years. The PIAMA score was also not contributory. Personal allergic diseases (eczema, allergic rhinitis, etc.) were relatively uncommon and non-discriminatory. There was a 2 to threefold lower prevalence of eczema in the NW than in the 3 other subgroups (NS). No other criteria (birth weight, gestational age, parental asthma, allergic SPT, and smoking) was significantly associated with a particular TUCSON wheezing profile. The “protective” role of p.Phe508del homozygote mutations representing 72.7% of the children in the NW group compared to 32% in the TW group (p = 0.009) and 41.7% in the PW group (p = 0.037) was not found by Ren et al. (19). Chronic colonization with P. aeruginosa at 3 years of age was not associated with “current” wheezing at 6 years of age (p = 0.044). Interestingly, McColley et al. (17) found that the presence of P. aeruginosa and/or Staphylococcus aureus seemed to be “protective” for the occurrence of crackles and chest congestion. They suggested that the treatments and more stringent follow-up when these bacteria were present may have played a “protective” role.
In our study, ppFEF25–75 was significantly greater in NW compared to PW (p = 0.027). Ren et al. also reported higher FEV1 and FVC values in NW compared to the other phenotypes (19). Preschool wheezing had a significant impact on lung function at school age in our study. In current wheezers at the age of 6 years ppFEV1 was impaired [91.5% vs. 100.9% in non-wheezers since 12 months (p = 0.047)], as shown in previous reports (16, 19). Preschool wheezing did not significantly affect Body Mass Index at 6 years of age even though “current” wheezers had a −0.52 lower BMI z-score compared to the “non-current” wheezers, or the microbiological status in the sputum.
We did not specifically study the prevalence of wheezing in school-age children. However, we did demonstrate that BDR was a common phenomenon involving approximately half of the patients studied. Mitchell et al. reported BHR after methacholine challenge in 51% of children with CF, compared to 98% in asthmatic control children (7). Mellis and Levison identified 24% of histamine responders among CF patients, compared to 90% in asthmatic control patients (27). In addition, there appears to be two categories of CF patients: those with symptoms of asthmatic disease, clinically diagnosed on the basis of recurrent acute exacerbations of wheezing, with a very high efficacy of bronchodilators and/or systemic corticosteroids; and those with some degree of “isolated” BHR (non-asthmatics) (9, 47).
Bronchial responsiveness to bronchodilators is an integrated physiological response involving airway epithelium, nerves, mediators and bronchial smooth muscle. In 1993, an ad hoc ATS committee recommended that the diagnosis of asthma be based either on methacholine/histamine challenges, or repeat spirometry after beta-adrenergic agonists or steroid trials (5). This suggests that bronchoconstrictor responsiveness (BCR) and BDR may be considered physiological opposites in chronic obstructive airways disease. In many LFT laboratories, provocation tests have been replaced by bronchodilator tests in the assessment of cases of airways obstruction. However, the correlation between bronchoconstriction and bronchodilator response is imperfect and it is not possible to infer with certainty the presence of one from the other (28). In COPD symptoms were more associated with the presence of a BCR, but not a BDR, indicating that they are two different phenotypic markers that are not interchangeable (29).
In the present study, a greater sum of allergic SPT HDM + Cat + Dog papules, lower lung function values and homozygous Phe508del genotypes were associated with a positive BDR in 6–8 year-old children. BDR+ patients aged 10–12 had lower blood eosinophil counts and Aspergillus precipitin arcs, as well as a higher body mass index, compared with BDR-children. In a similar study, Levin et al. showed that reversible obstruction (ΔppFEV1 ≥ 12%) was associated with younger age (p = 0.01) and a severe genotype (p = 0.02), but not with a family history of asthma, serum IgE, blood eosinophils, pancreatic status, ppFEV1 < 40%, Aspergillus or Pseudomonas infection (20). In a study by Van Haren et al. including 20 children with CF, 40% of patients had histamine BHR, mainly among the youngest, and this was correlated with positive allergic SPTs (30). In children with CF, the prevalence of positive markers of respiratory allergy is significantly higher than in the general population (9). In a study including 31 adult patients with CF, such sensitization was found in 65% of patients with BHR (31). In three other studies performed in adults after challenge testing, BHR was also not correlated with allergic SPT positivity (7, 27, 32). Our study allowed us to observe the BDR with a longitudinal perspective. Regardless of the criteria used, the prevalence of a BDR was greater in the youngest patients and had decreased by the age of 10–12 years. Overall, allergy appears to be inconsistently associated with BHR and BDR + in CF.
According to McCuaig and Martin, deficient ion transport across CFTR in patients with CF cannot be solely responsible for the altered ASM physiology, as there is as much smooth muscle hypertrophy in pediatric CF patients as in those with non-CF bronchiectasis (10). Both CF and non-CF bronchiectasis are diseases characterized by high levels of neutrophils in the lungs, suggesting an important contribution of the inflammatory environment to ASM alterations. The presence of a bacterial infection, particularly Pseudomonas, will promote the secretion of IL-8 and TNF-alpha, leading to bronchial remodeling. In addition, CFTR-deficient T cells will be directed to type 2 T-helper cells, which will cause a pro-allergic response (10). In addition, calcium responses are altered in CFTR-deficient ASM at a very early stage, as confirmed by studies in CFTR−/− neonatal pigs. Ca++ is a crucial second messenger in smooth muscle contraction, activating myosin light chain (MLC) kinase through the formation of a Ca++ and calmodulin complex. The release of Ca++ and the activation of Cl- channels in the sarcolemma of ASM may be of importance in the smooth muscle contraction in CF, in contrast to asthma (33). Other possible causes of wheezing include antenatal cigarette smoke exposure (16), small or floppy airways (34, 35), excess intraluminal mucus (34), gastro-intestinal reflux (36), and oxidant-antioxidant species imbalance (37). Gastro-esophageal reflux, one of the most common gastrointestinal manifestations of CF, probably plays a role in the pathogenesis of the airway disease by inducing repeated micro-aspiration and bronchospasm (36). An altered redox environment with a low concentration of antioxidants, in particular glutathione, contrasting with high levels of 8-isoprostane in the epithelial surface liquid contributes to progressive lung damage (37).
This study was retrospective and monocentric. This explains the small number of patients compared to other similar studies. However, missing data were limited since we have used prospective standardized national CF Registry procedures and dedicated software (MucoDomeos).1
Overall, based on the different data from the literature and from our own study the following hypothesis can be proposed: most children with CF behave like non-specific asthmatic patients, with a high prevalence of BHR and/or response to bronchodilators. They would then evolve toward a form of chronic obstructive airway disease with a lower prevalence of BHR. This raises the question as to which are the most appropriate treatments of BHR in CF. At the present time, the most promising agents are CFTR modulators. VX-809/770, reduces the ASM cell proliferation and normalizes calcium reuptake kinetics (38); Ivacaftor rapidly improves airflow obstruction, air trapping and airway distensibility (39); Lumacaftor-ivacaftor improves LCI (−1.6, 95% CI −2.6 to −0.5; P < 0.01), airway microbiota and inflammation, as well as MRI morphology (−1.3, 95% CI −2.3 to −0.3; P < 0.05) and perfusion score (−1.2, 95% CI −2.3 to −0.2; P < 0.05) (40). Ribeiro and Gentzsch have suggested that CF airway epithelial inflammation may enhance the efficacy of CFTR modulators, and this could have clinical implications regarding the presence of wheezing (41).
While wheezing is very common in children with CF, no major determinants in children below 6 years of age could be clearly identified. Current wheezing at age 6 years was significantly associated with lower lung function. In children older than 6 years, allergic factors, genetics (p.F508del homozygosity) and higher BMI were significantly associated with the presence of a positive BDR but this varied between age groups. In 6–8 years old children with a BDR, baseline lung function was significantly lower. Our results suggest that the “CF-Asthma” designation may be misleading in a vast majority of cases, and may lead to inappropriate treatment with inhaled steroids, especially in preschool wheezers.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethical approval was not provided for this study on human participants because not yet, processing. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
MF is affiliated (but not employed) by the INSERM, CIC 1401.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all members of the pediatric CF team, as well as the patients and their parents for having participated in this study.
ASM, airway smooth muscle; BDR, bronchodilatory response; BHR, bronchial hyperresponsiveness; IgE, type E immunoglobulin; API, Asthma Predictive Index; IS, induced sputum; LW, late (onset) wheezers; NW, never wheezers; PW, persistent wheezers; PIAMA, prevention and incidence of asthma and mite allergy; SPT, skin prick tests; TW, transient (earlier) wheezers.
1. Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, et al. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. (2005) 40:500–10. doi: 10.1002/ppul.20294
2. Bush A, Tiddens H, Silverman M. Clinical implications of inflammation in young children. Am J Respir Crit Care Med. (2000) 162:S11–4. doi: 10.1164/ajrccm.162.supplement_1.maic-3
3. Hays SR, Ferrando RE, Carter R, Wong HH, Woodruff PG. Structural changes to airway smooth muscle in cystic fibrosis. Thorax. (2005) 60:226–8. doi: 10.1136/thx.2004.028340
4. Regamey N, Ochs M, Hilliard TN, Muhlfeld C, Cornish N, Fleming L, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. (2008) 177:837–43. doi: 10.1164/rccm.200707-977OC
5. Miller A. Guidelines for the evaluation of impairment/disability in patients with asthma. Am J Respir Crit Care Med. (1994) 149(3 Pt 1):834–5. doi: 10.1164/ajrccm.149.3.8155167
6. Burdon JG, Cade JF, Sutherland PW, Pain MC. Cystic fibrosis and bronchial hyperreactivity. Concomitant defects or cause and effect ? Med J Aust. (1980) 2:77–8. doi: 10.5694/j.1326-5377.1980.tb76884.x
7. Mitchell I, Corey M, Woenne R, Krastins IR, Levison H. Bronchial hyperreactivity in cystic fibrosis and asthma. J Pediatr. (1978) 93:744–8. doi: 10.1016/s0022-3476(78)81070-1
8. Tobin MJ, Maguire O, Reen D, Tempany E, Fitzgerald MX. Atopy and bronchial reactivity in older patients with cystic fibrosis. Thorax. (1980) 35:807–13. doi: 10.1136/thx.35.11.807
9. Weinberger M. Airways reactivity in patients with CF. Clin Rev Allergy Immunol. (2002) 23:77–85. doi: 10.1385/CRIAI:23:1:077
10. McCuaig S, Martin JG. How the airway smooth muscle in cystic fibrosis reacts in proinflammatory conditions: implications for airway hyper-responsiveness and asthma in cystic fibrosis. Lancet Respir Med. (2013) 1:137–47. doi: 10.1016/S2213-2600(12)70058-9
11. Caudri D, Wijga A, Maarten C, Hoekstra M, Postma DS, Koppelman GH, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J Allergy Clin Immunol. (2009) 124:903–10. doi: 10.1016/j.jaci.2009.06.045
12. Hafkamp-de Groen E, Lingsma HF, Caudri D, Levie D, Wijga A, Koppelman GH, et al. Predicting asthma in preschool children with asthma-like symptoms: validating and updating the PIAMA risk score. J Allergy Clin Immunol. (2013) 132:1303–10. doi: 10.1016/j.jaci.2013.07.007
13. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. (2000) 162:1403–6. doi: 10.1164/ajrccm.162.4.9912111
14. Burgel PR. Evolutions épidémiologiques de la mucoviscidose en France: perspectives à 10 ans. Arch Pediatr. (2016) 23:S4–12.
15. Toulouse E, Masseguin C, Lafont B, McGurk G, Harbonn A, Roberts JA, et al. French legal approach to clinical research. Anaesth Crit Care Pain Med. (2018) 37:607–14. doi: 10.1016/j.accpm.2018.10.013
16. Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The group health medical associates. N Engl J Med. (1995) 332:133–8. doi: 10.1056/NEJM199501193320301
17. McColley SA, Ren CL, Schechter MS, Regelmann WE, Pasta DJ, Konstan MW. Risk factors for onset of persistent respiratory symptoms in children with cystic fibrosis. Pediatr Pulmonol. (2012) 47:966–72. doi: 10.1002/ppul.22519
18. Balfour-Lynn IM, Elborn JS. “CF asthma”: what is it and what do we do about it? Thorax. (2002) 57:742–8. doi: 10.1136/thorax.57.8.742
19. Ren CL, Konstan MW, Rosenfeld M, Pasta DJ, Millar SJ, Morgan WJ, et al. Early childhood wheezing is associated with lower lung function in cystic fibrosis. Pediatr Pulmonol. (2014) 49:745–50. doi: 10.1002/ppul.22894
20. Levine H, Cohen-Cymberknoh M, Klein N, Hoshen M, Mussaffi H, Stafler P, et al. Reversible airway obstruction in cystic fibrosis: common, but not associated with characteristics of asthma. J Cyst Fibros. (2016) 15:652–9. doi: 10.1016/j.jcf.2016.01.003
21. Vaincrelamuco. Registre de la Mucoviscidose. (2019). Available online at: https://www.vaincrelamuco.org/sites/default/files/registre_2019_vf.pdf.
22. Kent BD, Lane SJ, Van Beek EJ, Dodd JD, Costello RW, Tiddens HA. Asthma and cystic fibrosis: a tangled web. Pediatr Pulmonol. (2014) 49:205–13. doi: 10.1002/ppul.22934
24. Fayon M, Corvol H, Chiron R, Bui S. le groupe de travail inflammation de la société Française de la mucoviscidose. Consensus national sur les modalités de prescription des corticoïdes inhalés dans la mucoviscidose [National consensus regarding the prescription of inhaled corticosteroids in cystic fibrosis]. Arch Pediatr. (2014) 21:88–94. doi: 10.1016/j.arcped.2013.10.016
25. Smith S, Edwards CT. Long-acting inhaled bronchodilators for cystic fibrosis. Cochrane Database Syst Rev. (2017) 12:CD012102. doi: 10.1002/14651858.CD012102
26. Balfour-Lynn IM, Welch K, Smith S. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev. (2019) 7:CD001915.
28. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. (2005) 26:948–68.
29. Douma WR, de Gooijer A, Rijcken B, Schouten JP, Koëter GH, Weiss ST, et al. Lack of correlation between bronchoconstrictor response and bronchodilator response in a population-based study. Eur Respir J. (1997) 10:2772–7. doi: 10.1183/09031936.97.10122772
30. Van Asperen PP, Manglick P, Allen H. Mechanisms of bronchial hyperreactivity in cystic fibrosis. Pediatr Pulmonol. (1988) 5:139–44. doi: 10.1002/ppul.1950050304
31. Caudri D, Savenije OE, Smit HA, Postma DS, Koppelman GH, Wijga AH, et al. Perinatal risk factors for wheezing phenotypes in the first 8 years of life. Clin Exp Allergy. (2013) 43:1395–405. doi: 10.1111/cea.12173
32. Van Haren EH, Lammers JW, Festen J, van Herwaarden CL. Bronchial vagal tone and responsiveness to histamine, exercise and bronchodilators in adult patients with cystic fibrosis. Eur Respir J. (1992) 5:1083–8.
33. Michoud MC, Robert R, Hassan M, Moynihan B, Haston C, Govindaraju V, et al. Role of the cystic fibrosis transmembrane conductance channel in human airway smooth muscle. Am J Respir Cell Mol Biol. (2009) 40:217–22. doi: 10.1165/rcmb.2006-0444OC
34. Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. (2010) 182:1251–61. doi: 10.1164/rccm.201004-0643OC
35. McDermott S, Barry SC, Judge EP, Collins S, de Jong PA, Tiddens HA, et al. Tracheomalacia in adults with cystic fibrosis: determination of prevalence and severity with dynamic cine CT. Radiology. 2009 Aug;252(2):577-86. Erratum Radiol. (2015) 275:934. doi: 10.1148/radiol.2522081956
36. Bongiovanni A, Manti S, Parisi GF, Papale M, Mulè E, Rotolo N, et al. Focus on gastroesophageal reflux disease in patients with cystic fibrosis. World J Gastroenterol. (2020) 26:6322–34. doi: 10.3748/wjg.v26.i41.6322
37. Spicuzza L, Parisi GF, Tardino L, Ciancio N, Nenna R, Midulla F, et al. Exhaled markers of antioxidant activity and oxidative stress in stable cystic fibrosis patients with moderate lung disease. J Breath Res. (2018) 12:026010. doi: 10.1088/1752-7163/aa9b39
38. Jang JH, Panariti A, O’Sullivan MJ, Pyrch M, Wong C, Lauzon AM, et al. Characterization of cystic fibrosis airway smooth muscle cell proliferative and contractile activities. Am J Physiol Lung Cell Mol Physiol. (2019) 317:L690–701. doi: 10.1152/ajplung.00090.2019
39. Adam RJ, Hisert KB, Dodd JD, Grogan B, Launspach JL, Barnes JK, et al. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight. (2016) 1:e86183. doi: 10.1172/jci.insight.86183
40. Graeber SY, Vitzthum C, Pallenberg ST, Naehrlich L, Stahl M, Rohrbach A, et al. Effects of Elexacaftor/Tezacaftor/Ivacaftor therapy on CFTR function in patients with cystic fibrosis and one or two F508del alleles. Am J Respir Crit Care Med. (2022) 205:540–9.
41. Ribeiro CMP, Gentzsch M. Impact of airway inflammation on the efficacy of CFTR modulators. Cells. (2021) 10:3260. doi: 10.3390/cells10113260
42. Rodriguez-Martinez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Factors predicting persistence of early wheezing through childhood and adolescence: a systematic review of the literature. J Asthma Allergy. (2017) 10:83–98. doi: 10.2147/JAA.S128319
43. Midodzi WK, Rowe BH, Majaesic CM, Saunders LD, Senthilselvan A. Predictors for wheezing phenotypes in the first decade of life. Respirology. (2008) 13:537–45. doi: 10.1111/j.1440-1843.2008.01284.x
44. Sagel SD, Gibson RL, Emerson J, McNamara S, Burns JL, Wagener JS, et al. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J Pediatr. (2009) 154:183–8. doi: 10.1016/j.jpeds.2008.08.001
45. Colombo JL. Long-acting bronchodilators in cystic fibrosis. Curr Opin Pulm Med. (2003) 9:504–8. doi: 10.1097/00063198-200311000-00010
46. Donohue JF, van Noord JA, Bateman ED, Langley SJ, Lee A, Witek TJ Jr, et al. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest. (2002) 122:47–55. doi: 10.1378/chest.122.1.47
Keywords: asthma, wheezing, bronchial hyperresponsiveness, cystic fibrosis, respiratory function tests
Citation: Galodé F, Ladipo O, Andrieux A, Feghali H, Bui S and Fayon M (2022) Prevalence and Determinants of Wheezing and Bronchodilatation in Children With Cystic Fibrosis: A Retrospective Cohort Study. Front. Pediatr. 10:856840. doi: 10.3389/fped.2022.856840
Received: 17 January 2022; Accepted: 10 March 2022;
Published: 12 May 2022.
Edited by:
Francesca Santamaria, University of Naples Federico II, ItalyReviewed by:
Salvatore Leonardi, University of Catania, ItalyCopyright © 2022 Galodé, Ladipo, Andrieux, Feghali, Bui and Fayon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francois Galodé, ZnJhbmNvaXMuZ2Fsb2RlQGNodS1ib3JkZWF1eC5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.