- 1Division of Pediatric Urology, Department of Urology, Radboudumc Amalia Children's Hospital, Nijmegen, Netherlands
- 2Department of Pediatric Nephrology, Radboudumc Amalia Children's Hospital, Nijmegen, Netherlands
- 3Department of Pediatrics, Pediatric Nephrology, Beatrix Children's Hospital, University of Groningen, University Medical Center Groningen, Groningen, Netherlands
Worldwide, over 1,300 pediatric kidney transplantations are performed every year. Since the first transplantation in 1959, healthcare has evolved dramatically. Pre-emptive transplantations with grafts from living donors have become more common. Despite a subsequent improvement in graft survival, there are still challenges to face. This study attempts to summarize how our understanding of pediatric kidney transplantation has developed and improved since its beginnings, whilst also highlighting those areas where future research should concentrate in order to help resolve as yet unanswered questions. Existing literature was compared to our own data of 411 single-center pediatric kidney transplantations between 1968 and 2020, in order to find discrepancies and allow identification of future challenges. Important issues for future care are innovations in immunosuppressive medication, improving medication adherence, careful donor selection with regard to characteristics of both donor and recipient, improvement of surgical techniques and increased attention for lower urinary tract dysfunction and voiding behavior in all patients.
Introduction
The first successful pediatric kidney transplantation was performed in 1959 at the University of Oregon in Portland, USA (1, 2). The field of pediatric kidney transplantation (patient age 0–18 years) has continued to evolve ever since. Whereas, pediatric kidney recipients had worse outcome compared to adults in the earlier years, today outcomes are equal.
Both patient and graft survival improved dramatically; from a 1-year patient survival of 70% in 1970 into a current 1-year patient survival of 97% and 5-year graft survival of 89% (3–6). Consequently, kidney transplantation is the first choice of treatment for children suffering from end stage kidney disease (ESKD). Nowadays, over 1,300 pediatric kidney transplantations are performed each year (285 in Europe, 1,023 in the United States) (5, 7, 8).
Multiple factors led to improved graft survival and quality of life (QOL) in pediatric kidney recipients, for example new developments in immunosuppression protocols. Infections, both bacterial and viral, used to be responsible for high morbidity and mortality in the early years of transplantations (3, 9). Subsequently, clinicians became more cautious in using immunosuppressants resulting in higher rates of rejection (4, 10). A deeper understanding of the pediatric immune system and development of targeted immunosuppressive medication contributed to improved graft survival.
Pediatric transplantation differs from adult transplantation as unique pediatric concerns related to development, growth, viral infections, congenital disorders and adherence need to be managed (11, 12). Awareness of these differences, as well as optimization of multidisciplinary pre-, peri-, and post- operative care and surgical techniques, all contributed to improvement of outcome (13, 14).
Despite this increased survival rates, we aim to further optimize care for these patients. Considering the current allograft half-life of 12 to 15-years, most pediatric kidney recipients will require a re-transplantation during their lifetime (15, 16).
Moreover, since survival has increased, future research needs to focus on the long-term effects of kidney transplantation: maintaining QOL and minimizing the side effect of immunosuppressants. Due to relatively small numbers of transplantations per center, it takes time to gain expertise.
This non-systematic review presents a summary of the current available literature. The aim was to present an overview of lessons learned during the last 5 decades of pediatric kidney transplantation and to identify unresolved fields waiting to be unraveled. Analysis of still existing lacunas in pediatric kidney transplantation care are essential to further optimize outcome.
In addition we present our own results of 411 single center pediatric kidney recipients that were transplanted in our center in the time period 1968–2020.
Methods
A comprehensive literature search was conducted in the databases PubMed, Cochrane, EMBASE and MEDLINE for relevant English-language articles. In addition we followed citations from the primary references to relevant articles that the databases could not locate. The search was based on the following MESH-terms: pediatric kidney transplantation, donor selection, donor age, living related and unrelated kidney donation, post mortal kidney donation, prognostic factors, dialysis, pre-emptive transplantation, immunosuppressive drugs, corticosteroid withdrawal, long-term outcome/ graft survival, rejection, infections, and surgical techniques, complications, including ureteroneocystostomy. All abstracts were screened for relevant articles. Full text relevant articles were reviewed and included.
This article focused on pre-operative issues like donor selection, pre-emptive transplantation and screening for lower urinary tract dysfunction (LUTD). Besides this it covers peri-operative factors such as anastomosis technique and surgery for really small children. Eventually it describes post-operative factors like graft- and patient survival, immunosuppression and the need for transplantectomy.
In order to evaluate our own practice and to identify dissimilarities with previous research we compared outcomes retrieved from existing literature to outcomes of 411 single-center pediatric kidney recipients transplanted between 1968 and 2020 in our center. For this, population data were analyzed using SPSS Statistics 25.0 and Graphpad Prism 5.0. Differences were considered statistically significant at p < 0.05.
Results
In this section we will present an overview of current literature on the most important (modifiable) factors in pediatric kidney transplantation (age 0–18 years) and highlight existing controversies that remain to be clarified.
Survival
Patient Survival
Since 1959, patient survival increased significantly. Whereas, 5-years patient survival was 91% before 1990 it improved to up to 98% after 2010, mainly due to decline in infections (17). Although the number of infections decreased over time, it is still the most important cause of death in pediatric kidney recipients (28%) (18, 19).
As infection rates decreased other long-term factors became more important like malignant diseases and cardiopulmonary complications. Both these complications are responsible for respectively 12 and 15% of current 5- and 10-year patient survival (18).
Graft Survival
In 1990, 5-year graft survival was ~77% for the living donations (LD) and 57% for the deceased donations (DD) (20). In this period, rejection rates were as high as 80–90% and rejection (both acute and chronic) was the major cause of graft loss (4).
Nowadays, acute rejection rates have decreased to 10–15%, due to improvements in pre-operative donor selection, peri-operative management and immunosuppressive regimes. Although rates of acute rejection have immensely diminished, chronic rejection and acute rejection remain the leading causes of graft loss (21 and 15%, respectively) (17). Other important causes of graft loss are disease recurrence (10%) and vascular thrombosis (11%).
Radboudumc Amalia Children's Hospital
In our center, since 1968, 411 kidney transplantations have been performed in patient aging 0–18 years.
Similar to the literature, patient survival increased from a 5-year survival of 93% in recipients transplanted before 1990 to 98% when transplanted after 2010. Overall, infection was the most important cause of mortality (25%), followed by cardiovascular complications and malignancy (17 and 14%, respectively).
Graft survival increased significantly over ascending era's even when stratified for DD/LD [Figure 1 (p < 0.01)]. In general, LD resulted in better 5, 10, and 20 year graft survival compared to DD (p < 0.01). Majority of graft loss in our center was caused by both forms of rejection (75%). Other important causes were recurrence of primary disease (5%) and thrombosis (6%). Causes of graft loss did not change over time.
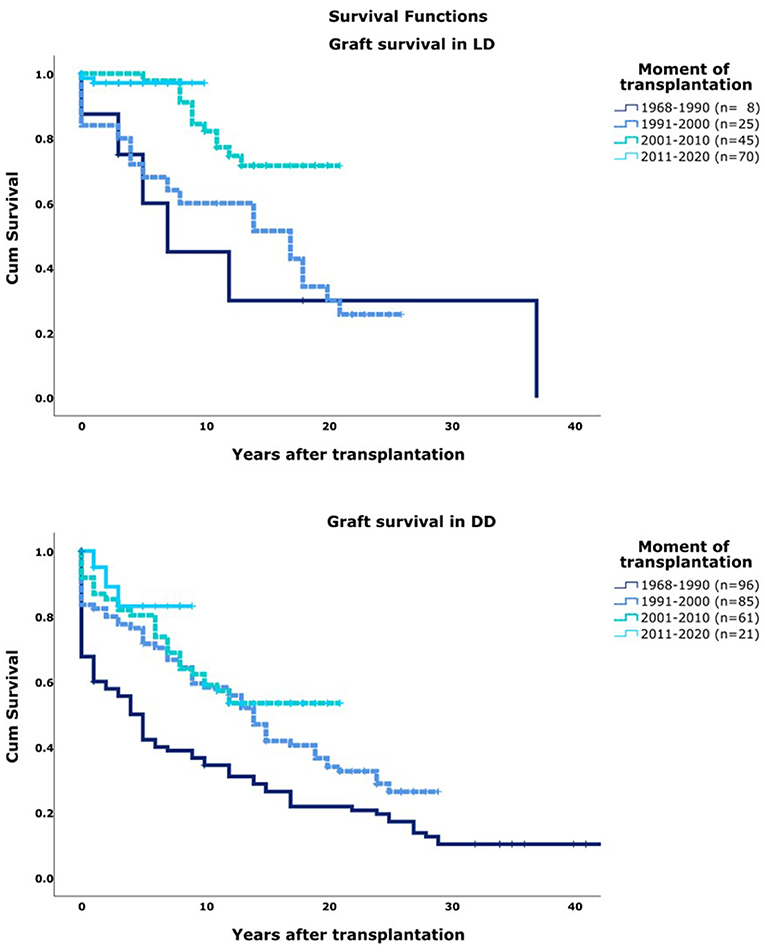
Figure 1. Graft survival per decade of transplantation. T0, moment of transplantation. DD, deceased donor; LD, Living Donor.
Pre-transplantation Dialysis vs. Pre-emptive Transplantation
Most pediatric kidney recipients are exposed to dialysis prior to their transplantation [51% to hemodialysis (HD) and 28% to peritoneal dialysis (PD)]. Current incidence of pre-emptive kidney transplantation (PKT) in children is 20% in Europe (7). However, rates of PKT vary greatly between countries with 2% PKT in Italy, 41% in the Netherlands and 61% in Norway (7, 21). This wide range might be partly due to differences in local allocation policies as these vary among countries (7). In adults, PKT was shown to be superior to post-dialysis transplantation as it results in favorable graft and patient outcome as well as improved QOL (22, 23). However, this is more controversial in pediatric patients.
PKT vs. Dialysis
In theory, dialysis has several disadvantages for children suffering from ESKD. Dialysis is associated with negative effects on growth, anemia, bone mineral regulation and cardiovascular status due to chronic volume overload and uremic toxins (24–26). Moreover, surgery for dialysis access makes patients more prone to infectious complications and avoiding dialysis might preserve the vessels for the future and increase graft survival (27).
Despite these theoretical objections to dialysis, literature showed conflicting results of PKT in children. Some studies report better graft and patient survival in PKT (28–30) whilst others found similar results for both pre-emptive and post-dialysis transplantation (31–33). However, some studies were performed after DD, others after LD and some after both. It should be noted that most of these studies had limited follow-up time.
There are several possible explanations for these conflicting results.
Undervaluation of PKT might be due to the relatively short duration of dialysis in children compared to adults. Period of dialysis is thought to predict survival since a longer time on dialysis was associated with increased risk of adverse events (23, 26, 34). In adults, average time on dialysis before transplantation is 5 years, whereas for children this is <1 year (5).
Amaral et al. showed significant graft survival benefits in pediatric recipients after PKT compared to those on dialysis for as little as 6 months (28). This was confirmed in a large adult cohort by Prezelin et al. (23) and advocates PKT regardless of the duration of dialysis.
Overvaluation of PKT might be caused by selection bias. Recipients of PKT are more likely to be healthier, better nourished, have better residual kidney function and more likely to receive a graft from a LD compared to those on dialysis (22, 35).
Another factor is lead time bias. The treatment of choice, in this case transplantation, was given at an earlier stage in PKT than after dialysis which results in a longer follow-up after transplantation. This can cause a perceived advantage in PKT, as the graft survival time is calculated from an earlier starting point than in post-dialysis transplantation (36).
Additionally, a possible explanation for the conflicting results is the limited follow-up time. As the risks of dialysis are mainly cardiovascular disease, consequences are expected later in life and affect patient survival rather than graft survival. More long-term research could provide better answers.
Promoting PKT
An important barrier to PKT is a lack of patient education as many patients and potential living donors are not aware of the possibilities of PKT (37, 38). Besides, it remains difficult for patients to address the topic of living donation with their loved ones (39, 40). In the Netherlands, a home-based educational program was introduced to increase knowledge and improve communication among patients who are yet to undergo renal replacement therapy (41). This resulted in increased rates of PKT, probably because of the involvement of patients social network to the program (42, 43).
Radboudumc Amalia Children's Hospital
In our center, the rate of PKT increased over time, from 6% before 1990 to a current number of 46% (Figure 2). Median time on dialysis was 15 months [IQR 9–32], the majority of patients (47%) were treated with HD vs. 28% with PD. When corrected for decade of transplantation, neither pre-transplantation treatment nor duration of dialysis significantly affected graft survival or patient survival.
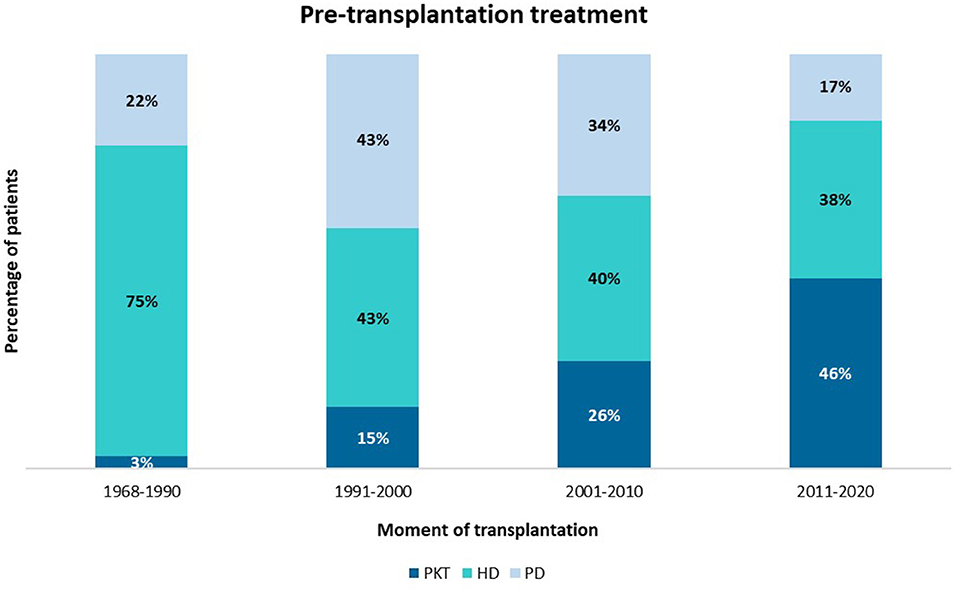
Figure 2. Mode of pre-transplantation treatment per era of transplantation. PKT, pre-emptive kidney transplantation; HD, hemodialysis; PD. peritoneal dialysis.
Donor and Recipient Selection
Donor Selection
Deceased Donors (DD)
Donor selection is an important factor for graft survival: LD is considered to be preferable to DD since it results in both better graft and recipient survival (18, 44). However, there is a disparity between supply and demand for grafts which necessitates DD. Besides, access to kidney transplantation and allocation procedures vary widely among countries. In most European countries, pediatric patients on a transplantation waiting list are given priority, which might have resulted in increased allocation of young DD kidneys to pediatric patients (21).
Living Related Donors (LRD)
Living related donation allows proper HLA-matching and limitation of ischemia time. And using living (un)related donors allows paired exchange.
In pediatric transplantation, LRD rates exceed those in the adult population since the donors are often the parents of the child.
Previous studies stated that maternal donation might be preferable to paternal donation since it results in decreased rate of acute rejection in the youngest recipients (<4 years) (45, 46). This phenomenon could be caused by microchimerism which is defined as the persistent presence of maternal cells in organs of the child due to bidirectional transfer of cells trough the placenta antenatally (47). However, this effect remains controversial as other studies found a negative association between graft survival and maternal donation. They stated that paternal grafts result in better long-term outcome because of the increased size and amount of nephrons of male kidneys (48–50).
Despite an overall increase in LD in Europe, the numbers of patients on donor kidney waiting lists are stabilizing (51). Impediments to find (living) donors include concerns on blood group incompatibility, donor age and health of the donor.
Living Unrelated Donors (LURD)
A possibility to expand the donor pool is the use of living unrelated donors. Graft outcome after LURD was shown to be superior to DD (52–54). In adults, rates of LURD are relatively high since donors are often the partner or a friend.
Although ethics of organ donation have always been a sensitive issue, this might be of more importance in (unrelated) living donation (4). Several countries in the Middle East prohibit LURD in order to avoid organ trafficking (55). On the other hand, the Iranian government operates a paid LURD kidney transplantation program also known as the Iranian model (56).
Non-commercial LURD is allowed in most Western Countries such as the Netherlands, following the recommendations of the Council of Europe (57).
Donor Age
Previous research in adults has shown advanced donor age to result in poor graft survival (58, 59).
This deleterious effect of high donor age seems less evident in pediatric recipients. Chesnaye et al. showed that the risk of graft failure in older living donors (50–75 years old) was similar to that of younger living donors (60). On the contrary, Trnka et al. showed that an increasing age difference between donor and recipient was associated with decreased graft survival (61). Allowing healthy elderly to donate their kidney remains debatable, however it might benefit against graft shortage.
HLA-(mis)match
Conflicting results have been published concerning the effect of HLA-matching. Whereas, some studies showed superior results for children receiving a poorly HLA-matched LD kidney compared to a well-matched DD kidney (62, 63) most studies showed the exact opposite (61, 64–66).
However, the definitions of poor and well-matched donation differ between studies. Additionally, geography might play a role in this context as cold ischemia times would be increased if DD grafts need to travel large distances.
Currently, the trade-off between time on a waiting list and HLA-mismatching remains unsolved and needs further exploration in the future.
Radboudumc Amalia Children's Hospital
In our center, the rate of LD and the donor age increased over time (Figure 3). Median graft survival after LD was longer compared to DD with a median survival of 20 years (95% CI 16–24) vs. 12 (95% CI 9–15) (p = 0.01) even when stratified for decade of transplantation.
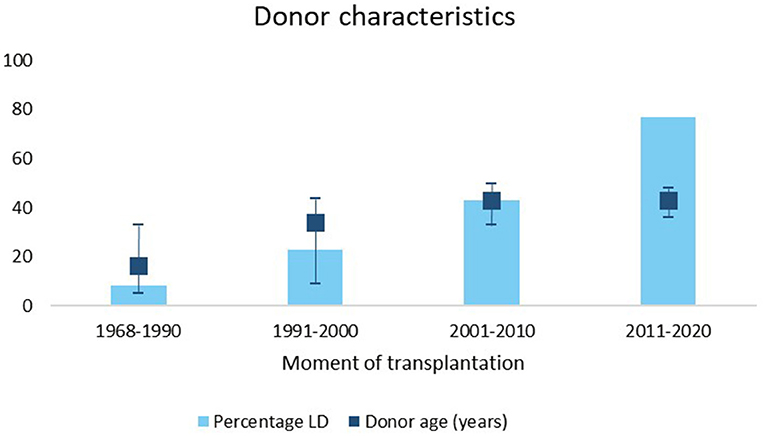
Figure 3. Percentage of living donors and IQR donor age stratified for different time periods. LD, living donor.
In total, 3% of the kidneys were from a LURD and 33% from a LRD, mostly parents (18% father and 11% mothers). There was no significant difference in graft survival between maternal and paternal donors.
In addition, median number of HLA mismatches significantly increased over time (p < 0.01).
Kidney Transplantation in Small Children
Kidney transplantation in children under the age of 1-year is rare (0.5–5% of all pediatric kidney transplantations) and poses surgical challenges in terms of size differences in body cavity and vessel diameters (67, 68). Previous studies use various definitions of “very small children” ranging from younger than 1 or 2 years of age to a weight below 10, 15, or 20 kg (69–73).
Special Considerations in Small Children
Trying to fit an adult-size graft in a small retroperitoneal space is challenging and might lead to increased abdominal pressure and impaired graft vascularization (74, 75). In addition, a relatively large graft demands an increase in renal blood flow which asks for aggressive fluid management in order to optimize renal perfusion (70, 76–78).
In adults, the graft is commonly placed retroperitoneal whereas in children graft placement depends on the size of both the abdomen and the graft. In the youngest children (generally <15 kg), many centers use intra-abdominal placement and implant the graft on the inferior vena cava and aorta rather than on the more commonly used iliac vessels (14, 72, 79, 80).
Despite the advantage of larger space for the graft, intra-abdominal placement has some disadvantages including risk on bowel injury and a more difficult access for future interventions such as graft biopsies or PD.
Previous studies showed small children (under 15 kg) to be at increased risk for thrombosis compared to older children (OR 0.11–0.85) (71, 81, 82).
Additionally, very specific individual management might be needed in case of rare and complex associated medical conditions associated with prematurity, severe anatomical anomalies, mental illnesses, syndromal anomalies or extensive urological, or surgical previous procedures. This care should include a medical point of view of all related specialties, nursing care experts and psychological support.
Innovations
Despite the relatively high complication rates, graft- and patient survival in small children have improved in the last decades (1-year graft survival of 50% 1978–2000 vs. 97% 2000–2016). Nowadays, outcomes of transplantation in infants are comparable to those in older children (71, 73, 77, 78, 83). These improvements might be due to the high rates of living donations, the operationalization of dedicated multidisciplinary teams and improved immunosuppressants and diagnostics.
Accurate imaging of recipients and potential donors allows health care providers to make well-educated choices regarding the favorable surgical technique and postoperative care.
Timing
Although kidney transplantation is shown to be safe and successful in very small children, there is still some controversy concerning the optimal timing of the transplantation. On the one hand, early transplantation could avoid dialysis and allows better physical and neurological growth in these young children. On the other hand, early exposure to immunosuppressive therapy might result in severe infections and long-term side effects. Besides, with current graft survival, transplantation at a very young age implicates multiple re-transplantations in a life time, which are known for decreased graft survival compared to primary transplantations (84–86).
Currently, there is no consensus on a minimal age or weight for transplantation. Whereas, some centers perform transplantations in children above 6 kg, others use a minimum age of 2 years (69, 72, 87). Further work is required to determine optimal timing with regard to long term outcome.
Radboudumc Amalia Children's Hospital
In our center, we started a program for transplantations in children weighting <20 kg in 2012. A multidisciplinary team including pediatric nephrologists, pediatric urologists, pediatric anesthesiologists, pediatric ICU specialists, vascular surgeons and pediatric surgeons as well as a paramedical team started this program with satisfying outcome (77).
Special attention is paid to the disparity between the size of the kidney graft and the length of the recipient and therefor the size of the body cavity.
Since the start of this special program in 2012, 13 children with a weight below 15 kg have been transplanted thus far (mean age 3.4 ± 1.6 years, mean weight 12.7 ± 1.5 kg). Up to this moment all recipients are alive with a functioning graft [median follow-up of 89 months (range 3–221)].
In contrast to other studies, we didn't find a difference in graft survival between age groups (p = 0.26) (Appendix).
Urological Work-Up and Follow-Up
Urological causes for ESKD are seen in 25–40% of the pediatric kidney recipients and encompass mainly posterior urethral valves (PUV), vesico-ureteral reflux (VUR) and neurogenic bladder (88). Nephrological causes include renal dysplasia, hereditary kidney diseases such as ciliopathies, focal segmental glomerulosclerosis and other types of chronic glomerulonephritis (7, 89).
Lower urinary tract dysfunction (LUTD) might affect pediatric kidney graft outcome (90). LUTD is an umbrella term that includes several urological items reflecting the function of the bladder and lower urinary tract. Exact definitions of LUTD vary widely across the literature which makes comparison of research data on LUTD challenging. Whereas, some diagnose LUTD using uroflowmetry and frequency voiding charts others include all children with for example a bladder augmentation and intermittent catherization without other diagnostics. By consequence, there is little consistency on the prevalence of LUTD (91, 92). Although LUTD was particularly thought to be a problem in urological patients, LUTD was found to be fairly common in all kidney recipients despite underlying cause for ESKD (93–96).
In children with PUV, myogenic changes in the bladder wall result in abnormal contractility and different sensations in for example a full bladder. This ultimately might induce abnormal voiding behavior and high intra-vesical pressures. The normal bladder cycle is interrupted in patients with PUV and high pressure might cause fibrosis in the bladder walls. The prevalence and severity depends on the severity of previous mentioned changes in the bladder wall (97–99). Therefore, long-term graft survival might be worse in recipients with PUV than in those with other forms of congenital anomalies (100).
There are several factors that could contribute to development of LUTD in pediatric renal recipients without an urological history. Long pre-transplantation polyuria may lead to overdistension of the bladder which results in diminished sensation and therefore abnormal voiding patterns (101). On the other hand, oliguria results in low-capacity bladders. These low-capacity bladders cannot adjust to a sudden increase of urine volume, e.g., after transplantation. This might result in high intra-vesical pressures that lead to LUTD and a subsequent deteriorating effect on graft function (93, 95, 96).
At this moment, little information is available on the effect of immunosuppressive medication on the bladder wall (102).
The general believe is to treat LUTD as much as possible before transplantation in order to protect the kidney graft from a high-pressure lower urinary tract (99). Although the occurrence of LUTD is associated with the risk on urinary tract infections (UTI), the effect on graft survival and graft function remains less clear (103–105). Some studies showed LUTD to negatively influence graft survival whereas the majority of previous research did not (90, 106, 107). As definitions of LUTD vary, it remains difficult to draw general conclusions from these studies.
Regardless of the actual effect on graft function, LUTD in children results in diminished quality of life and increased morbidity, especially in the case of urinary incontinence (108–110).
Screening for LUTD
Optimal timing and screening methods remain to be determined. Multiple diagnostic tools are available including (digital) frequency voiding charts (FVC), Urodynamic studies, Ultrasound (US), Voiding Cystourethrogram (VCUG), uroflowmetry (FR), and post voiding residual (PVR) measurement. Since most patients do not need invasive diagnostics, PVR was found to be the most accurate predictor for prognosis (111). However, a combination of multiple non-invasive diagnostics might be favorable to get a good understanding of voiding behavior and bladder function. In patients with PUV or neurogenic bladder a VCUG and urodynamic studies prior to transplantation are recommended (99, 112).
Urotherapy, which is defined as non-surgical, non-pharmacological treatment for LUTD, is considered to be the cornerstone in the treatment in otherwise healthy children (98, 113). It encompasses education, behavioral modification, registration of voiding habits and life-style advice (113). In this light, it seems reasonable to not only screen all children before kidney transplantation, but also start with urotherapy to allow earlier intervention. On the long term this might lead to subsequent decline in morbidity and better graft outcome.
Interventions
The timing of bladder augmentation remains controversial. If indicated, bladder augmentation is mostly performed before transplantation (114). An advantage of this timing is that the bladder can heal before starting immunosuppressive medication. Whereas, multiple studies show that pre-transplantation bladder augmentation is favorable over post-transplantation augmentation (115, 116), others reported equal outcome in children that were transplanted first (117). Arguments against pre-transplantation augmentation include the increased risk of infection and the scenario of a dry augmentation. In addition, peritoneal dialysis might be a relative contra-indication (114, 118). Augmentation concurrent with transplantation was discouraged by most authors because of the increased risk on surgical complications (118, 119).
The need of pre-transplantation bladder cycling remains unclear, although several authors argument that this would improve outcome, it was not shown to be beneficial (120, 121).
Asymptomatic Bacteriuria
Another controversial topic is the treatment of asymptomatic bacteriuria (AB) which occurs in 17–51% of adult kidney recipients (122). Multiple studies among which a recent Cochrane review stated there is insufficient evidence for treating asymptomatic bacteriuria with antibiotics, especially in the light of possible resistance (123–125). None of the included studies showed significant effects of antibiotic treatment on graft survival or graft function. Therefore, one can doubt if screening for asymptomatic bacteriuria is useful in this population.
The role of bladder rinsing with hyaluronic acid and chondroitin sulfate is unclear. Several studies have shown beneficial effects in individual pediatric patients, but those were limited in the number of participants (126, 127).
Radboudumc Amalia Children's Hospital
In our center 22% (n = 89) of the 411 pediatric kidney recipients had an urological cause for their ESKD. In a prospective study of 56 patients, we screened all recipients for LUTD and treated them if indicated. LUTD was diagnosed in the majority of patients (71%) regardless of the underlying cause of kidney failure. This indicates that most pediatric transplant recipients do not have adequate voiding behavior, normal bladder capacity, and micturition frequency.
Anesthetic Issues
Peri-operative care for pediatric kidney recipient differs from adult care and because of the rarity of pediatric kidney transplantation, there are no evidence-based guidelines available. There are multiple issues requiring special attention in this population.
Anesthesia Technique
Kidney transplantation requires general anesthesia, endotracheal intubation and controlled ventilation. There are various sedatives used, however no specific drugs were shown to be preferable. Sevoflurane might have a beneficial effect on hemodynamics although concerns have been raised about its nephrotoxicity (128, 129).
Many patients with ESKD have impaired long function because of fluid overload and leakage of alveolar membranes. Therefore, lung protective mechanical ventilation might be beneficial in pediatric kidney transplantation (130, 131).
Hemodynamic Challenges
One of the major challenges during kidney transplantation is the preservation of adequate graft perfusion. Although a minimum mean arterial pressure (MAP) of 70 mmHg is recommended in adults, administration of excessive fluids or vasopressors might be harmful in children. Therefore, adjustment of the target MAP to the donors MAP and visual judgement of perfusion is favored in this population.
Methods for managing hemodynamics are the administration of fluids and the use of vasoactive medication.
Norepinephrine is recommended in patients that do not respond to fluid administration. It prevents post reperfusion hypotension which is commonly seen in small pediatric patients that receive a kidney form an adult donor (76, 131).
In very young children (<5 years) hemodynamic challenges are even bigger. Because of large differences in vascular sizes between donor and recipient, renal arterial blood flow can be compromised. Additionally, a large kidney demands a persistent increase in cardiac output of the child in order to meet the flow demands of the graft. Therefore, close hemodynamic monitoring is of utmost importance in these patients and cardiac output measurement during anesthesia should be considered (76).
Radboudumc Amalia Children's Hospital
In our center, anesthesia for children below 40 kg is done by dedicated pediatric anesthesiologists.
During surgery, multiple monitoring tools are used including end tidal CO2 and oximetry. In children <20 kg we use Pulse Contour Cardiac Output (PiCCO) technique for advanced hemodynamic monitoring (131).
Of 411 transplants 4 had primary non-function. Median duration of cold ischemia was 16 h [IQR 2–26], median duration of second warm ischemia was 35 min [IQR 38–42].
Surgical Issues
Nephrectomy
Native kidneys are removed before transplantation if they are expected to be of short- or long-term risks to the kidney recipient or the graft. Indications for nephrectomy include high risk of recurrence of native disease (e.g., nephrotic syndrome, focal glomerular disease), congenital anomalies, chronic infection, refractory hypertension, and malignancy (132).
However, some of these indications are rather relative indications. Arguments against this procedure include the need of additional surgery and anesthetics, the risk of peritoneal laceration and the benefits of residual urine production.
Theptimal timing of nephrectomy has to be determined as well. Whereas, nephrectomy was commonly conducted before transplantation, some authors are in favor for post-transplantation nephrectomy because of the benefits of pre-emptive transplantation, minimization of sensitization and better clinical condition (133). Other studies showed favorable outcome for simultaneous transplantation, as this limit the amount of operations and anesthesia (134–136).
Currently, nephrectomy by means of a surgical intervention is the most common form of practice. However, various studies reported on alternatives like renal arterial embolization and medical nephrectomy by means of indomethacin or an ACE inhibitor (137–140). Although effectiveness of embolization was shown to be higher compared to medical nephrectomy, side effects like hemorrhage, postembolization syndrome, or non-target embolization were more severe.
However, only studies with limited patients were eligible, therefore future research should focus on these less invasive methods.
Radboudumc Amalia Children's Hospital
In the 100 most recent patients in our center, pre-transplantation nephrectomy was performed in 21%. Common indications were steroid-resistant nephrotic syndrome (38%) and large polycystic kidney volume (14%).
We conducted a successful medical nephrectomy using ACE inhibitor in 6 out of 8 patients with nephrotic syndrome (139).
Surgical Complications
Urological Complications
Urological complications after kidney transplantation can be divided in early (4%) and late complications (9%). Urinary leakage (2%) and early ureteral stenosis (1%) (due to limited ureteral perfusion) or lymphocele needing drainage occur in the 1st month after transplantations, whereas late complications are mostly UTI (15–58%) and late ureteral obstruction (5–8%) (75, 90, 124, 141–145). The latter is often the result of fibrosis, infection or rejection and therefore distinct from early stenosis (146).
The placement of a temporary ureteral stent remains controversial. Previous literature showed that stenting was associated with increased risk on BK viremia and UTIs (147, 148). This might be caused by the mechanical trauma induced by stent placement which activates latent BK virus (148). However, other studies showed ureteral stenting to be protective against urological complications such as stenosis or leakage (149, 150). In adults stents are commonly used and associated with a reduction in urological complications from 7 to 1.5% (151). Further work is required to determine the trade-offs between the positive effects of stents preventing post-operative complications and the negative effect of increased risk of BK nephropathy.
In adults, JJ stenting was shown to be preferable to percutaneous stents in terms of recovery, however duration of drainage remains debatable (152).
A Cochrane Review in the adult population showed that early removal (<15 days) of bladder indwelling and per-urethral stents might decrease the risk on UTI, however differences are small (153). The benefits of stenting in the pediatric populations remain unclear, currently many centers chose early stent removal in order to prevent urological complications and limit the number of infectious events (72, 149). The use of suprapubic bladder catheter or transurethral catheter is also based on individual preference and specific patient characteristics.
Additional clinical trials are needed to support this practice.
Vascular Complications
Vascular complications are an important cause for early graft loss and include mainly renal thrombosis (3–12%) and arterial stenosis (3–15%) (149, 154, 155).
Venous thrombosis is considered the most common cause of early graft loss and especially small children are at risk for developing thrombotic complications (73, 156). However, the benefits of anticoagulation should be balanced upon the risk on hemorrhage and practice differs between centers (75, 157, 158). Studies on thrombotic prophylaxis showed a reduction of thrombotic events for anticoagulant use, however because of the poor quality of the data and the diverse protocols no solid conclusion can be drawn (159).
Renal artery stenosis is associated with hypertension and progressive graft dysfunction and can be due to kinking or trauma of the artery, vascular rejection, inadequate suturing or atherosclerosis.
Lymphatic Complications
The most important lymphatic complication after transplantation is lymphocele, which occurs in 0.5–22% of the recipients (149, 160–162). It is caused by transection of lymphatic vessels of either donor or recipient and develops usually in the 1st week after transplantation.
Lymphocele can result in compression of the graft vessels, ureter or bladder outlet and therefore cause decreased graft function. Analysis of the aspired fluid can differentiate from hematoma, urinoma and seroma. In pediatric recipients, a higher age, BMI and number of transplantations were associated with the development of lymphoceles (160). In addition, several studies showed that sirolimus may is correlated with lymphocele formation (163–165).
Radboudumc Amalia Children's Hospital
According to our protocol, all children have a ureteral splint for 5 days in patients older than 4 years and 7 days in patients <4 years and a transurethral or suprapubic catheter for 7 and 9 days, respectively. Protocol antithrombotic prophylaxis for patients older than 12 years exists of daily 2500 IE dalteparine post-operative until good mobilization. Specific anticoagulants such as Direct Oral Anticoagulants (DOACs) or Vitamin K antagonists are given on indication. We don't use prophylaxis for arterial thrombosis. In total, 13 patients (3%) lost their graft due to thrombosis, all before 2005.
Ureteral-Bladder Anastomosis
The ureteroneocystostomy (UNC) technique is one of the surgical factors that might influence the urological complication rate (166).
UNC Methods
The method used for the neo-ureteral-bladder anastomosis has changed over the years in the adult population. Where an intra-vesical (anti-reflux) technique was common in the past, nowadays this has changed to an extra-vesical approach with or without anti-reflux technique (166).
Overall, UNC techniques can be divided in being either intra-vesical or extra-vesical and refluxing or anti-refluxing (Figure 4).
With the intra-vesical Leadbetter-Politano (PL) technique an anti-refluxing tunnel is created to prevent vesico-ureteral reflux (VUR) (167). This technique was originally performed in most transplantations and requires 2 cystostomies (166).
After some time the easier extra-vesical modified Lich-Gregoir (LG) technique gained more popularity. In the LG technique, a single cystotomy is performed and the distal ureter anastomosis to the bladder is covered by detrusor muscle with the intention to create a valve effect and prevent VUR (168). Over time, new methods were reported such as the “U-stitch” technique and the “full-thickness” technique. The latter is an extra-vesical refluxing technique in which the ureter is anastomosed to the bladder without coverage of detrusor (151, 166, 169, 170). Earlier research showed favorable outcome for the LG technique in terms of urinary leakage and hematuria compared to PL and U-stitch methods (169, 171).
Currently, the “full thickness” method is commonly used in the adult population which has no anti-reflux mechanism (151). This technique minimalizes any risk for ureteral obstruction with comparable outcomes as anti-refluxing techniques (172).
UNC in Pediatric Patients
Little is known about the optimal UNC technique in the pediatric population. Although an anti-reflux technique might be favorable regarding the increased risk of VUR in pediatric recipients (173), it could increase the risk for ureteral obstruction.
The importance of VUR as a complication after kidney transplantation is debatable since it is often asymptomatic and might not influence long-term outcome in adults (174, 175). The exact prevalence of VUR in children is unknown since routine VCUG after transplantation is not standard practice in most centers. Besides, the fact that a part of this population is not continent yet makes it difficult to compare to adult care. Whereas, Ranchin et al. showed VUR incidence up to 58% despite anti-refluxing methods (176), symptomatic VUR occurred in only 5–12% of the pediatric kidney recipients (177–179). Post-transplant obstruction was reported in 8% of cases (141, 142) and post-transplantation UTI in 15–58% (180–182).
Altogether, the choice of UNC technique in pediatric kidney recipients remains difficult and the long-term effects are still unknown. It would be worthwhile to compare outcomes of anti-reflux vs. reflux techniques in pediatric kidney recipients specifically.
Radboudumc Amalia Children's Hospital
In our center, from 2000 all anastomoses were created using a refluxing extra-vesical technique. In 100 recipients that received their graft between 2002 and 2018 55% had least one UTI, 20% had recurrent UTI's. VCUG was done in 11 symptomatic children of which five were diagnosed with VUR.
Transplantectomy
As stated before, graft failure is mainly the result of rejection or thrombosis. When a graft fails, one has to choose between either leaving the graft in situ or performing a graft nephrectomy (GN).
It is well–established that GN is indicated in cases of vascular thrombosis, hyperacute rejection, and therapy resistant malignancy (183–187). Relative indications include severe graft pyelonephritis, the wish of withdrawal of immunosuppressants and symptoms of the intolerance syndrome (185, 188, 189). The removal of an asymptomatic non-functioning graft remains controversial.
In adults, GN is performed in about 35% of patients with a failing graft (187, 190). In pediatric recipients over 50% of patients with a failing graft had GN, although data on the pediatric population are scarce (186, 191). This difference might be caused by the higher rates of acute rejection in children, which was thought to be caused by a more vigorous immune response in children (192). In both adults and children, recipients were most likely to have GN when graft failure occurred in the 1st year after transplantation (185–187, 191).
Considered benefits of GN include reduction of inflammation, discontinuation of immunosuppression and possible reduction of the number of donor specific antibodies. However, surgery for graft removal may cause considerable peri- and post-operative morbidity such as inflammation and hemorrhage. Post-operative mortality rates ranged from 1 to 39% and was mostly caused by sepsis (17%). Moreover, re-transplantation outcomes are worse after GN compared to no GN (187, 193, 194).
Moreover, GN is associated with higher donor specific antibodies because of the potential absorptive capacity of the graft (195). Previous studies showed a longer interval between graft loss and re-transplantation after GN (186, 187, 193). Moreover, minimal residual urine and erythropoietin production from the failed graft may be preserved when immunosuppression was continued (196).
Allosensitization After GN
To establish the impact of GN on both allosensitization and graft outcome remains challenging because of multiple confounding factors. However, timing of GN is thought to be an important factor. Sener et al. showed that patients that had GN in the 1st month after transplantation had lower panel reactive antibodies (PRA) and reduced risk on future graft failure compared to those who did not have GN (197).
In contrast, patients who had late GN (>1-year) were at increased risk for future graft failure and had increased PRA (187, 197, 198). Wang et al. reported no difference in patient and graft survival between those who underwent GN and those who did not, whereas Ayus et al. showed a 32% lower risk on morbidity after GN (190, 193).
The (dis)continuation of immunosuppressants after GN remains debatable, since continuation would prevent allosensitization while increasing the risk on infections, vascular disease and malignancy (144, 199). In children, considerations might be different than in adults since they are more likely to have a re-transplantation.
There is only limited research on GN in children. Whereas, high rates of morbidity and mortality were seen in adults, outcome in pediatric GN was shown to be good. No major complications, re-operations and blood transfusions were reported in the few studies on pediatric recipients (186, 191).
Alternatives
Renal artery embolization (RAE) was thought to be a minimal invasive alternative to GN as it results in less surgical complications. However, the risk on necrotic pyelonephritis and post-embolization syndrome are increased after RAE and RAE as monotherapy is not widely used (200). However, using RAE as neo-adjuvant intervention before GN was shown to reduce both blood loss and operating time (201–203).
Radboudumc Amalia Children's Hospital
Between 1977 and 2020, 379 transplantations were performed in our center. Graft failure occurred in 108 grafts so far of which 66 (53%) were removed. There was no operative mortality and 32% of the surgeries resulted in complications which were all resolved (191).
Acute Rejection
Graft Biopsies
Subclinical acute rejection was shown to be a cause for deterioration of graft function which implies that early diagnosis and treatment is favorable. Since subclinical rejection can only be diagnosed by means of graft biopsy, the practice of “protocol” or so called “surveillance” graft biopsies is under debate (204, 205). Although this allows early detection of rejection and tubular atrophy, the effect on long term graft survival remains unclear. Most studies showed comparable short term results in both patients that had protocol biopsies and those that had biopsies on indication of clinical symptoms (206). However, a recent prospective study revealed children who underwent protocol biopsies to have better renal function on the long term than the control group (205). Despite the prospective nature of this study, there are some limitations such as different immunosuppressive regimens and the lack of randomization.
Literature on protocol biopsies in pediatric kidney recipients is scarce and future (randomized controlled) trials or small group trials are needed to address significance of early subclinical rejection and therapeutic interventions.
Besides, possible benefits of protocol biopsies should be weighed against the potential risks such as arteriovenous fistulas and bleeding (207, 208). Because of the improved immunosuppressive regimens, rejection rates have decreased and protocol biopsies might be considered as disproportionate. Additionally, there is no consensus on timing and frequency for protocol biopsies (204, 209, 210).
Although new randomized controlled trials or dedicated small group trials could provide valuable insights in this debate, future research should also focus on developing non-invasive methods for detection of subclinical rejection.
Anti-rejection Therapy
Acute rejection of the kidney graft can be divided in either anti-body mediated rejection and T-cell mediated rejection (211). Therefore the treatment of rejection depends on accurate diagnosis and graft biopsy remains the gold standard. The Banff-classification is the international consensus method for the description of biopsies (212). However, in case of high clinical suspicion on rejection (within 6 months after transplantation, after reduction in immunosuppressive therapy and rapidly rising creatinine levels) one could consider treatment without a biopsy.
There are several strategies in the treatment of T-cell mediated rejection. Traditionally intravenous pulses of methylprednisolone are used. Other options are polyclonal antibodies such as ATG, monoclonal antibodies against lymphocyte receptors such as alemtuzumab and rituximab and a proteasome inhibitor such as Bortezomib (213).
Nowadays, the immediate use of polyclonal antibodies instead of methylprednisone is debatable. There is currently little evidence favoring one specific strategy and clinical decision making remains challenging.
There are various possible strategies to treat anti-body mediated rejection, however the optimal therapy remains controversial. Some of the treatment options are similar as in T-cell mediated rejection such as polyclonal antibodies and methylprednisolone. Other possibilities are plasma-exchange, administration of intravenous immunoglobulins (IVIG) and the monoclonal antibody rituximab (214).
In the past, plasmapheresis was common practice whereas this has a less prominent place in anti-rejection therapy nowadays. Billing at al. introduced the combination of IVIG and rituximab which reduced donor specific antibodies and stabilized renal function (215). Although a variety of studies was conducted on anti-body mediated rejection, they used diverse end-points which makes comparisons difficult (213).
Apart from anti-rejection therapy, changes in maintenance therapy should be considered (216).
Radboudumc Amalia Children's Hospital
In our center, graft biopsies are performed on clinical indications such as a deterioration of GFR. Five percent of our latest 100 pediatric kidney recipients had a graft biopsy during their transplantation admission. During a median follow-up of 47 months, 42 patients had at least one graft biopsy that mostly revealed calcineurin toxicity (32%) and acute rejection (30%). In total, 36 patients received pulsatile methyl prednisone during follow-up. We have reported one case of life-threatening respiratory failure after alemtuzumab administration (217).
Medication
Immunosuppressive Regimens
Historical Developments
One of the factors that improved graft survival is the substantial change in immunosuppressive strategies over time.
In the early days of pediatric kidney transplantation, immunosuppression consisted of total body irradiation and splenectomy which resulted in infection related mortality up to 72% (3, 4). This method was abandoned when corticosteroids were introduced in 1960. As a result, rejection rates increased to 85%. Consequently, the search for better protocols continued with the ultimate goal to minimize severe infections, organ rejections and prevent side effects. Novel immunosuppressive agents, and incorporation of newer prophylactic strategies contributes in achieving this holy grail hopefully in the near future.
In the late 60's more potent medication were available like 6-mercaptopurine and azathioprine (antimetabolites). After the introduction of calcineurin inhibitor (CNI) cyclosporine in the 1980's, graft survival increased substantially (4, 218, 219). In the past decades, various types of immunosuppressive drugs became accessible.
In the 90's CNI tacrolimus and mycophenolate mofetil (MMF, a prodrug of mycophenolic acid (MPA), an inhibitor of inosine-5′-monophosphate dehydrogenase) were introduced and the twenty-first century welcomed mammalian target of rapamycin (mTOR) inhibitors like sirolimus and everolimus (4, 10).
Current Practice
Today, maintenance immunosuppressive protocols combine multiple drugs with various modes of action. In this regime CNI, antimetabolites, mTOR-inhibitors and/or corticosteroids for anti-rejection maintenance prophylaxis are the cornerstone (11).
CNI-withdrawal was found to be deleterious for graft function and survival (18). Tacrolimus seems favorable over cyclosporine since it resulted in less acute rejection and improved graft survival (19). Moreover, tacrolimus has less cosmetic side effects than cyclosporine which might be important regarding medication adherence (220).
Similarly, MMF was shown to be a more potent immunosuppressant compared to azathioprine and therefore first choice in antimetabolites. On the other hand, MMF is known for multiple side effects including gastro-intestinal symptoms and anemia which might compromise medication adherence (221).
Corticosteroids are known for their multiple side effects like growth retardation, osteoporosis, hypertension, diabetes mellitus, obesity, dyslipidemia, impaired wound healing, and mental disorders. Currently, 90% of the immunosuppressive protocols contain corticosteroids, despite the demand for minimization. Several studies showed that late steroid-withdrawal is safe in terms of graft survival and rejection in patients with low immunological risks (222, 223).
Other studies showed that early steroid withdrawal is safe as well (224, 225).
On the other hand, earlier research suggested that steroid withdrawing protocols lead to higher incidences of viral infections and post-transplantation lymphoproliferative disease (PTLD) (79, 226). This was suggested to be caused by high dosages of other immunosuppressants in order to compensate for the loss of corticosteroids. In addition, the use of mycophenolate mofetil instead of glucocorticosteroids, is associated with more frequent and severe leukopenia, anemia, and gastrointestinal disturbances (79).
Although corticosteroid withdrawal seems safe in a selected population, long-term effects should be studied before general implementation.
Currently, there is no worldwide consensus on the use of induction therapy. Current literature showed no advantages in a standard, low-risk pediatric population (223, 224). If used, most common induction regimens are with antilymphocyte biological agents, T-lymphocyte-depleting rabbit-derived antithymocyte globulin (rATG), an IL-2 receptor antagonist (IL2RA) like Basiliximab (a chimeric (human/murine) monoclonal antibody) or Alemtuzumab (an anti-CD52 T-cell and B-cell–depleting monoclonal antibody) (11).
Radboudumc Amalia Children's Hospital
In our center, the use of cyclosporine and prednisone decreased over time, which is comparable to international literature (Figure 5). The Transplantation WIthout Steroids (TWIST) protocol, that limits use of prednisone to 5 days, was introduced in 2012 in recipients without additional risk factors like high sensitization or diseases that are known for their risk on recurrence (224).
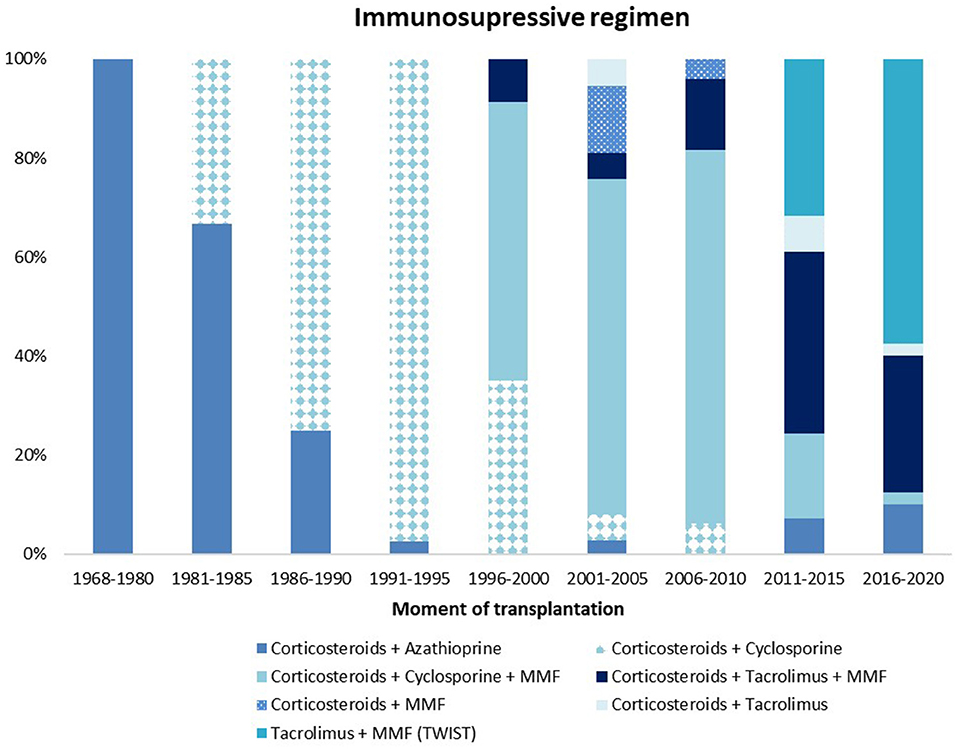
Figure 5. Immunosuppressive maintenance regimen at 3 months after transplantation. MMF, mycophenolate mofetil.
Of the most recent 100 recipients, 44 patients started the steroid-sparing TWIST regimen and 44% remained on this regimen during a follow-up period of 5-years. Patients on a steroid-based regimen had significantly more UTIs (63 vs. 25% p < 0.01), more CMV infections (11 vs. 0% p = 0.03) and more rejections (18 vs. 0% p = 0.02) than those on a steroid-sparing regimen. Other side effects did not differ between steroid-sparing and steroid-containing regimens (227). Steroid withdrawal was not associated with improved growth, increased incidence of PTLD, rejection or graft loss in this limited population.
Although the majority of patients started with MMF after transplantation, 45% needed to stop MMF due to side effects, despite the use of slow release Mycophenolate in such cases.
Anti-viral Therapy
Children on immunosuppressive therapy are prone to viral infections with potentially severe consequences. Live vaccines cannot be administered after transplantation and inactive vaccines might not be effective due to immunosuppressive agents (228). Therefore, vaccination status is an important issue to optimize before transplantation.
Viral infections that commonly cause morbidity in pediatric kidney recipients are Cytomegalovirus (CMV), Epstein-Barr virus (EBV) and BK-virus (BKV). The latter specifically harms the graft.
Without prophylaxis, these infections are most likely to occur in the early months after transplantation because of transmission trough the graft and high dosages immunosuppression during this period (9, 229).
The use of CMV prophylaxis is recommended in several guidelines, however which patients should receive prophylaxis and for how long remains debatable (229, 230). Valganciclovir is commonly used as prophylactic oral drug whereas ganciclovir is used for therapy.
Radboudumc Amalia Children's Hospital
In our center, high risk patients (D+R-) receive CMV prophylaxis until 3 months after transplantation. In the 100 most recent transplanted patients, 62% used CMV prophylaxis. During a median follow-up of 47 months 14% developed CMV disease regardless of prophylaxis.
Medication Adherence
Medication adherence is commonly defined as when the patient follows recommendations and instructions from health care professionals concerning the taking of medication that were previously agreed on (231).
Medication non-adherence (MNA) to immunosuppressant regimens is an important factor limiting graft survival. Moreover, in adolescents (aged 11–21 years) it is considered the most important modifiable risk factor for graft loss (232, 233). The risk of acute rejection is doubled when patients are non-adherent whereas the risk of graft loss increases with 80% (234).
The overall prevalence rate of MNA is considered between 30 and 50%, however numbers vary according to the characteristics of the patient population, definition of MNA used, timing of measurements and methods used to assess adherence (233, 235, 236). Despite different percentages mentioned in the literature, it is well-established that adherence is worse in adolescents than in younger children (233, 235, 237).
Measurement of MNA
Previous studies reported a wide range of assessment methods like pill counts, questionnaires, patients' diaries, and random measurements of blood drug concentrations. Despite the several methods available, it remains difficult to assess MNA because all methods have their own limitations (238). In general, health care providers underestimate MNA (239).
Despite its limitations, electronic monitoring is currently accepted as the most reliable measurement of adherence (240). In adult population, electronic monitoring results were directly associated with clinical outcome (241).
Risks for MNA
The WHO has classified the factors of MNA in 4 categories: individual level, family level, health-care system, and community level (242). Most studies focused on one or few determinants whereas multidimensional assessment might be desirable. Moreover, risk factors vary across countries, types of health care and ethnicities which makes it difficult to draw general conclusions.
At the individual level, recipient age was found to be one of the strongest factors affecting the risk for MNA (235, 243). Qualitative studies assessing barriers to adherence showed that in adolescents one of the main challenges was remembering to take medication, especially on days when there was no strict routine (like weekends and holidays) (232).
Transition of responsibility remains a difficult topic in this age category as these teenagers often desire more independence (244). In a Dutch study on transition, immigrant patients appeared to be particularly at risk for acute rejection during this period (245).
Improving MNA
Although many adherence-promoting methods were developed, single strategies were not shown to be effective (246, 247). Previous researchers suggested multi-component behavioral interventions to aim at multiple barriers to adherence (232, 248).
Although some trials with these multi-component interventions have been conducted successfully, these were labor-intensive and not easy to incorporate in daily life (232, 248, 249).
Studies with electronic pillboxes and eHealth interventions have shown promising results in studies among patients with chronic diseases. However, these should be tailor made for this specific population (233, 250–252). On the other side, concerns were raised about these interventions regarding privacy regulations and the large volumes of data health care providers have to deal with in limited time (232).
In addition, special attention should be drawn to the transition of recipients with delayed development. Since they will need extra support, they are prone to fall between two specialties due to their individual transition requirements, especially if they have comorbidities. Taken together, these results suggest that simple “one size fits all” interventions are not effective and that future interventions should be multidimensional and targeting risk levels on various levels in the healthcare system.
Radboudumc Amalia Children's Hospital
In our center, medication adherence is addressed every outpatient visit, especially in adolescents. To support transition to adult care, patients are actively prepared from the age of 12 years in a personalized manner. Aspects of this transition phase are education on their disease, the different drugs, outpatient clinic visits with the first part of the visit without their parents. These patients are guided to take responsibility for their treatment. However, actual data on adherence are missing. Little is known on the non-adherence rates in our center.
Discussion
In the past decades, innovations in pediatric kidney transplantation led to increased graft and patient survival. Despite these innovations, we endeavor to optimize clinical care for pediatric kidney recipients. In this article we provided a state of the art overview and identified paucity of evidence on several important issues in comparison with our results. Future possibilities to improve pre-, peri-, and post-operative care are discussed below.
Pre-operative Factors
Choosing between an old well-matched living donor and young poorly-matched deceased donor requires consideration of multiple aspects such as sensitization, existing waiting list time and the risk of graft failure (253). Future research should focus on combining these elements in order to make an appropriate trade off and achieve tailor made treatment. The development of validated algorithms would assist clinicians in their considerations for a suitable donor. Thresholds in developing such models include the relatively small numbers of pediatric kidney transplantations and the many changes in practice over time.
In order to fill in the knowledge gaps mentioned above, thorough and well-designed studies are needed. Previous literature is mainly based on retrospective studies in relatively small cohorts. Since the incidence of graft loss has impressively decreased, large volumes are needed to draw conclusions on the factors leading to graft loss.
Besides, research in such a rapid changing field is challenging since data are quickly outdated and confounding factors such as innovations in practice are difficult to correct for.
A possible solution for these problems might be collaboration between the different (inter)national registries. Currently, multiple organizations and registries are actively studying pediatric kidney transplantation such as the NOTR (national) and CERTAIN registry (international). However, each registry collects different data in a slightly different population. Collaboration between those registries allows studying large volumes of data and comparison of practices among different countries. A promising phenomenon is the development of European Reference Networks (ERN), which allows cooperation at the European level between clinicians with specialized expertise. They aim to improve diagnoses and treatment for patients with rare diseases and/or complex conditions. The ERN eUROGEN aims to improve diagnosis, create more equitable access to high-quality treatment and care for patients with rare uro-recto-genital diseases and complex conditions needing highly specialized surgery (254). ERKNet does the same for patients with rare kidney diseases.
Peri-operative Factors
Surgical techniques have improved over time which resulted a reduction of peri-operative complications. Transplantation in small children is possible although blood pressure and perfusion are vulnerable. Peri-operative monitoring enables strict regulation of those parameters and a multidisciplinary team working according well-defined protocols is mandatory (13, 14, 76).
Although in adults, a refluxing ureteroneocystostomy was shown to be comparable to anti-refluxing methods, less is known about the pediatric population. To reveal this topic future multicentered research should focus on differences in long-term outcome regarding graft function, UTIs and urological interventions. Thresholds for such studies are the confounding factors that differ between centers and the lack of routine VCUG to determine the rate of (asymptomatic) VUR.
Post-operative Factors
Immunosuppressive protocols have dramatically changed over time. The withdrawal of steroids was shown to be safe in low-risk patients regarding both graft survival and side effects. This remains uncertain for high-risk patients and long term outcome needs to be established.
It remains challenging to find the best combination of immunosuppressive agents, as the balance between preventing rejection while limiting side effects is precarious. Future research should focus on the long term effects of immunosuppressive medication, especially regarding long term side effects. Biotechnical advancements might result in withdrawal of conventional immunosuppression. Current studies focus on cell-based therapy, which aims at the induction of donor-specific unresponsiveness in the setting of either operational tolerance or mixed chimerism (255).
Medication non-adherence has been increasingly recognized as a cause of graft failure, especially in children and adolescents (12, 231). A better understanding of non-adherence is needed and current literature advocates to tailor interventions to each transplant recipients' unique needs, motivations, and barriers. However, previous studies mainly focused on patient- and family factors, the influence of health care providers and health systems are still to be determined. Future research should incorporate the pitfalls for clinicians and health systems in order to optimize medication adherence.
Future Perspective
Nowadays, artificial intelligence (AI) plays a major role in daily life. Application of AI in medicine and research is becoming more common, especially regarding risk assessment. Machine learning techniques are shown to be promising in processing biomedical data where they are successful for predictive models, image processing and genomic data analysis (256).
In adults, AI has been successfully implemented in the field of kidney diseases. Kuo et al. (257). designed an application that automatically estimates glomerular filtration rate using ultrasound images and multiple studies have shown the benefits of automatic analysis of histological or radiological images (258, 259).
However, such models should be based on a representative cohort of patients, which is a problem in pediatric kidney transplantation. Because of the small patient volumes, development of reliable algorithms and proper validation remains challenging. Although many countries do have their own databases, international collaboration is needed as well as standardization of data, identification of the patient and linking between the different registries (256).
Whereas, optimization of current care remains of utmost importance, recent technologies might offer new perspectives to renal replacement therapy. Current research focusses on developing a wearable artificial kidney that would improve both quality of life and quality of dialysis (260, 261).
Another promising field is that of regenerative medicine, the process of generating a human kidney de novo has been studied over the last decades (262–265). Several authors used pluripotent stem cells to form kidney precursors cells and eventually organoids (266, 267). Differentiation is shown to be limited in 3D cultures and those cultured organoids lack several important structures such as the loops of Henle. However, when placed into living animals these organoids develop capillary loops and connect to the hosts' vascularization. Nowadays, tissue derived from pluripotent stem cells is used to study the genetics aspects of kidney diseases (262, 268).
Despite these promising developments, there are many hurdles to take before one could generate a functioning human kidney. Among them are the possible tumorgenicity, the small scale of organoids and absence of potent vascularization and urinary drainage system (262, 269, 270). Therefore, using newly grown human kidney tissue for renal replacement therapy is still some time off.
Another solution for the graft shortage might be xenotransplantation with the kidney of genetically modified pigs. Recently, surgeons have placed such a kidney in a brain-dead patient for research sake (271). Both the kidney and the thymus were transplanted with good outcome in the first 54 h after transplantation. These findings have not been peer-reviewed and published yet and the procedure will not be available to patients any time soon. Both medical and ethical objections need to be considered first and long terms effects need to be studied.
Conclusion
This overview of 50 years care for pediatric kidney recipients revealed an impressive improvement of graft and patient survival. Important developments are the increased use of living donors, improved immunosuppressive therapy and better peri-operative care.
Still, many questions remain unanswered. In our center, pre-transplant treatment modality, donor age and HLA mismatching did not affect graft survival which might advocate donor pool expansion. More large scale, multicenter studies are needed to confirm these findings.
Since urological complications are more common in children, an active screening program for LUTD should be considered. Moreover, the optimal method for surgical vesico-ureteral anastomosis still needs to be established.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
LO: collected the data, performed the analysis, and wrote the paper. CB-R and LW: conceived and designed analysis, collected the data, and provided intellectual guidance. EC: collected the data and provided intellectual guidance. WdF: conceptualization, writing—review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.856630/full#supplementary-material
References
1. Papalois VE, Najarian JS. Pediatric kidney transplantation: historic hallmarks and a personal perspective. Pediatr Transplant. (2001) 5:239–45. doi: 10.1034/j.1399-3046.2001.005004239.x
2. Goodwin WE, Mims MM, Kaufman JJ. Human renal transplantation III: technical problems encountered in six cases of kidney homotransplantation. J Urol. (1963) 89:349–56. doi: 10.1016/S0022-5347(17)64556-7
3. Murray JE, Merrill JP, Dammin GJ, Dealy JB Jr, Walter CW, Brooke MS, et al. Study on transplantation immunity after total body irradiation: clinical and experimental investigation. Surgery. (1960) 48:272–84.
4. Verghese PS. Pediatric kidney transplantation: a historical review. Pediatr Res. (2017) 81:259–64. doi: 10.1038/pr.2016.207
5. USRDS. The United States Renal Data System (USRDS). 2020 Annual Data Report: Epidemiology of Kidney Disease in the United States. USRDS. (2020).
6. Lilly JR, Giles G, Hurwitz R, Schroter G, Takagi H, Gray S, et al. Renal homotransplantation in pediatric patients. Pediatrics. (1971) 47:548–57. doi: 10.1542/peds.47.3.548
7. Bonthuis M, Vidal E, Bjerre A, Aydog Ö, Baiko S, Garneata L, et al. Ten-year trends in epidemiology and outcomes of pediatric kidney replacement therapy in Europe: data from the ESPN/ERA-EDTA Registry. Pediatric Nephrol. (2021) 36:2337–48. doi: 10.1007/s00467-021-04928-w
8. Union E,. Eurostat Population. (2019). Available online at: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_pjan&lang=en: EU.
9. Fine RN, Grushkin CM, Anand S, Lieberman E, Wright HT Jr. Cytomegalovirus in children. Am J Dis Child. (1970) 120:197–202. doi: 10.1001/archpedi.1970.02100080081004
10. Pape L. State-of-the-art immunosuppression protocols for pediatric renal transplant recipients. Pediatric Nephrology. (2019) 34:187–94. doi: 10.1007/s00467-017-3826-x
11. Cho MH. Pediatric kidney transplantation is different from adult kidney transplantation. Korean J Pediatr. (2018) 61:205–9. doi: 10.3345/kjp.2018.61.7.205
12. Fernandez HE, Foster BJ. Long-term care of the pediatric kidney transplant recipient. Clin J Am Soc Nephrol. (2021) 2021:CJN.16891020. doi: 10.2215/CJN.16891020
13. Salvatierra O Jr, Millan M, Concepcion W. Pediatric renal transplantation with considerations for successful outcomes. Semin Pediatr Surg. (2006) 15:208–17. doi: 10.1053/j.sempedsurg.2006.03.007
14. Salvatierra O Jr, Singh T, Shifrin R, Conley S, Alexander S, Tanney D, et al. Successful transplantation of adult-sized kidneys into infants requires maintenance of high aortic blood flow. Transplantation. (1998) 66:819–23. doi: 10.1097/00007890-199810150-00001
15. Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, et al. OPTN/SRTR 2016 annual data report: kidney. Am J Transplant. (2018) 18(Suppl.1):18–113. doi: 10.1111/ajt.14557
16. Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, et al. OPTN/SRTR 2017 annual data report: kidney. Am J Transplant. (2019) 19(Suppl.2):19–123. doi: 10.1111/ajt.15274
17. Offner G, Latta K, Hoyer PF, Baum H-J, Ehrich JHH, Pichlmayr R, et al. Kidney transplanted children come of age. Kidney Int. (1999) 55:1509–17. doi: 10.1046/j.1523-1755.1999.00356.x
18. MD R. North American Pediatric Renal Transplant Cooperative Study (NAPRTCS): 2014 Annual Report. (2014).
19. Moudgil A, Martz K, Stablein DM, Puliyanda DP. Good outcome of kidney transplants in recipients of young donors: a NAPRTCS data analysis. Pediatr Transplant. (2011) 15:167–71. doi: 10.1111/j.1399-3046.2010.01432.x
20. Chua A, Cramer C, Moudgil A, Martz K, Smith J, Blydt-Hansen T, et al. Kidney transplant practice patterns and outcome benchmarks over 30 years: the 2018 report of the NAPRTCS. Pediatr Transplant. (2019) 23:e13597. doi: 10.1111/petr.13597
21. Harambat J, van Stralen KJ, Schaefer F, Grenda R, Jankauskiene A, Kostic M, et al. Disparities in policies, practices and rates of pediatric kidney transplantation in Europe. Am J Transplantat. (2013) 13:2066–74. doi: 10.1111/ajt.12288
22. Abramowicz D, Hazzan M, Maggiore U, Peruzzi L, Cochat P, Oberbauer R, et al. Does pre-emptive transplantation versus post start of dialysis transplantation with a kidney from a living donor improve outcomes after transplantation? A systematic literature review and position statement by the Descartes Working Group and ERBP. Nephrol Dial Transplant. (2016) 31:691–7. doi: 10.1093/ndt/gfv378
23. Prezelin-Reydit M, Combe C, Harambat J, Jacquelinet C, Merville P, Couzi L, et al. Prolonged dialysis duration is associated with graft failure and mortality after kidney transplantation: results from the French transplant database. Nephrol Dial Transplant. (2019) 34:538–45. doi: 10.1093/ndt/gfy039
24. Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. (2001) 344:726–31. doi: 10.1056/NEJM200103083441004
25. Falconi CA, Junho CVdC, Fogaça-Ruiz F, Vernier ICS, da Cunha RS, Stinghen AEM, et al. Uremic toxins: an alarming danger concerning the cardiovascular system. Front Physiol. (2021) 2021:12. doi: 10.3389/fphys.2021.686249
26. Chavers BM, Solid CA, Daniels FX, Chen S-C, Collins AJ, Frankenfield DL, et al. Hypertension in pediatric long-term hemodialysis patients in the United States. Clin J Am Soc Nephrol. (2009) 4:1363–9. doi: 10.2215/CJN.01440209
27. Shamszad P, Slesnick TC, Smith EO, Taylor MD, Feig DI. Association between left ventricular mass index and cardiac function in pediatric dialysis patients. Pediatr Nephrol. (2012) 27:835–41. doi: 10.1007/s00467-011-2060-1
28. Amaral S, Sayed BA, Kutner N, Patzer RE. Preemptive kidney transplantation is associated with survival benefits among pediatric patients with end-stage renal disease. Kidney Int. (2016) 90:1100–8. doi: 10.1016/j.kint.2016.07.028
29. Atkinson MA, Roem JL, Gajjar A, Warady BA, Furth SL, Muñoz A. Mode of initial renal replacement therapy and transplant outcomes in the chronic kidney disease in children (CKiD) study. Pediatr Nephrol. (2020) 35:1015–21. doi: 10.1007/s00467-019-04416-2
30. Vats AN, Donaldson L, Fine RN, Chavers BM. Pretransplant dialysis status and outcome of renal transplantation in North American children: a NAPRTCS Study. North American pediatric renal transplant cooperative study. Transplantation. (2000) 69:1414–9. doi: 10.1097/00007890-200004150-00035
31. Kim JK, Lorenzo AJ, Farhat WA, Chua ME, Ming JM, Dos Santos J, et al. A comparison of post-transplant renal function in pre-emptive and post-dialysis pediatric kidney transplant recipients. Pediatr Transplant. (2019) 23:e13377. doi: 10.1111/petr.13377
32. Cransberg K, Smits JM, Offner G, Nauta J, Persijn GG. Kidney transplantation without prior dialysis in children: the Eurotransplant experience. Am J Transplant. (2006) 6:1858–64. doi: 10.1111/j.1600-6143.2006.01405.x
33. Nevins TE, Danielson G. Prior dialysis does not affect the outcome of pediatric renal transplantation. Pediatr Nephrol. (1991) 5:211–4. doi: 10.1007/BF01095954
34. Goto N, Okada M, Yamamoto T, Tsujita M, Hiramitsu T, Narumi S, et al. Association of dialysis duration with outcomes after transplantation in a Japanese Cohort. Clin J Am Soc Nephrol. (2016) 11:497–504. doi: 10.2215/CJN.08670815
35. Kutner NG, Zhang R, Huang Y, Johansen KL. Impact of race on predialysis discussions and kidney transplant preemptive wait-listing. Am J Nephrol. (2012) 35:305–11. doi: 10.1159/000336891
36. Irish GL, Chadban S, McDonald S, Clayton PA. Quantifying lead time bias when estimating patient survival in preemptive living kidney donor transplantation. Am J Transplant. (2019) 19:3367–76. doi: 10.1111/ajt.15472
37. Gillis KA, Lees J, Ralston MR, Glen J, Stevenson K, McManus S, et al. Interaction between socioeconomic deprivation and likelihood of pr… emptive transplantation: influence of competing risks and referral characteristics@ a retrospective study. Transplant Int. (2019) 32:153–62. doi: 10.1111/tri.13336
38. Knight RJ, Teeter LD, Graviss EA, Patel SJ, DeVos JM, Moore LW, et al. Barriers to preemptive renal transplantation: a single center questionnaire study. Transplantation. (2015) 99:576–9. doi: 10.1097/TP.0000000000000357
39. Coorey GM, Paykin C, Singleton-Driscoll LC, Gaston RS. Barriers to preemptive kidney transplantation. Am J Nurs. (2009) 109:28. doi: 10.1097/01.NAJ.0000363348.29227.a9
40. Timmerman L. Exploring knowledge about dialysis, transplantation, and living donation among patients and their living kidney donors. Int J Behav Med. (2015) 22:580–9. doi: 10.1007/s12529-015-9461-7
41. Massey EK, Gregoor PJHS, Nette RW, van den Dorpel MA, van Kooij A, Zietse R, et al. Early home-based group education to support informed decision-making among patients with end-stage renal disease: a multi-centre randomized controlled trial. Nephrol Dialysis Transplant. (2015) 31:823–30. doi: 10.1093/ndt/gfv322
42. Ismail SY, Claassens L, Luchtenburg AE, Roodnat JI, Zuidema WC, Weimar W, et al. Living donor kidney transplantation among ethnic minorities in the Netherlands: a model for breaking the hurdles. Patient Educ Couns. (2013) 90:118–24. doi: 10.1016/j.pec.2012.08.004
43. Redeker S. Eindrapportage project ‘Nierteam aan Huis’ 2016-2020. Projectgroep nierteam aan huis. (2020).
44. Sigurjonsdottir VK, Grimm PC. Living or deceased donor kidney transplantation in children. Curr Opin Pediatr. (2019) 31:232–6. doi: 10.1097/MOP.0000000000000740
45. Engels G, Döhler B, Tönshoff B, Oh J, Kruchen A, Müller I, et al. Maternal versus paternal living kidney transplant donation is associated with lower rejection in young pediatric recipients: a Collaborative Transplant Study report. Pediatr Transplant. (2021) 2021:e14154. doi: 10.1111/petr.14154
46. Joo SY, Song EY, Shin Y, Ha J, Kim SJ, Park MH. Beneficial effects of pretransplantation microchimerism on rejection-free survival in HLA-haploidentical family donor renal transplantation. Transplantation. (2013) 95:1375–82. doi: 10.1097/TP.0b013e31828b10a1
47. Kinder JM, Stelzer IA, Arck PC, Way SS. Immunological implications of pregnancy-induced microchimerism. Nat Rev Immunol. (2017) 17:483–94. doi: 10.1038/nri.2017.38
48. Lim WH, McDonald SP, Coates PT, Chapman JR, Russ GR, Wong G. Maternal compared with paternal donor kidneys are associated with poorer graft outcomes after kidney transplantation. Kidney Int. (2016) 89:659–65. doi: 10.1016/j.kint.2015.11.016
49. Zeier M, Döhler B, Opelz G, Ritz E. The effect of donor gender on graft survival. J Am Soc Nephrol. (2002) 13:2570–6. doi: 10.1097/01.ASN.0000030078.74889.69
50. Kolonko A, Chudek J, Wiecek A. Nephron underdosing as a risk factor for impaired early kidney graft function and increased graft loss during the long-term follow-up period. Transplant Proc. (2013) 45:1639–43. doi: 10.1016/j.transproceed.2012.12.019
51. System URD. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. (2019). Available online at: https://www.usrds.org/media/2371/2019-executive-summary.pdf
52. Ishikawa N, Yagisawa T, Sakuma Y, Fujiwara T, Kimura T, Nukui A, et al. Kidney transplantation of living unrelated donor-recipient combinations. Transplant Proc. (2012) 44:254–6. doi: 10.1016/j.transproceed.2011.11.019
53. Van Arendonk KJ, Orandi BJ, James NT, Segev DL, Colombani PM. Living unrelated renal transplantation: a good match for the pediatric candidate? J Pediatr Surg. (2013) 48:1277–82. doi: 10.1016/j.jpedsurg.2013.03.023
54. Simforoosh N, Shemshaki H, Nadjafi-Semnani M, Sotoudeh M. Living related and living unrelated kidney transplantations: a systematic review and meta-analysis. World J Transplant. (2017) 7:152–60. doi: 10.5500/wjt.v7.i2.152
55. Hamid R, Khan M. Living-unrelated kidney donor transplantation: legalization in exceptional circumstances? Saudi J Kidney Dis Transplant. (2019) 30:1111–7. doi: 10.4103/1319-2442.270267
56. Ghahramani N. Paid living donation and growth of deceased donor programs. Transplantation. (2016) 100:1165–9. doi: 10.1097/TP.0000000000001164
57. Europe, Co,. Recommendation Rec(2006)16 of the Committee of Ministers to member states on quality improvement programmes for organ donatio. (2006). Available online at: www.CEO.int2006
58. Veroux M, Grosso G, Corona D, Mistretta A, Giaquinta A, Giuffrida G, et al. Age is an important predictor of kidney transplantation outcome. Nephrol Dial Transplant. (2012) 27:1663–71. doi: 10.1093/ndt/gfr524
59. Torreggiani M, Esposito C, Martinelli E, Jouve T, Chatrenet A, Rostaing L, et al. Outcomes in living donor kidney transplantation: the role of donor's kidney function. Kidney Blood Pressure Res. (2021) 46:84–94. doi: 10.1159/000512177
60. Chesnaye NC, van Stralen KJ, Bonthuis M, Groothoff JW, Harambat J, Schaefer F, et al. The association of donor and recipient age with graft survival in paediatric renal transplant recipients in a European Society for Paediatric Nephrology/European Renal Association-European Dialysis and Transplantation Association Registry study. Nephrol Dial Transplant. (2017) 32:1949–56. doi: 10.1093/ndt/gfx261
61. Trnka P, McTaggart SJ, Francis A. The impact of donor/recipient age difference and HLA mismatch on graft outcome in pediatric kidney transplantation. Pediatr Transplant. (2018) 22:e13265. doi: 10.1111/petr.13265
62. Marlais M, Hudson A, Pankhurst L, Fuggle SV, Marks SD. Living donation has a greater impact on renal allograft survival than HLA matching in pediatric renal transplant recipients. Transplantation. (2016) 100:2717–22. doi: 10.1097/TP.0000000000001159
63. Casey MJ, Wen X, Rehman S, Santos AH, Andreoni KA. Rethinking the advantage of zero-HLA mismatches in unrelated living donor kidney transplantation: implications on kidney paired donation. Transpl Int. (2015) 28:401–9. doi: 10.1111/tri.12495
64. Opelz G, Döhler B, Middleton D, Süsal C. HLA. Matching in pediatric kidney transplantation: HLA poorly matched living donor transplants versus HLA well-matched deceased donor transplants. Transplantation. (2017) 101:2789–92. doi: 10.1097/TP.0000000000001811
65. Shi X, Liu R, Xie X, Lv J, Han W, Zhong X, et al. Effect of human leukocyte antigen mismatching on the outcomes of pediatric kidney transplantation: a systematic review and meta-analysis. Nephrol Dial Transplant. (2017) 32:1939–48. doi: 10.1093/ndt/gfx259
66. Williams RC, West LJ, Opelz G. The risk of failure with HLA mismatch and recipient age in first pediatric (<18 years) kidney transplants. Transplant Direct. (2018) 4:e365. doi: 10.1097/TXD.0000000000000801
67. Van Stralen KJ, Borzych-Duzalka D, Hataya H, Kennedy SE, Jager KJ, Verrina E, et al. Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int. (2014) 86:168–74. doi: 10.1038/ki.2013.561
68. Smith JM, Martz K, Blydt-Hansen TD. Pediatric kidney transplant practice patterns and outcome benchmarks, 1987-2010: a report of the North American Pediatric Renal Trials and Collaborative Studies. Pediatr Transplant. (2013) 17:149–57. doi: 10.1111/petr.12034
69. Amesty MV, Fernandez C, Espinosa L, Rivas-Vila S, Lobato R, Monsalve S, et al. Long-term outcomes of adult-size and size-matched kidney transplants in small pediatric recipients. J Pediatr Urol. (2020) 16:481.e1–8. doi: 10.1016/j.jpurol.2020.05.012
70. Chavers B, Najarian JS, Humar A. Kidney transplantation in infants and small children. Pediatr Transplant. (2007) 11:702–8. doi: 10.1111/j.1399-3046.2007.00768.x
71. Chiodini B, Herman J, Lolin K, Adams B, Hennaut E, Lingier P, et al. Outcomes of kidney transplantations in children weighing 15 kilograms or less: a retrospective cohort study. Transpl Int. (2018) 31:720–8. doi: 10.1111/tri.13108
72. Etesami K, Hogen R, Lestz R. Pediatric kidney transplantation, a technical update. Curr Opin Organ Transplant. (2021) 26:356–9. doi: 10.1097/MOT.0000000000000898
73. Herthelius M, Celsi G, Edström Halling S, Krmar RT, Sandberg J, Tydén G, et al. Renal transplantation in infants and small children. Pediatr Nephrol. (2012) 27:145–50. doi: 10.1007/s00467-011-1962-2
74. Fontana I, Bertocchi M, Centanaro M, Varotti G, Santori G, Mondello R, et al. Abdominal compartment syndrome: an underrated complication in pediatric kidney transplantation. Transplant Proc. (2014) 46:2251–3. doi: 10.1016/j.transproceed.2014.07.045
75. Irtan S, Maisin A, Baudouin V, Nivoche Y, Azoulay R, Jacqz-Aigrain E, et al. Renal transplantation in children: critical analysis of age related surgical complications. Pediatr Transplant. (2010) 14:512–9. doi: 10.1111/j.1399-3046.2009.01260.x
76. Voet M, Nusmeier A, Lerou J, Luijten J, Cornelissen M, Lemson J. Cardiac output-guided hemodynamic therapy for adult living donor kidney transplantation in children under 20 kg: a pilot study. Paediatr Anaesth. (2019) 29:950–8. doi: 10.1111/pan.13705
77. Luijten JCHBM, Voet M, de Gier RPE, Nusmeier A, Scharbatke H, van der Vliet JA, et al. Transplantation of adult living donor kidneys in small children, a single-centre initial experience. Transplant Int. (2017) 30:640–2. doi: 10.1111/tri.12947
78. Chavers BM, Rheault MN, Matas AJ, Jackson SC, Cook ME, Nevins TE, et al. Improved outcomes of kidney transplantation in infants (age <2 years): a single-center experience. Transplantation. (2018) 102:284–90. doi: 10.1097/TP.0000000000001929
79. Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med. (2014) 371:549–58. doi: 10.1056/NEJMra1314376
80. Etesami K, Lestz R, Hogen R. Pediatric kidney transplantation in the United States. Curr Opin Organ Transplant. (2020) 25:343–7. doi: 10.1097/MOT.0000000000000783
81. Singh A, Stablein D, Tejani A. Risk factors for vascular thrombosis in pediatric renal transplantation. A special report of the North American Pediatric Renal Transplant Cooperative Study: 1. Transplantation. (1997) 63:1263–7. doi: 10.1097/00007890-199705150-00012
82. McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. (2004) 350:2654–62. doi: 10.1056/NEJMoa031643
83. Loubersac T, Roussey G, Dengu F, Langlois d'Estaintot H, Pere M, Glémain P, et al. Comparison of the outcomes of the pediatric kidney transplantation between recipients below and above 15 kg: a single center retrospective study. World J Urol. (2021) 39:2789–94. doi: 10.1007/s00345-020-03537-w
84. Tejani A, Sullivan EK. Factors that impact on the outcome of second renal transplants in children. Transplantation. (1996) 62:606–11. doi: 10.1097/00007890-199609150-00011
85. Gjertson DW. A multi-factor analysis of kidney regraft outcomes. Clin Transpl. (2002) 2002:335–49.
86. Van Arendonk KJ, Garonzik Wang JM, Deshpande NA, James NT, Smith JM, Montgomery RA, et al. Practice patterns and outcomes in retransplantation among pediatric kidney transplant recipients. Transplantation. (2013) 95:1360–8. doi: 10.1097/TP.0b013e31828c6d64
87. Muramatsu M, Mizutani T, Hamasaki Y, Takahashi Y, Itabashi Y, Kubota M, et al. Transplantation of adult-size kidneys in small pediatric recipients: a single-center experience. Pediatr Transplant. (2019) 23:e13401. doi: 10.1111/petr.13401
88. Power RE, Hickey DP, Little DM. Urological evaluation prior to renal transplantation. Transplant Proc. (2004) 36:2962–7. doi: 10.1016/j.transproceed.2004.11.006
89. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatric Nephrology. (2012) 27:363–73. doi: 10.1007/s00467-011-1939-1
90. Aki FT, Aydin AM, Dogan HS, Donmez MI, Erkan I, Duzova A, et al. Does lower urinary tract status affect renal transplantation outcomes in children? Transplant Proc. (2015) 47:1114–6. doi: 10.1016/j.transproceed.2014.10.069
91. Dannaway J, Ng H, Deshpande AV. Adherence to ICCS nomenclature guidelines in subsequent literature: a bibliometric study. Neurourol Urodyn. (2013) 32:952–6. doi: 10.1002/nau.22341
92. Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children's Continence Society. Neurourol Urodyn. (2016) 35:471–81. doi: 10.1002/nau.22751
93. Castagnetti M, Zhapa E, Berrettini A, Ghirardo G, Murer L, Zanon GF, et al. Lower urinary tract symptoms (LUTS) after renal transplant in non-urologic anuric patients. Pediatr Transplant. (2010) 14:859–62. doi: 10.1111/j.1399-3046.2010.01390.x
94. Walsh. Are non-invasive urodynamics indicated for all children requiring kidney transplantation? Pediatric Transplant. (2019) 23(Suppl.):13448.
95. Van der Weide MJ, Cornelissen EA, Van Achterberg T, de Gier RP, Feitz WF. Lower urinary tract symptoms after renal transplantation in children. J Urol. (2006) 175:297–302. doi: 10.1016/S0022-5347(05)00011-X
96. Herthelius M, Oborn H. Bladder dysfunction in children and adolescents after renal transplantation. Pediatr Nephrol. (2006) 21:725–8. doi: 10.1007/s00467-006-0018-5
97. Glassberg KI. The valve bladder syndrome: 20 years later. J Urol. (2001) 166:1406–14. doi: 10.1016/S0022-5347(05)65796-5
98. Deshpande AV. Current strategies to predict and manage sequelae of posterior urethral valves in children. Pediatr Nephrol. (2018) 33:1651–61. doi: 10.1007/s00467-017-3815-0
99. Thomas J. Etiopathogenesis and management of bladder dysfunction in patients with posterior urethral valves. Indian J Urol. (2010) 26:480–9. doi: 10.4103/0970-1591.74434
100. McKay AM, Kim S, Kennedy SE. Long-term outcome of kidney transplantation in patients with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol. (2019) 34:2409–15. doi: 10.1007/s00467-019-04300-z
101. De Wall LL, Oomen L, Glaap-Roeven F, Feitz WF, Bootsma-Robroeks C. Outcome of a thorough screening of lower urinary tract function in all pediatric kidney recipients. Pediatr Transplant. (2021) 25:e13929. doi: 10.1111/petr.13929
102. Dalton JT, Weintjes MG, Au JL. Effects of bladder resorption on pharmacokinetic data analysis. J Pharmacokinet Biopharm. (1994) 22:183–205. doi: 10.1007/BF02353328
103. Hebenstreit D, Csaicsich D, Hebenstreit K, Müller-Sacherer T, Berlakovich G, Springer A. Long-term outcome of pediatric renal transplantation in boys with posterior urethral valves. J Pediatr Surg. (2018) 53:2256–60. doi: 10.1016/j.jpedsurg.2018.07.003
104. Adams J, Mehls O, Wiesel M. Pediatric renal transplantation and the dysfunctional bladder. Transpl Int. (2004) 17:596–602. doi: 10.1111/j.1432-2277.2004.tb00392.x
105. Chinnakotla S, Verghese P, Chavers B, Rheault MN, Kirchner V, Dunn T, et al. Outcomes and risk factors for graft loss: lessons learned from 1,056 pediatric kidney transplants at the University of Minnesota. J Am Coll Surg. (2017) 224:473–86. doi: 10.1016/j.jamcollsurg.2016.12.027
106. Salomon L, Fontaine E, Guest G, Gagnadoux M-F, Broyer M, Beurton D, et al. Valves. J Urol. (2000) 163:1282–5. doi: 10.1016/S0022-5347(05)67761-0
107. Van der Weide MJ, Cornelissen EA, Van Achterberg T, Smits JP, Feitz WF. Dysfunction of lower urinary tract in renal transplant children with nephrologic disease. Urology. (2006) 67:1060–5. doi: 10.1016/j.urology.2005.11.065
108. Bower WF. Self-reported effect of childhood incontinence on quality of life. J Wound Ostomy Continence Nurs. (2008) 35:617–21. doi: 10.1097/01.WON.0000341476.71685.78
109. Natale N, Kuhn S, Siemer S, Stöckle M, von Gontard A. Quality of life and self-esteem for children with urinary urge incontinence and voiding postponement. J Urol. (2009) 182:692–8. doi: 10.1016/j.juro.2009.04.033
110. Gladh G, Eldh M, Mattsson S. Quality of life in neurologically healthy children with urinary incontinence. Acta Paediatr. (2006) 95:1648–52. doi: 10.1080/08035250600752458
111. Beksac AT, Koni A, Bozaci AC, Dogan HS, Tekgul S. Postvoidal residual urine is the most significant non-invasive diagnostic test to predict the treatment outcome in children with non-neurogenic lower urinary tract dysfunction. J Pediatr Urol. (2016) 12:215.e1-8. doi: 10.1016/j.jpurol.2016.04.011
112. Urology EAo. EAU Guideline of Peadiatric Urology. Congenital Lower Urinary Tract Obstruction (CLUTO). uroweborg (2021).
113. Nieuwhof-Leppink AJ, Hussong J, Chase J, Larsson J, Renson C, Hoebeke P, et al. Definitions, indications and practice of urotherapy in children and adolescents - a standardization document of the International Children's Continence Society (ICCS). J Pediatr Urol. (2021) 17:172–81. doi: 10.1016/j.jpurol.2020.11.006
114. Pereira PL, Urrutia MJM, Lobato R, Jaureguizar E. Renal transplantation in augmented bladders. Curr Urol Rep. (2014) 15:431. doi: 10.1007/s11934-014-0431-4
115. Taghizadeh AK, Desai D, Ledermann SE, Shroff R, Marks SD, Koffman G, et al. Renal transplantation or bladder augmentation first? A comparison of complications and outcomes in children. BJU Int. (2007) 100:1365–70. doi: 10.1111/j.1464-410X.2007.07096.x
116. Torricelli FCM, Watanabe A, Piovesan AC, David-Neto E, Nahas WC. Urologic issues in pediatric transplant recipients. Transl Androl Urol. (2019) 8:134–40. doi: 10.21037/tau.2018.06.17
117. Basiri A, Otookesh H, Hosseini R, Simforoosh N, Moghaddam SM. Kidney transplantation before or after augmentation cystoplasty in children with high-pressure neurogenic bladder. BJU Int. (2009) 103:86–8. doi: 10.1111/j.1464-410X.2008.08081.x
118. Eltemamy M, Crane A, Goldfarb DA. Urinary diversion in renal transplantation. Urol Clin North Am. (2018) 45:113–21. doi: 10.1016/j.ucl.2017.09.012
119. Coosemans W, Baert L, Kuypers D, Maes B, Messiaen T, Vanrenterghem Y, et al. Renal transplantation onto abnormal urinary tract: ileal conduit urinary diversion. Transplant Proc. (2001) 33:2493–4. doi: 10.1016/S0041-1345(01)02074-7
120. Zahran M, Osman Y, Elhefnawy A, Harraz A, Fakhreldin I, Kamal A, et al. Necessity of pre-transplant bladder cycling for patients with defunctionalized bladder: a prospective randomized trial. J Urol. (2017) 197:e1003. doi: 10.1016/j.juro.2017.02.2188
121. Rigamonti W, Capizzi A, Zacchello G, Capizzi V, Zanon GF, Montini G, et al. Kidney transplantation into bladder augmentation or urinary diversion: long-term results. Transplantation. (2005) 80:1435–40. doi: 10.1097/01.tp.0000174342.19265.f4
122. Amari EBE, Hadaya K, Bühler L, Berney T, Rohner P, Martin P-Y, et al. Outcome of treated and untreated asymptomatic bacteriuria in renal transplant recipients. Nephrol Dialysis Transplant. (2011) 26:4109–14. doi: 10.1093/ndt/gfr198
123. Coussement J, Scemla A, Abramowicz D, Nagler EV, Webster AC. Antibiotics for asymptomatic bacteriuria in kidney transplant recipients. Cochrane Database Syst Rev. (2018) 2:Cd011357. doi: 10.1002/14651858.CD011357.pub2
124. Coussement J, Kamar N, Matignon M, Weekers L, Scemla A, Giral M, et al. Antibiotics vs. no therapy in kidney transplant recipients with asymptomatic bacteriuria (BiRT): a pragmatic, multicentre, randomized, controlled trial. Clin Microbiol Infect. (2021) 27:398–405. doi: 10.1016/j.cmi.2020.09.005
125. Bonnéric S, Maisin A, Kwon T, Deschênes G, Niel O. Asymptomatic bacteriuria in pediatric kidney transplant recipients: to treat or not to treat? A retrospective study. Pediatric Nephrol. (2019) 34:1141–5. doi: 10.1007/s00467-019-04204-y
126. Cicek N, Yildiz N, Alpay H. Intravesical hyaluronic acid treatment in recurrent urinary tract infections in children with spina bifida and neurogenic bladder. J Pediatr Urol. (2020) 16:366.e1–5. doi: 10.1016/j.jpurol.2020.02.009
127. Fidan K, Büyükkaragöz B, Özen O, Demirogullari B, Söylemezoglu O. The use of intravesical hyaluronic acid for recurrent urinary tract infections in children: a case-series study. Ren Fail. (2015) 37:354–8. doi: 10.3109/0886022X.2015.1087863
128. Wodey E, Pladys P, Copin C, Lucas MM, Chaumont A, Carre P, et al. Comparative hemodynamic depression of sevoflurane versus halothane in infants: an echocardiographic study. Anesthesiology. (1997) 87:795–800. doi: 10.1097/00000542-199710000-00012
129. Ong Sio LCL, Dela Cruz RGC, Bautista AF. Sevoflurane and renal function: a meta-analysis of randomized trials. Med Gas Res. (2017) 7:186–93. doi: 10.4103/2045-9912.215748
130. Turcios NL. Pulmonary complications of renal disorders. Paediatr Respir Rev. (2012) 13:44–9. doi: 10.1016/j.prrv.2011.04.006
131. Voet M, Cornelissen EAM, van der Jagt MFP, Lemson J, Malagon I. Perioperative anesthesia care for the pediatric patient undergoing a kidney transplantation: an educational review. Pediatric Anesthesia. (2021) 31:1150–60. doi: 10.1111/pan.14271
132. Ghane Sharbaf F, Bitzan M, Szymanski KM, Bell LE, Gupta I, Tchervenkov J, et al. Native nephrectomy prior to pediatric kidney transplantation: biological and clinical aspects. Pediatric Nephrol. (2012) 27:1179–88. doi: 10.1007/s00467-012-2115-y
133. Chebib FT, Prieto M, Jung Y, Irazabal MV, Kremers WK, Dean PG, et al. Native nephrectomy in renal transplant recipients with autosomal dominant polycystic kidney disease. Transplant Direct. (2015) 1:e43-e. doi: 10.1097/TXD.0000000000000554
134. Grodstein EI, Baggett N, Wayne S, Leverson G, D'Alessandro AM, Fernandez LA, et al. An evaluation of the safety and efficacy of simultaneous bilateral nephrectomy and renal transplantation for polycystic kidney disease: a 20-year experience. Transplantation. (2017) 101:2774–9. doi: 10.1097/TP.0000000000001779
135. Ahmad SB, Inouye B, Phelan MS, Kramer AC, Sulek J, Weir MR, et al. Live donor renal transplant with simultaneous bilateral nephrectomy for autosomal dominant polycystic kidney disease is feasible and satisfactory at long-term follow-up. Transplantation. (2016) 100:407–15. doi: 10.1097/TP.0000000000000838
136. Elrggal M, Abd Elaziz H, Gawad M, Sheashaa H. Native nephrectomy in kidney transplantation, when, why, and how? J Egypt Soc Nephrol Transplant. (2018) 18:68–72. doi: 10.4103/jesnt.jesnt_8_18
137. Capozza N, Collura G, Falappa P, Caione P. Renal embolization as an alternative to surgical nephrectomy in children. Transplant Proc. (2007) 39:1782–4. doi: 10.1016/j.transproceed.2007.05.001
138. Sallam HE, El-Reshaid K, Varro J. Renal ablation using bilateral renal artery embolization for treatment of resistant nephrotic syndrome. Saudi J Kidney Dis Transpl. (2012) 23:1258–61. doi: 10.4103/1319-2442.100878
139. Vos E, Koster-Kamphuis L, van de Kar NCAJ, Bootsma-Robroeks CMHHT, Cornelissen EAM, Schreuder MF. Preparing for a kidney transplant: Medical nephrectomy in children with nephrotic syndrome. Pediatr Transplant. (2020) 24:e13703. doi: 10.1111/petr.13703
140. Pomeranz A, Wolach B, Bernheim J, Korzets Z, Bernheim J. Successful treatment of Finnish congenital nephrotic syndrome with captopril and indomethacin. J Pediatr. (1995) 126:140–2. doi: 10.1016/S0022-3476(95)70518-X
141. Routh JC Yu RN, Kozinn SI, Nguyen HT, Borer JG. Urological complications and vesicoureteral reflux following pediatric kidney transplantation. J Urol. (2013) 189:1071–6. doi: 10.1016/j.juro.2012.09.091
142. Rossi V, Torino G, Gerocarni Nappo S, Mele E, Innocenzi M, Mattioli G, et al. Urological complications following kidney transplantation in pediatric age: a single-center experience. Pediatr Transplant. (2016) 20:485–91. doi: 10.1111/petr.12691
143. Oomen L, de Wall LL, Cornelissen EAM, Feitz WFJ, Bootsma-Robroeks C. Prognostic factors on graft function in pediatric kidney recipients. Transplant Proc. (2021) 53:889–96. doi: 10.1016/j.transproceed.2020.10.017
144. Woodside KJ, Schirm ZW, Noon KA, Huml AM, Padiyar A, Sanchez EQ, et al. Fever, infection, and rejection after kidney transplant failure. Transplantation. (2014) 97:648–53. doi: 10.1097/01.TP.0000437558.75574.9c
145. Esezobor CI, Nourse P, Gajjar P. Urinary tract infection following kidney transplantation: frequency, risk factors and graft function. Pediatr Nephrol. (2012) 27:651–7. doi: 10.1007/s00467-011-2044-1
146. Rodríguez Faba O, Boissier R, Budde K, Figueiredo A, Taylor CF, Hevia V, et al. European association of urology guidelines on renal transplantation: update 2018. Eur Urol Focus. (2018) 4:208–15. doi: 10.1016/j.euf.2018.07.014
147. Dharnidharka VR, Araya CE, Wadsworth CS, McKinney MC, Howard RJ. Assessing the value of ureteral stent placement in pediatric kidney transplant recipients. Transplantation. (2008) 85:986–91. doi: 10.1097/TP.0b013e318169bf11
148. Hashim F, Rehman S, Gregg JA, Dharnidharka VR. Ureteral stent placement increases the risk for developing BK Viremia after kidney transplantation. J Transplant. (2014) 2014:459747. doi: 10.1155/2014/459747
149. Beetz O, Weigle CA, Nogly R, Klempnauer J, Pape L, Richter N, et al. Surgical complications in pediatric kidney transplantation-Incidence, risk factors, and effects on graft survival: a retrospective single-center study. Pediatr Transplant. (2021) 25:e13871. doi: 10.1111/petr.13871
150. Harza M, Baston C, Preda A, Olaru V, Ismail G, Domnisor L, et al. Impact of ureteral stenting on urological complications after kidney transplantation surgery: a single-center experience. Transplant Proc. (2014) 46:3459–62. doi: 10.1016/j.transproceed.2014.08.051
151. Wilson CH, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. (2013) 2013:Cd004925. doi: 10.1002/14651858.CD004925.pub3
152. Bruintjes MHD, Langenhuijsen JF, Kusters A, Hilbrands LB, d'Ancona FCH, Warlé MC. Double stent is superior to externally draining ureteric stent in enhancing recovery after kidney transplantation – a prospective cohort study. Int J Surg. (2019) 71:175–81. doi: 10.1016/j.ijsu.2019.09.031
153. Thompson ER, Hosgood SA, Nicholson ML, Wilson CH. Early versus late ureteric stent removal after kidney transplantation. Cochr Database Systemat Rev. (2018) 1:CD011455. doi: 10.1002/14651858.CD011455.pub2
154. Gargah T, Abidi K, Rajhi H, Ben Abdallah T, Chebil M, Lakhoua MR. Vascular complications after pediatric kidney transplantation. Tunis Med. (2011) 89:458–61.
155. Mehrabi A, Golriz M, Khajeh E, Ghamarnejad O, Kulu Y, Wiesel M, et al. Surgical outcomes after pediatric kidney transplantation at the University of Heidelberg. J Pediatric Urol. (2019) 15:221.e1–8. doi: 10.1016/j.jpurol.2019.01.007
156. McDonald RA, Smith JM, Stablein D, Harmon WE. Pretransplant peritoneal dialysis and graft thrombosis following pediatric kidney transplantation: a NAPRTCS report. Pediatr Transplant. (2003) 7:204–8. doi: 10.1034/j.1399-3046.2003.00075.x
157. Gander R, Asensio M, Royo GF, Molino JA, García L, Madrid A, et al. Vascular thrombosis in pediatric kidney transplantation: Graft survival is possible with adequate management. J Pediatr Urol. (2018) 14:222–30. doi: 10.1016/j.jpurol.2018.01.027
158. Esfandiar N, Otukesh H, Sharifian M, Hoseini R. Protective effect of heparin and aspirin against vascular thrombosis in pediatric kidney transplants. Iran J Kidney Dis. (2012) 6:141–5.
159. Bapistella S, Zirngibl M, Buder K, Toulany N, Laube GF, Weitz M. Prophylactic antithrombotic management in adult and pediatric kidney transplantation: a systematic review and meta-analysis. Pediatr Transplant. (2021) 25:e14021. doi: 10.1111/petr.14021
160. Giuliani S, Gamba P, Kiblawi R, Midrio P, Ghirardo G, Zanon GF. Lymphocele after pediatric kidney transplantation: Incidence and risk factors. Pediatr Transplant. (2014) 18:720–5. doi: 10.1111/petr.12341
161. Gander R, Asensio M, Royo GF, Molino JA, Vilalta R, Coma A, et al. Treatment of Post-transplant Lymphocele in Children. Urology. (2017) 103:218–23. doi: 10.1016/j.urology.2016.12.039
162. Choudhrie AV, Kumar S, Gnanaraj L, Devasia A, Chacko N, Kekre NS. Symptomatic lymphocoeles post renal transplant. Saudi J Kidney Dis Transpl. (2012) 23:1162–8.
163. Giessing M, Budde K. Sirolimus and lymphocele formation after kidney transplantation: an immunosuppressive medication as co-factor for a surgical problem? Nephrol Dial Transplant. (2003) 18:448–9. doi: 10.1093/ndt/18.2.448
164. Langer RM, Kahan BD. Incidence, therapy, and consequences of lymphocele after sirolimus-cyclosporine-prednisone immunosuppression in renal transplant recipients. Transplantation. (2002) 74:804–8. doi: 10.1097/00007890-200209270-00012
165. Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The rapamune US study group. Lancet. (2000) 356:194–202. doi: 10.1016/S0140-6736(00)02480-6
166. Kayler L, Kang D, Molmenti E, Howard R. Kidney transplant ureteroneocystostomy techniques and complications: review of the literature. Transplant Proc. (2010) 42:1413–20. doi: 10.1016/j.transproceed.2010.04.016
167. Politano VA, Leadbetter WF. An operative technique for the correction of vesicoureteral reflux. J Urol. (2017) 197:S94–100. doi: 10.1016/j.juro.2016.10.093
169. Alberts VP, Idu MM, Legemate DA, Laguna Pes MP, Minnee RC. Ureterovesical anastomotic techniques for kidney transplantation: a systematic review and meta-analysis. Transpl Int. (2014) 27:593–605. doi: 10.1111/tri.12301
170. Friedersdorff F, Weinberger S, Biernath N, Plage H, Cash H, El-Bandar N. The ureter in the kidney transplant setting: ureteroneocystostomy surgical options, double-J stent considerations and management of related complications. Curr Urol Rep. (2020) 21:3. doi: 10.1007/s11934-020-0956-7
171. Slagt IK, Dor FJ, Tran TC, Kimenai HJ, Weimar W, Ijzermans JN, et al. A randomized controlled trial comparing intravesical to extravesical ureteroneocystostomy in living donor kidney transplantation recipients. Kidney Int. (2014) 85:471–7. doi: 10.1038/ki.2013.464
172. Kayler L, Zendejas I, Molmenti E, Chordia P, Schain D, Magliocca J. Kidney transplant ureteroneocystostomy: comparison of full-thickness vs. Lich-Gregoir techniques. Clin Transplant. (2012) 26:E372–80. doi: 10.1111/j.1399-0012.2012.01655.x
173. Morrison CD, Shannon R, Rosoklija I, Nettey OS, Superina R, Cheng EY, et al. Ureteral complications of pediatric renal transplantation. J Urol. (2019) 201:810–4. doi: 10.1016/j.juro.2018.08.082
174. Margreiter M, Györi GP, Böhmig GA, Trubel S, Mühlbacher F, Steininger R. Value of routine voiding cystourethrography after renal transplantation. Am J Transplant. (2013) 13:130–5. doi: 10.1111/j.1600-6143.2012.04284.x
175. Jung GO, Chun JM, Park JB, Choi GS, Kwon CH, Joh JW, et al. Clinical significance of posttransplantation vesicoureteral reflux during short-term period after kidney transplantation. Transplant Proc. (2008) 40:2339–41. doi: 10.1016/j.transproceed.2008.06.027
176. Ranchin B, Chapuis F, Dawhara M, Canterino I, Hadj-Aïssa A, Saïd MH, et al. Vesicoureteral reflux after kidney transplantation in children. Nephrol Dialysis Transplant. (2000) 15:1852–8. doi: 10.1093/ndt/15.11.1852
177. Wang MK, Chuang KW, Li Y, Gaither T, Brakeman P, Gonzalez L, et al. Renal function outcomes in pediatric patients with symptomatic reflux into the transplanted kidney treated with redo ureteroneocystostomy. J Pediatr Urol. (2018) 14:275.e1–5. doi: 10.1016/j.jpurol.2018.01.024
178. Barrero R, Fijo J, Fernandez-Hurtado M, García-Merino F, León E, Torrubia F. Vesicoureteral reflux after kidney transplantation in children. Pediatr Transplant. (2007) 11:498–503. doi: 10.1111/j.1399-3046.2006.00668.x
179. Sheth KR, White JT, Stanasel I, Janzen N, Mittal A, Koh CJ, et al. Comparing treatment modalities for transplant kidney vesicoureteral reflux in the pediatric population. J Pediatr Urol. (2018) 14:554.e1–6. doi: 10.1016/j.jpurol.2018.07.006
180. John U, Kemper MJ. Urinary tract infections in children after renal transplantation. Pediatric Nephrol. (2009) 24:1129–36. doi: 10.1007/s00467-007-0690-0
181. John U, Everding AS, Kuwertz-Bröking E, Bulla M, Müller-Wiefel DE, Misselwitz J, et al. High prevalence of febrile urinary tract infections after paediatric renal transplantation. Nephrol Dial Transplant. (2006) 21:3269–74. doi: 10.1093/ndt/gfl464
182. Feber J, Spatenka J, Seeman T, Matousovic K, Zeman L, Dusek J, et al. Urinary tract infections in pediatric renal transplant recipients - a two center risk factors study. Pediatr Transplant. (2009) 13:881–6. doi: 10.1111/j.1399-3046.2008.01079.x
183. Olsburgh J, Zakri RH, Horsfield C, Collins R, Fairweather J, O'Donnell P, et al. TCC in transplant ureter–when and when not to preserve the transplant kidney. Am J Transplant. (2016) 16:704–11. doi: 10.1111/ajt.13533
184. Tillou X, Doerfler A, Collon S, Kleinclauss F, Patard JJ, Badet L, et al. De novo kidney graft tumors: results from a multicentric retrospective national study. Am J Transplant. (2012) 12:3308–15. doi: 10.1111/j.1600-6143.2012.04248.x
185. Phillips BL, Callaghan CJ. Graft nephrectomy in children. Pediatric Nephrol. (2018) 33:947–55. doi: 10.1007/s00467-017-3697-1
186. Minson S, Muñoz M, Vergara I, Mraz M, Vaughan R, Rees L, et al. Nephrectomy for the failed renal allograft in children: predictors and outcomes. Pediatric Nephrol. (2013) 28:1299–305. doi: 10.1007/s00467-013-2477-9
187. Gómez-Dos-Santos V, Lorca-Álvaro J, Hevia-Palacios V, Fernández-Rodríguez AM, Diez-Nicolás V, Álvarez-Rodríguez S, et al. The failing kidney transplant allograft. Transplant nephrectomy: current state-of-the-art. Curr Urol Rep. (2020) 21:4. doi: 10.1007/s11934-020-0957-6
188. Delgado P, Diaz F, Gonzalez A, Sanchez E, Gutierrez P, Hernandez D, et al. Intolerance syndrome in failed renal allografts: incidence and efficacy of percutaneous embolization. Am J Kidney Dis. (2005) 46:339–44. doi: 10.1053/j.ajkd.2005.04.024
189. Bunthof KLW, Verhoeks CM, van den Brand J, Hilbrands LB. Graft intolerance syndrome requiring graft nephrectomy after late kidney graft failure: can it be predicted? A retrospective cohort study. Transpl Int. (2018) 31:220–9. doi: 10.1111/tri.13088
190. Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol. (2010) 21:374–80. doi: 10.1681/ASN.2009050480
191. Zerouali F, Levtchenko EN, Feitz WF, Cornelissen EA, Monnens LA. Renal transplant nephrectomy in children: can an aggressive approach be recommended? Pediatr Transplant. (2004) 8:561–4. doi: 10.1111/j.1399-3046.2004.00228.x
192. Parada B, Figueiredo A, Nunes P, Bastos C, Macário F, Roseiro A, et al. Pediatric renal transplantation: comparative study with renal transplantation in the adult population. Transplant Proc. (2005) 37:2771–4. doi: 10.1016/j.transproceed.2005.05.046
193. Wang K, Xu X, Fan M, Qianfeng Z. Allograft nephrectomy vs. no-allograft nephrectomy for renal transplantation: a meta-analysis. Clin Transplant. (2016) 30:33–43. doi: 10.1111/ctr.12654
194. Pham PT, Everly M, Faravardeh A, Pham PC. Management of patients with a failed kidney transplant: dialysis reinitiation, immunosuppression weaning, and transplantectomy. World J Nephrol. (2015) 4:148–59. doi: 10.5527/wjn.v4.i2.148
195. Lachmann N, Schönemann C, El-Awar N, Everly M, Budde K, Terasaki PI, et al. Dynamics and epitope specificity of anti-human leukocyte antibodies following renal allograft nephrectomy. Nephrol Dial Transplant. (2016) 31:1351–9. doi: 10.1093/ndt/gfw041
196. Bargman JM Thorpe KE, Churchill DN, Group tCPDS. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. (2001) 12:2158–62. doi: 10.1681/ASN.V12102158
197. Sener A, Khakhar AK, Nguan CY, House AA, Jevnikar AM, Luke PP. Early but not late allograft nephrectomy reduces allosensitization after transplant failure. Can Urol Assoc J. (2011) 5:E142–7. doi: 10.5489/cuaj.10032
198. Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant. (2007) 7:1961–7. doi: 10.1111/j.1600-6143.2007.01884.x
199. Augustine JJ, Woodside KJ, Padiyar A, Sanchez EQ, Hricik DE, Schulak JA. Independent of nephrectomy, weaning immunosuppression leads to late sensitization after kidney transplant failure. Transplantation. (2012) 94:738–43. doi: 10.1097/TP.0b013e3182612921
200. Choe J, Shin JH, Yoon HK, Ko GY, Gwon DI, Ko HK, et al. Safety and efficacy of transarterial nephrectomy as an alternative to surgical nephrectomy. Korean J Radiol. (2014) 15:472–80. doi: 10.3348/kjr.2014.15.4.472
201. Yeast C, Riley JM, Holyoak J, Ross G Jr, Weinstein S, Wakefield M. Use of preoperative embolization prior to Transplant nephrectomy. Int Braz J Urol. (2016) 42:107–12. doi: 10.1590/S1677-5538.IBJU.2015.0052
202. Al-Geizawi SM, Singh RP, Zuckerman JM, Requarth JA, Farney AC, Rogers J, et al. Role of allograft nephrectomy following kidney graft failure: preliminary experience with pre-operative angiographic kidney embolization. J Nephrol. (2015) 28:379–85. doi: 10.1007/s40620-014-0145-1
203. Neschis DG, Gutta R, Al-Qudah HS, Bartlett ST, Philosophe B, Schweitzer EJ, et al. Intraoperative coil embolization reduces transplant nephrectomy transfusion requirement. Vasc Endovascular Surg. (2007) 41:335–8. doi: 10.1177/1538574407302845
204. Gordillo R, Munshi R, Monroe EJ, Shivaram GM, Smith JM. Benefits and risks of protocol biopsies in pediatric renal transplantation. Pediatr Nephrol. (2019) 34:593–8. doi: 10.1007/s00467-018-3959-6
205. Kanzelmeyer NK, Lerch C, Ahlenstiel-Grunow T, Bräsen JH, Haffner D, Pape L. The role of protocol biopsies after pediatric kidney transplantation. Medicine. (2020) 99:e20522. doi: 10.1097/MD.0000000000020522
206. Zachariah MS, Dwivedi AK, Yip CS, Chang SS, Gundroo A, Venuto RC, et al. Utility of serial protocol biopsies performed after 1 year in predicting long-term kidney allograft function according to histologic phenotype. Exp Clin Transplant. (2018) 16:391–400. doi: 10.6002/ect.2016.0323
207. Birk PE. Surveillance biopsies in children post-kidney transplant. Pediatr Nephrol. (2012) 27:753–60. doi: 10.1007/s00467-011-1969-8
208. Vidhun J, Masciandro J, Varich L, Salvatierra OJ, Sarwal M. Safety and risk stratification of percutaneous biopsies of adult-sized renal allografts in infant and older pediatric recipients. Transplantation. (2003) 76:552–7. doi: 10.1097/01.TP.0000076097.90123.21
209. Buchmann TN, Wolff T, Bachmann A, Guerke L, Steiger J, Mihatsch MJ, et al. Repeat true surveillance biopsies in kidney transplantation. Transplantation. (2012) 93:908–13. doi: 10.1097/TP.0b013e318248cab0
210. Fahmy LM, Massie AB, Muzaale AD, Bagnasco SM, Orandi BJ, Alejo JL, et al. Long-term renal function in living kidney donors who had histological abnormalities at Donation. Transplantation. (2016) 100:1294–8. doi: 10.1097/TP.0000000000001236
211. Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. (2009) 9(Suppl.3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x
212. Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, et al. A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation. (2018) 102:1795–814. doi: 10.1097/TP.0000000000002366
213. Webster AC, Wu S, Tallapragada K, Park MY, Chapman JR, Carr SJ. Polyclonal and monoclonal antibodies for treating acute rejection episodes in kidney transplant recipients. Cochr Database Systemat Rev. (2017) 7:CD004756. doi: 10.1002/14651858.CD004756.pub4
214. Heemann U, Abramowicz D, Spasovski G, Vanholder R. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) guidelines on kidney transplantation: a European Renal Best Practice (ERBP) position statement. Nephrol Dial Transplant. (2011) 26:2099–106. doi: 10.1093/ndt/gfr169
215. Billing H, Rieger S, Süsal C, Waldherr R, Opelz G, Wühl E, et al. IVIG and rituximab for treatment of chronic antibody-mediated rejection: a prospective study in paediatric renal transplantation with a 2-year follow-up. Transpl Int. (2012) 25:1165–73. doi: 10.1111/j.1432-2277.2012.01544.x
216. Seeman T. Immunosuppressive management of pediatric kidney transplant recipients. Curr Pharm Des. (2020) 26:3451–9. doi: 10.2174/1381612826666200708133429
217. Smeets NJL, Eijk RJR, de Wildt SN, Bootsma-Robroeks C. Assessing causality by means of the Naranjo scale in a paediatric patient with life threatening respiratory failure after alemtuzumab administration: a case report. BMC Pediatr. (2021) 21:229. doi: 10.1186/s12887-021-02698-w
218. Starzl TE, Weil R 3rd, Iwatsuki S, Klintmalm G, Schröter GP, Koep LJ, et al. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. (1980) 151:17–26.
219. Bökenkamp A, Offner G, Hoyer PF, Vester U, Wonigeit K, Brodehl J. Improved absorption of cyclosporin A from a new microemulsion formulation: implications for dosage and monitoring. Pediatr Nephrol. (1995) 9:196–8. doi: 10.1007/BF00860745
220. Krejci K, Zadrazil J, Lackova E, Zilinska Z, Roland R, Dedinska I. Clinical experience of conversion from cyclosporine to tacrolimus prolonged-release in stabilized kidney transplant patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2016) 160:407–11. doi: 10.5507/bp.2016.026
221. Hardinger KL, Brennan DC, Lowell J, Schnitzler MA. Long-term outcome of gastrointestinal complications in renal transplant patients treated with mycophenolate mofetil. Transpl Int. (2004) 17:609–16. doi: 10.1111/j.1432-2277.2004.tb00394.x
222. Opelz G, Döhler B, Laux G. Long-term prospective study of steroid withdrawal in kidney and heart transplant recipients. Am J Transplant. (2005) 5:720–8. doi: 10.1111/j.1600-6143.2004.00765.x
223. Höcker B, Weber LT, Feneberg R, Drube J, John U, Fehrenbach H, et al. Improved growth and cardiovascular risk after late steroid withdrawal: 2-year results of a prospective, randomised trial in paediatric renal transplantation. Nephrol Dial Transplant. (2010) 25:617–24. doi: 10.1093/ndt/gfp506
224. Grenda R, Watson A, Trompeter R, Tönshoff B, Jaray J, Fitzpatrick M, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant. (2010) 10:828–36. doi: 10.1111/j.1600-6143.2010.03047.x
225. Matas AJ. Early steroid cessation after kidney transplant. J Am Med Assoc Surg. (2021) 156:314. doi: 10.1001/jamasurg.2020.6959
226. McDonald RA, Smith JM, Ho M, Lindblad R, Ikle D, Grimm P, et al. Incidence of PTLD in pediatric renal transplant recipients receiving basiliximab, calcineurin inhibitor, sirolimus and steroids. Am J Transplant. (2008) 8:984–9. doi: 10.1111/j.1600-6143.2008.02167.x
227. Oomen L, de Wall LL, Cornelissen EAM, Feitz WFJ, Charlotte BR. Steroid withdrawal in pediatric kidney recipients: is it safe? Int J Transplantat Plastic Surg. (2021) 5:16000159. doi: 10.23880/IJTPS-16000159
228. Danziger-Isakov L, Kumar D. Vaccination in solid organ transplantation. Am J Transplant. (2013) 13(Suppl.4)311–7. doi: 10.1111/ajt.12122
229. Tanné C, Roy P, Frobert É, Duncan A, Laurent A, Cochat P. Cytomegalovirus infection in the first year after pediatric kidney transplantation. Néphrol Thérapeutique. (2019) 15:44–50. doi: 10.1016/j.nephro.2018.04.003
230. Weikert BC, Blumberg EA. Viral infection after renal transplantation: surveillance and management. Clin J Am Soc Nephrol. (2008) 3(Suppl.2):S76–86. doi: 10.2215/CJN.02900707
231. Nevins TE, Nickerson PW, Dew MA. Understanding medication nonadherence after kidney transplant. J Am Soc Nephrol. (2017) 28:2290–301. doi: 10.1681/ASN.2017020216
232. Nguyen C, Dew MA, Irizarry T, McNulty M, Rennick J, Knäuper B, et al. Promoting medication adherence from the perspective of adolescent and young adult kidney transplant recipients, parents, and health care professionals: a TAKE-IT TOO study. Pediatr Transplant. (2020) 24:e13709. doi: 10.1111/petr.13709
233. Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. (2010) 14:603–13. doi: 10.1111/j.1399-3046.2010.01299.x
234. Connelly J, Pilch N, Oliver M, Jordan C, Fleming J, Meadows H, et al. Prediction of medication non-adherence and associated outcomes in pediatric kidney transplant recipients. Pediatr Transplant. (2015) 19:555–62. doi: 10.1111/petr.12479
235. Hoegy D, Bleyzac N, Robinson P, Bertrand Y, Dussart C, Janoly-Dumenil A. Medication adherence in pediatric transplantation and assessment methods: a systematic review. Patient Prefer Adherence. (2019) 13:705–19. doi: 10.2147/PPA.S200209
236. Manickavasagar R, Wong G, Alexander SI, Francis A, Prestidge C, Larkins NG, et al. Allograft outcome following repeat transplantation of patients with non-adherence-related first kidney allograft failure: a population cohort study. Transplant Int. (2019) 32:1247–58. doi: 10.1111/tri.13492
237. Shaw RJ, Palmer L, Blasey C, Sarwal M. A typology of non-adherence in pediatric renal transplant recipients. Pediatr Transplant. (2003) 7:489–93. doi: 10.1046/j.1397-3142.2003.00117.x
238. Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertension. (2013) 62:218–25. doi: 10.1161/HYPERTENSIONAHA.113.00687
239. Prendergast MB, Gaston RS. Optimizing medication adherence: an ongoing opportunity to improve outcomes after kidney transplantation. Clin J Am Soc Nephrol. (2010) 5:1305–11. doi: 10.2215/CJN.07241009
240. FDA. US Food and Drug Administration: Draft Guidance for Industry Enrichment Strategies for Clinical Trials to Support Approval of Human Drugs and Biological Products. FDA (2019). Available online at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm332181.pdf
241. Nevins TE, Robiner WN, Thomas W. Predictive patterns of early medication adherence in renal transplantation. Transplantation. (2014) 98:878–84. doi: 10.1097/TP.0000000000000148
242. Kisa A, Sabaté E, Nuño-Solinís R. Adherence to Long-Term Therapies: Evidence for Action (2003).
243. Neuberger JM, Bechstein WO, Kuypers DR, Burra P, Citterio F, De Geest S, et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the consensus on managing modifiable risk in transplantation (COMMIT) group. Transplantation. (2017) 101(4S Suppl.2):S1–56. doi: 10.1097/TP.0000000000001651
244. Killian MO, Schuman DL, Mayersohn GS, Triplett KN. Psychosocial predictors of medication non-adherence in pediatric organ transplantation: a systematic review. Pediatr Transplant. (2018) 22:e13188. doi: 10.1111/petr.13188
245. Heuvel M, van der Lee J, Cornelissen E, Bemelman F, Hoitsma A, Geskus R, et al. Transition to the adult nephrologist does not induce acute renal transplant rejection. Nephrol Dial Transplant. (2009) 25:1662–7. doi: 10.1093/ndt/gfp684
246. Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. (2013) 95:333–40. doi: 10.1097/TP.0b013e3182725532
247. Duncan S, Annunziato RA, Dunphy C, LaPointe Rudow D, Shneider BL, Shemesh E. A systematic review of immunosuppressant adherence interventions in transplant recipients: decoding the streetlight effect. Pediatr Transplant. (2018) 22:10.1111/petr.13086. doi: 10.1111/petr.13086
248. Foster BJ, Pai ALH, Zelikovsky N, Amaral S, Bell L, Dharnidharka VR, et al. A randomized trial of a multicomponent intervention to promote medication adherence: the teen adherence in kidney transplant effectiveness of intervention trial (TAKE-IT). Am J Kidney Dis. (2018) 72:30–41. doi: 10.1053/j.ajkd.2017.12.012
249. Holmberg C. Nonadherence after pediatric renal transplantation: detection and treatment. Curr Opin Pediatr. (2019) 31:219–25. doi: 10.1097/MOP.0000000000000734
250. Jeminiwa R, Hohmann L, Qian J, Garza K, Hansen R, Fox BI. Impact of eHealth on medication adherence among patients with asthma: a systematic review and meta-analysis. Respir Med. (2019) 149:59–68. doi: 10.1016/j.rmed.2019.02.011
251. Rathbone AL, Prescott J. The use of mobile apps and SMS messaging as physical and mental health interventions: systematic review. J Med Internet Res. (2017) 19:e295. doi: 10.2196/jmir.7740
252. Tang J, James L, Howell M, Tong A, Wong G. eHealth interventions for solid organ transplant recipients: a systematic review and meta-analysis of randomized controlled trials. Transplantation. (2020) 104:e224–35. doi: 10.1097/TP.0000000000003294
253. Van Arendonk KJ, Chow EK, James NT, Orandi BJ, Ellison TA, Smith JM, et al. Choosing the order of deceased donor and living donor kidney transplantation in pediatric recipients: a Markov decision process model. Transplantation. (2015) 99:360–6. doi: 10.1097/TP.0000000000000588
254. Oomen L, Leijte E, Shilhan DE, Battye M, Waltregny D, Van der Aa F, et al. Rare and complex urology: clinical overview of ERN eUROGEN. Eur Urol. (2021). 81:204–12. doi: 10.1016/j.eururo.2021.02.043
255. Lim MA, Kohli J, Bloom RD. Immunosuppression for kidney transplantation: where are we now and where are we going? Transplant Rev. (2017) 31:10–7. doi: 10.1016/j.trre.2016.10.006
256. Thongprayoon C, Kaewput W, Kovvuru K, Hansrivijit P, Kanduri SR, Bathini T, et al. Promises of big data and artificial intelligence in nephrology and transplantation. J Clin Med. (2020) 9:1107. doi: 10.3390/jcm9041107
257. Kuo CC, Chang CM, Liu KT, Lin WK, Chiang HY, Chung CW, et al. Automation of the kidney function prediction and classification through ultrasound-based kidney imaging using deep learning. NPJ Digit Med. (2019) 2:29. doi: 10.1038/s41746-019-0104-2
258. Hermsen M, de Bel T, den Boer M, Steenbergen EJ, Kers J, Florquin S, et al. Deep learning-based histopathologic assessment of kidney tissue. J Am Soc Nephrol. (2019) 30:1968–79. doi: 10.1681/ASN.2019020144
259. Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. (2017) 542:115–8. doi: 10.1038/nature21056
260. Topfer LA. Wearable Artificial Kidneys for End-Stage Kidney Disease. CADTH Issues in Emerging Health Technologies. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health. Copyright © CADTH 2017. You Are Permitted to Reproduce This Document for Non-Commercial Purposes, Provided It Is Not Modified When Reproduced and Appropriate Credit Is Given to CADTH. (2017).
261. Gura V, Rivara MB, Bieber S, Munshi R, Smith NC, Linke L, et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. (2016) 1:e86397. doi: 10.1172/jci.insight.86397
262. Woolf AS. Growing a new human kidney. Kidney Int. (2019) 96:871–82. doi: 10.1016/j.kint.2019.04.040
263. Lindström NO, McMahon JA, Guo J, Tran T, Guo Q, Rutledge E, et al. Conserved and divergent features of human and mouse kidney organogenesis. J Am Soc Nephrol. (2018) 29:785–805. doi: 10.1681/ASN.2017080887
264. Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. (2014) 16:118–26. doi: 10.1038/ncb2894
265. Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. (2014) 25:1211–25. doi: 10.1681/ASN.2013080831
266. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. (2015) 33:1193–200. doi: 10.1038/nbt.3392
267. Taguchi A, Nishinakamura R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell. (2017) 21:730–46.e6. doi: 10.1016/j.stem.2017.10.011
268. van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem cell reports. (2018) 10:751–65. doi: 10.1016/j.stemcr.2018.01.041
269. Shukrun R, Pode-Shakked N, Pleniceanu O, Omer D, Vax E, Peer E, et al. Wilms' tumor blastemal stem cells dedifferentiate to propagate the tumor bulk. Stem Cell Rep. (2014) 3:24–33. doi: 10.1016/j.stemcr.2014.05.013
Keywords: pediatric kidney transplantation, graft survival, pediatric urology, pediatric nephrology, immunosuppression, donor selection, lower urinary tract dysfunction
Citation: Oomen L, Bootsma-Robroeks C, Cornelissen E, de Wall L and Feitz W (2022) Pearls and Pitfalls in Pediatric Kidney Transplantation After 5 Decades. Front. Pediatr. 10:856630. doi: 10.3389/fped.2022.856630
Received: 17 January 2022; Accepted: 15 February 2022;
Published: 08 April 2022.
Edited by:
Juan Manuel Moldes, Italian Hospital of Buenos Aires, ArgentinaReviewed by:
Lyndsay Harshman, The University of Iowa, United StatesMara Medeiros, Federico Gómez Children's Hospital, Mexico
Andréa Exeni, Hospital Universitario Austral, Argentina
Copyright © 2022 Oomen, Bootsma-Robroeks, Cornelissen, Wall and Feitz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wout Feitz, d291dC5mZWl0eiYjeDAwMDQwO3JhZGJvdWR1bWMubmw=
 Loes Oomen
Loes Oomen Charlotte Bootsma-Robroeks
Charlotte Bootsma-Robroeks Elisabeth Cornelissen2
Elisabeth Cornelissen2 Wout Feitz
Wout Feitz