- 1Department of Pediatrics, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 2Department of Neonatology, King Saud University Medical City, Riyadh, Saudi Arabia
- 3Department of Pediatrics, King Saud University Medical City, Riyadh, Saudi Arabia
- 4Department of Pediatrics, King Fahad Medical City, Riyadh, Saudi Arabia
- 5Department of Pediatrics, Pediatric Infectious Disease, Prince Sultan Military Medical City, Riyadh, Saudi Arabia
- 6Global Center for Mass Gatherings Medicine, Ministry of Health, Riyadh, Saudi Arabia
- 7Department of Emergency Medicine, College of Medicine, King Saud University, Riyadh, Saudi Arabia
- 8Department of Pediatrics, Mount Sinai Hospital, Toronto, ON, Canada
- 9Department of Pediatrics, Toronto University, Toronto, ON, Canada
- 10Maternal-Infant Care Research Centre, Mount Sinai Hospital, Toronto, ON, Canada
Background: Data on SARS-CoV-2 in infants ≤ 90 days are limited with conflicting reports regarding its presentation and outcomes.
Methods: We conducted an ambispective cohort study using prospectively collected Health Electronic Surveillance Network Database by the Ministry of Health, Saudi Arabia. Infants of ≤ 90 days of age who had a positive RT-PCR test for SARS-CoV-2 virus were included. Patients were divided in Early neonatal (0–6 days), late neonatal (7–27 days), and post- neonatal (28–90 days) groups and were compared for clinical characteristics and outcomes by contacting parents and collecting information retrospectively.
Results: Of 1,793 infants, 898 infants were included for analysis. Most infants in the early neonatal group had no features of infection (tested based on maternal positivity), whereas most infants in the late and post- neonatal groups were tested because of clinical features of infection. Fever and respiratory signs were the most common presenting feature in the late and post-neonatal groups. Hospitalization was higher in the early neonatal group (80%), compared to the two other groups. The overall mortality in the cohort was 1.6%.
Conclusion: SARS-CoV-2 infection in infants ≤ 90 days might not be as rare as previously reported. The clinical presentation varies based on age at positive RT-PCR result.
Introduction
The first reported pediatric case of coronavirus diseases of 2019 (COVID-19) was a 10-year-old boy from Shenzhen, China, diagnosed with the condition in January 2020 (1). Available literature suggests that pediatric population may be less affected from COVID-19 than adult population, (2) and that a high percentage of infected children remain asymptomatic. The majority of children develop a milder disease and have a lower mortality rate (3, 4). However, the newly mutated variants seem to affect children more than what has been reported earlier (5).
Approximately a third of hospitalized children due to COVID-19 were ≤ 90 days of age (6) and half of the patients in this age group and in neonates when presented to hospital required admission (7, 8). Infants ≤ 90 days of age are at a higher risk of developing severe COVID-19 disease (9–11). Furthermore, the reported mortality rate of infants in this age group has ranged between 0 and 2.9% (6, 12, 13). However, data specifically reporting on infants of ≤ 90 days of age have been limited (14).
The objective of this study is to describe the clinical features, presentation, and outcomes of infants of ≤ 90 days of age who were identified to be severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive from a national cohort from Saudi Arabia.
Methods
Design, Setting, and Inclusion Criteria
We conducted an ambispective cohort study from a prospectively collected database by the Ministry of Health in Saudi Arabia. We also obtained detailed information from families via phone interviews if we identified missing information. The study included infants of ≤ 90 days of age who had a positive SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) test results and presented to health care facilities between March 12, 2020, and February 8, 2021, in Saudi Arabia. No exclusion criteria were applied. We collected data only on the first infection, as re-infection in this age group has not been reported.
Procedure
After obtaining the required permits and ethics committees' approval, data were extracted from the Health Electronic Surveillance Network (HESN) Database housed within the Ministry of Health in Saudi Arabia. The database contains demographic, clinical features, and outcome data of patients who were tested positive for SARS-CoV-2 via RT-PCR testing. The data were entered in the system by trained health care workers from all regions of Saudi Arabia (15). We extracted infants from the database with positive RT-PCR for SARS-CoV-2 and then called those who were ≤ 90 days of age into a datasheet. From this parent database, data on infants of ≤ 90 days of age were extracted by two abstractors involved in this study in separate electronic datasheets. These abstracted data were checked by a third investigator to ensure completeness and to remove duplicate records. During the study period, the positivity of SARS-CoV-2 was assessed using RT-PCR test from nasopharyngeal samples in accordance with protocols set by the World Health Organization throughout Saudi Arabia (16). Using a standardized questionnaire, one of the investigators or research associates made a phone call to parents/caregivers of all infants with positive results. The phone calls were made between 22/02/2021 and 29/04/2021 to complement information obtained from HESN. The additional information included: demographics, gestational age, mother's COVID-19 status at the time of birth, feeding method at time of testing, contact with any other COVID-19 patients, clinical presentation, hospital/ICU admission, and mortality. Before labeling a phone number as “no response” the phone number was tried four times on different occasions. The HESN data items were also checked with infants' caregivers to remove duplicate information if an infant attended multiple facilities. The data collected from parents were inputted on a google sheet form which was then exported to an excel sheet. The data sheet was reviewed daily by two of the investigators to ensure completeness of the data collected.
Outcomes and Groups
Infants in the study were divided into early neonatal (0–6 days), late neonatal (7–27 days), and post-neonatal (28–90 days) groups, according to their age at first positive RT-PCR test for SARS-CoV-2 as per the AAP definition (14). The status on hospitalization was collected as hospitalized or not hospitalized.
Ethics
The study was approved by the Central Ministry of Health Institutional Review Board Committee (Approval number 20−198M), Saudi Arabia. During the phone interview, a verbal consent was obtained first from the parents/caregivers and if parents declined participation, the patient was excluded from the study. Throughout the study, data were stored on a computer with a strong password and access was restricted to authorized investigators after signing non-disclosure agreement forms.
Statistical Analysis
Descriptive statistics were performed at first. Three groups, based on timing of testing of positivity, were compared against each other. We also evaluated the outcomes of three groups based on illness severity (asymptomatic, symptomatic, hospitalized, and non-hospitalized). Finally, we compared outcomes based on the status of hospitalization; hospitalized vs. not hospitalized. We reported continuous variable outcomes as mean and SD (for normally distributed data) or median and IQR (for non- normally distributed data), and categorical variable outcomes as percentages. We used Student t test, chi-square test or Wilcoxon Rank Sum test as appropriate for these comparisons. P < 0.05 were considered significant. All analyses were conducted using SPSS software (SPSS Statistics for Windows, Version 26.0. Armonk, NY).
Results
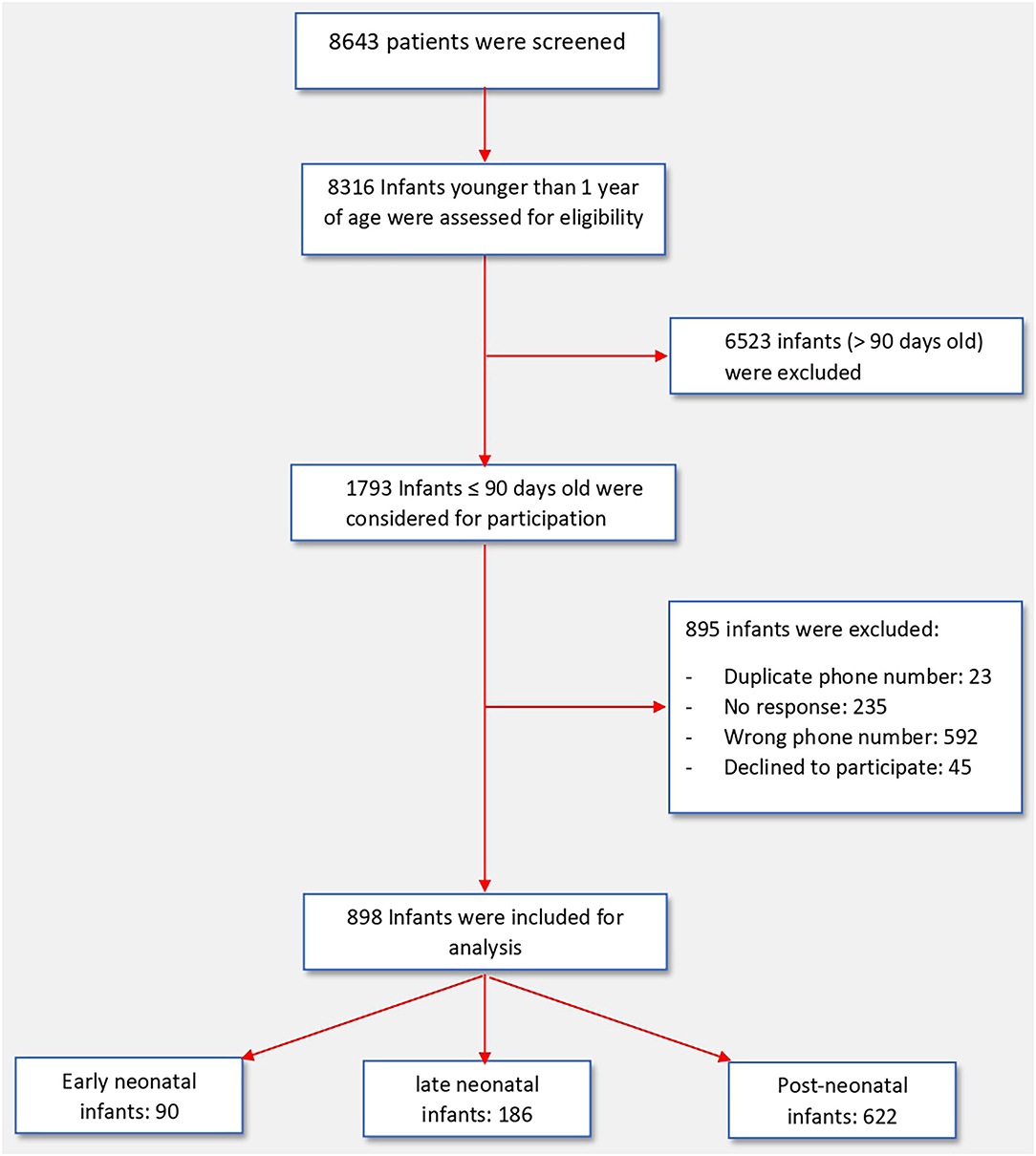
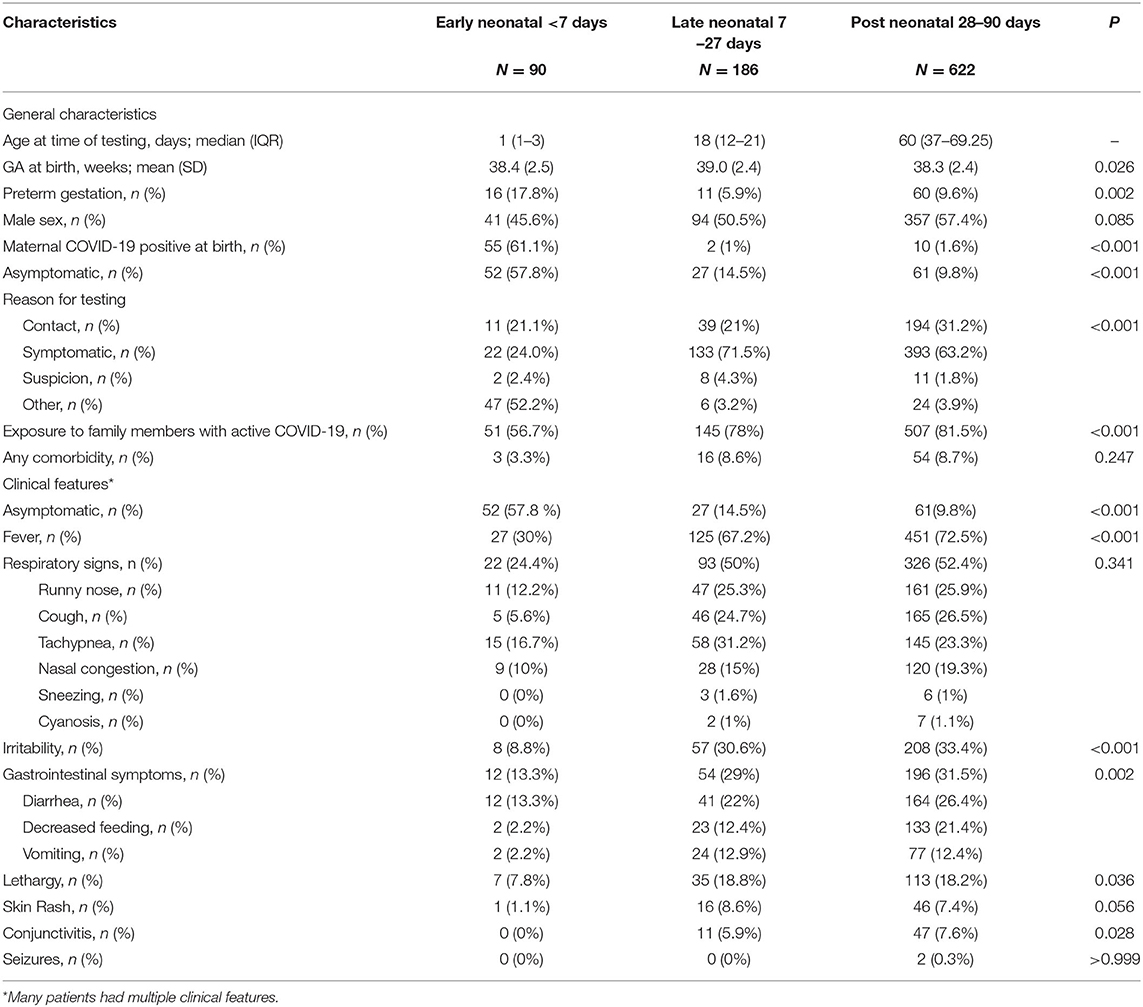
On the initial scan of the HESN database, a total of 1,793 infants of ≤ 90 days of age were identified, of them, 895 infants were excluded due to duplicate records, incorrect contact details, no response or refusal to participate. Thus, we included 898 infants in this study (Figure 1). The baseline characteristics of all three groups of infants are presented in Table 1. Included were 90 patients in the early neonatal group, 186 patients in the late neonatal group and 622 patients in the post neonatal group. Early neonatal group patients were mostly tested due to maternal positivity, whereas late neonatal and post-neonatal patients were tested either because of symptoms or being in contact with a person with positive RT-PCR test. Fever and respiratory signs were the most common presenting features in late neonatal and post-neonatal groups; however, nearly one third of them presented with irritability or gastrointestinal signs.
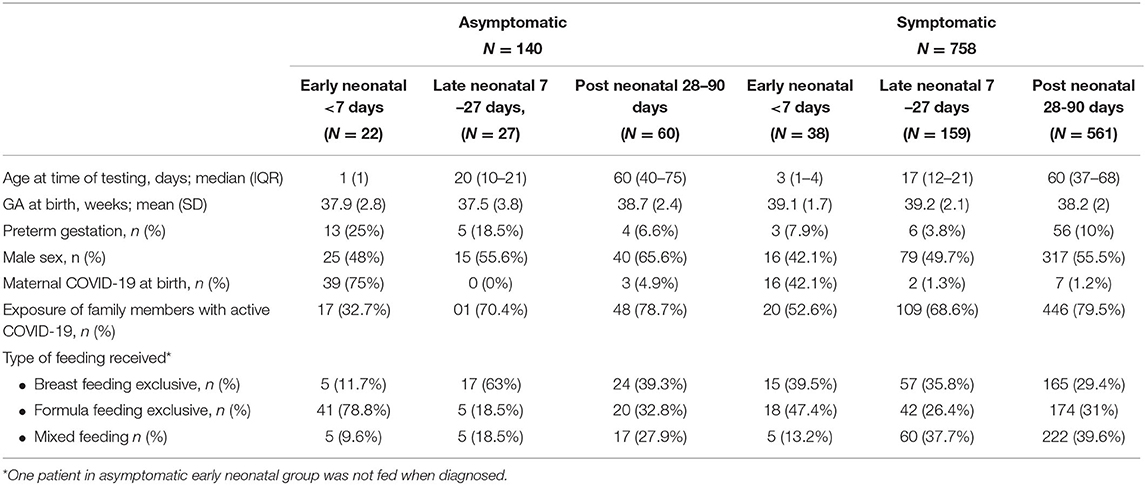
As seen in Table 2, there were 140 asymptomatic infants and 758 symptomatic infants. About two fifths of infants in the early neonatal group had features suggestive of infection whereas the majority of the infants in the late neonatal and post neonatal groups had features suggestive of infection, 58, 85, and 90%, respectively. The percentage of infants with clinical features who were born at preterm gestation was higher in the post-neonatal group (10%) compared to both early and late neonatal groups (7.9 and 3.8% respectively). Exclusive formula feeding was higher in early neonatal period (78.8%) (Table 2).
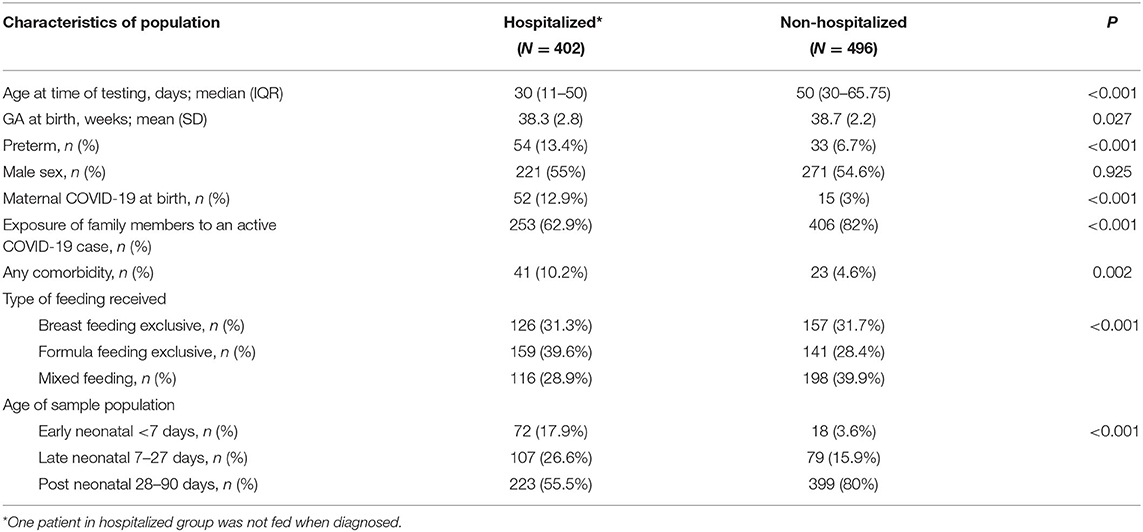
Of the included infants 402 were hospitalized while 496 were not hospitalized. Among the hospitalized infants, the median age at testing was 30 days. Being born at preterm gestation was more prevalent among hospitalized infants (13%) vs. (7%) in non-hospitalized infants. Infants with comorbidity, or those with a positive maternal COVID-19 swab at birth, were more likely to be hospitalized than non-hospitalized, however, this may reflect the initial birth hospitalization. More infants had a history of exposure to family member with active COVID-19 at presentation in the non-hospitalized group (82%) compared to the hospitalized group (62.9%). The characteristics of hospitalized patients are detailed in Table 3.
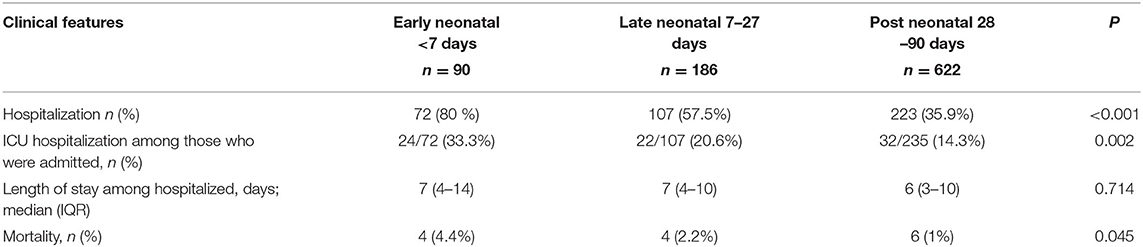
As presented in Table 4. Infants of the early neonatal period were more likely (80%) to be hospitalized, as compared to those of late neonatal (57.5%), or post neonatal (35.9%) periods.
Furthermore, infants in the early neonatal period were more likely to be admitted to the ICU as compared with infants of the other two groups. The median length of hospital stay was not significantly different among the groups. The overall mortality of the whole cohort was 14 (1.56%) infants, with a higher rate of death among infants of the early neonatal period (Table 4).
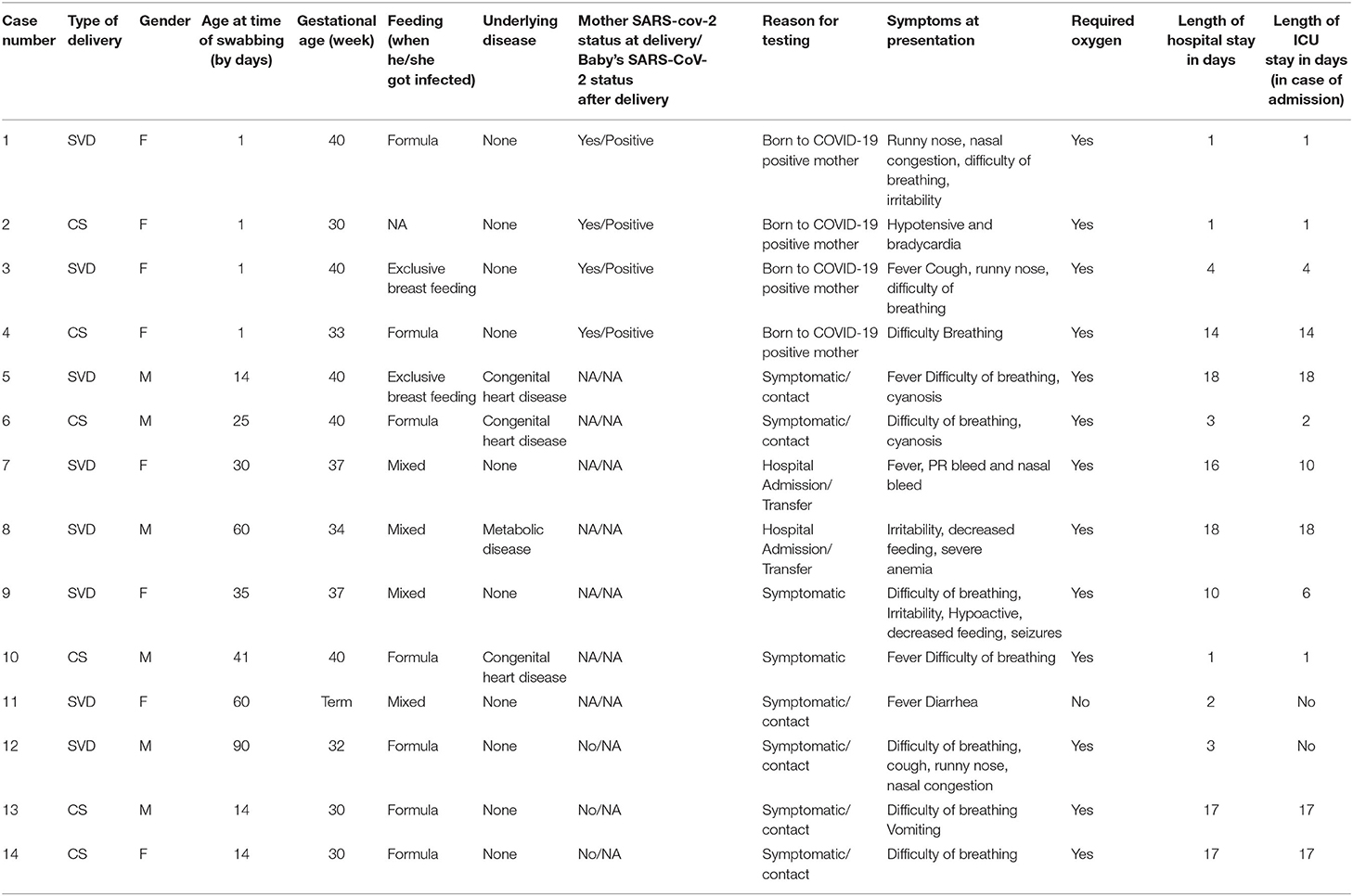
Table 5 describes the details of the infants who died. Four infants were born to COVID-19 positive mothers and were tested positive after delivery. Most of the infants who died had respiratory distress (10/14 = 71.4%), five of them presented with fever, and two presented with GI symptoms of vomiting and diarrhea. Six of the non-survivor infants were born preterm, and four of them had a comorbidity including metabolic disorder or a congenital heart disease.
Discussion
We report in this study on one of the largest nation-wide cohorts with a diverse population, which allows for a balanced epidemiological profile of SARS-CoV-2 infection in infant ≤ 90 days of age. Our study suggests that while only two fifth of early neonatal cases of COVID-19 disease were symptomatic, most infants who are < 90 days old presented with symptoms when infected with SARS-CoV-2 virus. Symptoms' frequency, however, varied among groups; in early neonatal group, the most common presenting sign was fever followed by respiratory symptoms, whereas in late and post neonatal groups respiratory symptoms were more common followed by fever.
In a case series, Shaiba et al. (17) reported that most (94%) infants of ≤ 90 days old presented to a local hospital for SARS-CoV-2 testing were symptomatic. This is almost similar to what was observed in this study where 85% of all infants presented with symptoms (42, 85, and 90% in early, late and post neonatal groups, respectively). In the aforementioned case series, the most common presenting sign was fever (70%) followed by respiratory signs (59%), which is comparable to our study's findings, where fever was encountered in 67% of infants followed by respiratory signs in 50% of them. Gastrointestinal symptoms, however, presented in only 30% of our study's population, in contrast to what has been published in a Canadian study, where GI symptoms were more prevalent (85%) (8). As for the cutaneous and neurological manifestations of COVID-19 disease in infants ≤ 90 days, our study has corroborated previous reports, which indicated that these signs and symptoms are very rare (8, 9, 13, 15).
In a recent systematic review of SARS-CoV-2 infection in neonates, they have reported that 50% of neonates were symptomatic upon presentation (18). The most prominent clinical feature in this systematic review was respiratory signs and symptoms.
As expected, most of the infants in the early neonatal group had a mother who tested positive just prior to birth, this finding suggests the possibility of maternal-fetal transmission in some if not all of these cases. This possibility of maternal-fetal transmission is further supported by the positive SARS-CoV-2 results of infants born to a mother with SARS-CoV-2 infection despite the strict and immediate isolation of the infant from the mother upon birth (as per the Saudi National regulation by the Saudi Ministry of Health). Contrary to the published literature, our study reports a higher percentage of infants tested positive with a family member who is affected by COVID-19 (8, 13, 19).
Breastfeeding and Kangaroo mother care (KMC) are integral parts of neonatal care and has been a standard of care in recent years (20, 21). Neonatologists all over the world had confusion in the initial days of the pandemic to advocate for breastfeeding and KMC as some initial guidelines early in the pandemic advised immediate mother and infant separation for neonates born to mothers infected with SARS-CoV-2. This recommendation seems unnecessary especially with current evidence suggesting only 2.16% of breastmilk samples tested for SARS-CoV-2 RT-PCR were positive and 5% of breastmilk samples contained viral genome with low infectivity rate (22, 23). In our population exclusive breastfeeding rate was low (24). It has been noted that the rate of breastfeeding has gone even lower as only 32.9% of asymptomatic mothers were exclusively breastfeeding and 31.3% of symptomatic mothers were exclusively breastfeeding. Many of neonates and infants were separated from their mothers after being diagnosed with SARS-CoV-2 which prevented proper KMC which might have contributed to the lower rate of breastfeeding. Of note, one of the neonates (neonate number 1 in Table 5) who died on day one of life although the mother expressed breastmilk the neonate was never fed due to his critical condition.
Hospitalized infants tended to be younger (11–50 days of age) than the non-hospitalized ones (30–66 days), this higher rate of admission in younger infants is similar to what was observed in previous studies. Panetta et al. reported that infants who were ≤ 90 days were more likely to be hospitalized (57%) compared to older infants (15%) (8). In a study investigating severe cases of SARS-CoV-2 infection in children 0–18 years of age, Ouldali et al. reported that infants <90 days of age accounted for 37% of admissions with SARS-CoV-2 (6). In our study, the majority of early neonatal infection cases were hospitalized (80%), and of them, one third required ICU admission. Policy aside, we speculate that these infants were admitted to the ICU due to reasons other than SARS-CoV-2 infection, such as prematurity. Of note, a recent study from Saudi Arabia derived from the same pool of patients reported on infants 2 months of age and older suggested that hospitalization rate of infants 2 month of age-−1 year is 11% (3/27 infants) (4). Of note the period of data collection in the study cited was only 1 month and reported cases during the first wave of SARS-CoV-2, when the pediatric patients were less.
COVID-19 infected infants (<1year of age) comprise 17% of all pediatric cases <18 years of age. Also, in infants <1 year of age critical cases were seen in 14% compared to 5% in children older than 1 year of age (25). The neonatal mortality rate (early and late) in our study was 2.9%, which is higher than the rate reported in a national cohort from the UK (2.4%) (9). Notably, the investigators have not specified in this study whether the mortality was directly related to COVID-19 infection (12). This finding is further supported with another national cohort study in the UK (10). In our study included were 8 neonates who died, five of them were preterm and 2 had underlying congenital heart disease. The presence of these comorbidities might have contributed to the high mortality rate in this age group. The lack of a formal detailed medical report to assess the exact cause of death in our study population makes it difficult to ascertain SARS-CoV-2 infection as the primary cause of death.
Assessing the mortality rate of infants ≤ 90 days of age due to SARS-CoV-2 infection is difficult due to the limited number of studies reporting on the disease in this age group and their outcomes. The overall mortality rate in infants ≤ 90 days of life ranged in some studies from 0% (26) up to 2.9% (6, 9) in our study population, this rate is 1.6%, which is in concordance with the above studies.
Our study has many limitations. This includes the potential of selection bias, nonetheless, we strived to include all infants included in the registry by repeated calls (up to 4 times) before labeling a number as a non- responding number. Given it is a partly questionnaire-based study, there is a possibility for recall bias, however, we tried to mitigate this possibility by standardizing the way we applied the questionnaire, and by providing multiple sessions of training to the research associates contacting the families, as well as the availability of one-to-one advice around the clock by an expert physician. Lastly, we could not collect detailed clinical, laboratory and imaging data, as the HESN database is collected primarily for administrative purposes.
In conclusion, although previously reported that neonates and infants have a favorable outcome when infected with SARS-CoV-2, healthcare providers might need to be cautious when counseling parents. We report a higher rate of severe cases of SARS-CoV-2 infection in this age group. We also report a mortality rate in this vulnerable age group that is higher than some of the previously published figures. SARS-CoV-2 continues to be a threat for infants ≤ 90 days old given their susceptibility.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by King Saud University Medical City. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LS and AH elicit the main idea. KA, MA, and AAAlm wrote the proposal. AAlh, AH, KMA, NAlS, and RM collected the data. WA, NAld, MB, and RA data analysis. YA, AAla, YA, FA, AK, and PS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. doi: 10.1016/S0140-6736(20)30154-9
2. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease2019 (COVID-19) Outbreak in China. JAMA. (2020) 323:1239–1242. doi: 10.1001/jama.2020.2648
3. Rabinowicz S, Leshem E, Pessach IM. Covid-19 in the pediatric population review and current evidence. Curr Infect Dis Rep. (2020) 22:29. doi: 10.1007/s11908-020-00739-6
4. Alsohime F, Almuzaini Y, Subaie SA, Temsah MH, Alsofayan Y, Alamri F, et al. Clinical profiles associated with SARS-COV-2 infection and complications from coronavirus disease-2019 in children from a national registry in Saudi Arabia. Annals Thoracic Med. (2021) 16:280. doi: 10.4103/atm.atm_709_20
5. Covid-19 pandemic. American Academy of Pediatrics. (2021). Available online at: http://bit.ly/AAPNewsCOVID19 (accessed Oct, 272021)
6. Ouldali N, Yang DD, Madhi F, Levy M, Gaschignard J, Craiu I, et al. Factors associated with severe SARS-COV-2 infection. Pediatrics. (2020) 147:e2020023432. doi: 10.1542/peds.2020-023432
7. Fernández Colomer B, Sánchez-Luna M, de Alba Romero C, Alarcón A, Baña Souto A, Camba Longueira F, et al. Neonatal infection due to SARS-COV-2: an epidemiological study in Spain. Front Pediatr. (2020) 8:580584. doi: 10.3389/fped.2020.580584
8. Panetta L, Proulx C, Drouin O, Autmizguine J, Luu TM, Quach C, et al. Clinical characteristics and disease severity among infants with SARS-COV-2 infection in Montreal, Quebec, Canada. JAMA Network Open. (2020) 3:e2030470. doi: 10.1001/jamanetworkopen.2020.30470
9. Gale C, Quigley MA, Placzek A, Knight M, Ladhani S, Draper ES, et al. Characteristics and outcomes of neonatal SARS-COV-2 infection in the UK: a prospective national cohort study using active surveillance. Lancet Child & Adolescent Health. (2021) 5:113–21. doi: 10.1016/S2352-4642(20)30342-4
10. Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatrics. (2020) 174:e202430. doi: 10.1001/jamapediatrics.2020.2430
11. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in Real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
12. Papapanou M, Papaioannou M, Petta A, Routsi E, Farmaki M, Vlahos N, et al. Maternal and neonatal characteristics and outcomes of COVID-19 in pregnancy: an overview of systematic reviews. Int J Environ Res Public Health. (2021) 18:596. doi: 10.3390/ijerph18020596
13. Mithal LB, Machut KZ, Muller WJ, Kociolek LK. SARS-COV-2 infection in infants less than 90 days old. J Pediatr. (2020) 224:150–2. doi: 10.1016/j.jpeds.2020.06.047
14. Barfield WD. Standard terminology for fetal, infant, and perinatal deaths. Pediatr J. (2016) 137:1143–51.
15. Ministry Of Health Saudi Arabia. Dr. Al-Rabeeah Launches HESN System. Retrieved from: https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2012-10-24-011.aspx (accessed October 23, 2021).
16. World Health Organization. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: Interim guidance, 19 March 2020. World Health Organization (1970). Available online at: https://apps.who.int/iris/handle/10665/331501 (accessed Oct 27, 2021).
17. Shaiba LA, Altirkawi K, Hadid A, Alsubaie S, Alharbi O, Alkhalaf H, et al. Covid-19 disease in infants less than 90 days: case series. Front Pediatr. (2021) 9:674899. doi: 10.3389/fped.2021.674899
18. Dhir SK, Kumar J, Meena J, Kumar P. Clinical Features and Outcome of SARS-CoV-2 infection in neonates: a systematic review. J Trop Pediatr. (2021) 67:fmaa059. doi: 10.1093/tropej/fmaa059
19. Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, et al. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine. (2021) 39:667–77. doi: 10.1016/j.vaccine.2020.11.078
20. World Health Organization. Kangaroo Mother Care. World Health Organization (2019). Retrieved from: https://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9241590351/en/ (accessed February 7, 2022).
21. World Health Organization. Protecting, Promoting and Supporting Breastfeeding: The Baby-Friendly Hospital Initiative for Small, Sick and Preterm Newborns. World Health Organization. Retrieved from: https://www.who.int/publications/i/item/9789240005648 (accessed February 7, 2022).
22. Kumar J, Meena J, Yadav A, Kumar P. SARS-CoV-2 detection in human milk: a systematic review. J Matern Fetal Neonatal Med. (2021). 8:1–8. doi: 10.1080/14767058.2021.1882984
23. Zhu F, Zozaya C, Zhou Q, De Castro C, Shah PS. SARS-CoV-2 genome and antibodies in breastmilk: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2021) 106:514–521. doi: 10.1136/archdischild-2020-321074
24. Al Juaid DA, Binns CW, Giglia RC. Breastfeeding in Saudi Arabia: a review. Int Breastfeed J. (2014) 9:1. doi: 10.1186/1746-4358-9-1
25. Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta- analysis of children with coronavirus disease 2019 (Covid- 19). J Med Virol. (2020) 93:1057–69. doi: 10.1002/jmv.26398
26. Saudi MOH protocol for patients suspected of/confirmed (n.d.). Retrieved from: https://www.moh.gov.sa/Ministry/MediaCenter/Publications/Docum ents/MOH-therapeutic-protocol-for-COVID-19.pdf (accessed February 7, 2022).
Keywords: SARS-CoV-2, positivity, early infancy, national, cohort, Saudi Arabia
Citation: Shaiba LA, Hadid A, Altirkawi K, Alnamnakani MA, Almutayliq AA, Alharbi AT, Hijazi AM, AlMoosa KM, AlSaud NF, Murshid RE, AlMuhanna WS, Aldawsari NA, Bin Hadyan MF, Almaghrabi R, Alsofayan YM, Alahmari AA, Almuzaini YS, Alamri FA, Khan AA and Shah PS (2022) SARS-CoV-2 Positivity in Early Infancy: A National Cohort From Saudi Arabia. Front. Pediatr. 10:849659. doi: 10.3389/fped.2022.849659
Received: 06 January 2022; Accepted: 24 February 2022;
Published: 28 March 2022.
Edited by:
Jogender Kumar, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Shashi Kant Dhir, Guru Gobind Singh Medical College and Hospital, IndiaLiviana Da Dalt, University of Padua, Italy
Copyright © 2022 Shaiba, Hadid, Altirkawi, Alnamnakani, Almutayliq, Alharbi, Hijazi, AlMoosa, AlSaud, Murshid, AlMuhanna, Aldawsari, Bin Hadyan, Almaghrabi, Alsofayan, Alahmari, Almuzaini, Alamri, Khan and Shah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lana A. Shaiba, bHNoYWliYUBrc3UuZWR1LnNh
 Lana A. Shaiba1,2*
Lana A. Shaiba1,2* Khalid Altirkawi
Khalid Altirkawi Rana Almaghrabi
Rana Almaghrabi Yousef M. Alsofayan
Yousef M. Alsofayan Ahmed A. Alahmari
Ahmed A. Alahmari Yasir S. Almuzaini
Yasir S. Almuzaini Fahad A. Alamri
Fahad A. Alamri Anas A. Khan
Anas A. Khan Prakesh S. Shah
Prakesh S. Shah