- 1Child Development and Education Unit, Laboratory of Applied Psychology, Department of Psychology, University of Crete, Rethymno, Greece
- 2Department of Neonatology, Neonatal Intensive Care Unit (NICU), School of Medicine, University of Crete, Rethymno, Greece
This review aims to discuss the factors that may affect maternal mental health and infant development in COVID-19 pandemic condition. Toward this direction, the two objectives of this review are the following: (a) to discuss possible factors that may have affected negatively perinatal mental health through the pandemic-related restrictions; and (b) to present the implications of adversely affected maternal emotional wellbeing on infant development. We conclude that the pandemic may has affected maternal mental health with possible detrimental effects for the infants of the COVID-19 generation. We highlight the need for evidence-based interventions to be integrated within the health system for prenatal and postpartum care in an effort to promote maternal mental health and infant development.
Introduction
COVID-19 pandemic constitutes a major threat to global human health and a worldwide traumatic experience (1, 2). During the 2 years of the COVID-19 pandemic, million infants were born to mothers and families who have experienced tremendous stress and change in their daily lives and environments due to the pandemic (3–5)].
According to the World Health Organization (WHO), maternal mental health is defined as “a state of wellbeing in which a mother realizes her own abilities, can cope with the normal stresses of life, can work productively and fruitfully, and is able to make a contribution to her community” [as cited in Engle (6)]. Perinatal mental disorders constitute the commonest complications of child-bearing, and they are associated with high levels of maternal and fetal/infant morbidity and mortality (7). For women in the perinatal period, research has identified two major-pandemic–related stress domains: stress associated with feeling unprepared for birth and stress related to fears of perinatal infection (8). What is more, in the first months of COVID-19 pandemic, evidence from around the world1 indicated that pregnant women suffer from high prevalence of anxiety (ranging from 21.7 to 78.4%), depression (ranging from 17 to 56.3%) and post-traumatic stress disorder (9–13). During COVID-19, the overall prevalence of anxiety and depression among pregnant women was 40 and 27%, respectively. Though the levels of anxiety and depression during pregnancy vary across different countries, in general prenatal depression is estimated to affect 9–18% of pregnant women at any given time during pregnancy (14, 15) while 5–13% of pregnant women suffer from anxiety symptoms (10, 11). Thus, evidence suggests that symptoms of maternal mental disorders have become more common during the pandemic (9–13). In the meanwhile, prenatal psychopathological diagnoses have been rarely investigated (13) and only a limited number of studies analyzed longitudinally anxiety and depression symptoms of pregnant women in the course of the lockdown. Despite contrasting results, findings show that ongoing COVID-19 pandemic may aggravate anxiety and depression symptoms of pregnant women (9, 12, 16).
COVID-19 pandemic coincides with sensitive time windows of heightened plasticity, such as pregnancy and neonatal life (1, 2). The significance of adversely affected perinatal maternal mental health as a potential risk factor for infant development has been emphasized (17). Prenatal stress affects the development of fetal systems (14, 15, 18–21). These fetal systems are also potentially related factors and causes of neuropsychiatric disorders (depression, anxiety, behavioral dysfunction, attention-deficit hyperactivity disorder, autism spectrum disorder) in children (21). Further, symptoms of maternal mental disorders have been linked with delays and poor infant motor, social, cognitive, and language development and difficulties in emotional self-regulation [see (22, 23) for reviews].
In addition, face mask wearing by caregivers in daily interactions with their infants may affect negatively: (a) infants' abilities related to social and emotional reciprocity and interpersonal engagement (24, 25) and (b) infant speech perception by interfering in the way basic features of infant-directed speech are expressed and transferred to infants and by obscuring, or reducing infant perception of intersensory coherence, speech intelligibility and maintainance of infant attention [(26), as cited in (27, 28)].
On this ground, as the pandemic continues, the adverse impact on maternal mental health may has long-term effects and powerful influence not only for the generation of infants born during the pandemic, but also for future generations to come (5). We are seriously concerned on this issue because the results of a limited number of the first relevant studies confirm the impact of negatively affected perinatal maternal stress on infant development in the pandemic condition (2, 29–32).
The WHO considers maternal mental health as a global health priority (33). Despite that, in this unprecedented time, mental health issues may have been overshadowed by more pressing issues in health care (34). What is more, relevant literature has mainly focused on the impact as an outcome of the pandemic on postpartum maternal emotional wellbeing. A growing body of research investigates women's mental health also during pregnancy (35). In the meanwhile, the factors that may affect perinatal maternal mental health and their connection with infant development have been discussed only in fragments. From an holistic perspective, this review comes to fill in this gap in the literature by discussing the possible factors that may affect perinatal maternal mental health through pandemic-related restrictions, and by highlighting the adverse implications of negatively affected maternal emotional wellbeing on infant development of the COVID-19 generation (see Figure 1).
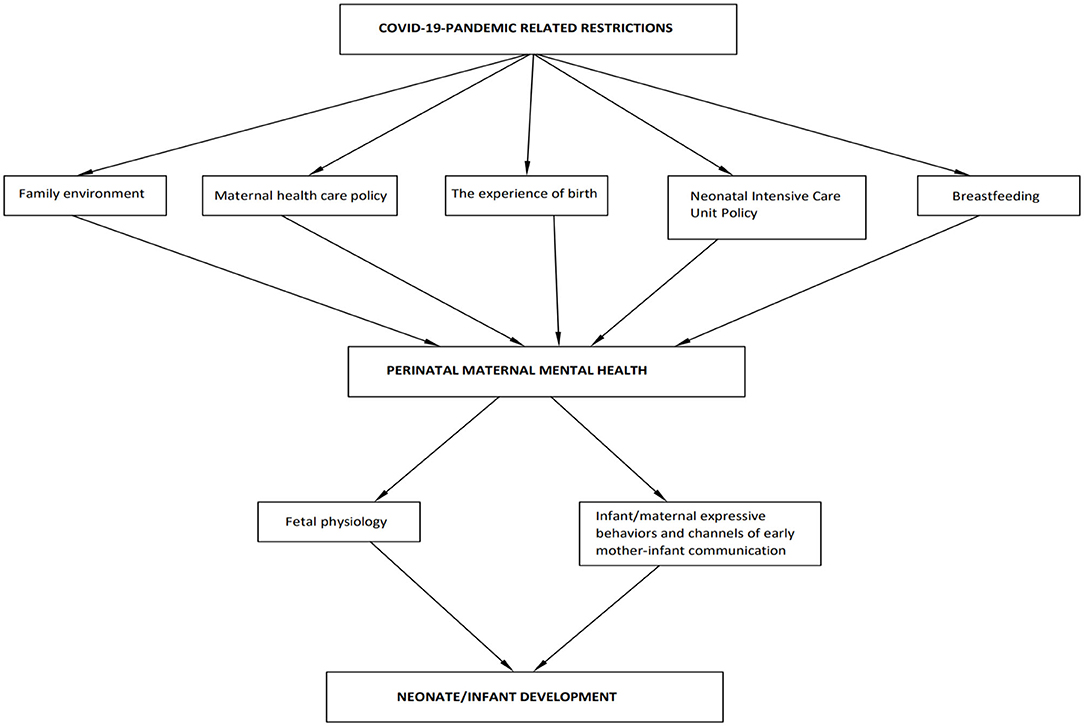
Figure 1. Factors that may affect perinatal maternal mental health through pandemic-related restrictions and implications for neonate and infant development.
Search Strategy
A strategy was formulated and literature searches were conducted from March 2020 to March 2022. The following electronic databases were searched: Web of Science, APA PsycINFO, Academic Search Ultimate, and JSTOR. Search was restricted to papers published in English language, with no restriction on the year of publication. Editorials, opinion papers, empirical studies, reviews, systematic reviews, and meta-analyses were eligible. In this review, we excluded publications which investigated: (a) the way the pandemic may has affected maternal mental health of preschoolers, school-aged children and adolescents and (b) research with a focus on maternal mental health of children, or adolescents with atypical development. Thus, we included only articles with a focus on the way the pandemic has affected perinatal maternal mental health of typically developing neonates and infants. For the first five subsections of the first objective of this review, literature search included the combined use of the keywords: “COVID-19 or coronavirus or 2019-ncov or sars-cov-2 or cov-19” with each of the following terms: “maternal mental health or postnatal mental health or perinatal mental health,” “family environment family relationships or family dynamics or family functioning”/“family cohesion,” “maternal care or maternal health or reproductive health,” “maternal health or pregnancy or perinatal,” “breastfeeding or breast-feeding or infant feeding or lactation or lactating,” “birth experience or womens' feelings about birth or birth satisfaction,” and “neonatal intensive care unit or nicu or baby unit or newborn intensive care.” For the sixth subsection of the first objective of this review, literature search included the combined use of “COVID-19 or coronavirus or 2019-ncov or sars-cov-2 or cov-19” with “maternal mental health or postnatal mental health or perinatal mental health” and “infant development.” Thus, for the coherence of this review, we restricted the presentation of these studies only to those that provided evidence on infant developmental outcomes in relation to perinatal maternal mental health in the first months of life. For the second objective of this review, search terms included the combined use of “maternal depression or postpartum depression or perinatal depression” or “maternal anxiety or postnatal anxiety or perinatal anxiety” or “maternal stress during pregnancy” with each of the following terms: “infant development,” “fetal systems,” “fetal physiology,” “mother-infant interaction or early interaction,” “mother-infant attachment,” “maternal sensitivity or parental sensitivity or caregiver sensitivity.”
Possible Factors That may Have Affected Negatively Perinatal Mental Health Through the Pandemic-Related Restrictions
Family Environment and Parental Mental Health
The lockdowns and shutdown policies related to COVID-19 have led to economic difficulties, instability or loss of job and uncertainty for the future economic status. The introduction of strict measures changed considerably the daily routine of citizen. Further, COVID-19 has been posing a threat to interpersonal interactions and relationships due to both a limited physical proximity with one's not cohabiting family members/friends, and at the same time, a forced and prolonged cohabitation with members of one's family (1, 36). Family environment/function has a significant impact on the mental health of its members. Under a crisis condition, the family environment may affect the interaction of negative emotion among its members (37). The unusual family environment has been associated with mental disorders among pregnant women. The mother's perinatal mental health is influenced by her family environment and compatibility between them is very important (38). Parental depression can be passed on to children through poor family function (39).
In connection to these, findings coming from limited studies in different regions provide evidence of a negative impact of the COVID-19 pandemic on the family environment and parental (maternal and paternal) mental health (1, 38, 40–46).
In particular, in China, Xie et al. (38) showed that, compared to the pre-pandemic period, the scores of cohesion2 and independence in families were much lower during the pandemic. The scores for family cohesion were negatively related with depression, anxiety, and hostility symptoms. Similarly, Li et al. (45) confirmed negative correlations between depressive symptoms and family cohesion/adaptability. In Singapore, parents with greater COVID-19-related concerns reported higher stress and less closeness to their children (41). In USA, Feinberg et al. (42) reported deteriorations in family wellbeing and parent depression in the first months of the pandemic, compared to the period preceding it, and a moderate decline in co-parenting quality. Bate et al. (40) confirmed increased levels of parents' self-reported depression and anxiety symptoms during the pandemic. Higher conflict in the parent-child relationship strengthened the positive links between parent and child emotional health issues. In Canada, since the onset of the COVID-19, compared to respondents without children (35.6%), a significantly higher proportion of parents reported worse mental health (44.3%) with high levels of anxiety, worry, stress and boredom. 28.3% of parents reported being stressed while facing challenges in the relationship with their partner. Regarding sources of support, 47.6% of parents reported connecting with those in the household (43). In the same cultural context, COVID-19 health anxiety was found to impair family engagement because of the increased emotion suppression and the lack of psychological need fulfillment (47). In Spain (one of the worst affected European countries), Gunther-Bel's et al. qualitative analysis (44) confirmed elevated levels of state anxiety during lockdown and provide mixed results on perceived changes in family dynamics. In particular, prevalence of improvement themes (61.7%) outnumbered deterioration themes (41.0%). For parents with children at home, family (re)connection was cited most often among the improvement themes while unbalanced needs were most frequent among deterioration themes. Despite the fact that marital functioning for couples with children systematically improved with days in lockdown, this was not the case for parenting functions. In Australia, Westrupp et al. (46) showed that, compared to estimates in the pre-pandemic period, during the COVID-19 parents reported higher levels of depression, anxiety and stress, higher parenting irritability and lower levels of family positive expressiveness. In Italy, Donato et al. (1) confirmed that COVID-19 concerns threaten individuals' psychological wellbeing and showed that explicit stress communication3 in the couple and responses in dyadic coping mediated the link between COVID-19-related concerns and parents' mental health.
Health Maternity Care Policy
Basic health services worldwide have been heterogeneously affected by the COVID-19 pandemic. There is a variation in strategies adopted to maintain continuity of maternal health services (49). There is a complex organizational response to COVID-19 in maternal services. In some cases these responses are in direct contraversion of COVID-19 recommendations from relevant organizations. These practices may affect negatively, both physically and psychologically, mothers along with their infants as well as medical staff caring for childbearing women and their families (50–53).
In particular, as a response to COVID-19 crisis, limits have been placed on antenatal classes' attendance by pregnant women, decrease of presentations of obstetric-related conditions to the emergency departments, restrictions on health care tests and treatments availability, in both ante- and postnatal care while companionship for birth and postnatal visiting have been prohibited. Giving birth in hospitals full of SARS-CoV-2 infected patients increase further maternal worries for the possibility of their and their infants' infection with adverse physical and psychological implications for both of them. Expectant mothers' reluctance to attend and delay in seeking treatment, due to the fear of being exposed to the virus, may result in poorer outcomes. Moreover, in cases of maternal SARS-CoV-2 infection, forced separation of mothers and infants for up to 14 days has been reported. This prohibited immediate and uninterrupted skin-to-skin contact. Further, a lack of opportunity to support mothers to initiate breastfeeding in the first hour after birth along with non-acceptance of breastmilk donation have been evidenced. These restrictions have a negative effect on mother's mood, self-esteem, self-confidence and confidence in their abilities related to their infant's care. Standard precautions (such as hand hygiene, use of medical mask, routine disinfection) applied by mothers with suspected, or confirmed COVID-19, who must take care of their infants by themselves, may impose psychological demands on new mothers and may complicate the early mother-infant relationship (51–56).
The Experience of Birth
During the pandemic, and especially during the lockdown period, limited access to formal, and informal support network along with medical conditions and risk factors may have shaped adversely the experience of birth (35).
In connection to these, limitations set by the pandemic may cause a shift in the way mothers and fathers experience birth. Thus, birth experience may shift from being a “couple event”—based on “togetherness”—to being in “singleness,” placing a barrier within the couple and within the newly born family. Father's stress combined with that of mother in the perinatal period may has implications for infant development (36).
Pregnant mental health is at the core of the following interrelated factors that have been identified as affecting the subjective childbirth experience: pregnant psychological wellbeing, personal history of maternal mental illness, pregnancy complications, fear of childbirth, support and relationship with the partner, fear of health, the first moments with the baby/mother-infant bond (skin-to-skin contact, breastfeeding), previous birth experiences, perceived control, birth plan compliance and medical-obstetric dimensions (33, 35, 57, 58).
In particular, COVID-19 pandemic has affected adversely all above interconnected factors that contribute to the subjective childbirth experience (33, 35, 57, 58). Women who gave birth, or pregnant women during the current pandemic, are at greater risk of reporting general stress, isolation and frustration at all phases of pregnancy, birthing and infant care. They are also at greater risk of manifesting depressive, anxiety, or post-traumatic symptoms (59, 60). In the pandemic condition, depression and anxiety have been negatively related to birth satisfaction (61). New-mothers with earlier psychological disorders and complications during pregnancy were more likely to suffer from trait anxiety and postpartum depression, to develop a postpartum post-traumatic stress disorder and to have perceived childbirth as a negative experience (35). In the period preceding the pandemic, the fear of childbirth was associated with anticipation, impatience, joy and encounter. However, during the pandemic fear was correlated with sadness, loneliness, inability, sense of isolation and constriction (58). For more than half of expectant mothers, fear of childbirth is above the cutoff value while 32% of women reported a negative childbirth experience (35). Lack of support from the partner has arisen as a major issue affecting adversely women's pregnancy and childbirth experience under the restrictions imposed due to the pandemic. Pregnant women were more likely to suffer from state anxiety and to have intense fear of childbirth if they believed that their partner could not be present at childbirth, or had not been present during delivery (35). During the earliest months of the pandemic, higher birth satisfaction has been associated with having a birth partner present (61). COVID-19 has intensified the protective response of women for those around them (13, 16, 62). This shift of focus to others' health may increase the risk of maternal mental health problems (13). Regarding the first moments with their infants, Del Rio et al. (50) showed that 43.5% of infants did not receive maternal skin-to-skin contact after birth. After the quarantine termination, 49.1% of SARS-CoV-2 infected mothers chose to prolong mother-baby separation. In the first months of the pandemic, separation from the infant has been negatively associated with birth satisfaction (61). The combined impact of isolation with the feeling of the newborn as “fragile” caused tension and mistrust (59). Ravaldi et al. (58) showed that before the pandemic, previous birth experiences were associated with positive expectations for birth but during the pandemic the same experiences changed to feelings of danger, anxiety and loneliness. Pandemic situation has been associated with birth plan changes (e.g., place of birth, presence of birth partner) (63) which may negatively affect their birth experience and the sense of personal achievement and control (33). Regarding medical-obstetric dimensions, pandemic-related health care policy and maternity care practices have a negative impact on birthing women's perceptions of safety and support (64). Women who gave birth during the pandemic gave a worse rating of the quality of care they received (65).
Neonatal Intensive Care Unit Policy
The pandemic-related restrictions in NICU vary widely depending on local infection rates, availability of personal protective equipment and the structure and layout of the NICU (66). In the meanwhile, restrictions in NICU parental presence have been widely adopted (67). COVID-19-related policies that impose restrictions on components of parent presence in NICU (e.g., who can be present, how many people can be present, when they can be present) may inhibit the concept of parents as “partners in care” against the Family Centered Care (FCC)/Family Integrated Care (FIC) model concepts (66, 68–70) with adverse effects on the components of FCC, on infants, new parents and stress-related consequences in health professionals (68).
FCC/FIC—the gold standard in healthcare—has been incorporated into neonatal intensive care units. This kind of care encourages and empowers parents to play an active role in the caregiving of their child, while cooperating with staff and taking part in the decision making for their infants. Providing parents with the opportunity to exercise their role of primary caregivers brings benefits in their emotional health with short- and long-term positive implications for infant development such as: improved weight gain, increase in the incidence of breastfeeding, decreased parental stress and increased parental satisfaction rates, improved neurodevelopmental outcomes (through parent-infant skin-to-skin contact) and the development of parent-infant bonding. Further, promoting parental mental health will also support health professional's wellbeing. In this context, FCC requires that parents are not labeled as “visitors” but rather as “partners in care,” or they provide most of the care for their infant (66, 68–70).
Limiting parent presence may contribute in additionally aggravating the psychological distress of NICU family, a vulnerable population due to trauma of separation from the infant along with stress for their medical condition (54, 67, 71). The experience of parenthood in NICU has been negatively affected by the restrictions in parental presence time and physical contact of parents with their infant and additional concerns for their child's health. Parent presence due to isolation recommendations are connected with restrictions on parent-newborn contact, loss of opportunities for interaction and for provision of parental care. Restrictions may impact adversely breastfeeding and may cause additional emotional disturbance on parental pandemic-related preexisting heightened anxiety with adverse effects on parent-infant bonding and infant neurodevelopment. What is more, the restriction to fathers' access to the NICU acted as a significant obstacle to early infant-father bonding and led to loneliness and isolation by the mothers (66, 68, 71–73).
Travel restrictions due to the pandemic constituted an obstacle for family members' presence to provide postpartum social support. Thus, many women are left feeling isolated and alone, a condition that potentially contributes to risk of developing perinatal anxiety and mood disorders (74). Less support from family and friends and loneliness have been frequently reported by parents in NICU (71).
The majority of the newborns born by SARS-CoV-2 infected mothers were followed in isolation rooms in the NICU, others were monitored with a distance of 2 m away from the mother, or cared by family members in a separate room (Yekta 72). Policies that impose limitations in early neonate-mother tactile interaction may disrupt the previously established fetus-mother communication and may have detrimental effects on all domains of infant development, parental mental health and on the quality of spouses' relationship (75–83).
Early skin-to-skin care has been related to positive influences on maternal mental health and sensitivity, oxytocin levels (which facilitates mother-infant bonding with long-term effects), lower pain perception, improved self-efficacy of mothers of premature infants, growth of parental self-esteem, and higher maternal satisfaction (77, 84–86). Early skin-to-skin care has also beneficial effects for father-infant attachment scores/interactive experiences, for fathers themselves at a biochemical level, and on their mental health which can have an indirect positive impact on maternal domains (exclusive breastfeeding and care-giving-related hormones), and increases spouses' mutual understanding (87–94).
In addition, NICU staff members experience a sudden and continuous environmental stressor since they are further affected by a number of factors that seem to increase even more their psychological stress such as: moral distress when limitations beyond their control make them unable to take decisions according to their own values, the values of the patient's family, or the values of FCC; and difficulties in finding a balance between meeting the emotional needs of hospitalized infants and their families while also safeguarding their own health (52, 54, 68).
Further, it has to be emphasized that personal protection equipment wearing, virtual consultations and online antenatal education cause disruption to interpersonal communication and limit supportive touch between NICU staff and parents (52). Face-to-face psychological support of pregnant and new mothers by mental health professional is equally important as physical checks. A trusting relationship between professionals and families is a prerequisite for good quality maternal and family care. Dynamics of interpersonal communication, such as good eye contact, touch, and tone, are essential elements of care (52). Under conditions of substantial mental health burden of both healthcare workers and new parents, limitation of interpersonal engagement may aggravate even more the risk of emotional exhaustion of pregnant women/new mothers and their families.
Breastfeeding
The nutritional and physical health benefits of breastfeeding for infants and children are well-established. Accumulating research shows the long-term effects of breastfeeding on brain, cognitive and socio-emotional development of children and on mental health of mothers (95).
In the course of COVID-19 pandemic, the main scientific and public institutions (e.g., WHO, UNICEF) advice to facilitate mother-infant interactions and to support breastfeeding initiation even in cases in which a mothers has been virus infected as long as clinical conditions permit it (63, 96, 97). In the meanwhile, other institutions highlight the risk of virus transmission and recommend maternal separation precluding breastfeeding (98). So far, though emerging evidence suggests that vertical transmission is possible, there is not enough scientific evidence to unequivocally state the possibility of SARS-CoV-2 mother-infant transmission via breastmilk. However, infection transmission risk is attributed to close contact between neonate and mother with suspected, or confirmed infection during breastfeeding [see (99–101) for reviews].
Maternal mental health constitutes a core factor related to face-to-face breastfeeding support, skin-to-skin contact after birth and partner's support, all interrelated factors connected to breastfeeding, one aspect of the gold standard infant care (102–104). There is evidence that most of these factors have been affected adversely by the COVID-19 pandemic.
Regarding maternal mental health, under the pandemic condition, a limited number of studies shows that breastfeeding mothers reported that they experienced anxiety, depression, isolation, loneliness and distress for not being able to see their family during the lockdown (63, 105–107). Worries about the safety of breastfeeding were commonly mentioned but, at the same time, exclusive breastfeeding was a protective factor to maternal mental health (102, 106). Continued breastfeeding support is a key for breastfeeding success while the quality of breastfeeding support is important for both breastfeeding promotion and maternal mental health (63, 103, 108). The lack of face-to-face health services and lack/decrease of support for breastfeeding has been mentioned as one of the main concerns of breastfeeding mothers, the most common reason for breastfeeding cessation, a frequent maternal response related to feeding plans changes and as a factor that negatively impacted breastfeeding experience (59, 63, 102, 105). However, the absence of recommendations on breastfeeding support and lack of support has led to reduced compliance to the recommendations of main scientific institutions suggesting breastfeeding initiation. This may has affected adversely both maternal mental health and the rate of breastfeeding (50, 51, 109). However, mothers experience breastfeeding heterogeneously since 41.8% felt that breastfeeding was protected due to the lockdown but 27% of mothers reported a negative impact of lockdown upon their breastfeeding experience. An intense focus on feeding which made them feeling overwhelmed by the breastfeeding experience and a lack of face-to-face support were some of the barriers placed in their way (102). As for partner support, both women who delivered before and during the lockdown reported that the main source of infant feeding support is the partner (63). Mothers who reported a positive impact of the pandemic on breastfeeding mentioned greater partner support. Shared care was felt to strengthen the new parent relationship and to increase bonds between partner and baby. Mothers who reported an adverse effect of the pandemic on breastfeeding talked about the isolation they felt which had a negative impact on their wellbeing and mental health (102). Regarding the connection of skin-to-skin contact and breastfeeding, in the course of COVID-19 pandemic, there is evidence for a strong negative correlation between exclusive breastfeeding at discharge and mother-newborn separation at birth (50). The implications of early mother-neonate separation have been discussed above.
COVID-19 Pandemic, Maternal Mental Health, and Infant Development: First Results
To our knowledge, the results of a limited number of relevant studies from different regions confirm our concerns on the negative effect of adversely affected perinatal maternal stress on infant development in the pandemic condition.
In Italy, the first longitudinal study (32) that documented the short-term implications of COVID-19 pandemic-related stress on infant's temperament at 3 months, showed that infant's regulatory capacity was linked with less parenting stress and more mother-infant bonding. In Japan, mother-infant bonding was worse one month after birth among mothers who gave birth during the COVID-19 pandemic compared to those who gave birth in the same period of the previous year (110). Maternal mental health problems have been related to long-term risks for the establishment of mother-infant bonding (30). In Portugal, compared to mothers who gave birth before the pandemic, mothers of 0–12 month-old infants who gave birth during the COVID-19 pandemic presented lower levels of emotional awareness of the child and a more impaired mother-infant bonding. A more impaired mother-infant bonding was associated with higher levels of parenting stress and lower levels of mindful parenting dimensions. Maternal mental health problems may prevent maternal adoption of a mindful parenting practice (30).
An online survey, mainly in European countries, showed an acute decrease in sleep quality (which plays a crucial role in brain maturation) in 0–35-month-old infants and 36–71-month preschool children in April 2020. At two-follow up assessments (May/June 2020), this effect largely disappeared. Caregiver's stress due to the confinement was identified as the dominant factor with a negative impact on children's sleep. In the meanwhile, protective factors influencing children's sleep quality included caregiver's mindful techniques, childcare and the presence of siblings/pets (111). What is more, children (aged 0–4) with parents scoring higher on separation anxiety showed more distress after child care center reopening. There was a positive correlation between concurrent child and parental distress after reopening (29).
In Serbia, Jelicic's et al. follow up study (31) showed medium and high levels of maternal anxiety among 142 third-semester pregnant women during the COVID-19 pandemic, and a high level of perceived social support. The study showed a positive correlation between maternal trait anxiety and child's socio-emotional status at 12 months.
Lastly, there is evidence that COVID-19-related prenatal stress was significantly correlated with higher infants' SLC6A4 methylation (which occurs at the level of stress-related genomic portions). SLC6A4 methylation was negatively associated with the infants' positive affect at 3 months (2).
Possible Effects of Maternal Depression and Anxiety During the COVID-19 Pandemic on Fetal and Infant Development
There is evidence that maternal stress impacts fetal central and autonomic nervous system function (ANS) (15) and both maternal hypothalamic-pituitary-adrenocortical axis (HPA) activity and fetal HPA development (19, 20). The early structures of the developing limbic system (e.g., amygdala and hippocampus) may also be influenced by the maternal stress (21). What is more, heightened CpG-specific SLC6A4 methylation has been evidenced for infants exposed to prenatal maternal depression and stress. This is important given that heightened SLC6A4 methylation constitutes a potential biomarker of early adverse experiences (2). Prenatal stress exposure has also been related to physical health-related outcomes [(14, 18, 112–114), as cited in (20, 115, 116)].
Taken the above evidence together, it is critical that the development of fetal systems [ANS, HPA development and brain structures of the limbic system (amygdala and the hippocampus)] that have been reported to be affected by maternal prenatal stress are also potentially related factors and causes of neuropsychiatric disorders [depression, anxiety, behavioral dysfunction, attention-deficit hyperactivity disorder (ADHD), autism spectrum disorder] in children (21). Though most children are not affected by prenatal stress, partly due to differential genetic susceptibilities (117), we express our concern that the COVID-19 pandemic may contribute into a further increase in the incidence of neuropsychiatric disorders in children, and later in life, as this may be reported in the coming years.
What is more, research provides evidence that maternal depression negatively affects both infant and maternal expressive behaviors. Maternal depression disrupts all channels of early infant-mother communication and parameters of fine-grained interactive temporal coordination along with the dyad's capacity to mutually regulate the interaction (118–125). What is more, maternal depression has a negative effect on mother-infant affective and behavioral synchrony, bonding, attachment, mutual attunement and negatively affects positive enrichment activities and care for the infant, infant health and sleep, breastfeeding and its parameters (e.g., duration, timing exclusivity, satisfaction, confidence and weaning) [(22, 23, 123) for recent reviews]. On this ground, maternal depression has been linked with delays and poor infant motor, social, cognitive and language development and difficulties in emotional self-regulation. Maternal depression has been associated with short and long-term adverse consequences for mothers' physical and psychological health, partner relationships, sexuality and social relationships [see (22, 23) for recent reviews].
Recent evidence on the impact of maternal anxiety on mother-infant interactions shows that high levels of maternal state anxiety significantly predicted a lower score on the sensitivity scale (126), reduced emotional tone and increased level of non-contingent maternal comments during interaction to their infants (127). Anxious mothers present greater intrusiveness (128) and they do not adapt to the infant's moment-by-moment signals (129). Infants of anxious mothers seem less communicative, less emotional during social challenges (130) and they score less optimally on social engagement (129). Maternal anxiety has been positively associated with infant negativity and with mismatches in which infant was in positive affect and mother was in negative affect, or infant expressed negative emotion and mother was in a neutral state (131).
What is more, face masks may aggravate even more the already disrupted channels of early infant-mother face-to-face interaction. In connection to this, in relation to non-depressed mothers, depressed mothers manifest more flat and negative affect, less positive affect and increased gaze focus at the infants (132). Infants of depressed and anxious mothers are likely to encounter even fewer opportunities to observe and imitate facial expressions of emotion (133). Infants of depressed mothers need more trials and take almost twice as long to habituate to their mother's face and voice compared to infants of non-depressed mothers (134).
Discussion
We reviewed possible factors that may have affected negatively perinatal mental health through the pandemic-related restrictions. We presented the implications of adversely affected maternal emotional wellbeing on infant development.
On the basis of the above review, we would like to note the following:
1. It is our obligation to emphasize that evidence-based promotion of new family mental health during the COVID-19 pandemic is needed to be integrated within the health system at a multi-layered level in the prenatal, intrapartum, postnatal period and in infancy/early parenthood (135, 136). Protective factors, including partner/social support (137) must be taken into consideration. All healthcare providers involved with birth and NICU staff must give even more active support to new families (36, 54). What is more, maternal mental health screening during the pandemic condition has been highlighted as an essential issue (137). It is important for nurses in obstetric units to identify stressors of pregnant women in the course of prenatal care and provide resources to manage/reduce their impact. Providers in outpatient clinics should consider synchronous group prenatal telehealth care visits that may provide support for pregnant women by creating a sense of community (74).
2. High priority should be given to the preservation of family-centered care principles with emphasis on parents' presence in the NICU, parent-infant physical and emotional closeness and parental involvement in the infant's care (54). Toward this direction, while strict restrictions on parental presence were initially adopted to prevent infection spread in the NICU, recently there is a relaxation of such restrictions in favor of parent-infant contact (71). It is the responsibility of hospital systems to ensure that family-infant communication can continue to be supported in the safest manner possible (138). Current recommendations are being modified on a case-by case basis. For mothers in good clinical condition, the separation of the mother-child pair might be not recommended. The recommendations for infected neonates vary from isolated admission without caregivers to strategies adapted to the clinical situation of the infant, but with parental accompaniment (68). When parents cannot be in the NICU, it is crucial that they must be supported to see their baby via video (54). Video-technology interventions showed parental appreciation of being able to see their infant when they could not be in the NICU. Parental ability to visualize their infant reduced stress and anxiety. Videoconferencing seems to be helpful and meaningful to parents [see (72) for a full discussion]. Further, NICU systems should implement evidence-based assessment and treatment for parental distress while providing peer support for parents and voice calls [(54); see in (72) for a discussion on the benefits and the drawbacks of online support groups].
3. We are seriously concerned that the COVID-19 healthcare emergency may be “…a hidden pandemic of developmental psychopathology” [(32), p. 7]. There is an urgent need for more investment to research with the aim to evaluate the way the pandemic is affecting maternal mental health and on the impact of poor maternal mental health on young infants' developing brains. Also, there is a need to follow up these children and their families in order to mitigate the COVID-19 pandemic effects in the long-term. Health authorities and government have to treat this as a public health issue, and not as a condition with short-term effect (34, 139).
Conclusion
We highlighted the possible factors that may have affected negatively perinatal mental health through the pandemic-related restrictions. We presented the implications of adversely affected maternal emotional wellbeing on infant development. It is critical to extend with more research our understanding of the way the pandemic is affecting maternal mental health and the impact of poor maternal mental health on infant development. The implications of the adverse maternal wellbeing on infant development under the pandemic condition call for nationwide policies and evidence-based interventions. These interventions have to be integrated within the health system for prenatal and postpartum care in an effort to promote new family wellbeing and infant development. Interventions for improving perinatal maternal mental health is needed to be adapted in the “new normal” of the current situation. Maternal wellbeing and the implications of it on infant development should be priority areas to be included in COVID-19 related policy guidelines.
Author Contributions
TK and EH contributed equally to the writing of this review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^These studies compared mental health between pregnant and non-pregnant women, or mental health of pregnant women during the pandemic with pre-pandemic period, or they provided quantitative evidence of anxiety and depression symptomatology of pregnant women in different countries.
2. ^The Olson Circumplex Model explains family function and the way family systems will change in response to a crisis. It includes two dimensions: family cohesion and family adaptability. “Family cohesion is defined as the emotional bond that family members have toward one another” [(37), p.145]. “Family adaptability is the amount of change in its leadership, role relationships and relationship rules” [(37), p. 147]. The four levels of cohesion range from disengaged (very low) to separated (low to moderate) to connected (moderate to high) to enmeshed (very high). The four levels of adaptability range from rigid (very low) to structured (low to moderate) to flexible (moderate to high) to chaotic (very high). The balanced levels of cohesion (separated and connected) and adaptability (structured and flexible) are considered as optimal family functioning while the unbalanced (disengaged or enmeshed/ rigid or chaotic) are seen as problematic for relationships in the long-term. Balanced types of couples and families will have more positive communication compared to unbalanced systems) (37).
3. ^According to Bodenmann's Systemic-Transactional Model of dyadic coping (48), dyadic stress is observed when partners are affected by a stressor and the source of stress is defined as common. In order to cope against dyadic stress, partners initiate a dyadic coping process. A dyadic coping process is the interplay between both partners' stress, their coping reactions along with proper common responses to the dyadic stressor. Stress communication is constitutes the first step in the dyadic coping process (1).
References
1. Donato S, Parise M, Pagani AF, Lanz M, Regalia C, Rosnati R, et al. Together against COVID-19 concerns: the role of the dyadic coping process for partners; psychological well-being during the pandemic. Front Psychol. (2021) 11:578395. doi: 10.3389/fpsyg.2020.578395
2. Provenzi L, Mambretti F, Villa M, Crumi S, Citterio A, Bertazzoli E, et al. Hidden pandemic: COVID-19-related stress, SLC6A4 methylation, and infants' temperament at 3 months. Sci Rep. (2021) 11:15658. doi: 10.1038/s41598-021-95053-z
3. UNICEF. Pregnant mothers and babies born during COVID-19 pandemic threatened by strained health systems and disruptions in services. (2020). Available online at: https://www.unicef.org/press-releases/pregnant-mothers-and-babies-born-during-covid-19-pandemic-threatened-strained-health (accessed July 01, 2020).
4. Crimmins Easterlin M, Crimmins EM, Finch CE. Will prenatal exposure to SARS-CoV-2 define a birth cohort with accelerated aging in the century ahead? J Dev Orig Health Dis. (2020) 12:683–7. doi: 10.1017/S204017442000104X
5. McDonald AJ, Mew EJ, Hawley NL, Lowe SR. Anticipating the long-term neurodevelopmental impact of the Covid-19 pandemic on newborns and infants: a call for research and preventive policy. J Affect Disord Rep. (2021) 6:100213. doi: 10.1016/j.jadr.2021.100213
6. Engle PL. Maternal mental health: program and policy implications. Am J Clin Nutr. (2009) 89:963S−6S. doi: 10.3945/ajcn.2008.26692G
7. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. (2020) 19:313–27. doi: 10.1002/wps.20769
8. Preis H, Mahaffey B, Heiselman C, Lobel M. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. (2020) 266:113348. doi: 10.1016/j.socscimed.2020.113348
9. Dagklis T, Tsakiridis I, Mamopoulos A, Athanasiadis A, Pearson R, Papazisis G. Impact of the Covid-19 lockdown on antenatal mental health in Greece. Psychiatry Clin Neurosci. (2020) 74:602–31. doi: 10.1111/pcn.13135
10. Dong H, Hu R, Lu C, Huang D, Cui D, Huang G, et al. Investigation on the mental health status of pregnant women in China during the pandemic of Covid-19. Arch Gynecol Obstet. (2021) 303:463–9. doi: 10.1007/s00404-020-05805-x
11. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. (2020) 277:5–13. doi: 10.1016/j.jad.2020.07.126
12. López-Morales H, Del Valle MV, Canet-Juric L, Andrés ML, Galli JI, Poó F, et al. Mental health of pregnant women during the Covid-19 pandemic: a longitudinal study. Psychiatry Res. (2021) 295:113567. doi: 10.1016/j.psychres.2020.113567
13. Ravaldi C, Ricca V, Wilson A, Homer C, Vannacci A. Previous psychopathology predicted severe COVID-19 concern, anxiety, and PTSD symptoms in pregnant women during ‘lockdown' in Italy. Arch Womens Ment Health. (2020) 23:783–6. doi: 10.1007/s00737-020-01086-0
14. Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Hum Dev. (2009) 85:65–70. doi: 10.1016/j.earlhumdev.2008.07.002
15. Kinsella MT, Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin Obstet Gynecol. (2009) 52:425–40. doi: 10.1097/GRF.0b013e3181b52df1
16. Mortazavi F, Ghardashi F. The lived experiences of pregnant women during COVID-19 pandemic: a descriptive phenomenological study. BMC Childbirth. (2021) 21:193. doi: 10.1186/s12884-021-03691-y
17. Porter E, Lewis AJ, Watson SJ, Galbally M. Perinatal maternal mental health and infant socio-emotional development: a growth curve analysis using the MPEWS cohort. Infant Behav Dev. (2019) 57:101336. doi: 10.1016/j.infbeh.2019.101336
18. Class QA, Khashan AS, Lichtenstein P, Långström N, D'Onofrio BM. Maternal stress and infant mortality: the importance of the preconception period. Psychol Sci. (2013) 24:1309–16. doi: 10.1177/0956797612468010
19. Coussons-Read ME. The psychoneuroimmunology of stress in pregnancy. Curr Dir Psychol Sci. (2012) 21:323–8. doi: 10.1177/0963721412453720
20. Coussons-Read M.-E. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet Med. (2013) 6:52–7. doi: 10.1177/1753495x12473751
21. Mulkey SB, du Plessis AJ. Autonomic system development and its impact on neuropsychiatric outcome. Pediatr Res. (2019) 85:120–6 doi: 10.1038/s41390-018-0155-0
22. Slomian J, Honvo G, Emonts P, Reginster JY, Bruyère O. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes [published correction appears in Womens health (Lond). 2019 Jan-Dec;15:1745506519854864]. Womens Health. (2019) 15:1745506519844044. doi: 10.1177/1745506519844044
23. Sliwerski A, Kossakowska K, Jarecka K, Switalska J, Bielawska-Batorowicz E. The effect of maternal depression on infant attachment: a systematic review. Int J Environ Res Public Health. (2020) 17:2675. doi: 10.3390/ijerph17082675
24. Gori M, Schiatti L, Amadeo MB. Masking emotions: face masks impair how we read emotions. Front Psychol. (2021) 12:669432. doi: 10.3389/fpsyg.2021.669432
25. Kastendieck T, Zillmer S, Hess U. (Un)mask yourself! Effects of face masks on facial mimicry and emotion perception during the Covid-19 pandemic. Cogn Emot. (2021) 36:59–69. doi: 10.1080/02699931.2021.1950639
26. Campbell R. The processing of audio-visual speech: empirical and neural bases. Philos Trans R Soc Lond B Biol Sci. (2008) 363:1001–10. doi: 10.1098/rstb.2007.2155
27. Yi H, Pingsterhaus A, Song W. Effects of wearing face masks while using different speaking styles in noise on speech intelligibility during the COVID-19 pandemic. Front Psychol. (2021) 12:682677. doi: 10.3389/fpsyg.2021.682677
28. Havy M, Foroud A, Fais L, Werker JF. The role of auditory and visual speech in word learning at 18 months and in adulthood. Child Dev. (2017) 8:2043–59. doi: 10.1111/cdev.12715
29. De Vet SM, Vrijhof CI, van der Veek SMC, Pieplenbosch JM, van Bakel HJA, Vermeer HJ. Child care in times of Covid-19: predictors of distress in dutch children and parents when re-entering center-based child care after a 2-month lockdown. Front Psychol. (2021) 12:718898. doi: 10.3389/fpsyg.2021.718898
30. Fernandes DV, Canavarro MC, Moreira H. Postpartum during COVID-19 pandemic: Potuguese mothers; mental health, mindful parenting, mother-infant bonding. J Clin Psychol. (2021) 77:1997–2010. doi: 10.1002/jclp.23130
31. Jeličic L, Sovilj M, Bogavac I, Drobnjak A, Gouni O, Kazmierczak M, et al. The impact of maternal anxiety on early child development during the Covid-19 pandemic. Front Psychol. (2021) 12:792053. doi: 10.3389/fpsyg.2021.792053
32. Provenzi L, Grumi S, Altieri L, Bensi G, Betazzoli E, Biasucci G, et al. Prenatal maternal stress during the Covid-19 pandemic and infant regulatory capacity at 3 months: a longitudinal study. Dev Psychopathol. (2021) 2:1–9. doi: 10.1017/S0954579421000766
33. Rodríguez-Almagro J, Hernández-Martínez A, Rodríguez-Almagro D, Quirós-García JM, Martínez-Galiano JM, Gómez-Salgado J. Women's perceptions of living a traumatic childbirth experience and factors related to a birth experience. Int J Environ Res Public Health. (2019) 16:1654. doi: 10.3390/ijerph16091654
34. Thapa SB, Mainali A, Schwank SE, Acharya G. Maternal mental health in the time of the COVID-19 pandemic. Spec Edit Acta Obstetr Gynecol Scand. (2020) 99:817–8. doi: 10.1111/aogs.13894
35. Molgora S, Accordini M. Motherhood in the time of coronavirus: the impact of the pandemic emergence on expectant and postpartum women's psychological well-being. Front Psychol. (2020) 11:567155. doi: 10.3389/fpsyg.2020.567155
36. Lista G, Bresesti I. Fatherhood during the COVID-19 pandemic: an unexpected turnaround. Early Hum Dev. (2020) 144:105048. doi: 10.1016/j.earlhumdev.2020.105048
37. Olson DH. Circumplex model of marital and family systems. J Fam Ther. (2000) 22:144–67. doi: 10.1111/1467-6427.00144
38. Xie M, Wang X, Zhang J, Wang Y. Alternation in the psychologic status and family environment of pregnant womens before and during the COVID-19 pandemic. Int J Gynecol Obstet. (2020) 153:71–5. doi: 10.1002/ijgo.13575
39. Daches S, Vine V, Layendecker KM, George CJ, Kovacs M. Family functioning as perceived by parents and young offspring at high and low risk for depression. J Affect Disord. (2018) 226:355–60. doi: 10.1016/j.jad.2017.09.031
40. Bate J, Pham P, Borelli JL. Be my safe haven: parent-child relationships and emotional health during COVID-19. J Pediatr Psychol. (2021) 46:624–34. doi: 10.1093/jpepsy/jsab046
41. Chung G, Lanier P, Wong PYJ. Mediating effects of parental stress on harsh parenting during the Coronavirus (COVID-19) pandemic in Singapore. J Fam Violence. (2021) 2:1–12. doi: 10.1007/s10896-020-00200-1
42. Feinberg M, Mogle JA, Lee JK, Tornello SL, Hostetler ML, Cifelli JA, et al. Impact of the Covid-19 pandemic on parent, child and family functioning. Family Proc. (2021) 61:361–74. doi: 10.1111/famp.12649
43. Gadermann AC, Thomson KC, Richardson CG, Gagné M, McAuliffe C, Hirani S, et al. Examining the impact of the COVID-19 pandemic on family mental health in Canada: findings from a national cross-sectional study. BMJ. (2021) 11:e042871. doi: 10.1136/bmjopen-2020-042871
44. Gunther-Bel C, Vilaregut A, Carratala E, Torras-Garat S, Perez-Testor C. A mixed-method study of individual, couple and parental functioning during the state – regulated covid-19 lockdown in Spain. Fam Process. (2020) 59:1060–79. doi: 10.1111/famp.12585
45. Li M, Li L, Wu F, Cao Y, Zhang H, Li X, et al. Perceived family adaptability and cohesion and depressive symptoms: a comparison of adolescents and parents during COVID-19 pandemic. J Affect Disord. (2021) 287:255–60. doi: 10.1016/j.jad.2021.03.048
46. Westrupp EM, Bennett C, Berkowitz T, Youssef GJ, Toumbourou JW, Tucker R, et al. Child, parent, and family mental health and functioning in Australis during COVID-19: comparison to pre-pandemic data. Euro Child Adolesc Psychiatry. (2021) 21:1–14. doi: 10.1007/s00787-021-01861-z
47. Trougakos JP, Chawla N, McCarthy JM. Working in a pandemic: exploring the impact of Covid-19 anxiety on work, family, health outcomes. J Appl Psychol. (2020) 105:1234–45. doi: 10.1037/apl0000739
48. Bodenmann G. Dyadic coping and its significance for marital functioning. In: Revenson T, Kayser K, Bodenmann G, editors. Couples Coping With Stress: Emerging Perspectives on Dyadic Coping. American Psychological Association (2005). p. 33–50. doi: 10.1037/11031-002
49. Aranda Z, Binde T, Tashman K, Tadikonda A, Mawindo B, Boley EJ, et al. Disruptions in maternal health service use during the COVID-19 pandemic in 2020: experiences from 37 health facilities in low-income and middle-income countries. BMJ Glob Health. (2022) 7:e007247. doi: 10.1136/bmjgh-2021-007247
50. Del Río R Pérez ED Marín Gabriel MA the Neo-COVID-19 Research Group. Multi-centre study showed reduced compliance with the World Health Organization recommendations on exclusive breastfeeding during COVID-19. Acta Paediatr. (2020). 110:935–6. doi: 10.1111/apa.15642
51. Gonçalves-Ferri WA, Pereira-Cellini FM, Coca K, Aragon DC, Nader P, Lyra JC, et al. The impact of coronavirus outbreak on breastfeeding guidelines among Brazilian hospitals and maternity services: a cross- sectional study. Int Breastfeed J. (2021) 16:30. doi: 10.1186/s13006-021-00377-1
52. Horsch A, Lalor J, Downe S. Moral and mental health challenges faced by maternity staff during the Covid-19 pandemic. Psychol Trauma Theory Res Pract Policy. (2020) 12:S141–2. doi: 10.1037/tra0000629
53. Iqbal A, Burrin C, Aydin E, Beardsall K, Wong H, Austin T. Generation COVID-19 – should the fetus be worried? Acta Paediatr. (2020) 110:759–64. doi: 10.1111/apa.15693
54. Cena L, Biban P, Janos J, Lavelli M, Langfus J, Tsai A, et al. The collateral impact of Covid-19 emergency on neonatal intensive care units and family-centered care: challenges and opportunities. Front Psychol. (2021) 12:630594. doi: 10.3389/fpsyg.2021.630594
55. Caparros-Gonzalez RA, Alderdice F. The Covid-19 pandemic and perinatal mental health. J Reprod Infant Psychol. (2020) 38:223–5. doi: 10.1080/02646838.2020.1786910
56. Morelius E, Theodorsson E, Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. (2005) 116:1105–13. doi: 10.1542/peds.2004-2440
57. Carquillat P, Vendittelli F, Perneger T, Guittler MJ. Development of a questionnaire for assessing the childbirth experience (QACE). BMC Preg Childbirth. (2017) 17:279. doi: 10.1186/s12884-017-1462-x
58. Ravaldi C, Wilson A, Ricca V, Homer C, Vannacci A. Pregnant women voice their concerns and birth expectations during the COVID-19 pandemic in Italy. Women Birth. (2020) 34:335–43. doi: 10.1016/j.wombi.2020.07.002
59. DeYoung SE, Magnum M. Pregnancy, birthing and postpartum experiences during COVID-19 in the United States. Front Sociol. (2021) 6:611212. doi: 10.3389/fsoc.2021.611212
60. Mayopoulos GA, Ein-Dor T, Dishy GA, Nandru R, Chan S, Hanley LE, et al. COVID-19 is associated with traumatic childbirth and subsequent mother-infant bonding problems. J Affect Disord. (2021) 282:122–5. doi: 10.1016/j.jad.2020.12.101
61. Mollard E, Kupzyk K. Birth satisfaction during the early months of the Covid-19 pandemic in the United States. MCN. (2022) 47:6–12. doi: 10.1097/NMC.0000000000000777
62. Barbosa-Leiker C, Smith LC, Crespi EJ, Brooks O, Burduli E, Ranjo S, et al. Stressors, coping, and resources needed during the COVID-19 pandemic in a sample of perinatal women. BMC Preg Childbirth. (2021) 21:171. doi: 10.1186/s12884-021-03665-0
63. Vazquez-Vazquez A, Dib S, Rougeaux E, Wells JC, Fewtrell MS. The impact of the Covid-19 lockdown on the experiences and feeding practices of new mothers in the UK: preliminary data from the COVID-19 new mum study. Appetite. (2021) 156:104985. doi: 10.1016/j.appet.2020.104985
64. Mollard E, Wittmaack A. Experiences of women who gave birth in US hospitals during the Covid-10 pandemic. J Pat Exp. (2021) 8:1–6. doi: 10.1177/2374373520981492
65. Mariño-Narvaez C, Puertas-Gonzalez JA, Romero-Gonzalez B, Isabel M, Peralta-Ramirez MI. Giving birth during the COVID-19 pandemic: the impact on birth satisfaction and postpartum depression. Int J Gynecol Obstetr. (2021) 153:83–8. doi: 10.1002/ijgo.13565
66. Muniraman H, Ali M, Cawley P, Hillyer J, Heathcote A, Ponnusamy V, et al. Parental perceptions of the impact of neonatal unit visitation policies during Covid-19 pandemic. BMJ Pediatr Open. (2020) 4:e000899. doi: 10.1136/bmjpo-2020-000899
67. Mahoney AD, White RD, Velasquez A, Barrett TS, Clark RH, Ahmad KA. Impact of restrictions on parental presence in neonatal intensive care units related to coronavirus disease 2019. J Perinatol. (2020) 40:36–46. doi: 10.1038/s41372-020-0753-7
68. Arnaez J, Montes MT, Herranz-Rubia N, Garcia-Alix A. The impact of the current SARS-CoV-2 pandemic on neonatal Care. Front Psychol. (2020) 8:247. doi: 10.3389/fped.2020.00247
69. Griffin T. A family-centered visitation policy in the neonatal intensive care unit that welcomes parents as partners. J Perinatal Neonatal Nurs. (2013) 27:160–5. doi: 10.1097/JPN.0b013e3182907f26
70. O'Brien K, Bracht M, Macdonell K, McBride T, Robson K, O'Leary L, et al. A pilot cohort analytic study of family integrated care in a canadian neonatal intensive care unit. BMC Pregn Childbirth. (2013) 13 (Suppl. 1):512. doi: 10.1186/1471-2393-13-S1-S12
71. Polloni L, Cavallin F, Lolli E, Schiavo R, Bua M, Volpe B, et al. Psychological wellbeing of parents with infants admitted to the neontal intensive care unit during SARS-CoV-2 pandemic. Children. (2021) 8:755. doi: 10.3390/children8090755
72. Murray PD, Swanson JR. Visitation restrictions: is it right and how do we support families in the NICU during COVID-19? J Perinatol. (2020) 40:1576–81. doi: 10.1038/s41372-020-00781-1
73. Gunes AO, Dincer E, Karadag N, Topcuoglu S, Karatekin G. Effects of COVID-19 pandemic on breastfeeding rates in a neonatal intensive care unit. J Perinat Med. (2021) 49:500–5. doi: 10.1515/jpm-2020-0462
74. Goyal D, Selix NW. Impact of the Covid-19 on maternal mental health. MCN. (2021) 46:103–9. doi: 10.1097/NMC.0000000000000692
75. Rolland Souza AS, Cavalcante E, Macedo CA, Freitas SG, Figueiredo A, Alves SA, et al. The impact of maternal touch of the abdomen on cardiotocography fetal patterns. Brain Behav. (2019) 9:e01345. doi: 10.1002/brb3.1345
76. Agudelo S, Gamboa O, Rodríguez F, Cala S, Gualdón N, Obando E, et al. The effect of skin-to-skin contact at birth, early versus immediate, on the duration of exclusive human lactancy in full-term newborns treated at the Clinica Universidad de La Sabana: study protocol for a randomized clinical trial. Trials. (2016) 17:521. doi: 10.1186/s13063-016-1587-7
77. Anderzén-Carlsson A, Lamy ZC, Tingvall M, Eriksson M. Parental experiences of providing skin-to-skin care to their newborn infant - Part 2: a qualitative meta-synthesis. Int J Qual Stud Health Well Being. (2014) 9:24907. doi: 10.3402/qhw.v9.24907
78. Cecchini M, Baroni E, Di Vito C, Piccolo F, Aceto P, Lai C. Effects of different types of contingent tactile stimulation on crying, smiling and sleep in newborns: an observational study. Dev Psychobiol. (2012) 62:591–99. doi: 10.1002/dev.21054
79. Field T. Touch for socioemotional and physical well-being: a review. Dev Rev. (2010) 30:367–83. doi: 10.1016/j.dr.2011.01.001
80. Karimi FZ, Miri HH, Khadivzadeh T, Maleki-Saghooni N. The effect of mother-infant skin-to-skin contact immediately after birth on exclusive breastfeeding: a systematic review and meta-analysis. J Turk German Gynecol Assoc. (2020) 21:46–56. doi: 10.4274/jtgga.galenos.2019.2018.0138
81. Marx V, Nagy E. Fetal behavioural responses to maternal voice and touch. PLoS ONE. (2015) 10:e0129118. doi: 10.1371/journal.pone.0129118
82. Mercuri M, Stack DM, Trojan S, Giusti L, Morandi F, Mantis I, et al. Mothers' and fathers' early tactile contact behaviors during triadic and dyadic parent-infant interactions immediately after birth and at 3-months postpartum: implications for early care behaviors and intervention. Infant Behav Dev. (2019) 57:101347. doi: 10.1016/j.infbeh.2019.101347
83. Severi FM, Prattichizzo D, Casarosa E, Barbagli F, Ferretti C, Altomare A, et al. Virtual fetal touch through a haptic interface decreases maternal anxiety and salivary cortisol. J Soc Gynecol Invest. (2005) 12:37–40. doi: 10.1016/j.jsgi.2004.07.006
84. Bystrova K, Ivanova V, Edhborg M, Matthiesen AS, Ransjö-Arvidson B, Mukhamedrakhimov R, et al. Early contact versus separation: effects on mother-infant interaction one year later. Birth. (2009) 36:97–109. doi: 10.1111/j.1523-536X.2009.00307.x
85. Gupta N, Deieri A, Hills E, Banerjee J. Systematic review confirmed the benefits of early skni-to-skin contact but highlighted lack of studies on very and extremely preterm infants. Acta Paediatr. (2021) 110:2310–15. doi: 10.1111/apa.15913
86. Scime NV, Gavarkovs AG, Chaput KH. The effect of skin-to-skin care on postpartum depression among mothers of preterm and low birthweight infants: a systematic review and meta-analysis. J Affect Disord. (2019) 253:376–84. doi: 10.1016/j.jad.2019.04.101
87. Blomqvist YT, Rubertsson C, Kylberg E, Jöreskog K, Nyqvist KH. Kangaroo mother care helps fathers of preterm infants gain confidence in the paternal role. J Adv Nurs. (2011) 68:1988–96. doi: 10.1111/j.1365-2648.2011.05886.x
88. Filippa M, Saliba S, Esseily R, Gratier M, Grandjean D, Kuhn P. Systematic review shows the benefits of involving the fathers of preterm infants in early interventions in neonatal intensive care units. Acta Paediatr. (2021) 110:2509–20. doi: 10.1111/apa.15961
89. Gettler LT, Kuo PX, Sarma MS, Trumble BC, Lefever JEB, Braungart-Rieker JM. Fathers' oxytocin responses to first holding their newborns: interactions with testosterone reactivity to predict later parenting behavior and father-infant bonds. Dev Psychobiol. (2021) 63:1384–98. doi: 10.1002/dev.22121
90. Morelius E, Ortenstrand A, Theodorsson E, Frostell A. A randomized trial of continuous skin-to-skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression and breastfeeding. Early Hum Dev. (2015) 91:63–70. doi: 10.1016/j.earlhumdev.2014.12.005
91. Toprak FU, Erenel AS. Impact of kangaroo care after caesarean section on paternal-infant attachment and involvement at 12 months: a longitudinal study in Turkey. Health Soc Care the Commun. (2020) 29:1502–10. doi: 10.1111/hsc.13210
92. Varela N, Tessier R, Tarabulsy G, Pierce T. Cortisol and blood pressure levels decreased in fathers during the first hour of skin-to-skin contact with their premature babies. Acta Paediatr. (2018) 107:628–32. doi: 10.1111/apa.14184
93. Vittner D, McGrath J, Robinson J, Lawhon G, Cusson R, Eisenfeld L, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biol Res Nurs. (2018) 20:54–62. doi: 10.1177/1099800417735633
94. Vogl JL, Dunne EC, Liu C, Bradley A, Rwei A, Lonergan EK, et al. Kangaroo father care: a pilot feasibility study of physiologic, biologic, and psychosocial measures to capture the effects of father-infant and mother-infant skin-to-skin contact in the neonatal intensive care unit. Dev Psychobiol. (2021) 63:1521–33. doi: 10.1002/dev.22100
95. Krol KM, Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2018) 61:977–85. doi: 10.1007/s00103-018-2769-0
96. Giuliani C, Li Volsi P, Brun E, Chiambretti A, Giandalia A, Tonutti L, et al. Breastfeeding during the COVID-19 pandemic: suggestions on behalf of woman study group of AMD. Diabetes Res Clin Pract. (2020) 165:108239. doi: 10.1016/j.diabres.2020.108239
97. Pramana C, Suwantoro J, Sumarni N, Kumalasari MLF, Selasih Putri Isnawati H, Supinganto A, et al. Breastfeeding in postpartum women infected with Covid-19. Int J Pharm Res. (2020) 12:1857–62. doi: 10.31838/ijpr/2020.12.04.265
98. Rollins N, Minckas N, Jehan F, Lodha R, Raiten D, Thorne C, et al. A public health approach for deciding policy on infant feeding and mother–infant contact in the context of COVID-19. Lancet Glob Health. (2021) 9:e552–7. doi: 10.1016/S2214-109X(20)30538-6
99. Centeno-Tablante E, Medina-Rivera M, Finkelstein JL, Rayco-Solon P, Garcia-Casal MN, Rogers L, et al. Transmission of SARS-CoV-2 through breastmilk and breastfeeding: A living systematic review. Ann N Y Acad Sci. (2021) 1484:32–54. doi: 10.1111/nyas.14477
100. Rodrigues C, Baía I, Domingues R, Barros H. Pregnancy and breastfeeding during COVID-19 pandemic: a systematic review of published pregnancy cases. Front Public Health. (2020) 8:558144. doi: 10.3389/fpubh.2020.558144
101. Singh V, Trigunait P, Majumdar S, Ganeshan R, Sahu R. Managing pregnancy in COVID-19 pandemic: a review article. J Fam Med Prim Care. (2020) 9:5468–73. doi: 10.4103/jfmpc.jfmpc_950_20
102. Brown A, Shenker N. Experiences of breastfeeding during COVID-19: lessons for future practical and emotional support. Mater Child Nutr. (2020) 17:e13088. doi: 10.1111/mcn.13088
103. Chaput KH, Nettel-Aguirre A, Musto R, Adair CE, Tough SC. Breastfeeding difficulties and supports and risk of postpartum depression in a cohort ofwomen who have given birth in Calgary: a prospective cohort study. CMAJ Open. (2016) 4:E103. doi: 10.9778/cmajo.20150009
104. Mannion CA, Hobbs AJ, McDonald SW, Tough SC. Maternal perceptions of partner support during breastfeeding. Int Breastfeed J. (2013) 4:8. doi: 10.1186/1746-4358-8-4
105. Hull N, Kam RL, Gribble KD. Providing breastfeeding support during the COVID-19 pandemic: concerns of mothers who contacted the Australian Breastfeeding Association. medRvix [Preprint]. (2020). doi: 10.1101/2020.07.18.20152256
106. Zanardo V, Tortora D, Guerrini P, Garani G, Severino L, Soldera G, et al. Infant feeding initiation practices in the context of COVID-19 lockdown. Early Hum Dev. (2021) 152:105286. doi: 10.1016/j.earlhumdev.2020.105286
107. Wei Z, Gao MY, Fewtrell M, Wells J, Yu JY. Maternal mental health and well-being during the COVID-19 pandemic in Beijing, China. World J Pediatr. (2021) 17:280–9. doi: 10.1007/s12519-021-00439-8
108. Pérez-Escamilla R. Impact of the baby-friendly hospital initiative on breastfeeding and child health outcomes: a systematic review. Mater Child Nutr. (2016) 12:402–17. doi: 10.1111/mcn.12294
109. Oncel MY, Akin IM, Kanburoglu MK, Tayman C, Coskun S, Narter F, et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with COVID-19 by Turkish neonatal society. Eur J Pediatr. (2021) 180:733–42. doi: 10.1007/s00431-020-03767-5
110. Suzuki S. Psychological status of postpartum women under the COVID-19 pandemic in Japan. J Matern Fetal Neonatal Med. (2020) 3:1–2. doi: 10.1080/14767058.2020.1814250
111. Markovic A, Muhlematter C, Beaugrand M, Camos V, Kurth S. Severe effects of the Covid-19 confinement on young children's sleep: a longitudinal study identifying risk and protective factors. J Sleep Res. (2021) 30:e13314. doi: 10.1111/jsr.13314
112. Entringer S, Kumsta R, Nelson EL, Hellhammer DH, Wadhwa PD, Wust S. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev. Psychobiol. (2008) 50:579–87. doi: 10.1002/dev.20316
113. Entringer S, Wust S, Kumsta R, Layes IM, Nelson EL, Hellhammer DH, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am. J. Obstet. Gynecol. (2008) 199:e491–7. doi: 10.1016/j.ajog.2008.03.006
114. Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr Opin Clin Nutr Metab Care. (2013) 16:320–7. doi: 10.1097/MCO.0b013e32835e8d80
115. Entringer S, Buss C, Wadhwa P. Prenatal stress, development, health and disease risk: a psychobiological perspective – 2015 curt richter award paper. Psychoneuroendocrinology. (2015) 62:366–75. doi: 10.1016/j.psyneuen.2015.08.019
116. Kwon EJ, Kim YJ. What is fetal programming?: a lifetime health is under the control of in utero health. Obstetr Gynecol Sci. (2017) 60:506–19. doi: 10.5468/ogs.2017.60.6.506
117. Glover V, Ahmed-Salim Y, Capron L. Maternal anxiety, depression, and stress during pregnancy: Effects on the fetus and the child and underlying mechanisms. In: Nadja RN, Kisilevsky BS, editors. Fetal Development: Research on Brain and Behavior, Environmental Influences and Emerging Technologies. Cham: Springer International Publishing (2016). p. 213–27. doi: 10.1007/978-3-319-22023-9_12
118. Whiffen VE, Gotlib IH. Infants of postpartum depressed mothers: temperament and cognitive status. J Abnorm Psychol. (1989) 98:274–9. doi: 10.1037/0021-843X.98.3.274
119. Arteche A, Joormann J, Harvey A, Craske M, Gotlib IH, Lehtonen A, et al. The effects of postnatal maternal depression and anxiety on the processing of infant faces. J Affect Disord. (2011) 133:197–203. doi: 10.1016/j.jad.2011.04.015
120. Tronick E, Reck C. Infants of depressed mothers. Harv Rev Psychiatry. (2009) 17:147–56. doi: 10.1080/10673220902899714
121. Hakanen H, Flykt M, Sinervä E, Nolvi S, Kataja EL, Pelto J, et al. How maternal pre- and postnatal symptoms of depression and anxiety affect ear;y mother-infant interaction? J Affect Disord. (2019) 257:83–90. doi: 10.1016/j.jad.2019.06.048
122. Defelipe RP, de Resende BD, Vinicius Frayze DV, Raad Bussab V. S. Postpartum depression in high-risk Brazilian women: psychosocial predictors and effects on maternal vocalization. Early Child Dev Care. (2019) 189:1480–93. doi: 10.1080/03004430.2017.1389918
123. Lam-Cassettari C, Kohlhoff J. Effect of maternal depression on infant-directed speech to prelinguistic infants: implications for language development. PLoS ONE. (2020) 15:e0236787. doi: 10.1371/journal.pone.0236787
124. Bettes BA. Maternal depression and motherese: temporal and intonational features. Child Dev. (1988) 59:1089–96. doi: 10.2307/1130275
125. Herrera E, Reissland N, Shepherd J. Maternal touch and maternal child-directed speech: effects of depressed mood in the postnatal period. J Affect Disord. (2004) 81:29–39. doi: 10.1016/j.jad.2003.07.001
126. Ierardi E, Ferro V, Trovato A, Tambelli R, Crugnola CR. Maternal and paternal depression and anxiety: their relationship with mother-infant interactions at 3 months. Arch Womens Ment Health. (2019) 22:527–33. doi: 10.1007/s00737-018-0919-x
127. Nicol-Harper R, Harvey AG, Stein A. Interactions between mothers and infants: impact of maternal anxiety. Infant Behav Dev. (2007) 30:161–7. doi: 10.1016/j.infbeh.2006.08.005
128. Pelizzon Dib E, Pereira Padovani FH, Benzaquen Perosa G. Mother-child interaction: implications of chronic maternal anxiety and depression. Psicologia. (2019) 32:10. doi: 10.1186/s41155-019-0123-6
129. Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, stress reactivity. J Am Acad Child Adolesc Psychiatry. (2009) 48:919–27. doi: 10.1097/CHI.0b013e3181b21651
130. Kaitz M, Maytal HR, Devor N, Bergman L, Mankuta D. Maternal anxiety, mother-infant interactions and infants' response to challenge. Infant Behav Dev. (2010) 33:136–48. doi: 10.1016/j.infbeh.2009.12.003
131. Riva Crugnola C, Ierardi E, Ferro V, Gallucci M, Parodi C, Astengo M. Mother-infant emotion regulation at three months: the role of maternal anxiety, depression and parenting stress. Psychopathology. (2016) 49:285–94. doi: 10.1159/000446811
132. Braarud HC, Skotheim S, Høie K, Markhus MW, Kjellevold M, Graff IE, et al. Affective facial expression in sub-clinically depressed and non-depressed mothers during contingent and non-contingent face-to-face interactions with their infants. Infant Behav Dev. (2017) 48:98–104. doi: 10.1016/j.infbeh.2017.05.004
133. Meiser S, Zietlow AL, Reck C, Träuble B. The impact of postpartum depression and anxiety disorders on children's processing of facial emotional expressions at pre-school age. Arch Womens Ment Health. (2015) 18:707–16. doi: 10.1007/s00737-015-0519-y
134. Pickens J, Field T. Facial expressions and vagal tone in infants of depressed and non-depressed mothers. Early Dev Parent. (1995) 4:83–9. doi: 10.1002/edp.2430040205
135. Choi KR, Records K, Low LK, Alhusen JL, Kenner C, Bloch JR, et al. Promotion of maternal-infant mental health and trauma-informed care during the COVID-19 pandemic. J Obstetr Gynecol Neonatal Nurs. (2020) 49:409–15. doi: 10.1016/j.jogn.2020.07.004
136. Shidhaye R, Madhivanan P, Shidhaye P, Krupp K. An integrated approach to improve maternal mental health and well-being during the COVID-19 crisis. Front Psychiatry. (2020) 11:598746. doi: 10.3389/fpsyt.2020.598746
137. Suwalska J, Napierała M, Bogdański P, Łojko D, Wszołek K, Suchowiak S, et al. Perinatal mental health during Covid-19 pandemic: an integrative review and implications for clinical practice. J Clin Med. (2021) 10:2406. doi: 10.3390/jcm10112406
138. Carter BS, Willis T, Knackstedt A. Neonatal family-centered care in a pandemic. J Perinatol. (2021) 41:1177–9. doi: 10.1038/s41372-021-00976-0
139. de Figueiredo CS, Sandre PC, Portugal LCL, Mázala-de-Oliveira T, da Silva Chagas L, Raony Í, et al. COVID-19 pandemic impact on children and adolescents' mental health: biological, environmental, social factors. Progr Neuropsychopharmacol Biol Psychiatry. (2021) 106:1101171. doi: 10.1016/j.pnpbp.2020.110171
Keywords: COVID-19 pandemic, maternal mental health, neonate/infant development, family functioning, maternal health care policy, birth experience, NICU, breastfeeding
Citation: Kokkinaki T and Hatzidaki E (2022) COVID-19 Pandemic-Related Restrictions: Factors That May Affect Perinatal Maternal Mental Health and Implications for Infant Development. Front. Pediatr. 10:846627. doi: 10.3389/fped.2022.846627
Received: 31 December 2021; Accepted: 30 March 2022;
Published: 12 May 2022.
Edited by:
Carole Ann Kenner, The College of New Jersey, United StatesReviewed by:
Carol Jaeger, The Ohio State University, United StatesNorsham Juliana, Universiti Sains Islam Malaysia, Malaysia
Copyright © 2022 Kokkinaki and Hatzidaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theano Kokkinaki, a29ra2luYWtpQHVvYy5ncg==
†Theano Kokkinaki orcid.org/0000-0002-5136-4619
Eleftheria Hatzidaki orcid.org/0000-0001-6256-3309
 Theano Kokkinaki
Theano Kokkinaki Eleftheria Hatzidaki
Eleftheria Hatzidaki