- 1Division of Neonatal-Perinatal Medicine, Department of Pediatrics, Medical University of South Carolina, Charleston, SC, United States
- 2Division of Pediatric Nephrology, Department of Pediatrics, Medical University of South Carolina, Charleston, SC, United States
Acute kidney injury (AKI) is a common occurrence in the neonatal intensive care unit (NICU). In recent years, our knowledge of the incidence and impact of neonatal AKI on outcomes has expanded exponentially. Neonatal AKI has been shown to be associated with adverse outcomes including increased length of mechanical ventilation, prolonged length of stay, and rise in mortality. There has also been increasing work suggesting that neonates with AKI are at higher risk of chronic kidney disease (CKD). In the past, AKI had been defined multiple ways. The utilization of the neonatal modified Kidney Disease: Improving Global Outcomes (KDIGO) criteria as the standard definition for neonatal AKI in research and clinical care has driven the advances in our understanding of neonatal AKI over the last 10 years. This definition has allowed researchers and clinicians to better understand the incidence, risk factors, and outcomes associated with neonatal AKI across populations through a multitude of single-center studies and the seminal, multicenter Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study. As the impacts of neonatal AKI have become clear, a shift in efforts toward identifying those at highest risk, protocolizing AKI surveillance, improving prevention and diagnosis, and expanding kidney support therapy (KST) for neonates has occurred. These efforts also include improving risk stratification (identifying high risk populations, including those with nephrotoxic medication exposure) and diagnostics (novel biomarkers and diagnostic tools). Recent work has also shown that the targeted use of methylxanthines may prevent AKI in a variety of high-risk populations. One of the most exciting developments in neonatal AKI is the advancement in technology to provide KST to neonates with severe AKI. In this comprehensive review we will provide an overview of recent work and advances in the field of neonatal AKI. This will include a detailed review of (1) the definition of neonatal AKI, (2) the epidemiology, risk factors, and outcomes associated with neonatal AKI, (3) improvements in risk stratification and diagnostics, (4) mitigation and treatment, (5) advancements in the provision of KST to neonates, and (6) the incidence and risk of subsequent CKD.
Introduction
Acute kidney injury (AKI) occurs commonly in the neonatal intensive care unit (NICU) and is associated with increase morbidity and mortality. Furthermore, those who develop neonatal AKI may be at increased risk for the development of chronic kidney disease (CKD). With ongoing study, the definition of neonatal AKI has evolved and been standardized, improving our ability to quantify and describe the epidemiology and outcomes associated with neonatal AKI. Here we will review the definition of neonatal AKI, the epidemiology, risk factors, and outcomes associated with neonatal AKI, strategies to improve risk stratification, diagnostics, mitigation, and treatment, recent advances in kidney support therapy (KST) for neonates, and the incidence and risk for CKD following AKI in the NICU.
Definition
Over the last 20 years the field of critical care nephrology and the study of AKI have been driven by the utilization of agreed upon standardized definitions of AKI. In 2012, Jetton et al. first put forth a modification of the Acute Kidney Injury Network AKI definition and subsequently the Kidney Disease: Improving Global Outcomes (KDIGO) definition was developed and modified for neonates (1). This definition has since been adopted as the neonatal modified KDIGO definition and has served as the consensus definition that should be utilized in research and clinically to diagnose and stage AKI (Table 1) (2, 3). This definition was agreed upon as the consensus definition of neonatal AKI at a multidisciplinary 2013 National Institutes of Health workshop dedicated to neonatal AKI (2). The neonatal, modified KDIGO definition represents the first consensus definition of neonatal AKI and has been validated in the international multicenter, Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study (1).
While the development and utilization of the neonatal modified KDIGO definition has driven the advancements in the field over the last 5–10 years, it has also become clear that there are short-comings of the current definition that will warrant future refinement. The most obvious issues relate to utilizing the current thresholds of changes in serum creatinine (SCr) to define neonatal AKI. A recent secondary analysis of the AWAKEN study shows that the ideal definition of neonatal AKI may not be the same across gestational age (GA) groups (4). Similarly, work by Gupta et al. interrogated the impact of the failure of SCr to drop over the first postnatal week, which does not qualify as AKI by the current neonatal modified KDIGO definition (5). In this study of 106 neonates with hypoxic ischemic encephalopathy (HIE), a failure of SCr to drop by 50% or fall below 0.6 mg/dL was associated with adverse outcomes. Additionally, the optimal definition of oliguria that defines AKI in neonates and the incorporation of novel biomarkers (discussed later) into the definitions of AKI remain active areas of research need in neonatal AKI. As with all definitions in medicine, the definition of neonatal AKI represents an iterative process that will be refined over time with future consensus conferences. At this time, the neonatal modified KDIGO definition remains the gold-standard to define neonatal AKI for clinical and research purposes.
Epidemiology, Risk Factors, and Outcomes of Neonatal AKI
To understand the unique epidemiology, risk factors, and outcomes associated with neonatal AKI, it is important to appreciate the nuances of neonatal renal development and physiology. Neonatal kidneys are particularly susceptible to hypoperfusion and ischemia secondary to the dynamic changes in renal blood flow that occur postnatally. These perfusion alterations are driven by changes in the renin-angiotensin system and prostaglandins making neonates susceptible to medications such as angiotensin-converting enzyme (ACE) inhibitors and non-steroidal anti-inflammatory drugs (NSAIDS).
In utero nephron development begins at 5 weeks’ gestation and continues to 34–36 weeks of gestation (6). The majority of nephrogenesis occurs late in pregnancy with up to 60% of nephrogenesis occurring in the third trimester (6, 7). While final nephron number is highly variable, each additional kilogram (kg) increase in birth weight (BW) confers nearly 200,000 additional nephrons (8–10). As a result, premature birth and low birth weight (LBW) both alter final nephron number and development increasing the risk for AKI and subsequent CKD (11–18). The neonatal kidney continues to mature over the first two postnatal years as renal vascular resistance falls, cardiac output to the kidney increases, and the adult-level glomerular filtration rate (GFR) is established (19).
Over the last decade, our understanding of the epidemiology of neonatal AKI has expanded exponentially with the utilization of the neonatal modified KDIGO consensus definition of neonatal AKI. In the largest study to date, the AWAKEN study, investigators evaluated the incidence and impact of neonatal AKI in an international, multicenter cohort study of 2022 neonates admitted to 24 NICUs over a 3-month period. In this study, incidence of AKI was 30%, with variation by GA (≥22 to <29 weeks’: 48%; ≥29 to <36 weeks’: 18%; ≥36 weeks’: 37%) (1). This study showed that AKI in neonates was independently associated with increased mortality (9.7 vs. 1.4%; p < 0.001; adjusted odds ratio (aOR) 4.6 [95% confidence interval (CI) 2.5–8.3; p < 0.0001] and length of stay [adjusted parameter estimate 8.8 days (95% CI 6.1–11.5); p < 0.0001] after adjustment for 16 variables.
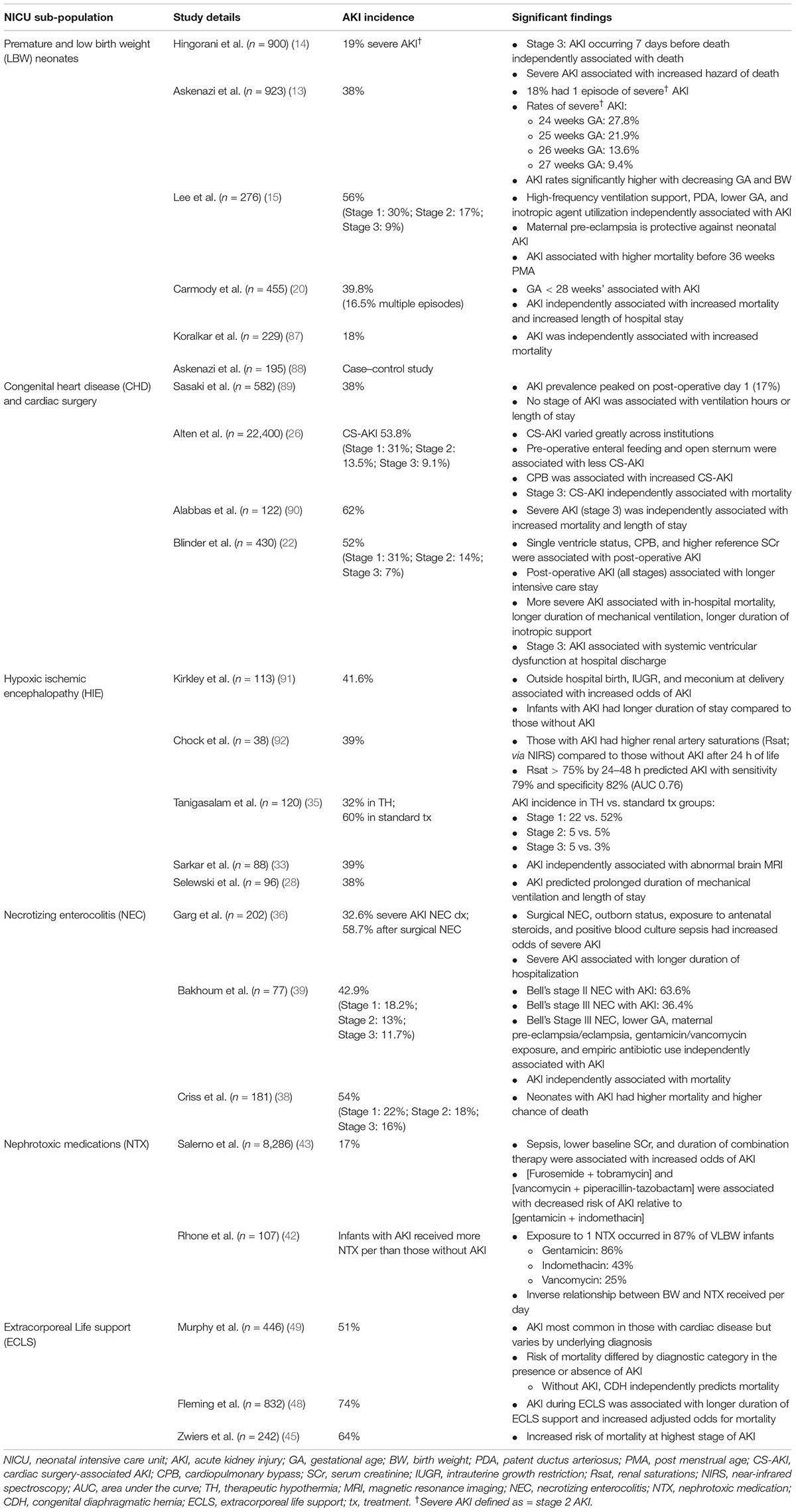
Though the incidence of neonatal AKI is high among NICU patients, specific sub-populations are at particularly high risk and warrant further discussion (Table 2). Several of these populations are discussed in detail below.
Prematurity and Low Birth Weight
There has been a considerable amount of work highlighting the high incidence and impact of AKI in premature infants. This work has confirmed a significant association between LBW, early GA, and AKI (11–16). In a single retrospective study of 455 very LBW neonates (VLBW, i.e., ≤1,500 g), Carmody et al. showed that AKI occurred in 40% of neonates with 16.5% of infants experiencing multiple episodes of AKI (20). In this cohort, AKI was associated with increased mortality (aOR, 4.0; 95% CI 1.4–11.5) and length of stay (11.7 days; 95% CI 5.1–18.4 days). This group also noted that AKI was only present as a diagnosis in 13.5% of discharges, highlighting the need for continued education of providers. More recently, a secondary analysis of the Preterm Erythropoietin Neuroprotection Trial evaluated the incidence and impact of neonatal AKI in a cohort of 932 extremely low GA neonates (13). In this study the incidence of AKI was 38% and differed significantly by GA, with AKI rates being significantly higher with decreasing GA and BW. In 2021, Wu et al. published a meta-analysis of 50 articles including over 10,000 premature and LBW neonates evaluating the incidence and impact of neonatal AKI (21). This study showed a pooled incidence of AKI of 25% (95% CI 20–30%), and those with AKI had significantly higher odds of mortality (OR 7.1; 95% CI 5.9–8.6; p < 0.01).
Congenital Heart Disease
Neonates and infants undergoing cardiac surgery are among the highest risk patients for developing AKI (22, 23). This results from the complex interaction of a multitude of risk factors including but not limited to low cardiac output, single ventricle physiology, ischemic-reperfusion injury, fluid overload (FO), and nephrotoxic medication (NTX) receipt (17, 24, 25). Despite these factors, the incidence and impact of AKI in this population remains varied in the literature. In a single center retrospective study of 430 infants < 90 days of age undergoing surgical correction, AKI occurred in 52% of the cohort (22). In this study, stage 2 and 3 AKI were associated with increased mortality. More recently the Neonatal and Pediatric Heart and Renal Outcomes in Newborns (NEPHRON) collaborative evaluated the incidence and impact of AKI in a multicenter (22 centers) cohort of neonates (n = 2,240) undergoing congenital heart surgery (26). The overall incidence of AKI in this study was 53.8%, and the prevalence of AKI peaked on postoperative day 1; only stage 3 AKI was associated with increased mortality (aOR 2.44; 95% CI 1.3–4.61). A novel finding in this study is the incidence of AKI amongst infants undergoing cardiac surgery varied by institution from 27 to 86%, and a significant amount of AKI was transient in nature. The authors suggest the current definition of neonatal AKI may need to be adjusted to capture truly meaningful phenotypes of cardiac surgery-associated AKI in neonates.
Hypoxic Ischemic Encephalopathy
Neonates with perinatal asphyxia, also known as HIE, often develop multiorgan failure, which impacts virtually every organ system. AKI has been shown to occur commonly in infants with HIE with an incidence ranging from 38 to 72% (27–32). In a single center study of 96 neonates with HIE undergoing therapeutic hypothermia, AKI occurred in 38% of neonates and independently predicted prolonged duration of mechanical ventilation and NICU stay (28). In a follow-up study, AKI during therapeutic hypothermia was found to be associated with the development of abnormal magnetic resonance imaging (MRI) findings at 7–10 postnatal days (33). The impact of therapeutic hypothermia on the incidence of AKI in HIE remains unclear, with trials and retrospective studies demonstrating conflicting results (34, 35).
Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) occurs in 2–5% of NICU admissions and represents a significant cause of morbidity and mortality. Infants with NEC have multiple risk factors for AKI including sepsis, hemodynamic instability, NTX receipt, systemic inflammation, increased intrabdominal pressure, and frequently prematurity (36, 37). In a single center retrospective study of 202 neonates with NEC, the overall incidence of severe AKI was 32.6% and in those requiring surgical intervention, the incidence was 58.7% (36). This study also showed that severe AKI was associated with a longer length of stay {124 days [interquartile range (IQR) 88–187] vs. 82 days (42–126); p < 0.001}. Criss et al. reported a similar incidence of any AKI of 54% in a single center cohort of 181 neonates with NEC (38). Studies in neonates with NEC have consistently shown that AKI occurs commonly and is associated with increased morbidity and mortality (11, 38, 39).
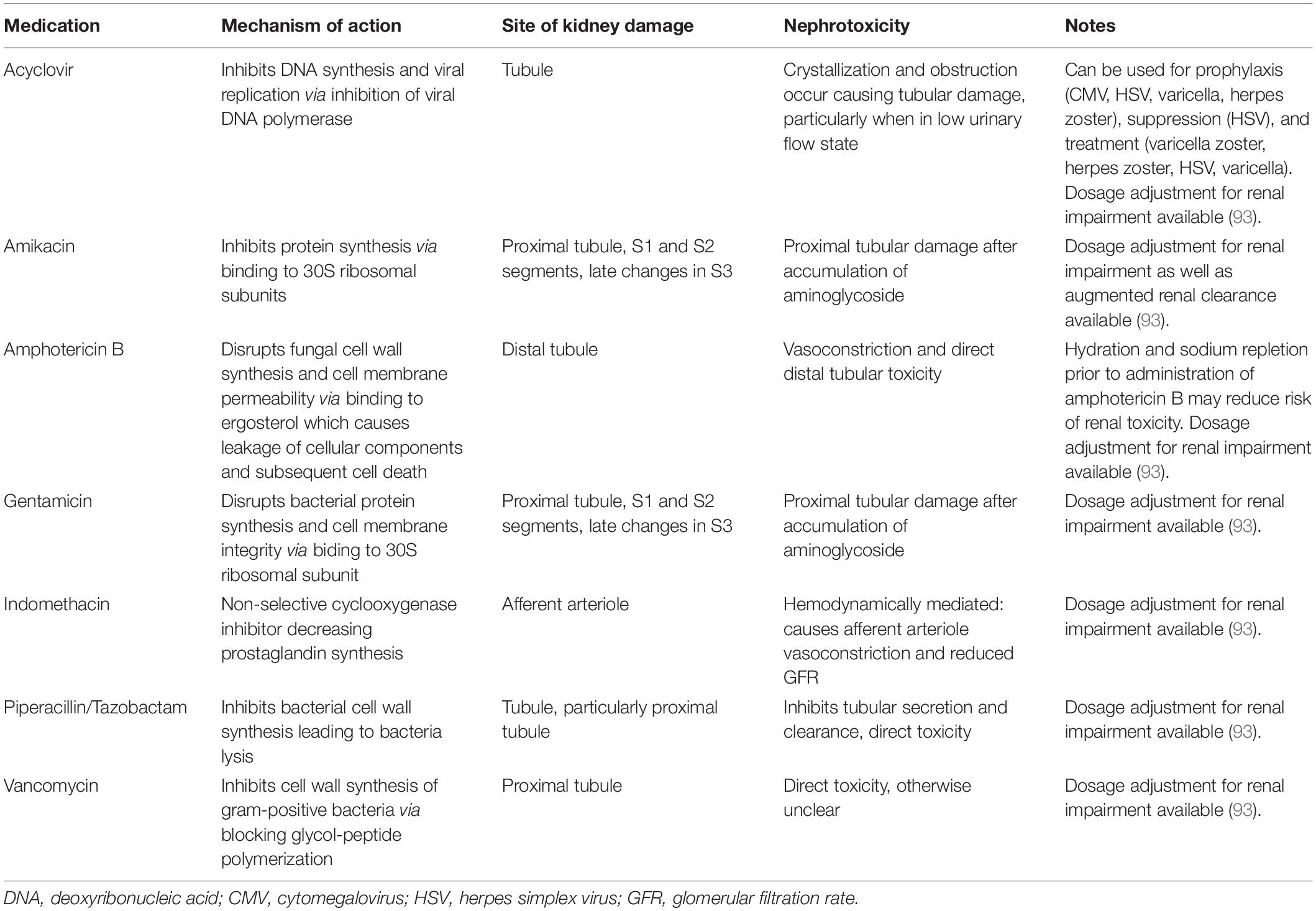
Nephrotoxic Medications
Critically ill neonates are frequently exposed to NTX during their NICU stay. Table 3 outlines the mechanism and nephrotoxicity of commonly encountered NTX in neonates. In older children NTX exposure has been shown to be a potentially modifiable cause of AKI (40, 41). As a result, there has been an increased interest in understanding the epidemiology of NTX exposures in neonates. Rhone et al. evaluated 107 VLBW neonates and described the epidemiology of NTX exposure (42). In this study 87% of all neonates received at least one NTX, with gentamicin (86%), indomethacin (43%) and vancomycin (25%) most commonly administered. Neonates in this study received NTX for a median 8 days (IQR 3–21) with a significant difference in mean days of NTX exposure in those with AKI compared to those without AKI (23.9 vs. 9.9 days; p < 0.001).
Since the publication of this seminal work, there have been further studies evaluating NTX exposure in neonates. In 2021, Salerno et al. evaluated the impact of combinations of NTX in a database including 268 NICUs (43). In this study of 8,286 neonates exposed to NTX, the incidence of AKI was 17%, and increased duration of NTX exposure was associated with increased risk of AKI. An interesting finding in this study is that 23,399 neonates were exposed to NTX during the study period, but 15,113 neonates were excluded from the analysis because they did not have 2 SCr measured. This highlights the potential need for improved surveillance strategies, such as Nephrotoxic Injury Negated by Just-in-time Action (NINJA) (discussed below) (44).
Extracorporeal Life Support
Neonates treated with extracorporeal life support (ECLS) are the sickest patients in the NICU and are at increased risk of AKI for a multitude of reasons including hypotension, underlying disease etiology, systemic inflammation, hemolysis and hemoglobinuria, micro-emboli, non-pulsatile flow, and NTX exposure. The incidence of AKI in this population is as high as 70% (45–48). In a retrospective single-center study of 242 neonates treated with ECLS, Zwiers et al. found the incidence of AKI was 64% with the most severe stage of AKI associated with increased mortality (45). In a recent report from the multicenter Kidney Interventions During Membrane Oxygenation (KIDMO) study group about 446 neonates treated with ECLS, the incidence of AKI in the overall cohort was 51%, but the AKI incidence varied by underlying diagnosis (cardiac 68%, congenital diaphragmatic hernia 38%, respiratory 33%) (49). The association of AKI with outcomes varied significantly by underlying diagnosis as well.
Impact of Acute Kidney Injury on Other Organ Systems
Across medicine, the paradigm surrounding AKI has shifted from an isolated disease process impacting one organ to a multisystem disease process that impacts distant organs. Recent work in neonates has begun to highlight this. Studies in neonates report associations between neonatal AKI and intraventricular hemorrhage (IVH) and abnormal brain MRI (33, 50). In a secondary evaluation of 866 premature neonates from the AWAKEN study, AKI was associated with increased odds of IVH (aOR, 95% CI 1.04–2.56) (50). Multiple recent single center studies have confirmed this association of AKI with IVH in premature infants (51, 52). AKI during HIE has also been shown to be associated with increased odds of abnormal brain MRI findings at 7–10 postnatal days (33). In a recent 2-year follow-up study of 101 neonates with HIE, AKI was associated with an unfavorable outcome (death or disability according to Griffiths Mental Development Scales) at 24 months (53).
Recent work has also begun to establish a link between AKI in neonates and respiratory outcomes including length of mechanical ventilation and bronchopulmonary dysplasia (BPD). Starr et al. reported findings from the AWAKEN study which showed that neonates 29–32 weeks’ GA with AKI were more likely to have a poor composite outcome of moderate to severe BPD and/or death (aOR 4.2, 95% CI: 2.1–8.6; p < 0.001) (54). This confirmed previous single center work by Askenazi et al. which showed a higher risk of oxygen requirement or of dying at 28 days of life [relative risk (RR) 1.7, 95% CI 1.2–2.4; p < 0.002] in a single center cohort of 122 premature infants with AKI (55).
Advances in Diagnosis, Prevention and Mitigation, and Treatment of Sequelae
There are currently no proven treatments for established AKI. Despite multiple clinical trials across critical care nephrology, no therapeutic interventions have been shown to be effective in patients once AKI has occurred. As a result, efforts to advance the field have shifted toward improved diagnostics, prevention and mitigation strategies, and treatment of sequelae in neonatal AKI.
Diagnostics
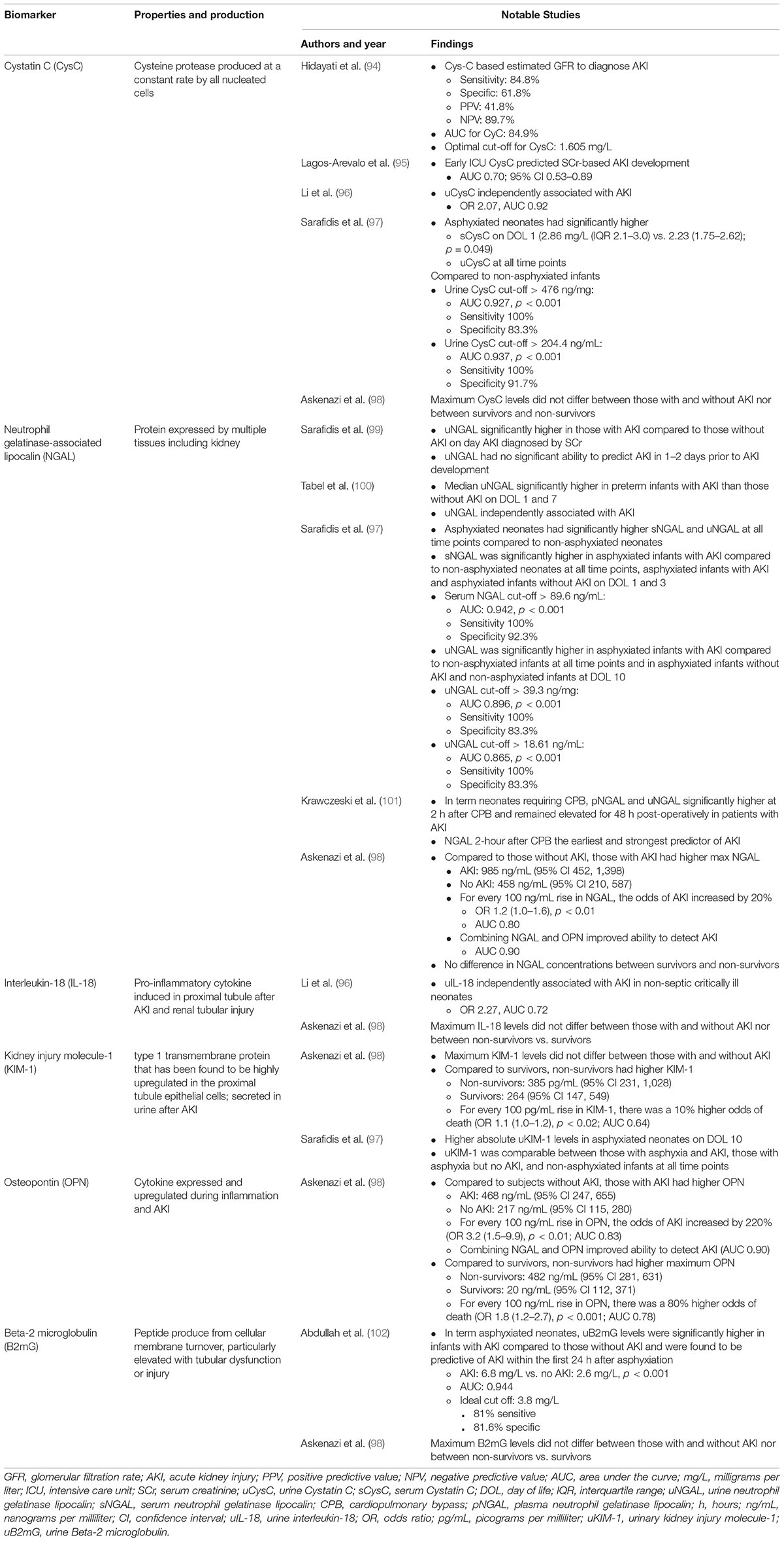
SCr is currently the “gold-standard” of biomarkers to identify AKI, but there are a multitude of challenges with SCr as a biomarker. Most importantly, SCr serves as a measure of kidney function, rather than injury (56). Furthermore, SCr is a delayed (up to 48–72 h) marker of kidney function, which may not change until 25–50% of the kidney function has been lost (6, 57). These impediments taken together may explain the challenges faced with the development of successful clinical trials and interventions in AKI. Efforts to detect AKI earlier have led to the development of novel biomarkers that lead to the timely diagnosis of AKI, improved clinical trials, and improved outcomes.
These novel biomarkers were identified and developed initially in high-risk populations such as those undergoing cardiac surgery, where the incidence of AKI is high and the timing of the insult is known. Many of these biomarkers show promise in the early and accurate diagnosis of neonatal AKI (Table 4). Neutrophil gelatinase associated lipocalin (NGAL) is perhaps the most studied novel biomarker in neonates and as described in Table 4, may predict AKI earlier than changes in SCr in a variety of neonatal populations. Studies to validate its use are ongoing, and consensus on how best to utilize NGAL is lacking. Understanding how to best utilize these novel biomarkers in clinical practice is the subject of ongoing research.
Prevention and Mitigation
Risk Stratification
A critical step in improving outcomes in AKI is the early identification of populations that are at the highest risk for the development of AKI. The concept of risk stratification is an active area of research across critical care nephrology and is embodied by the concept of “renal angina.” In pediatric patients, the “Renal Angina Index” has been developed based on clinical risk factors and signs of injury. This work has parlayed into thoughtful utilization of biomarkers to identify patients early on that are at the highest risk of developing severe AKI (58). Recently, a similar scoring system has been developed in a multicenter cohort of critically ill neonates in India termed the “STARZ” study (59). Utilization of this scoring system allowed for the successful prediction of AKI in the first 7 postnatal days. Further research is needed to develop and validate such scoring tools in other neonatal populations.
Nephrotoxic Medication
The contribution of NTX receipt to the incidence of AKI has been increasingly recognized among hospitalized children. This led to the development of the NINJA study designed to mitigate NTX-associated AKI in older children. This was initially studied as a single center experience and validated in a multicenter study in hospitalized children (40, 41). This strategy utilizes the electronic medical record to identify patients at high risk of nephrotoxic AKI (based on receipt of intravenous aminoglycosides ≥3 days or ≥3 NTX given concurrently) and subsequently trigger kidney function monitoring with daily SCr. Stoops et al. has extended this work to critically ill neonates in the “Baby NINJA” study (44). This study identified neonates at high risk of nephrotoxic AKI (defined as ≥3 NTX within 24 h or ≥4 calendar days of an intravenous aminoglycoside) and triggered daily SCr measurement until 2 days after end of exposure or end of AKI. This study showed implementation was feasible and associated with improved AKI metrics including reduced NTX exposure, reduced nephrotoxic AKI, and reduced AKI intensity.
Methylxanthine Therapy: Theophylline and Caffeine
Methylxanthines are adenosine-receptor antagonists. In high risk populations methylxanthines have been shown to prevent the development of AKI by preventing adenosine driven pre-glomerular vasoconstriction and post-glomerular vasodilation (60).
Theophylline has been extensively studied in neonates with HIE. There are now nine randomized controlled trials in term neonates with HIE comparing a single dose of theophylline (5–8 mg/kg) vs. placebo (60–67). These studies have consistently shown that theophylline is safe and reduces AKI rates, protects the renal tubule, and improves fluid balance, GFR and urine output. The current KDIGO clinical practice guidelines “suggest that a single dose of theophylline may be given in neonates with severe perinatal asphyxia who are at high risk for AKI” (3). To date there have not been any studies evaluating the impact of theophylline in neonates undergoing therapeutic hypothermia.
In premature neonates, caffeine is often utilized to prevent or treat apnea of prematurity. Carmody et al. first evaluated the impact of caffeine administration during the first postnatal week on the incidence of AKI in a single center cohort of 140 VLBW neonates (68). This study showed that caffeine exposure in the first postnatal week was associated with decreased odds of AKI (aOR 0.22, 95% CI 0.07–0.75; p = 0.02). These findings were subsequently confirmed in a secondary analysis of the AWAKEN study (69). More recently, caffeine has been shown to be associated with lower incidence of AKI in neonates with NEC (70).
Treatment of Sequelae: Kidney Support Therapy
The indications for KST in neonates are similar to those in older children and include uremia, electrolyte abnormalities, metabolic syndromes, inability to provide adequate nutrition, and the pathologic state of FO. The two modalities of KST commonly utilized in neonates are peritoneal dialysis (PD) and continuous kidney support therapy (CKST). The choice between these two modalities is often driven by clinical scenario, institutional expertise, and available resources. PD is generally less resource intensive and may be performed with the placement of a chronic catheter or a temporary catheter. A detailed discussion of the utilization of PD in AKI has recently been published by the International Society for Peritoneal Dialysis (71).
In recent years there have been extraordinary advances in the ability to provide CKST to critically ill neonates. Prior to this time, CKST was provided utilizing machines and filters that were designed for adults, and significant hemodynamic instability was common and often prohibitive. More recently, smaller filters such as the Prismaflex HF-20 (Baxter Healthcare Corporation, Deerfield, IL; extracorporeal volume 60 mL) have been developed which can be utilized to provide CKST in neonates. One of the most exciting advancements has been the development of CKST devices designed specifically for utilization in neonates. In 2014, Ronco et al. first reported the use of the Cardio-Renal Pediatric Dialysis Emergency Machine (CARPEDIEM®; Bellco Medtronic, Mirandola, Italy) device (72). Since, then, similar devices include the Nidus® (Allmed, London, England) and the Aquadex® (CHF Solutions, Eden Prarie, MN) have been developed with circuit volumes less than half of that of other available CKST circuits (73, 74). These devices are specifically designed for neonatal patients to decrease hemodynamic instability and improve CKST delivery and tolerance.
Long-Term Follow Up
In the US, a 21% increase in preterm birth rates was noted from 1980 to 2000 (75–77). Survival rates have similarly improved with those born as early as 25 weeks’ GA now having a >80% chance of survival (78). With these improved survival rates, risks for long-term complications of prematurity, including develop of CKD after AKI, have increased and warrant follow-up. CKD is increasingly prevalent, particularly in infants born <2.5 kg, presenting as decreased renal volume, hypertension (HTN), and/or microalbuminuria (78).
Data from both animal and human models suggest AKI likely leads to permanent kidney damage (78–83). In a review of eight longitudinal studies from 1978 to 2014 examining long-term kidney outcomes following neonatal AKI, rates of CKD in survivors of neonatal AKI were as high at 66% (78). In a follow-up study of AKI in VLBW infants at median age of 5 years, children with a history of AKI had a higher risk of kidney dysfunction than those who never had AKI (65 vs. 14%, RR 4.5 (1.2–17.1); p = 0.01) (84). Subjects with kidney dysfunction were more likely to have had a higher stage of AKI, msore episodes of AKI, higher peak SCr, and more days with SCr >1 mg/dl. In a long term follow-up study of extremely premature infants with significant AKI (SCr > 2.0 mg/dl), Abitbol et al. found that at median follow up of 6.6 years, 85% of patients had either a reduced GFR or an elevated urine protein:creatinine ratio (PCR); a SCr > 0.6 mg/dl and urine PCR > 0.6 mg/mg at 1 year of age were most predictive of CKD progression (85). They also found an association with body mass index (BMI) > 85th percentile at 3 years of age and increased risk of kidney dysfunction.
Although current evidence of significant risk of CKD following neonatal AKI is largely based on small observational studies, it is expert opinion that all neonates with an identified AKI episode should have longitudinal follow-up (78). KDIGO guidelines recommend patients with a history of AKI have a CKD evaluation 3 months after their AKI event (3). Aside from this and recommendations from the American Academy of Pediatrics to begin blood pressure monitoring earlier in infants born LBW (prior to the typical 3 years of age in infants born at term), there are currently no evidence-based guidelines governing who should follow neonates after AKI or how frequently they should be screened for HTN and CKD (86). Chaturvedi et al. recommends screening of all patients with history of AKI for HTN and albuminuria at least annually, with more invasive testing (e.g., SCr) recommended for patients at higher risk (e.g., more severe AKI, KDIGO stages 2 and 3) (78). Counseling should also be performed regarding healthy weight and lifestyle choices given the association between elevated BMI and kidney dysfunction. These recommendations underscore the importance of proper AKI diagnosis in the neonatal period to identify patients in need of follow-up prior to discharge from the NICU.
Conclusive Results
Neonatal AKI is prevalent, particularly in high risk populations, including those born prematurely or with LBW, those with congenital heart disease, HIE, NEC, and in neonates who receive NTX or require ECLS. With ever-increasing study and the utilization of the expert-endorsed, modified, neonatal KDIGO criteria, the consequences of neonatal AKI are becoming clear. Identification of those infants at highest risk for AKI using protocolized surveillance and novel biomarkers is an area of active study. Avoidance of NTX and treatment of specific, at-risk populations with methylxanthines may improve AKI rates or mitigate AKI that has already occurred. With a lack of specific treatments currently available, prevention and prompt diagnosis are key. Emerging evidence suggests innovative KST technologies may improve survival and that long-term follow-up is necessary given the risk of HTN and potentially CKD after neonatal AKI.
Author Contributions
DS, CC, ATP, and HS each developed the abstract, developed the outline, and wrote specific sections of the manuscript. All authors edited the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AKI, acute kidney injury; NICU, neonatal intensive care unit; CKD, chronic kidney disease; KST, kidney support therapy; KDIGO, Kidney Disease: Improving Global Outcomes; AWAKEN, Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates; SCr, serum creatinine; GA, gestational age; HIE, hypoxic ischemic encephalopathy; ACE, angiotensin converting enzyme; NSAIDs, non-steroidal anti-inflammatory drugs; kg, kilograms; BW, birth weight; LBW, low birth weight; GFR, glomerular filtration rate; aOR, adjusted odds ratio; CI, confidence interval; VLBW, very low birth weight; FO, fluid overload; NTX, nephrotoxic medication; MRI, magnetic resonance imaging; NEC, necrotizing enterocolitis; IQR, interquartile range; NINJA, Nephrotoxic Injury Negated by Just-in-time Action; ECLS, extracoporeal life support; KIDMO, Kidney Intervention during Extracorporeal Membrane Oxygenation; IVH, intraventricular hemorrhage; BPD, bronchopulmonary dysplasia; RR, relative risk; NGAL, neutrophil gelatinase associated lipocalin; PD, peritoneal dialysis; CKST, continuous kidney support therapy; PCR, protein-to-creatinine ratio; BMI, body mass index; HTN, hypertension; tx, treatment.
References
1. Jetton J, Boohaker LJ, Sethi S, Wazir S, Rohatgi S, Soranno D, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolsc Health. (2017) 1:184–94. doi: 10.1016/S2352-4642(17)30069-X
2. Zappitelli M, Ambalavanan N, Askenazi D, Moxey-Mims M, Kimmel P, Star R, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res. (2017) 82:569–73. doi: 10.1038/pr.2017.136
3. Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. (2012) 2:1–138.
4. Askenazi D, Abitbol C, Boohaker L, Griffin R, Raina R, Dower J, et al. Optimizing the AKI definition during first postnatal week using assessment of worldwide acute kidney injury epidemiology in neonates (AWAKEN) cohort. Pediatr Res. (2019) 85:329–38. doi: 10.1038/s41390-018-0249-8
5. Gupta C, Massaro AN, Ray P. A new approach to define acute kidney injury in term newborns with hypoxic ischemic encephalopathy. Pediatr Nephrol. (2016) 31:1167–78. doi: 10.1007/s00467-016-3317-5
6. Charlton J, Guillet R. Neonatal acute kidney injury: diagnosis, exposures, and long-term outcomes. Neoreviews. (2018) 19:e322–36. doi: 10.1542/neo.19-6-e322
7. Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, et al. Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine. (2018) 27:275–83. doi: 10.1016/j.ebiom.2017.12.016
8. Douglas-Denton RM, McNamara BJ, Hoy WE, Hughson MD, Bertram JF. Does nephron number matter in the development of kidney disease? Ethin Dis. (2006) 16:S2–40.
9. Trnka P, Hiatt MJ, Tarantal A, Matsell D. Congenital urinary tract obstruction: defining markers of developmental kidney injury. Pediatr Res. (2012) 72:446–54. doi: 10.1038/pr.2012.113
10. Manalich R, Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. (2000) 58:770–3. doi: 10.1046/j.1523-1755.2000.00225.x
11. Hu Q, Li SJ, Chen Q, Chen H, Li Q, Wang M. Risk factors for acute kidney injury in critically ill neonates: a systematic review and meta-analysis. Front Pediatr. (2021) 9:666507. doi: 10.3389/fped.2021.666507
12. Perico N, Askenazi D, Cortinovis M, Remuzzi G. Maternal and environmental risk factors for neonatal AKI and its long-term consequences. Nat Rev Nephrol. (2018) 14:688–703. doi: 10.1038/s41581-018-0054-y
13. Askenazi D, Heagerty PJ, Schmicker R, Griffin R, Brophy P, Juul S, et al. Prevalence of acute kidney injury (AKI) in extremely low gestaional age neonates (ELGAN). Pediatr Nephrol. (2020) 2020:9. doi: 10.1007/s00467-020-04563-x
14. Hingorani S, Schmicker RH, Brophy P, Heagerty P, Juul S, Goldstein S, et al. Severe acute kidney injury and mortality in extremely low gestational age neonates. Clin J Am Soc Nephrol. (2021) 16:862–9. doi: 10.2215/CJN.18841220
15. Lee C, Chan OW, Lai M, Hsu K, Wu T, Lim W, et al. Incidence and outcomes of acute kidney injury in extremely low birth weight infants. PLoS One. (2017) 12:e0187764. doi: 10.1371/journal.pone.0187764
16. Mian A, Guillet R, Ruck L, Wang H, Schwartz G. Acute kidney injury in premature, very low birth weight infants. J Pediatr Intensive Care. (2016) 5:69–78. doi: 10.1055/s-0035-1564797
17. Luyckx V, Perico N, Somaschini M, Manfellotto D, Valensise H, Cetin I, et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the low birth weight and nephron number working group. Lancet. (2017) 390:424–8. doi: 10.1016/S0140-6736(17)30576-7
18. Gurusinghe S, Tambay A, Sethna C. Developmental origins and nephron endowment in hypertension. Front Pediatr. (2017) 5:151. doi: 10.3389/fped.2017.00151
19. Sadler TW. Urogenital system. 14th ed. In: Thomas W, Sadler-Redmond SL, Tosney K, Byrne J, Imseis H, editors. Langman’s Medical Embryology. Philadelphia, PA: Wolters Kluwer (2019). p. 14.
20. Carmody J, Swanson JR, Rhone E, Charlton J. Recognition and reporting of AKI in very low birth weight infants. Clin J Am Soc Nephrol. (2014) 12:2036–43. doi: 10.2215/CJN.05190514
21. Wu Y, Wang H, Pei J, Jiang X, Tang J. Acute kidney injury in premature and low birth weight neonates: a systematic review and meta-analysis. Pediatr Nephrol. (2022) 37:275–87. doi: 10.1007/s00467-021-05251-0
22. Blinder J, Goldstein SL, Lee V, Baycroft A, Fraser C, Nelson D, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. (2012) 143:368–74. doi: 10.1016/j.jtcvs.2011.06.021
23. Sharma A, Chakraborty R, Sharma K, Sethi SK, Raina R. Development of acute kidney injury following pediatric cardiac surgery. Kidney Res Clin Pract. (2020) 39:259–68. doi: 10.23876/j.krcp.20.053
24. Carmody J, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. (2013) 131:1168–79. doi: 10.1542/peds.2013-0009
25. Luyckx V, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes – a global concern. Nat Rev Nephrol. (2015) 11:135–49. doi: 10.1038/nrneph.2014.251
26. Alten J, Cooper DS, Blinder J, Selewski D, Tabbutt S, Sasaki J, et al. Epidemiology of acute kidney injury after neonatal cardiac injury: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med. (2021) 49:e941–51. doi: 10.1097/CCM.0000000000005165
27. Durkan A, Alexander RT. Acute kidney injury post neonatal asphyxia. J Pediatr. (2011) 158(2 Suppl.):e29–33. doi: 10.1016/j.jpeds.2010.11.010
28. Selewski D, Jordan BK, Askenazi D, Dechert R, Sarkar S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J Pediatr. (2013) 162:725–9. doi: 10.1016/j.jpeds.2012.10.002
29. Karlowicz M, Adelman RD. Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol. (1995) 9:718–22. doi: 10.1007/BF00868721
30. Kaur S, Jain S, Saha A, Chawla D, Parmar VR, Basu S, et al. Evaluation of glomerular and tubular renal function in neonates with birth asphyxia. Ann Trop Paediatr. (2011) 31:129–34. doi: 10.1179/146532811X12925735813922
31. Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischmic encephalopathy. Arch Dis Child Fetal Neonatal Ed. (2004) 89:F152–5. doi: 10.1136/adc.2002.023093
32. Hankins G, Koen S, Gei A, Lopez S, Van Hook J, Anderson G. Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol. (2002) 99:688–91. doi: 10.1016/s0029-7844(02)01959-2
33. Sarkar S, Askenazi DJ, Jordan B, Bhagat I, Bapuraj J, Dechert R, et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res. (2014) 75:431–5. doi: 10.1038/pr.2013.230
34. Nour I, Elmaghraby R, Shehata R, El-Refaey A, Aldomiaty H, Mosbah A, et al. Selective head cooling and acute kidney injury in neonates with hypoxic ischemic encephalopathy. J Neonatal Perinat Med. (2020) 13:21–30. doi: 10.3233/NPM-180200
35. Tanigasalam V, Bhat V, Adhisivam B, Sridhar M. Does therapeutic hypothermia reduce acute kidney injury among term neonates with perinatal asphyxia? – a randomized controlled trial. J Mater Fetal Neonatal Med. (2016) 29:2545–8. doi: 10.3109/14767058.2015.1094785
36. Garg P, Britt AB, Ansari M, Sobisek S, Block D, Paschal J, et al. Severe acute kidney injury in neonates with necrotizing enterocolitis: risk factors and outcomes. Pediatr Res. (2021) 90:642–9. doi: 10.1038/s41390-020-01320-6
37. Garg P, Tatum R, Ravisankar S, Shekhawat P, Chen Y. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr Res. (2015) 78:527–32. doi: 10.1038/pr.2015.146
38. Criss C, Selewski DT, Sunkara B, Gish J, Hsieh L, McLeod J, et al. Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr Nephrol. (2018) 33:503–10. doi: 10.1007/s00467-017-3809-y
39. Bakhoum C, Basalely A, Koppel R, Sethna C. Acute kidney injury in preterm infants with necrotizing enterocolitiis. J Matern Fetal Neonatal Med. (2019) 32:3185–90. doi: 10.1080/14767058.2018.1459553
40. Goldstein S, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. (2013) 132:e756–67. doi: 10.1542/peds.2013-0794
41. Goldstein S, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int. (2016) 90:212–21. doi: 10.1016/j.kint.2016.03.031
42. Rhone E, Carmody JB, Swanson J, Charlton J. Nephrotoxic medication exposure in very low birth weight infants. J Matern Fetal Neonatal Med. (2014) 27:1485–90. doi: 10.3109/14767058.2013.860522
43. Salerno S, Liao Y, Jackson W, Greenberg R, McKinzie C, McCallister A, et al. Association between nephrotoxic drug combinations and acute kidney injury in the neonatal intensive care unit. J Pediatr. (2021) 228:213–9. doi: 10.1016/j.jpeds.2020.08.035
44. Stoops C, Stone S, Evans E, Dill L, Henderson T, Grriffin R, et al. Baby NINJA (nephrotoxic injury negated by just-in-time action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J Pediatr. (2019) 215:223–8. doi: 10.1016/j.jpeds.2019.08.046
45. Zwiers A, de Wildt SN, Hop W, Dorresteijn E, Gischler S, Tibboel D, et al. Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14-year cohort study. Crit Care. (2013) 17:R151. doi: 10.1186/cc12830
46. Smith A, Hardison DC, Worden C, Fleming G, Taylor M. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. (2009) 55:412–6. doi: 10.1097/MAT.0b013e31819ca3d0
47. Gadepalli S, Selewski DT, Drongowski R, Mychaliska G. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. (2011) 46:630–5. doi: 10.1016/j.jpedsurg.2010.11.031
48. Fleming G, Sahay R, Zappitelli M, King E, Askenazi D, Bridges B, et al. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: a multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatric Crit Care Med. (2016) 17:1157–69. doi: 10.1097/PCC.0000000000000970
49. Murphy H, Gien J, Sahay R, King E, Selewski DT, Bridges BC, et al. Acute kidney injury, fluid overload, and renal replacement therapy differ by underlying diagnosis in neonatal extracorporeal support and impact mortality disparately. Blood Purif. (2021) 18:1–10. doi: 10.1159/000512538
50. Stoops C, Boohaker L, Sims B, Griffin R, Selewski D, Askenazi D. Intraventricular hemorrhage and acute kidney injury in premature infants from the assessment of the worldwide acute kidney injury epidemiology in neonates (AWAKEN) study. Neonatology. (2019) 116:321–30. doi: 10.1159/000501708
51. Adcock B, Carpenter S, Bauer J, Giannone P, Schadler A, Chishti A, et al. Acute kidney injury, fluid balance and risks of intraventricular hemorrhage in premature infants. J Perinatol. (2020) 40:1296–300. doi: 10.1038/s41372-020-0613-5
52. Al-Mouqdad M, Huseynova R, Khalil T, Asfour Y, Asfour S. Relationship between intraventricular hemorrhage and acute kidney injury in premature infants and its effect on neonatal mortality. Sci Rep. (2021) 11:13262. doi: 10.1038/s41598-021-92746-3
53. Cavallin F, Rubin G, Vidal E, Cainelli E, Bonadies L, Suppiej A, et al. Prognostic role of acute kidney injury on long-term outcome in infants with hypoxic-ischemic encephalopathy. Pediatr Nephrol. (2020) 35:477–83. doi: 10.1007/s00467-019-04406-4
54. Starr M, Boohaker L, Eldredge L, Menon S, Griffin R, Mayock D, et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks. Am J Perinatol. (2020) 37:341–8. doi: 10.1055/s-0039-3400311
55. Askenazi D, Patil NR, Ambalavanan N, Balena-Borneman J, Lozano D, Ramani M, et al. Acute kidney injury is associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr Nephrol. (2015) 30:1511–8. doi: 10.1007/s00467-015-3087-5
56. Kastl JT. Renal function in the fetus and neonate – the creatinine enigma. Semin Fetal Neonatal Med. (2017) 22:83–9. doi: 10.1016/j.siny.2016.12.002
57. Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr. (2021) 24:192–6. doi: 10.1097/MOP.0b013e32834f62d5
58. Basu R, Kaddourah A, Goldstein S. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicenter, multinational, prospective observational study. Lancet Child Adolesc Health. (2018) 2:112–20. doi: 10.1186/s13054-016-1208-6
59. Wazir S, Sethi SK, Agarwal G, Tibrewal A, Dhir R, Bajaj N, et al. Neonatal acute kidney injury risk stratification score: STARZ study. Pediatr Res. (2021). doi: 10.1038/s41390-021-01573-9 [Epub ahead of print].
60. Raina A, Pandita A, Harish R, Yachha M, Jamwal A. Treating perinatal asphyxia with theophylline at birth helps to reduce severity of renal dysfunction in term neonates. ACTA Paediatr. (2016) 105:e448–51. doi: 10.1111/apa.13469
61. Bakr A. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia – a study in a developing country. Pediatr Nephrol. (2005) 20:1249–52. doi: 10.1007/s00467-005-1980-z
62. Bhat M, Shah ZA, Makhdoomi M, Mufti M. Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J Pediatr. (2006) 149:180–4. doi: 10.1016/j.jpeds.2006.03.053
63. Jenik A, Ceriani Cernadas JM, Gorenstein A, Ramirez J, Vain N, Armadans M, et al. Randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics. (2000) 105:e45. doi: 10.1542/peds.105.4.e45
64. Eslami Z, Shajari A, Kheirandish M, Heidary A. Theophylline for prevention of kidney dysfunction in neonates with severe asphyxia. Iran J Kidney Dis. (2009) 3:222–6.
65. Merrikhi A, Ghaemi S, Gheissari A, Shokrani M, Madihi Y, Mousavinasab F. Effects of aminophylline preventing renal failure in premature neonates with asphyxia in Isfahan-Iran. J Pak Med Assoc. (2012) 62:S48–51.
66. Axelrod D, Sutherland SM, Anglemyer A, Grimm PC, Roth SJ. A double-blinded, randomized, placebo-controlled clinical trial of aminophylline to prevent acute kidney injury in children following congenital heart surgery with cardiopulmonary bypass. Pediatr Crit Care Med. (2016) 17:135–43. doi: 10.1097/PCC.0000000000000612
67. Khurshid G, Khurshid S, Mehmood H, Afshan S. Efficacy of theophylline for prevention of kidney dysfunction in neonates with severe birth asphyxia. Pak J Med Health. (2017) 11:1346–8. doi: 10.1136/archdischild-2018-315805
68. Carmody J, Harer MW, Denotti A, Swanson J, Charlton J. Caffeine exposure and risk of acute kidney injury in a retrospective cohort of very low birth weight neonates. J Pediatr. (2016) 172:63–8. doi: 10.1016/j.jpeds.2016.01.051
69. Harer M, Askenazi DJ, Boohaker L. Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates. JAMA Pediatr. (2018) 172:e180322. doi: 10.1001/jamapediatrics.2018.0322
70. Aviles-Otero N, Kumar R, Khalsa D, Green G, Carmody J. Caffeine exposure and acute kidney injury in premature infants with necrotizing enterocolitis and spontaneous intestinal perforation. Pediatr Nephrol. (2019) 34:729–36. doi: 10.1007/s00467-018-4140-y
71. Nourse P, Cullis B, Finkelstein F, Numanoglu A, Warady B, Antwi S, et al. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update (pediatrics). Perit Dial Int. (2021) 41:139–57. doi: 10.1177/0896860820982120
72. Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, et al. Continuous renal replacement therapy in neonates and small infants: development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet. (2014) 383:1807–13. doi: 10.1016/S0140-6736(14)60799-6
73. Coulthard M, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, et al. Haemodialysing babies weighing <8kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol. (2014) 29:1873–81. doi: 10.1007/s00467-014-2923-3
74. Menon S, Broderick J, Munshi R, Dill L, DePaoli B, Fathallah-Shaykh S, et al. Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol. (2019) 14:1432–40. doi: 10.2215/CJN.03240319
75. Blencowe H, Cousens S, Oestergaard M, Chou D, Moller A, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. doi: 10.1016/S0140-6736(12)60820-4
76. Hamilton B, Hoyert DL, Martin J, Strobino D, Guyer B. Annual summary of vital statistics: 2010-2011. Pediatrics. (2013) 131:548–58. doi: 10.1542/peds.2012-3769
77. Martin J, Kung HC, Mathews T, Hoyert D, Strobino D, Guyer B, et al. Annual summary of vital statistics: 2006. Pediatrics. (2008) 121:788–801. doi: 10.1542/peds.2007-3753
78. Chaturvedi S, Ng KH, Mammen C. The path to chronic kidney disease following acute kidney injury: a neonatal perspective. Pediatr Nephrol. (2017) 32:227–41. doi: 10.1007/s00467-015-3298-9
79. Amdur R, Chawla LS, Amodeo S, Kimmel P, Palant C. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int. (2009) 76:1089–97. doi: 10.1038/ki.2009.332
80. Chawla L, Eggers PW, Star R, Kimmel P. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. (2014) 371:58–66. doi: 10.1056/NEJMra1214243
81. Coca S, Singanamala S, Parikh C. Chronic kidney disease after acute kidney injury: a systematic revew and meta-analysis. Kidney Int. (2012) 81:442–8. doi: 10.1038/ki.2011.379
82. Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet J, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. (2012) 59:523–30. doi: 10.1053/j.ajkd.2011.10.048
83. Wald R, Quinn RR, Adhikari N, Burns K, Friedrich J, Garg A, et al. Risk of chronic dialysis and death following acute kidney injury. Am J Med. (2012) 125:585–93. doi: 10.1016/j.amjmed.2012.01.016
84. Harer M, Pope CF, Conaway M, Charlton J. Follow-up of acute kidney injury in neonates during childhood years (FANCY): a prospective cohort study. Pediatr Nephrol. (2017) 32:1067–76. doi: 10.1007/s00467-017-3603-x
85. Abitbol C, Bauer CR, Montane B, Chandar J, Suara S, Zilleruelo G. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol. (2003) 18:887–93. doi: 10.1007/s00467-003-1186-1
86. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. (2004) 114:555–76. doi: 10.1542/peds.114.2.s2.555
87. Koralkar R, Ambalavanan N, Levitan E, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. (2011) 69:354–8. doi: 10.1203/PDR.0b013e31820b95ca
88. Askenazi D, Griffin R, McGwin G, Carlo W, Ambalavanan N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. (2009) 24:991–7. doi: 10.1007/s00467-009-1133-x
89. Sasaki J, Rodriguez Z, Alten JA, Rahman AF, Reichle G, Lin P, et al. Epidemiology of neonatal acute kidney injury after cardiac surgery without cardiopulmonary bypass. Ann Thorac Surg. (2021). doi: 10.1016/j.athoracsur.2021.09.032 [Epub ahead of print].
90. Alabbas A, Campbell A, Skippen P, Human D, Matsell D, Mammen C. Epidemiology of cardiac surgery associated acute kidney injury in neonates: a retrospective study. Pediatr Nephrol. (2013) 7:1127–34. doi: 10.1007/s00467-013-2454-3
91. Kirkley M, Boohaker L, Griffin R, Soranno D, Gien J, Askenazi D, et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr Nephrol. (2019) 34:169–76. doi: 10.1007/s00467-018-4068-2
92. Chock V, Frymoyer A, Yeh C, Van Meurs K. Renal saturation and acute kidney injury in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. J Pediatr. (2018) 200:232–9.e1. doi: 10.1016/j.jpeds.2018.04.076
93. Wolters Kluwer Health. Lexicomp. (2022). Available online at: https://online.lexi.com (accessed March 14, 2022).
94. Hidayati E, Utami MD, Rohsiswatmo R, Tridjaja B. Cystatin C compared to serum creatinine as a marker of acute kideny injury in critically ill neonates. Pediatr Nephrol. (2021) 36:181–6. doi: 10.1007/s00467-020-04668-3
95. Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P, Bennet M, Sabbisetti V, et al. Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol. (2015) 30:665–76. doi: 10.1007/s00467-014-2987-0
96. Li Y, Fu C, Zhou X, Xiao Z, Zhu X, Jin M, et al. Urine interleukin-18 and cystatin-C as biomarkers of acute kidney injury in critically ill neonates. Pediatr Nephrol. (2012) 27:851–60. doi: 10.1007/s00467-011-2072-x
97. Sarafidis K, Tsepkentzi E, Agakidou E, Diamanti E, Taparkou A, Soubasi V, et al. Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol. (2012) 27:1575–82. doi: 10.1007/s00467-012-2162-4
98. Askenazi D, Montesanti A, Hunley H, Koralkar R, Pawar P, Shuaib F, et al. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr. (2011) 159:907–12. doi: 10.1016/j.jpeds.2011.05.045
99. Sarafidis K, Tsepkentzi E, Diamanti E, Agakidou E, Taparkou A, Soubasi V, et al. Urine neutrophil gelatinase-associated lipocalin to predict acute kidney injury in preterm neonates. A pilot study. Pediatr Nephrol. (2014) 29:305–10. doi: 10.1007/s00467-013-2613-6
100. Tabel Y, Elmas A, Ipek S, Karadag A, Elmas O, Ozyalin F. Urinary neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury in preterm infants. Am J Perinatol. (2014) 31:167–74. doi: 10.1055/s-0033-1343770
101. Krawczeski C, Woo JG, Wang Y, Bennett M, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. (2011) 158:1009–15. doi: 10.1016/j.jpeds.2010.12.057
Keywords: acute kidney injury, neonatal, continuous renal replacement therapy, fluid overload, premature (babies), NICU, renal failure, kidney support therapy
Citation: Coleman C, Tambay Perez A, Selewski DT and Steflik HJ (2022) Neonatal Acute Kidney Injury. Front. Pediatr. 10:842544. doi: 10.3389/fped.2022.842544
Received: 23 December 2021; Accepted: 01 March 2022;
Published: 07 April 2022.
Edited by:
Michael L. Moritz, University of Pittsburgh, United StatesReviewed by:
Enrico Eugenio Verrina, Giannina Gaslini Institute (IRCCS), ItalyJuan C. Kupferman, Maimonides Medical Center, United States
Copyright © 2022 Coleman, Tambay Perez, Selewski and Steflik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi J. Steflik, c3RlZmxpa2hAbXVzYy5lZHU=, c2VsZXdza2lAbXVzYy5lZHU=
 Cassandra Coleman
Cassandra Coleman Anita Tambay Perez
Anita Tambay Perez David T. Selewski
David T. Selewski Heidi J. Steflik1*
Heidi J. Steflik1*