- 1Department of Pediatric Surgery, West China of Hospital, Sichuan University, Chengdu, China
- 2Department of Neonatal Surgery, Shenzhen Children's Hospital, Shenzhen, China
- 3Department of Ultrasound, West China of Hospital, Sichuan University, Chengdu, China
Background: The failed clearance of jaundice (CJ) in patients with biliary atresia (BA) after the Kasai procedure (KP) often leads to a shorter native liver survival (NLS) time and earlier liver transplantation. We aimed to investigate risk factors of failed CJ and establish a novel nomogram model to predict the status of CJ.
Methods: We retrospectively reviewed institutional medical records from January 2015 to April 2020 and enrolled BA patients post-KP, randomly divided into training and testing cohorts at a ratio of 7:3, and further subdivided into cleared and uncleared jaundice groups. Univariate and multiple logistic regression analyses were used to select risk factors to establish the nomogram in the training cohort. The performance of the nomogram was evaluated by calculating the areas under the receiver operating curve (AUC) in both cohorts.
Results: This study included 175 BA patients post-KP. After univariate and multiple logistic regression analyses, Cytomegalovirus IgM +ve associated BA (OR = 3.38; 95% CI 1.01–11.32; P = 0.04), ln γ-glutamyl transpeptidase (GGT) (OR = 0.41; 95% CI 0.22–0.80; P = 0.009), thickness of the fibrous portal plate (OR = 0.45; 95% CI 0.27–0.76; P = 0.003), liver stiffness measurement (LSM) (OR = 1.19; 95% CI 1.06–1.34; P = 0.002), and multiple episodes of cholangitis (OR = 1.65; 95% CI 1.13–2.41; P = 0.01) were identified as independent risk factors of unsuccessful CJ to construct the nomogram. The receiver operating characteristic curve (ROC) analysis suggested good nomogram performance in both the training (AUC = 0.96) and testing cohorts (AUC = 0.91).
Conclusion: Our nomogram model including several risk factors effectively predicts CJ in patients post-KP, which could aid in clinical decision-making.
Introduction
Biliary atresia (BA) is a rare and severe cholangiopathy that leads to liver failure in infants and is characterized by a progressive fibro-inflammatory process affecting the intrahepatic and extrahepatic bile ducts (1). The pathogenesis of BA remains unclear, and the majority of untreated patients die before the age of 1 year due to liver failure (2–4). Five- and ten-year native liver survival (NLS) rates in BA patients have been documented as 46–58% and 30–40%, respectively (5–8). Despite advances in surgical techniques and perioperative management, liver transplantation is required for these patients to achieve long-term survival (9).
Some studies have indicated that a rapid, early, and complete clearance of jaundice (CJ) after the Kasai procedure (KP) was the most valuable prognostic factor for long-term NLS (10, 11). Moreover, unsuccessful CJ in BA patients was always associated with a shorter NLS and an earlier time for pediatric liver transplantation (12). Several studies reported that the post-KP CJ widely varied from 36 to 61% (3, 8, 13). Although previous studies have identified some clinical risk factors for unsuccessful CJ (14–16), it is still difficult to make an early and precise prediction of CJ in clinical practice. However, an early and precise prediction of CJ is essential for clinicians to asses risk stratification, estimate the prognosis, develop individualized treatment, and prepare for further liver transplantation, such as ethics application, implementation of intensified nutritional support, and monitoring of potential complications related to progressive liver disease (17, 18). We hypothesized that a visual prediction model based on clinical factors can accurately predict the CJ. Therefore, the purpose of this study was to investigate risk factors of failed CJ and establish a novel nomogram model based on these selected factors to predict the CJ in BA patients post-KP.
Patients and Methods
Patients
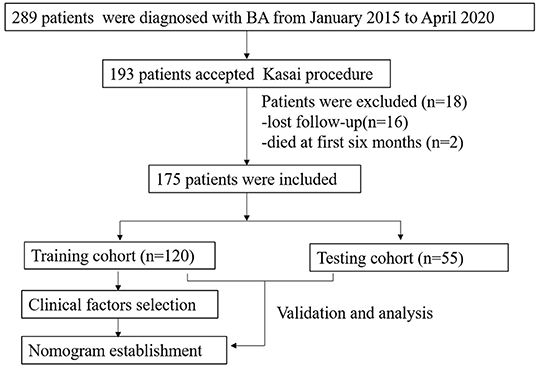
This retrospective study was approved by the Ethical Committee of the West China Hospital of Sichuan University (ChiCTR1800017017). From January 2015 to April 2020, 289 patients who underwent intraoperative cholangiography and liver biopsy were diagnosed with BA. Patients who underwent the KP were included in this study. In all patients, methylprednisolone was administered intravenously 5 days postoperatively at a dose of 4- mg/kg/day initially, reduced by 1 mg/kg/day every 3 days for 2 weeks or even longer until the normal value of total bilirubin reached. Cephalosporin antibiotics were orally taken alternately weekly until 1 year of age. Ursodeoxycholic acid and hepatoprotective tablets were used until 3 years of age. Patients with incomplete clinical data, such as those who died within the first 6 months post-operation and those lost to follow-up, were excluded from the study. The workflow of this study is illustrated in Figure 1. Enrolled patients with cleared and uncleared jaundice were randomly divided into training and testing cohorts at a ratio of 7:3 by random number. Additionally, patients were divided into cleared and uncleared jaundice groups according to their achievement of CJ. CJ was defined as a reduction in total bilirubin (TBIL) level to <20 μmol/L at 6 months post-KP (19).
Clinical data were collected from each patient's medical records, including age at surgery, sex, weight, surgical method used, other malformations, blood loss, Davenport classification (1), Metavir scores, and times of postoperative cholangitis. CMV (cytomegalovirus) IgM +ve associated BA was determined based on the CMV-specific IgM results, which are immunoenzymatic assays based on the ELISA technique. Metavir scores were estimated from the results of the liver biopsy (20). Cholangitis was defined as unexplained fever (>38°C), reappearance of white stools, increase in TBIL levels, and increase in inflammatory parameters. The multiple episodes of cholangitis were calculated from the first day after surgery to 3 months postoperatively. An episode of cholangitis was defined as the time from diagnosis to discharge from the hospital. Preoperative liver function and coagulation results, including TBIL, alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin, alkaline phosphatase (ALP), total bile acid (TBA), and γ-glutamyl transpeptidase (GGT) levels, international normalized ratio (INR), and prothrombin time (PT), were also collected. The preoperative thickness of the fibrous portal plate and liver stiffness measurement (LSM) values were examined by a pediatric sonographer with 20 years of experience. Thickness of the fibrous portal plate was defined as the thickness of the echogenic anterior wall of the anterior branch of the right portal vein just distal to the right portal vein in a transverse oblique plane (21). LSM was measurement by shear wave elastography (SWE) technique. More than five valid measurements were recorded for each patient, and the median value was considered as the ultimate LSM value in each individual.
Establishment and Evaluating Performance of the Nomogram
A univariate analysis was performed to identify significant (P < 0.05) clinical factors in the training cohort to establish a multivariate logistic regression model. Clinical factors with a P < 0.05 in the logistic regression model were used to establish the nomogram, and the predictive performance of the nomogram was evaluated by calculating the area under the receiver operating curve (AUC) in both the training and testing cohorts.
Statistical Analysis
SPSS 26.0 statistical software and R programming (3.2 version) were used for all statistical analyses. Ln-transformation was applied to normalize the ALP and GGT values. Normally distributed data were represented as x ± s, and the skewed measurement data were represented as M. Differences were evaluated using the Student's t-test for continuous parametric data, Wilcoxon test for continuous non-parametric data, and Pearson's chi-squared test for non-continuous data. Statistical significance was set at P < 0.05. The “rms” package was used to establish the nomogram. The “pROC” package was used to plot the receiver operating characteristics (ROC) curve of the prediction model.
Results
Patient Characteristic
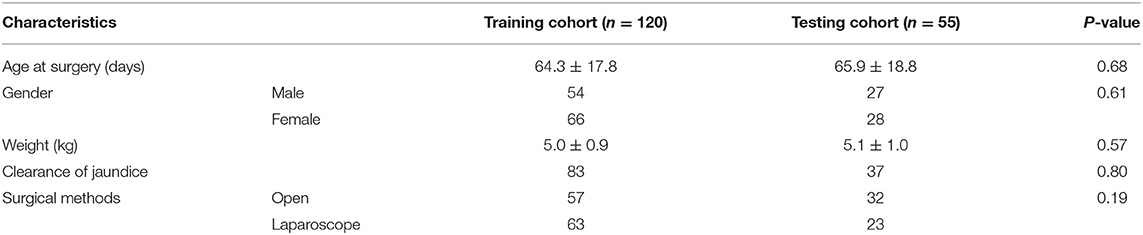
From January 2015 to April 2020, 193 BA patients underwent the KP at our center. Sixteen patients who were lost to follow-up and two patients who died in the first 6 months post-KP were excluded from this study. Therefore, 175 BA patients were included in this study. The distributions of the Davenport classification were as follows: 8 (4.6%) cases with syndromic BA, 5 (2.8%) cases with cystic BA, 48 (27.4%) cases with CMV-IgM +ve associated BA, and 114 (65.2%) cases with isolated BA. A total of 120 and 55 patients were randomly enrolled in the training and testing cohorts, respectively. The baseline characteristics of both the training and testing cohorts were presented in Table 1. There was no significant difference in age at surgery (P = 0.32), gender (P = 0.61), weight (P = 0.57), outcomes of CJ (P = 0.80), or surgical methods (P = 0.19).
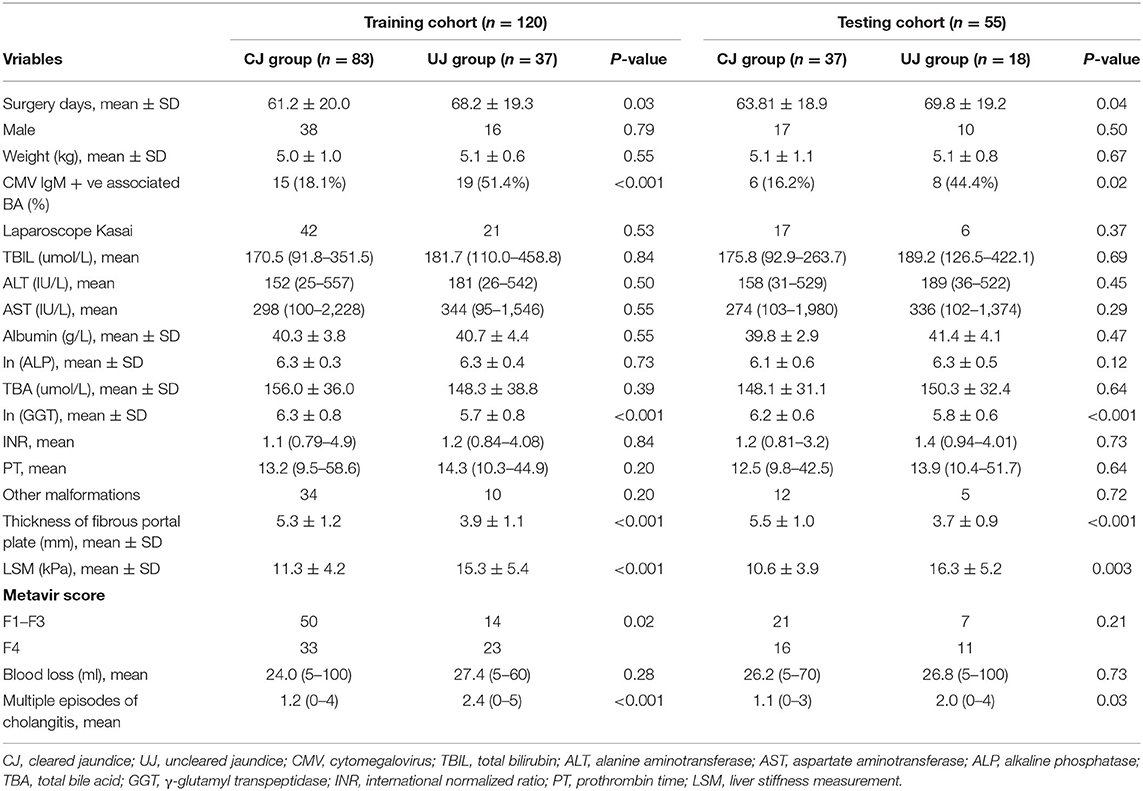
The characteristics of the patients and univariate analysis of the training and testing cohorts were shown in Table 2. In training cohort, the univariate analysis identified significant differences in surgery days (P = 0.03), CMV IgM +ve associated BA (P < 0.001), ln (GGT) (P < 0.001), thickness of the fibrous portal plate (P < 0.001), LSM value (P < 0.001), Metavir score (P = 0.02), and multiple episodes of cholangitis (P < 0.001).
Establishment and Validation of the Nomogram Model
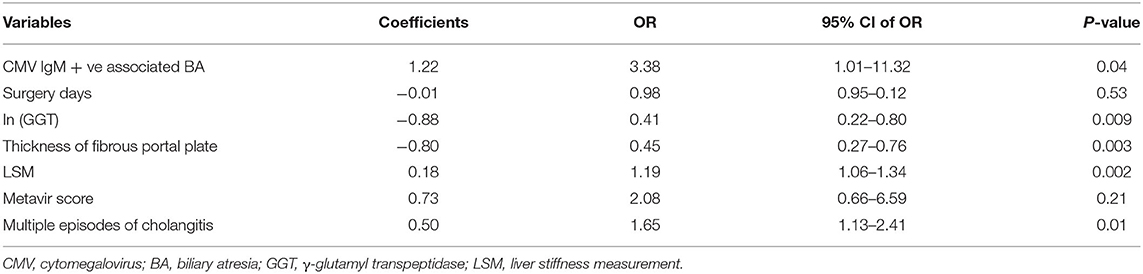
The results of the multiple logistic regression model were presented in Table 3. CMV IgM +ve associated BA (OR = 3.38; 95% CI 1.01–11.32; P = 0.04), ln (GGT) (OR = 0.41; 95% CI 0.22–0.80; P = 0.009), thickness of the fibrous portal plate (OR = 0.45; 95% CI 0.27–0.76; P = 0.003), LSM (OR = 1.19; 95% CI 1.06–1.34; P = 0.002), and multiple episodes of cholangitis (OR = 1.65; 95% CI 1.13–2.41; P = 0.01) were identified as independent predictors for CJ post-KP, which was used to construct the clinical nomogram model (Figure 2). Based on the area under the ROC curve (Figure 3), the AUC of the nomogram in the training and testing cohorts were 0.96 and 0.91, respectively.
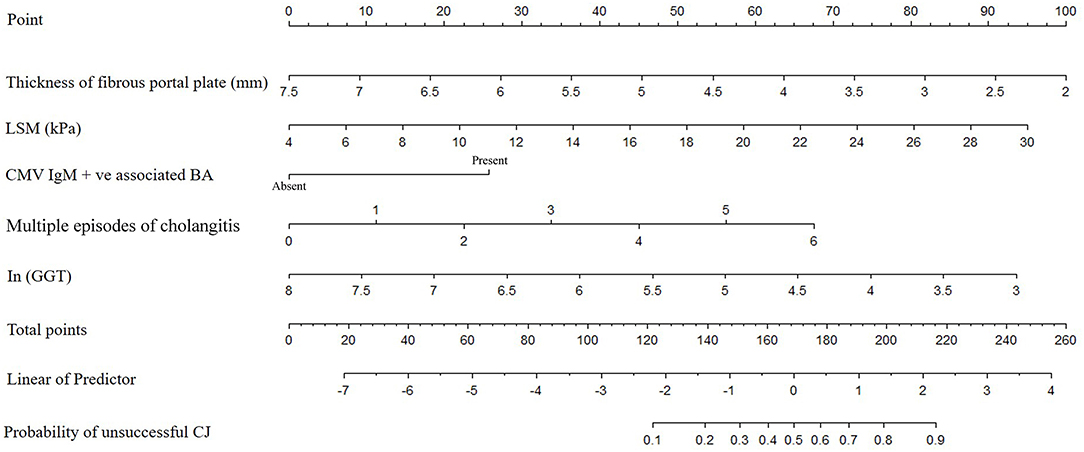
Figure 2. The nomogram for prediction the possibility of unsuccessful CJ in patients with KP. (Each clinical factor with different status would correspond to a score in “Point”. The scores for each clinical factor were added to obtain an overall score in “Total point”. Then, “Total point” would correspond to risk of “Probability of unsuccessful CJ”.) CMV, cytomegalovirus; GGT, γ-glutamyl transpeptidase; LSM, liver stiffness measurement; CJ, clearance of jaundice; BA, biliary atresia; KP, Kasai procedure.
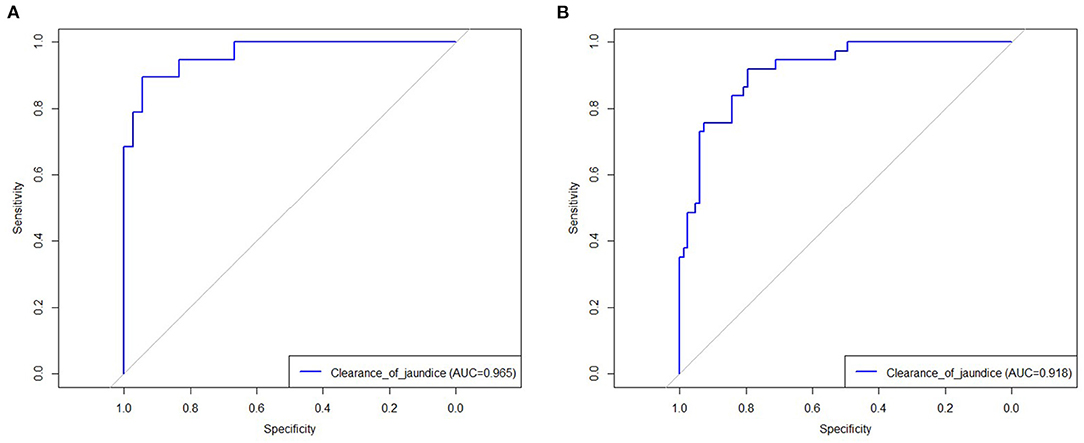
Figure 3. The receive operating characteristic of established model in training (A) and testing cohort (B).
Discussion
Post-KP CJ is crucial for the prognosis of BA patients. Several studies have indicated that an unsuccessful KP reduces the NLS time and requires early liver transplantation (6, 12). Wang et al. reported that a successful KP enabled patients to achieve long-term event-free survival and a rapid CJ rate within 4 weeks that exhibited a good predictive value (6). Additionally, Ge et al. found that uncleared post-KP jaundice was a risk factor for a shorter NLS time and earlier liver transplantation (12). In this study, we found that several clinical factors, including CMV IgM +ve associated BA, ln (GGT), thickness of the fibrous portal plate, LSM value, and multiple episodes of cholangitis, were associated with the status of CJ in BA patients post-KP. We then constructed a visual nomogram model using logistical regression analysis to accurately predict CJ in BA patients post-KP. Further, the AUC of the nomogram revealed a robust predictive performance in both the training and testing cohorts, which may help clinicians to predict CJ more accurately and aid in clinical decision-making.
Our results showed that the rates of CMV IgM +ve associated Davenport classification in the uncleared jaundice group were significantly higher than those in the cleared jaundice group in both the training and testing cohorts. Further, the results of multivariate logistic regression revealed that it was an independent risk factor for uncleared jaundice, which was similar to the results of previous studies. Shen et al. found that BA patients with CMV infection were associated with a lower rate of CJ and a higher possibility of cholangitis (22). Zhao et al. also reported that Biliary atresia patients who were also infected with CMV had a poorer prognosis, particularly with respect to jaundice clearance (23). Additionally, Zani et al. showed that infants with CMV IgM +ve Davenport classification were always associated with more jaundiced, worse liver function, and a greater degree of inflammation and fibrosis in histology compared with CMV IgM-ve isolated BA (24). They believed that CMV IgM +ve BA often led to a reduced rate of JC, shorter NLS time, and higher mortality, which is consistent with our study.
We revealed that the preoperative GGT level of the uncleared jaundice group was significantly lower than that of the cleared jaundice group, and our multivariate analysis results suggested that patients with a lower preoperative level of GGT were more inclined to develop post-KP failure CJ. Some studies also have investigated the relationship between preoperative GGT levels and the outcome of the KP. Sun et al. found that the preoperative GGT level in BA patients who survived for >5 years with normal liver function was significantly higher than in those who died from liver failure within a year post- KP (25). Besides, Shankar et al. retrospectively analyzed 113 BA patients and found that 12.3% of patients with preoperative GGT <200 IU/L experienced a shorter time from the KP to liver transplantation and poorer transplant-free survival than those with preoperative GGT > 200 IU/L (26). These results seemed to suggest that a low preoperative level of GGT may be associated with poor prognosis after KP. However, no exact results were proposed to explain it. Besides, limited sample size and retrospective data collection may also contribute to this result. Further studies may be needed.
As a non-invasive tool, the abdominal ultrasound has been widely used to help evaluate infants for BA (27), but few studies investigated the correlation between ultrasound signs and CJ post-KP. This study emphasized the importance of the thickness of the fibrous portal plate and LSM value, which were collected by ultrasound for this novel prediction model. Additionally, we investigated the correlation between the thickness of the fibrous portal plate and CJ. Our results suggested that a thicker fibrous portal plate was related to higher rates of CJ. It is well known that sufficient bile flow through the bile capillary is essential for the achievement of CJ post-KP. Good bile flow often leads to a rapid reduction in bilirubin levels in BA patients. A thicker fibrous portal plate may contain more bile capillaries, which may contribute to sufficient flow of bile and a higher possibility of CJ. Additionally, the LSM value mainly reflects the degree of liver fibrosis, which is one of the main pathological features of BA (28). Previous studies have applied the LSM assessment for the diagnostic and prognostic prediction of BA (29–31). Wu et al. found that an LSM value > 7.7 kPa was a predictive factor for discriminating BA patients from other cholestatic patients, and patients with an LSM value > 16 kPa often require early liver transplantation (29). Liu et al. also showed that the LSM value at 3 months was associated with 2-years NLS post- KP (31). In our study, the preoperative LSM values in the uncleared jaundice group were significantly higher. A higher LSM value is always suggestive of more severe inflammatory fibrosis (28), this progressive inflammatory fibrosis often deteriorates the injury narrowing the bile duct (32), which may contribute to a poor CJ rate.
It has been recognized that postoperative recurrent cholangitis is a critical clinical factor that leads to the failure of the KP, a shorter NLS, and the requirement for earlier liver transplantation (33, 34). Our study also suggested that cholangitis was an independent risk factor of successful CJ. Therefore, cholangitis was a significant portion of prediction model.
Although many studies have indicated several risk factors associated with the short- and long-term outcomes of BA patients post-KP (17, 35, 36), there are few established visual predictive models to determine its prognosis. Liu et al. constructed a nomogram to predict the 2-year NLS in BA patients post-KP and showed good performance in a validation cohort (31). However, there was a difference compared with the results of our study. The clinical factors they included were not completely consistent with those of our study, the thickness of the fibrous portal plate and times of cholangitis were not included in their study. The majority of the clinical factors they included were postoperative, but the clinical factors selected in our study were mainly preoperative. Thus, we aimed to screen high-risk patients who may require more frequent follow-ups and early preparation of liver transplantation. Additionally, the clinical factors of our model were all accessible by regular non-invasive examinations, which may improve the feasibility of clinical practice.
There are several limitations to our study. First, this was a single-center retrospective study, which may reduce the generalizability of our results. Second, the sample size of this study was small. Finally, the performance of the established models may be affected by the lack of multicenter external validation. Therefore, further studies with larger sample sizes and multicenter external validation are required. Despite these limitations, we firstly investigated that preoperative thickness of the fibrous portal plate and LSM value measured by ultrasound were related to CJ post-KP. Besides, this is the first study to establish a visual nomogram to predict CJ post-KP and to present good predictive performance. In the context of precision medicine, this model may help clinical decision-making and improve the management of BA patients.
Conclusion
Our nomogram model including several clinical factors effectively predicts CJ in patients post-KP, which could aid in clinical decision-making.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The study was approved by the Ethics Committee of the West China Hospital of Sichuan University. Written informed consent was obtained from the patients' parents, according to the provisions of the Declaration of Helsinki.
Author Contributions
YZ: data curation, formal analysis, and writing–original draft. QW: methodology and writing–original draft. SP: data curation, resources, and software. JW: data curation and formal analysis. BX: methodology. JL: conceptualization and data curation. SJ: conceptualization, funding acquisition, supervision, and writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (81571473).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BA, biliary atresia; NLS, native liver survival; CJ, clearance of jaundice; KP, Kasai procedure; CMV, cytomegalovirus; TBIL, total bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SA, serum albumin; ALP, alkaline phosphatase; TBA, total bile acid; GGT, γ-glutamyl transpeptidase; INR, international normalized ratio; PT, prothrombin time; LSM, liver stiffness measurement; ROC, receiver operating characteristics curve; AUC, area under the receiver operating curve.
References
1. Lakshminarayanan B, Davenport M. Biliary atresia: a comprehensive review. J Autoimmun. (2016) 73:1–9. doi: 10.1016/j.jaut.2016.06.005
2. Nizery L, Chardot C, Sissaoui S, Capito C, Henrion-Caude A, Debray D, et al. Biliary atresia: clinical advances and perspectives. Clin Res Hepatol Gastroenterol. (2016) 40:281–7. doi: 10.1016/j.clinre.2015.11.010
3. Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet. (2009) 374:1704–13. doi: 10.1016/S0140-6736(09)60946-6
4. Wang J, Xu Y, Chen Z, Liang J, Lin Z, Liang H, et al. Liver immune profiling reveals pathogenesis and therapeutics for biliary atresia. Cell. (2020) 183:1867–83.e26. doi: 10.1016/j.cell.2020.10.048
5. Davenport M, Ong E, Sharif K, Alizai N, McClean P, Hadzic N, et al. Biliary atresia in England and Wales: results of centralization and new benchmark. J Pediatr Surg. (2011) 46:1689–94. doi: 10.1016/j.jpedsurg.2011.04.013
6. Wang Z, Chen Y, Peng C, Pang W, Zhang T, Wu D, et al. Five-year native liver survival analysis in biliary atresia from a single large Chinese center: the death/liver transplantation hazard change and the importance of rapid early clearance of jaundice. J Pediatr Surg. (2019) 54:1680–5. doi: 10.1016/j.jpedsurg.2018.09.025
7. Kelay A, Davenport M. Long-term outlook in biliary atresia. Semin Pediatr Surg. (2017) 26:295–300. doi: 10.1053/j.sempedsurg.2017.09.003
8. Chardot C, Buet C, Serinet MO, Golmard JL, Lachaux A, Roquelaure B, et al. Improving outcomes of biliary atresia: French national series 1986-2009. J Hepatol. (2013) 58:1209–17. doi: 10.1016/j.jhep.2013.01.040
9. Jain V, Burford C, Alexander EC, Sutton H, Dhawan A, Joshi D, et al. Prognostic markers at adolescence in patients requiring liver transplantation for biliary atresia in adulthood. J Hepatol. (2019) 71:71–7. doi: 10.1016/j.jhep.2019.03.005
10. Hukkinen M, Kerola A, Lohi J, Heikkila P, Merras-Salmio L, Jahnukainen T, et al. Treatment policy and liver histopathology predict biliary atresia outcomes: results after national centralization and protocol biopsies. J Am Coll Surg. (2018) 226:46–57.e1. doi: 10.1016/j.jamcollsurg.2017.09.009
11. Pakarinen MP, Johansen LS, Svensson JF, Bjornland K, Gatzinsky V, Stenstrom P, et al. Outcomes of biliary atresia in the Nordic countries - a multicenter study of 158 patients during 2005-2016. J Pediatr Surg. (2018) 53:1509–15. doi: 10.1016/j.jpedsurg.2017.08.048
12. Ge L, Zhan J, Gao W, Zhao S, Xu X, Dou R. Relevant factors for early liver transplantation after Kasai portoenterostomy. BMC Pediatr. (2020) 20:484. doi: 10.1186/s12887-020-02355-8
13. Tyraskis A, Davenport M. Steroids after the Kasai procedure for biliary atresia: the effect of age at Kasai portoenterostomy. Pediatr Surg Int. (2016) 32:193–200. doi: 10.1007/s00383-015-3836-3
14. Russo P, Magee JC, Anders RA, Bove KE, Chung C, Cummings OW, et al. Key histopathologic features of liver biopsies that distinguish biliary atresia from other causes of infantile cholestasis and their correlation with outcome: a multicenter study. Am J Surg Pathol. (2016) 40:1601–15. doi: 10.1097/PAS.0000000000000755
15. Goda T, Kawahara H, Kubota A, Hirano K, Umeda S, Tani G, et al. The most reliable early predictors of outcome in patients with biliary atresia after Kasai's operation. J Pediatr Surg. (2013) 48:2373–7. doi: 10.1016/j.jpedsurg.2013.08.009
16. Ihn K, Ho IG, Chang EY, Han SJ. Correlation between gamma-glutamyl transpeptidase activity and outcomes after Kasai portoenterostomy for biliary atresia. J Pediatr Surg. (2018) 53:461–67. doi: 10.1016/j.jpedsurg.2017.10.001
17. Hukkinen M, Pihlajoki M, Pakarinen MP. Predicting native liver injury and survival in biliary atresia. Semin Pediatr Surg. (2020) 29:150943. doi: 10.1016/j.sempedsurg.2020.150943
18. Huang CY, Chang MH, Chen HL, Ni YH, Hsu HY, Wu JF. Bilirubin level 1 week after hepatoportoenterostomy predicts native liver survival in biliary atresia. Pediatr Res. (2020) 87:730–734. doi: 10.1038/s41390-019-0610-6
19. Davenport M, De Ville DGJ, Stringer MD, Mieli-Vergani G, Kelly DA, McClean P, et al. Seamless management of biliary atresia in England and Wales (1999-2002). Lancet. (2004) 363:1354–7. doi: 10.1016/S0140-6736(04)16045-5
20. Intraobserver interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. (1994) 20:15–20. doi: 10.1002/hep.1840200104
21. Zhou LY, Wang W, Shan QY, Liu BX, Zheng YL, Xu ZF, et al. Optimizing the US diagnosis of biliary atresia with a modified triangular cord thickness and gallbladder classification. Radiology. (2015) 277:181–91. doi: 10.1148/radiol.2015142309
22. Shen C, Zheng S, Wang W, Xiao XM. Relationship between prognosis of biliary atresia and infection of cytomegalovirus. World J Pediatr. (2008) 4:123–6. doi: 10.1007/s12519-008-0024-8
23. Zhao Y, Xu X, Liu G, Yang F, Zhan J. Prognosis of biliary atresia associated with cytomegalovirus: a meta-analysis. Front Pediatr. (2021) 9:710450. doi: 10.3389/fped.2021.710450
24. Zani A, Quaglia A, Hadzic N, Zuckerman M, Davenport M. Cytomegalovirus-associated biliary atresia: an aetiological and prognostic subgroup. J Pediatr Surg. (2015) 50:1739–45. doi: 10.1016/j.jpedsurg.2015.03.001
25. Sun S, Zheng S, Lu X, Chen G, Ma Y, Chen L, et al. Clinical and pathological features of patients with biliary atresia who survived for more than 5 years with native liver. Pediatr Surg Int. (2018) 34:381–6. doi: 10.1007/s00383-018-4231-7
26. Shankar S, Bolia R, Foo HW, D'Arcy CE, Hardikar N, Wensing M, et al. Normal gamma glutamyl transferase levels at presentation predict poor outcome in biliary atresia. J Pediatr Gastroenterol Nutr. (2020) 70:350–5. doi: 10.1097/MPG.0000000000002563
27. Dike PN, Mahmood N, Harpavat S. Recent advances in the use of ultrasound and related techniques in diagnosing and predicting outcomes in biliary atresia. Curr Opin Pediatr. (2021) 33:515–20. doi: 10.1097/MOP.0000000000001048
28. Raizner A, Shillingford N, Mitchell PD, Harney S, Raza R, Serino J, et al. Hepatic inflammation may influence liver stiffness measurements by transient elastography in children and young adults. J Pediatr Gastroenterol Nutr. (2017) 64:512–7. doi: 10.1097/MPG.0000000000001376
29. Wu JF, Lee CS, Lin WH, Jeng YM, Chen HL, Ni YH, et al. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology. (2018) 68:616–24. doi: 10.1002/hep.29856
30. Shen Q, Tan SS, Wang Z, Cai S, Pang W, Peng C, et al. Combination of gamma-glutamyl transferase and liver stiffness measurement for biliary atresia screening at different ages: a retrospective analysis of 282 infants. BMC Pediatr. (2020) 20:276. doi: 10.1186/s12887-020-02172-z
31. Liu JQ, Chen WJ, Zhou MJ, Li WF, Tang J, Zhou QC. A nomogram predicting the prognosis of children with biliary atresia after hepatoportoenterostomy. Front Pediatr. (2021) 9:641318. doi: 10.3389/fped.2021.641318
32. Bezerra JA, Wells RG, Mack CL, Karpen SJ, Hoofnagle JH, Doo E, et al. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology. (2018) 68:1163–1173. doi: 10.1002/hep.29905
33. Cheng K, Molleston JP, Bennett WJ. Cholangitis in patients with biliary atresia receiving hepatoportoenterostomy: a national database study. J Pediatr Gastroenterol Nutr. (2020) 71:452–8. doi: 10.1097/MPG.0000000000002836
34. Ginstrom DA, Hukkinen M, Kivisaari R, Pakarinen MP. Biliary atresia-associated cholangitis: the central role and effective management of bile lakes. J Pediatr Gastroenterol Nutr. (2019) 68:488–94. doi: 10.1097/MPG.0000000000002243
35. Garcia AV, Ladd MR, Crawford T, Culbreath K, Tetteh O, Alaish SM, et al. Analysis of risk factors for morbidity in children undergoing the Kasai procedure for biliary atresia. Pediatr Surg Int. (2018) 34:837–44. doi: 10.1007/s00383-018-4298-1
Keywords: biliary atresia, Kasai, clearance of jaundice, nomogram, prediction model
Citation: Zhang Y, Wang Q, Pu S, Wang J, Xiang B, Liu J and Jin S (2022) A Novel Model for Predicting the Clearance of Jaundice in Patients With Biliary Atresia After Kasai Procedure. Front. Pediatr. 10:837247. doi: 10.3389/fped.2022.837247
Received: 16 December 2021; Accepted: 06 January 2022;
Published: 31 January 2022.
Edited by:
Zenon Pogorelić, University Hospital of Split, CroatiaReviewed by:
Timo Jahnukainen, Helsinki University Hospital, FinlandSachit Anand, Kokilaben Dhirubhai Ambani Hospital and Medical Research Institute, India
Copyright © 2022 Zhang, Wang, Pu, Wang, Xiang, Liu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuguang Jin, 18482379056@163.com; Juxian Liu, ljxhuaxip1@126.com
†These authors have contributed equally to this work and share first authorship
 Yimao Zhang1†
Yimao Zhang1† Siyu Pu
Siyu Pu Bo Xiang
Bo Xiang Shuguang Jin
Shuguang Jin