- Department of Ophthalmology, The People’s Hospital of Leshan, Leshan, China
Epidemiological data about the prevalence of amblyopia around the world vary widely among regions and periods. This meta-analysis aimed to determine the global prevalence of amblyopia in children. PubMed, Embase, and the Cochrane Library were searched for prevalence studies published up to 5 November 2021. The outcome was the prevalence of amblyopia, analyzed as pooled estimates with 95% confidence intervals (CI). A total of 97 studies were included, including 4,645,274 children and 7,706 patients with amblyopia. The overall worldwide pooled prevalence of amblyopia was 1.36% (95%CI: 1.27–1.46%). The prevalence of amblyopia was higher in males (1.40%, 95%CI: 1.10–1.70%) than in females (1.24%, 95%CI: 0.94–1.54%) (OR = 0.885, 95%CI: 0.795–0.985, P = 0.025). The results of the meta-regression analysis showed that there were no significant associations between the prevalence of amblyopia and geographical area, publication year, age, sample size, and whether it was carried out in a developed or developing country (all P > 0.05). Begg’s test (P = 0.065) and Egger’s test (P < 0.001) showed that there was a significant publication bias in the prevalence of amblyopia. In conclusion, amblyopia is a significant vision problem worldwide, and public health strategies of early screening, treatment, and management are important.
Introduction
Amblyopia is a common vision disorder among children and is defined as decreased vision due to abnormal development of the visual cortex in infancy or childhood. Amblyopia is a reduction in best-corrected visual acuity (BCVA) (2-line difference between the two eyes) secondary to neurological deficits in visual output caused by abnormal brain stimulation during critical periods of visual development (1–3). It is usually unilateral and is the most common cause of vision loss and mononuclear blindness in children (1–3). It can be caused by any condition that creates a disparity in vision between the two eyes (1), and 90% of the cases are reportedly caused by strabismus and/or anisometropia (1–3). The treatment is characterized by the occlusion and penalization of the better-seeing eye while forcing the use of the amblyopic eye (3). The initial treatment includes refractive correction of visual impairment in the affected eye(s) with eyeglasses, the correction of any strabismus with glasses or surgery if severe, and the removal of any obstacle to vision, such as cataract or local hemangioma (3). Possible complications include the permanent loss of vision in the affected eye (1, 2), estimated at 1.2% lifetime risk (4). If detected early, most patients will have normal vision restored (2).
The reported incidence is 1–5% worldwide and 2–4% in North America (2, 3), but the reported prevalence varies widely among studies, from 0.05 to 7.54% (5–21). Those studies are from various countries and different periods and included children of different age groups. Therefore, this data must be considered to be highly fragmented. A meta-analysis of 73 studies, published in 2018, showed that the pooled prevalence of amblyopia was 1.75%, varying from 0.51% in Africa to 3.67% in Europe (22). A meta-analysis of 60 studies, published in 2019, reported a pooled prevalence of 1.44% among children and young adults, with 0.72% in Africa, 1.09% in Asia, 2.41% in America, and 2.90% in Europe (23). Since the publication of these meta-analyses, novel studies have been published that could help improve the global estimates.
Therefore, the purpose of this meta-analysis was to determine the global prevalence of amblyopia in children. Improvements in screening methods and policies might lead to changes in the prevalence of amblyopia, which could be important for public health and decision-makers. More accurate estimates might be important for policymakers in public health.
Methods
Literature Search
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (24). Since no original clinical raw data was collected or used, ethical approval was not requested for this meta-analysis.
Three recognized electronic databases, PubMed, Embase, and the Cochrane Library, were searched for studies published from inception up to 5 November 2021, using the MeSH terms of “child,” “amblyopia,” “prevalence,” and “epidemiology” combined with relevant key words. The eligibility criteria were (1) study type: prevalence study (because this study aimed to summarize the global prevalence), (2) population: since amblyopia occurs in childhood, only studies reporting data in juveniles/children were included (because the definition of children or juvenile varies among countries, a study could be included as long as the authors considered their study population to be underage), (3) outcome: prevalence of amblyopia, and (4) full text published in English. Special groups, such as hospitalized patients or patients with certain ocular or systemic diseases, were excluded. If prevalence data or overlapping groups of participants were reported in multiple papers, the original paper was selected for inclusion.
Data Extraction and Quality Assessment
Potentially relevant publications were screened and evaluated by two reviewers (Budan Hu and Li Zeng) in a double-blind manner, with a third reviewer (Gengsheng Hao, Dan Shui, or Ke Mao) resolving any disagreement. A structured data collection form was developed. Two researchers (Zongshun Liu and Jiao Zhao) independently extracted the data, including authors, year of publication, country, study design, sample size, age, percentage of males, the definition of amblyopia, number of cases, number of subjects, and prevalence of amblyopia. “Mixed country” referred to studies that included countries from different continents.
The cross-sectional studies were evaluated using the Healthcare Research and Quality (AHRQ) tool (25). The cohort studies were evaluated according to the Newcastle-Ottawa scale (NOS) (26).
Statistical Analysis
All analyses were performed using STATA MP 14.0 (StataCorp, College Station, TX, United States). Prevalence with the 95% confidence interval (CI) was combined for statistical analysis. Prevalence estimates were converted using the Freeman-Tukey transformation and back-transformed after quantitative data synthesis (27). Statistical heterogeneity among studies was calculated using Cochran’s Q-test and the I2 index. An I2 > 50% and Q-test P < 0.10 indicated high heterogeneity, and the random-effects model was used; otherwise, the fixed-effects model was applied. In the case of the random-effects model, the tau square was calculated as instructed in the Cochrane Handbook (28). P-values = 0.05 were considered statistically significant. Potential publication bias (resulting from the publication or non-publication of relevant trials) was assessed by funnel plots, Egger’s test, and Begg’s test (28–30). Univariable meta-regression models were used to investigate the effect of age, sample size, publication year, developed or developing region, and geographical location as factors affecting the prevalence of amblyopia. Subgroups were compared using odds ratios (OR) and 95%CI.
Results
Selection of the Studies
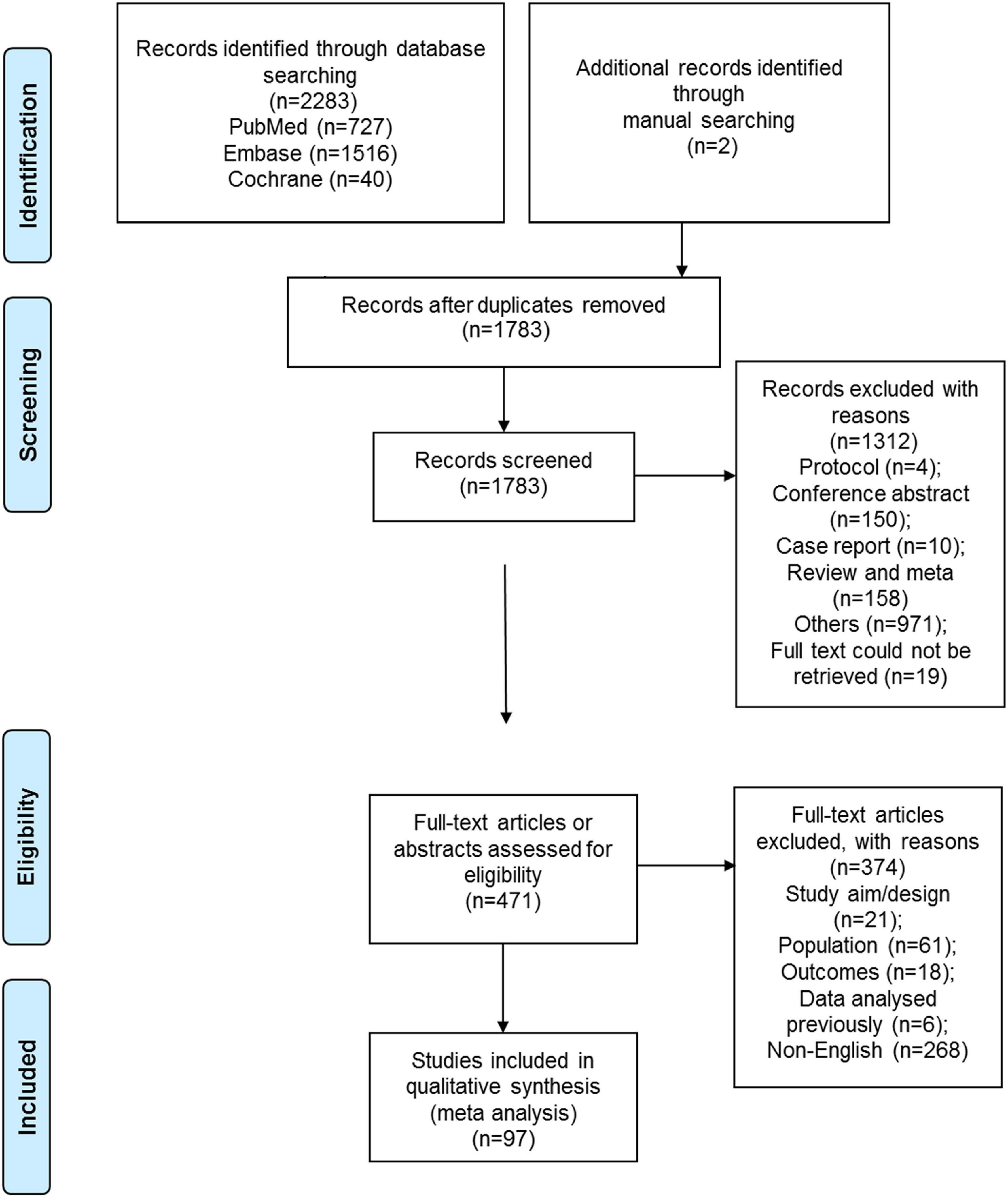
Figure 1 shows the selection process. A total of 2,283 records were retrieved, and 1,783 were left after removing the duplicates. Supplementary Figure 1 presents the search strings for PubMed. From these, 1,312 were excluded after screening the titles and abstracts, and 471 full-text papers were assessed for eligibility. From them, 21 were excluded because of study aim or design, 61 because of the study population, 18 because of the outcomes, 6 because of previously analyzed data, and 268 for non-English full-text papers. Finally, 97 studies were included in the present meta-analysis (Supplementary Table 2). A total of 4,645,274 children were included, including 7,706 patients with amblyopia. The study quality assessments are presented in Supplementary Tables 3a,b.
Pooled Prevalence of Amblyopia
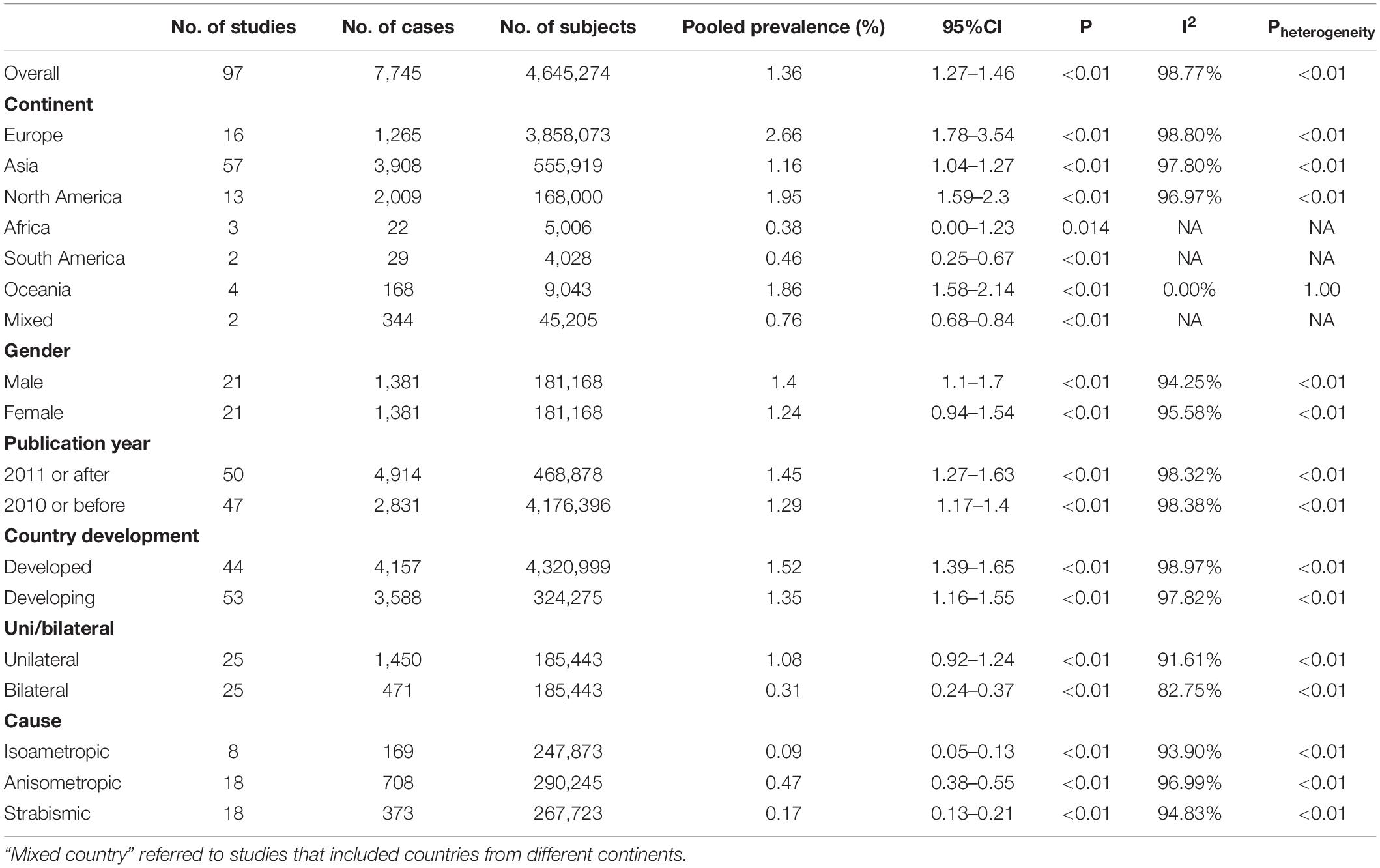
The overall worldwide pooled prevalence of amblyopia was 1.36% (95%CI: 1.27–1.46%; I2 = 98.8%, Pheterogeneity < 0.01). When considering each continent, the pooled prevalence of amblyopia was 2.66% (95%CI: 1.78–3.54%; I2 = 98.8%, Pheterogeneity < 0.01) in Europe, 1.95% (95%CI: 1.59–2.30%; I2 = 97.0%, Pheterogeneity < 0.01) in North America, 1.86% (95%CI: 1.58–2.14%; I2 = 0.0%, Pheterogeneity > 0.99) in Oceania, 1.16% (95%CI: 1.04–1.27%; I2 = 97.8%, Pheterogeneity < 0.01) in Asia, 0.46% (95%CI: 0.25–0.67%) in South America, 0.38% (95%CI: 0.00–1.23%) in Africa, and 0.76% (95%CI: 0.68–0.84%) in mixed countries (Figures 2, 3, Supplementary Figure 1, and Table 1).
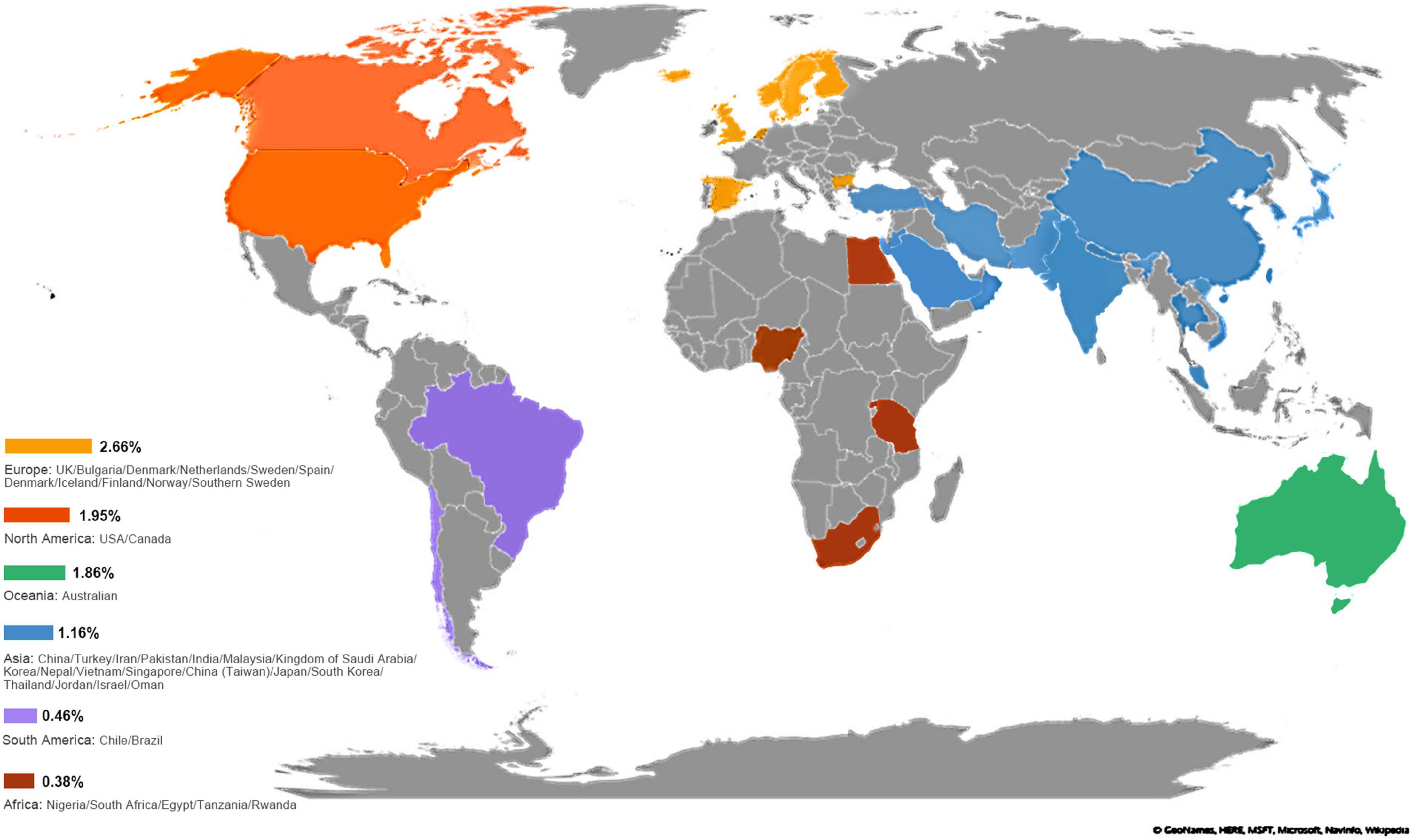
Figure 2. Pooled prevalence of amblyopia based on countries. The reported prevalence values were pooled for each country. A darker shade of blue indicates higher prevalence. No data were available for the countries in gray.
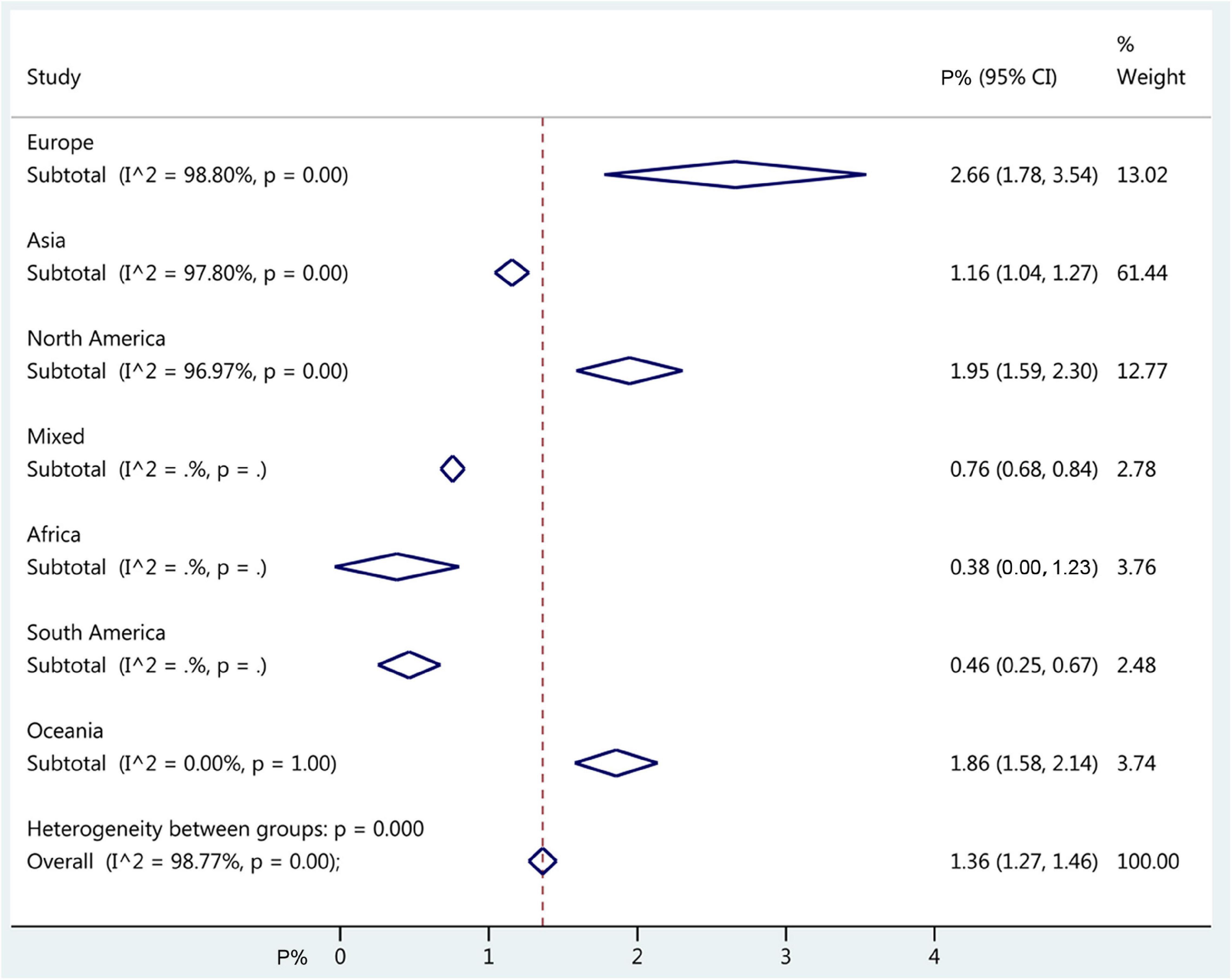
Figure 3. Forest plot of pooled continent prevalence of amblyopia. The diamonds represent the prevalence estimates with 95% confidence intervals (CIs). The dashed red line represents the global estimate. The x-axis represents the prevalence of amblyopia, in%. “Mixed country” referred to studies that included countries from different continents.
Subgroup Analyses
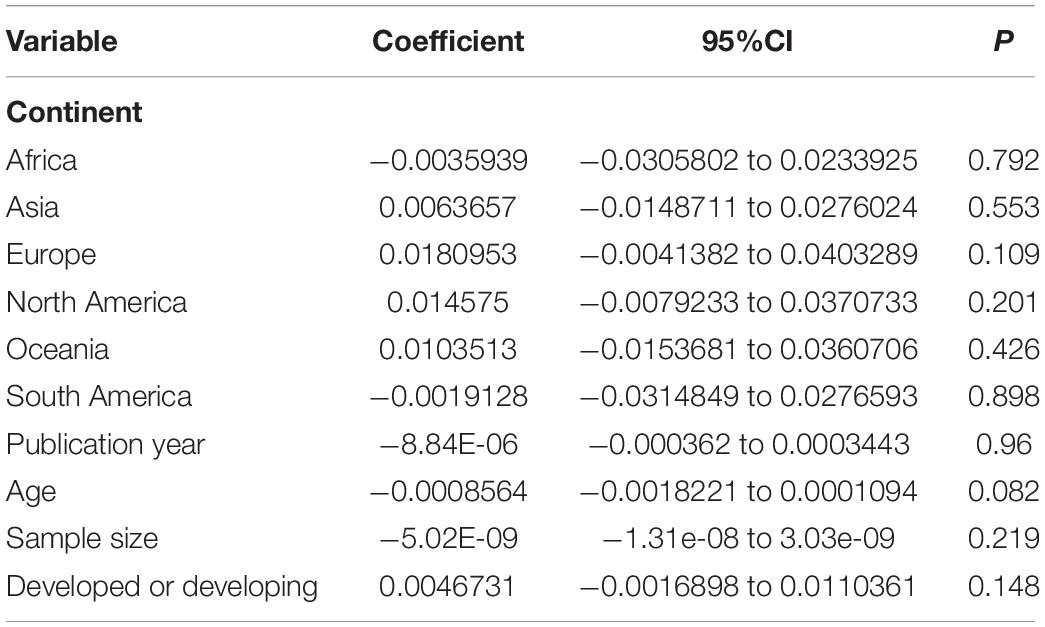
The prevalence of amblyopia was higher in males (1.40%, 95%CI: 1.10–1.70%; I2 = 94.3%, Pheterogeneity < 0.01) than in females (1.24%, 95%CI: 0.94–1.54%; I2 = 95.6%, Pheterogeneity < 0.01) (OR = 0.885, 95%CI: 0.795–0.985, P = 0.025) (Figure 4 and Table 1). The results of the meta-regression analysis showed that there were no significant associations between the prevalence of amblyopia and geographical area, publication year, age, sample size, and whether it was carried out in a developed or developing country (Table 2).
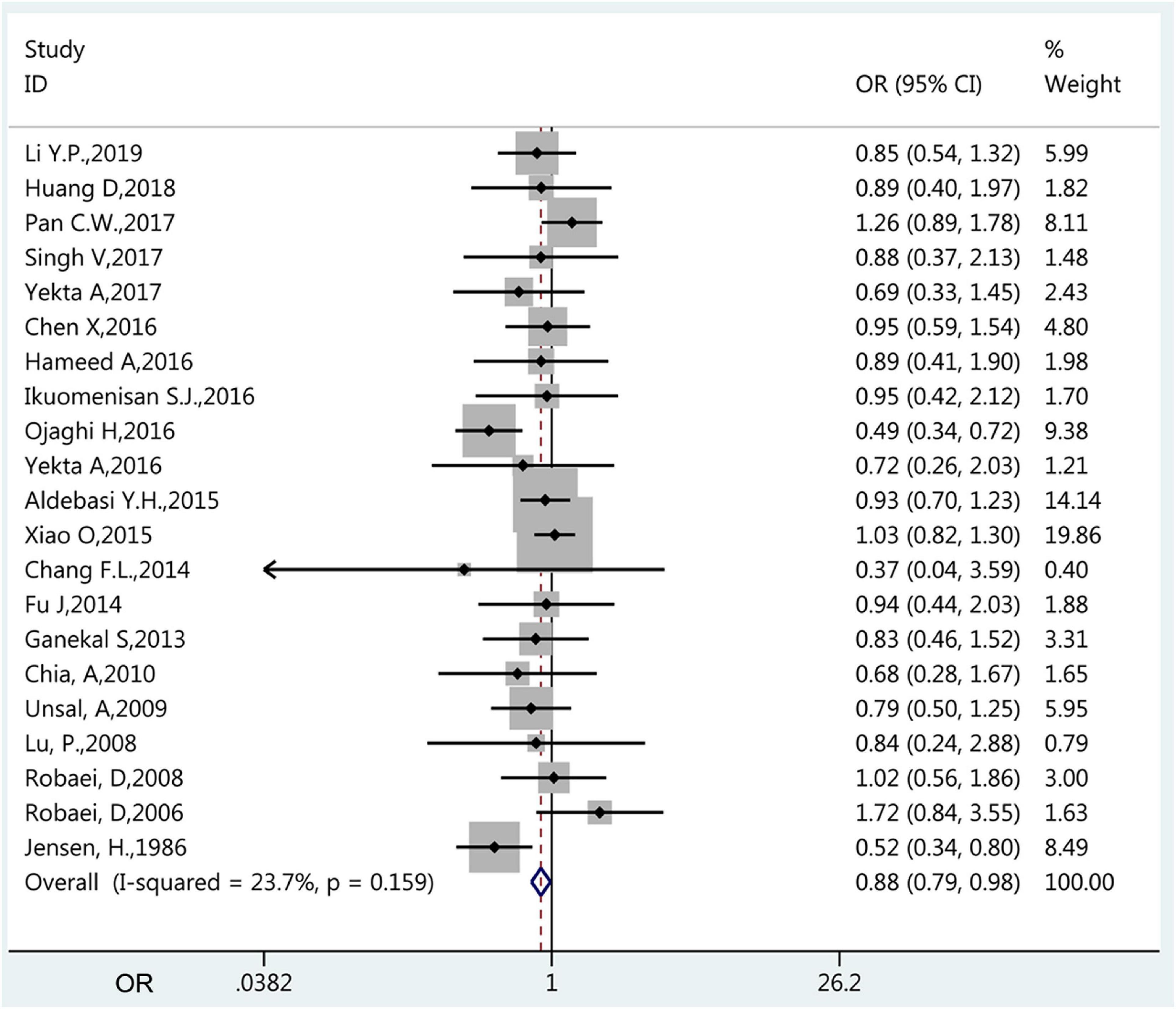
Figure 4. Forest plot of prevalence of amblyopia of female vs. male. The small diamonds are the odds ratios (ORs), the line represents the 95% confidence intervals (CIs), and the gray boxes represent the proportional sample size. The x-axis represents the ORs. This analysis only included the studies (n = 20) that compared males vs. females.
Assessment of Publication Bias
Begg’s test (P = 0.065) and Egger’s test (P < 0.001) showed that there was a significant publication bias in the prevalence of amblyopia.
Discussion
Epidemiological data about the prevalence of amblyopia around the world vary widely among regions and periods. Therefore, the present meta-analysis aimed to determine the global prevalence of amblyopia in children. The results indicate that the prevalence of amblyopia varies between boys and girls, but not according to the geographical area, publication year, age, sample size, and economic status. Nevertheless, it still is a significant vision problem worldwide, and public health strategies of early screening, treatment, and management are important.
In the present study, the worldwide pooled prevalence of amblyopia was 1.36%. It is supported by previous meta-analyses as it is within the range of the reported pooled prevalence rates, with 1.44% for Fu et al. (23) and 1.75% for Hashemi et al. (22). Indeed, Simons et al. (31) reported in 2005 a worldwide prevalence of 1.6–3.6%.
Xiao et al. (32) reported a prevalence of 0.625%, while other studies in China reported a prevalence of around 1.19% (11, 33, 34). High prevalence in Europe has been reported (35, 36) and the United States of America (37–39). Hashemi et al. (22) reported that the pooled prevalence of amblyopia varied from 0.51% in Africa to 3.67% in Europe, while Fu et al. (23) reported that the pooled prevalence of amblyopia was 0.72% in Africa, 1.09% in Asia, 2.41% in America, and 2.90% in Europe. The present meta-analysis observed similar trends, with 2.66% in Europe, 1.95% in North America, 1.86% in Oceania, 1.16% in Asia, 0.46% in South America, and 0.38% in Africa. The meta-regression analysis showed that those differences were not statistically significant. Nevertheless, those apparent differences might be explained by the socio-economic status of the continents, leading to different access to screening. It is in contrast with the study by Hashemi et al. (22), whose meta-regression analysis revealed differences among continents, and by other studies as well (32, 38, 40–42), but Simons et al. (31) rejected the presence of ethnic differences in the prevalence of amblyopia, supporting the present study.
The present meta-analysis revealed a significant difference in the prevalence of amblyopia between boys and girls, with a higher prevalence in boys. It is in contradiction with Fu et al. (23), who reported a higher prevalence in girls. A study from Nigeria reported that all cases were males (43). Nevertheless, sex is not recognized as a risk factor for amblyopia (1–3), and those differences warrant further study.
In the present study, there were no significant differences among the causes of amblyopia. Previous studies reported that anisometropia was the most common cause of amblyopia (22, 31, 36), which is fortunate because it is the most easily treatable form of amblyopia (31), involving mostly optical correction without occlusion or penalization. Nevertheless, a source of bias that could explain the lack of differences among the causes of amblyopia in the present study could be that the causes vary according to ethnicity. Indeed, strabismus might be more common in non-Hispanic Caucasians than in Asians (44, 45), while anisometropic amblyopia could be more prevalent in the Middle East (46, 47).
Previous studies reported differences in the prevalence of amblyopia among periods, probably due to more or less attention given to the disease, public awareness, and screening programs (23, 48, 49). Such a difference was not observed in the present meta-analysis.
In the present study, the publication bias was high. It is supported by the recent previous meta-analyses on the prevalence of amblyopia worldwide (22, 23).
Of course, the results of the present meta-analysis must be considered alongside its limitations. Indeed, despite the high numbers of included studies and children, nearly all analyses suffered from significantly high heterogeneity. This high heterogeneity is probably rooted in the different countries, periods, diagnostic tools, screening policies, and economic status. Nevertheless, the subgroup results must be taken with caution since some subgroups contained only a small number of studies/participants. Second, the definition of juvenile/child varies among countries, and the studies were included if their authors considered their study population to be a child. Therefore, some patients were included but would be considered young adults by some authors. It had to be done because, without access to the raw data, it would be impossible to exclude older participants specifically. Third, not all studies reported differences between boys and girls, which could bias the results.
In conclusion, the present meta-analysis indicates that the prevalence of amblyopia varies between boys and girls, but not according to the geographical area, publication year, age, sample size, and economic status. Nevertheless, it still is a significant vision problem worldwide, and public health strategies of early screening, treatment, and management are important.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
BH and LZ carried out the studies, participated in collecting data, and drafted the manuscript. ZL and JZ performed the statistical analysis and participated in its design. GH, DS, and KM helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.819998/full#supplementary-material
References
1. Gunton KB. Advances in amblyopia: what have we learned from pedig trials? Pediatrics. (2013) 131:540–7. doi: 10.1542/peds.2012-1622
3. Pescosolido N, Stefanucci A, Buomprisco G, Fazio S. Amblyopia treatment strategies and new drug therapies. J Pediatr Ophthalmol Strabismus. (2014) 51:78–86. doi: 10.3928/01913913-20130107-01
4. Rahi J, Logan S, Timms C, Russell-Eggitt I, Taylor D. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet. (2002) 360:597–602. doi: 10.1016/s0140-6736(02)09782-9
5. Abolfotouh MA, Badawi I, Faheem Y. Prevalence of amblyopia among schoolboys in Abha City, Asir Region, Saudi Arabia. J Egypt Public Health Assoc. (1994) 69:19–30.
6. Aldebasi YH. Prevalence of amblyopia in primary school children in qassim province, kingdom of Saudi Arabia. Middle East Afr J Ophthalmol. (2015) 22:86–91. doi: 10.4103/0974-9233.148355
7. Al-Rowaily MA. Prevalence of refractive errors among pre-school children at King Abdulaziz Medical City, Riyadh, Saudi Arabia. Saudi J Ophthalmol. (2010) 24:45–8. doi: 10.1016/j.sjopt.2010.01.001
8. Azizoglu S, Crewther SG, Serefhan F, Barutchu A, Goker S, Junghans BM. Evidence for the need for vision screening of school children in turkey. BMC Ophthalmol. (2017) 17:230. doi: 10.1186/s12886-017-0618-9
9. Caca I, Cingu AK, Sahin A, Ari S, Dursun ME, Dag U, et al. Amblyopia and refractive errors among school-aged children with low socioeconomic status in Southeastern Turkey. J Pediatr Ophthalmol Strabismus. (2013) 50:37–43. doi: 10.3928/01913913-20120804-02
10. Chang FL, Lee YC, Chen N, Hsieh HP, Li EH, Yang YY, et al. The prevalence of ocular diseases in primary and junior high school students on orchid Island. Tzu Chi Med J. (2014) 26:166–9. doi: 10.1016/j.tcmj.2014.08.002
11. Chen X, Fu Z, Yu J, Ding H, Bai J, Chen J, et al. Prevalence of amblyopia and strabismus in Eastern China: results from screening of preschool children aged 36-72 months. Br J Ophthalmol. (2016) 100:515–9. doi: 10.1136/bjophthalmol-2015-306999
12. Chia A, Dirani M, Chan YH, Gazzard G, Au Eong KG, Selvaraj P, et al. Prevalence of amblyopia and strabismus in young Singaporean Chinese children. Invest Ophthalmol Vis Sci. (2010) 51:3411–7. doi: 10.1167/iovs.09-4461
13. Chia A, Lin X, Dirani M, Gazzard G, Ramamurthy D, Quah BL, et al. Risk factors for strabismus and amblyopia in young Singapore Chinese children. Ophthalmic Epidemiol. (2013) 20:138–47. doi: 10.3109/09286586.2013.767354
14. Dandona R, Dandona L, Srinivas M, Sahare P, Narsaiah S, Munoz SR, et al. Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci. (2002) 43:615–22.
15. de Koning HJ, Groenewoud JH, Lantau VK, Tjiam AM, Hoogeveen WC, de Faber JT, et al. Effectiveness of screening for amblyopia and other eye disorders in a prospective birth cohort study. J Med Screen. (2013) 20:66–72. doi: 10.1177/0969141313497355
16. Dikova SP, Dragoev SA, Chernodrinska VS. Prevalence of amblyopia in Bulgaria. Strabismus. (2018) 26:163–7. doi: 10.1080/09273972.2018.1530266
17. Donnelly UM, Stewart NM, Hollinger M. Prevalence and outcomes of childhood visual disorders. Ophthalmic Epidemiol. (2005) 12:243–50. doi: 10.1080/09286580590967772
18. Drover JR, Kean PG, Courage ML, Adams RJ. Prevalence of amblyopia and other vision disorders in young newfoundland and Labrador children. Can J Ophthalmol. (2008) 43:89–94. doi: 10.3129/i07-187
19. Eibschitz-Tsimhoni M, Friedman T, Naor J, Eibschitz N, Friedman Z. Early screening for amblyogenic risk factors lowers the prevalence and severity of amblyopia. J AAPOS. (2000) 4:194–9. doi: 10.1067/mpa.2000.105274
20. Faghihi M, Ostadimoghaddam H, Yekta AA. Amblyopia and strabismus in Iranian schoolchildren, Mashhad. Strabismus. (2011) 19:147–52. doi: 10.3109/09273972.2011.622341
21. Flom MC, Neumaier RW. Prevalen‘ce of amblyopia. Public Health Rep. (1966) 81: 329–41. doi: 10.1097/00006324-196611000-00003
22. Hashemi HM, Pakzad RM, Yekta AP, Bostamzad PM, Aghamirsalim MM, Sardari SM, et al. Global and regional estimates of prevalence of amblyopia: a systematic review and meta-analysis. Strabismus. (2018) 26:168–83. doi: 10.1080/09273972.2018.1500618
23. Fu Z, Hong H, Su Z, Lou B, Pan CW, Liu H. Global prevalence of amblyopia and disease burden projections through 2040: a systematic review and meta-analysis. Br J Ophthalmol. (2019) 104:1164–70. doi: 10.1136/bjophthalmol-2019-314759
24. Selcuk AA. A guide for systematic reviews: Prisma. Turk Arch Otorhinolaryngol. (2019) 57:57–8. doi: 10.5152/tao.2019.4058
25. Smetana GW, Umscheid CA, Chang S, Matchar DB. Editorial: methods guide for authors of systematic reviews of medical tests: a collaboration between the agency for healthcare research and quality (AHRQ) and the journal of general internal medicine. In: Chang SM, Matchar DB, Smetana GW, Umscheid CA editors. Methods Guide for Medical Test Reviews. (Rockville, MD: Agency for Healthcare Research and Quality) (2012).
26. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
27. Lin L, Xu C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci Rep. (2020) 3:e178. doi: 10.1002/hsr2.178
28. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1. London: Cochrane Collaboration (2020).
29. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
30. Sedgwick P. What is publication bias in a meta-analysis? BMJ. (2015) 351:h4419. doi: 10.1136/bmj.h4419
31. Simons K. Amblyopia characterization, treatment, and prophylaxis. Surv Ophthalmol. (2005) 50:123–66. doi: 10.1016/j.survophthal.2004.12.005
32. Xiao O, Morgan IG, Ellwein LB, He M. Refractive error study in children study G, prevalence of amblyopia in school-aged children and variations by age, gender, and ethnicity in a multi-country refractive error study. Ophthalmology. (2015) 122:1924–31. doi: 10.1016/j.ophtha.2015.05.034
33. Huang D, Chen X, Zhu H, Ding H, Bai J, Chen J, et al. Prevalence of amblyopia and its association with refraction in Chinese preschool children aged 36-48 months. Br J Ophthalmol. (2018) 102:767–71. doi: 10.1136/bjophthalmol-2016-310083
34. Fu J, Li SM, Li SY, Li JL, Li H, Zhu BD, et al. Prevalence, causes and associations of amblyopia in year 1 students in central China : the Anyang childhood eye study (aces). Graefes Arch Clin Exp Ophthalmol. (2014) 252:137–43. doi: 10.1007/s00417-013-2451-z
35. Williams C, Northstone K, Howard M, Harvey I, Harrad RA, Sparrow JM. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. (2008) 92:959–64. doi: 10.1136/bjo.2007.134700
36. Elflein HM. [Amblyopia. Epidemiology, causes and risk factors]. Ophthalmologe. (2016) 113:283–8. doi: 10.1007/s00347-016-0247-3
37. Pascual M, Huang J, Maguire MG, Kulp MT, Quinn GE, Ciner E, et al. Risk factors for amblyopia in the vision in preschoolers study. Ophthalmology. (2014) 121: 622–9.e1. doi: 10.1016/j.ophtha.2013.08.040
38. Multi-ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia and strabismus in African American and Hispanic children ages 6 to 72 months the multi-ethnic pediatric eye disease study. Ophthalmology. (2008) 115: 1229–1236.e1. doi: 10.1016/j.ophtha.2007.08.001
39. Ohlsson J, Villarreal G, Sjostrom A, Abrahamsson M, Sjostrand J. Visual acuity, residual amblyopia and ocular pathology in a screened population of 12-13-year-old children in Sweden. Acta Ophthalmol Scand. (2001) 79:589–95. doi: 10.1034/j.1600-0420.2001.790609.x
40. Pan CW, Chen X, Zhu H, Fu Z, Zhong H, Li J, et al. School-based assessment of amblyopia and strabismus among multiethnic children in rural China. Sci Rep. (2017) 7:13410. doi: 10.1038/s41598-017-13926-8
41. McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, Wen G, Kim J, Borchert M, et al. Prevalence of amblyopia or strabismus in Asian and non-hispanic white preschool children: Multi-ethnic pediatric eye disease study. Ophthalmology. (2013) 120:2117–24. doi: 10.1016/j.ophtha.2013.03.001
42. Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence and causes of visual impairment in African-American and hispanic preschool children: the multi-ethnic pediatric eye disease study. Ophthalmology. (2009) 116:1990–2000e1. doi: 10.1016/j.ophtha.2009.03.027
43. Megbelayin EO. Prevalence of amblyopia among secondary school students in Calabar, South-South Nigeria. Niger J Med. (2012) 21:407–11.
44. Lithander J. Prevalence of amblyopia with anisometropia or strabismus among schoolchildren in the sultanate of Oman. Acta Ophthalmol Scand. (1998) 76:658–62. doi: 10.1034/j.1600-0420.1998.760604.x
45. Ying GS, Maguire MG, Cyert LA, Ciner E, Quinn GE, Kulp MT, et al. Prevalence of vision disorders by racial and ethnic group among children participating in head start. Ophthalmology. (2014) 121:630–6. doi: 10.1016/j.ophtha.2013.09.036
46. Yekta A, Fotouhi A, Hashemi H, Dehghani C, Ostadimoghaddam H, Heravian J, et al. The prevalence of anisometropia, amblyopia and strabismus in schoolchildren of Shiraz, Iran. Strabismus. (2010) 18:104–10. doi: 10.3109/09273972.2010.502957
47. Jamali P, Fotouhi A, Hashemi H, Younesian M, Jafari A. Refractive errors and amblyopia in children entering school: Shahrood, Iran. Optom Vis Sci. (2009) 86:364–9. doi: 10.1097/OPX.0b013e3181993f42
48. Rafiei M, Rivakani F, Torabi L, Alaeddini F, Safiri S. Community-based amblyopia screening program for early detection in iran: a repeated cross-sectional study from 1996 to 2013. Public Health. (2017) 142:196–200. doi: 10.1016/j.puhe.2015.06.011
49. Shapira Y, Machluf Y, Mimouni M, Chaiter Y, Mezer E. Amblyopia and strabismus: trends in prevalence and risk factors among young adults in Israel. Br J Ophthalmol. (2018) 102:659–66. doi: 10.1136/bjophthalmol-2017-310364
50. Harrington S, Breslin K, O’Dwyer V, Saunders K. Comparison of amblyopia in schoolchildren in Ireland and Northern Ireland: a population-based observational cross-sectional analysis of a treatable childhood visual deficit. BMJ Open. (2019) 9:e031066. doi: 10.1136/bmjopen-2019-031066
51. Li YP, Zhou MW, Forster SH, Chen SY, Qi X, Zhang HM, et al. Prevalence of amblyopia among preschool children in central South China. Int J Ophthalmol. (2019) 12:820–5. doi: 10.18240/ijo.2019.05.19
52. Ugurbas SC, Kucuk N, Isik I, Alpay A, Buyukuysal C, Ugurbas SH. Objective vision screening using plusoptix for children aged 3-11 years in rural turkey. BMC Ophthalmol. (2019) 19:73. doi: 10.1186/s12886-019-1080-7
53. Zhu H, Pan C, Sun Q, Huang D, Fu Z, Wang J, et al. Prevalence of amblyopia and strabismus in Hani school children in rural southwest china: a cross-sectional study. BMJ Open. (2019) 9:e025441. doi: 10.1136/bmjopen-2018-025441
54. Ghaderi S, Hashemi H, Jafarzadehpur E, Yekta A, Ostadimoghaddam H, Mirzajani A, et al. The prevalence and causes of visual impairment in seven-year-old children. Clin Exp Optom. (2018) 101:380–5. doi: 10.1111/cxo.12646
55. Hansen MH, Munch IC, Li XQ, Skovgaard AM, Olsen EM, Larsen M, et al. Visual acuity and amblyopia prevalence in 11- to 12-year-old Danish children from the Copenhagen child cohort 2000. Acta Ophthalmol. (2019) 97:29–35. doi: 10.1111/aos.13842
56. Khan TP, Humayun F. Amblyopic risk factors and its prevalence among growing children in our population. Pakistran J Med Health Sci. (2018) 12:309–11.
57. Magdalene D, Bhattacharjee H, Choudhury M, Multani PK, Singh A, Deshmukh S, et al. Community outreach: an indicator for assessment of prevalence of amblyopia. Indian J Ophthalmol. (2018) 66:940–4. doi: 10.4103/ijo.IJO_1335_17
58. Min FCL, Thavaratnam LK, Shukor INCB, Tamasamy S, Rahmat J, Reidpath DD, et al. Visual impairment and amblyopia in Malaysian pre-school children – the segpaeds study. J Med Malaysia. (2018) 73:25–30. doi: 10.1177/026461969601400105
59. Sandfeld L, Weihrauch H, Tubaek G, Mortzos P. Ophthalmological data on 4.5- to 7-year-old Danish children. Acta Ophthalmol. (2018) 96:379–83. doi: 10.1111/aos.13650
60. Singh V, Malik KPS, Malik VK, Jain K. Prevalence of ocular morbidity in school going children in west Uttar Pradesh. Indian J Ophthalmol. (2017) 65:500–8. doi: 10.4103/ijo.IJO_676_15
61. Uddin M, Omar R, Feizal V, Alam K. Ocular morbidity among preschool children in urban area of Chittagong in Bangladesh. Int Eye Sci. (2017) 17:16–20.
62. Yekta A, Hashemi H, Norouzirad R, Ostadimoghaddam H, Nabovati P, Dadbin N, et al. The prevalence of amblyopia, strabismus, and ptosis in schoolchildren of Dezful. Eur J Ophthalmol. (2017) 27:109–12. doi: 10.5301/ejo.5000795
63. Griffith JF, Wilson R, Cimino HC, Patthoff M, Martin DF, Traboulsi EI. The use of a mobile van for school vision screening: results of 63 841 evaluations. Am J Ophthalmol. (2016) 163: 108–114.e1. doi: 10.1016/j.ajo.2015.11.026
64. Hameed A. Screening for refractive errors and visual impairment among school children in Kohat, Pakistan. Rawal Med J. (2016) 41:437–40.
65. Hendler K, Mehravaran S, Lu X, Brown SI, Mondino BJ, Coleman AL. Refractive errors and amblyopia in the Ucla preschool vision program; first year results. Am J Ophthalmol. (2016) 172:80–6. doi: 10.1016/j.ajo.2016.09.010
66. Ikuomenisan SJ, Musa KO, Aribaba OT, Onakoya AO. Prevalence and pattern of amblyopia among primary school pupils in Kosofe town, Lagos state, Nigeria. Niger Postgrad Med J. (2016) 23:196–201. doi: 10.4103/1117-1936.196261
67. Mehravaran S, Duarte PB, Brown SI, Mondino BJ, Hendler K, Coleman AL. The Ucla preschool vision program, 2012-2013. J AAPOS. (2016) 20:63–7. doi: 10.1016/j.jaapos.2015.10.018
68. Ojaghi H, Moghaddar R, Ahari SS, Bahadoram M, Amani F. Amblyopia prevention screening program in northwest Iran (Ardabil). Int J Prev Med. (2016) 7:45. doi: 10.4103/2008-7802.177887
69. Pan CW, Liu H. School-based myopia prevention effort. JAMA. (2016) 315:819. doi: 10.1001/jama.2015.17133
70. Yekta A, Hashemi H, Ostadimoghaddam H, Haghighi B, Shafiee H, Mehravaran S, et al. Strabismus and near point of convergence and amblyopia in 4-6 year-old children. Strabismus. (2016) 24:113–9. doi: 10.1080/09273972.2016.1205103
71. Jeong SH, Kim US. Ten-year results of home vision-screening test in children aged 3-6 years in Seoul, Korea. Semin Ophthalmol. (2015) 30:383–8. doi: 10.3109/08820538.2014.912335
72. Maqsud MA, Arblaster GE. The incidence and visual acuity outcomes of children identified with ametropic amblyopia by vision screening. J AAPOS. (2015) 19:104–7. doi: 10.1016/j.jaapos.2014.10.023
73. Yamamah GA, Talaat Abdel Alim AA, Mostafa YS, Ahmed RA, Mohammed AM. Prevalence of visual impairment and refractive errors in children of south Sinai, Egypt. Ophthalmic Epidemiol. (2015) 22:246–52. doi: 10.3109/09286586.2015.1056811
74. Fu J, Li SM, Liu LR, Li JL, Li SY, Zhu BD, et al. Prevalence of amblyopia and strabismus in a population of 7th-grade junior high school students in central China: the Anyang childhood eye study (aces). Ophthalmic Epidemiol. (2014) 21:197–203. doi: 10.3109/09286586.2014.904371
75. Hashemi H, Yekta A, Jafarzadehpur E, Nirouzad F, Ostadimoghaddam H, Eshrati B, et al. The prevalence of amblyopia in 7-year-old schoolchildren in Iran. Strabismus. (2014) 22:152–7. doi: 10.3109/09273972.2014.971824
76. Paudel P, Ramson P, Naduvilath T, Wilson D, Phuong HT, Ho SM, et al. Prevalence of vision impairment and refractive error in school children in Ba Ria – Vung Tau province, Vietnam. Clin Exp Ophthalmol. (2014) 42:217–26. doi: 10.1111/ceo.12273
77. Ganekal S, Jhanji V, Liang Y, Dorairaj S. Prevalence and etiology of amblyopia in southern India: Results from screening of school children aged 5-15 years. Ophthalmic Epidemiol. (2013) 20:228–31. doi: 10.3109/09286586.2013.809772
78. Gursoy H, Basmak H, Yaz Y, Colak E. Vision screening in children entering school: Eskisehir, turkey. Ophthalmic Epidemiol. (2013) 20:232–8. doi: 10.3109/09286586.2013.808672
79. Moraes Ibrahim F, Moraes Ibrahim M, Pomepo de Camargo JR. Veronese Rodrigues Mde L, Scott IU, Silva Paula J. visual impairment and myopia in Brazilian children: a population-based study. Optom Vis Sci. (2013) 90:223–7. doi: 10.1097/OPX.0b013e31828197fd
80. Sapkota K, Pirouzian A, Matta NS. Prevalence of amblyopia and patterns of refractive error in the amblyopic children of a tertiary eye care center of Nepal. Nepal J Ophthalmol. (2013) 5:38–44. doi: 10.3126/nepjoph.v5i1.7820
81. Wu JF, Bi HS, Wang SM, Hu YY, Wu H, Sun W, et al. Refractive error, visual acuity and causes of vision loss in children in Shandong, china. The Shandong children eye study. PLoS One. (2013) 8:e82763. doi: 10.1371/journal.pone.0082763
82. Pai AS, Rose KA, Leone JF, Sharbini S, Burlutsky G, Varma R, et al. Amblyopia prevalence and risk factors in Australian preschool children. Ophthalmology. (2012) 119:138–44. doi: 10.1016/j.ophtha.2011.06.024
83. Pi LH, Chen L, Liu Q, Ke N, Fang J, Zhang S, et al. Prevalence of eye diseases and causes of visual impairment in school-aged children in Western China. J Epidemiol. (2012) 22:37–44. doi: 10.2188/jea.je20110063
84. Polling JR, Loudon SE, Klaver CC. Prevalence of amblyopia and refractive errors in an unscreened population of children. Optom Vis Sci. (2012) 89:e44–9. doi: 10.1097/OPX.0b013e31826ae047
85. Sherpa D, Panta CR, Joshi N. Ocular morbidity among primary school children of Dhulikhel, Nepal. Nepal J Ophthalmol. (2011) 3:172–6. doi: 10.3126/nepjoph.v3i2.5272
86. Marasini S, Sharma R, Sthapit PR, Sharma D, Koju U, Thapa G, et al. Refractive errors and visual anomalies in school children in the Kavrepalanchowk District. Kathmandu Univ Med J (KUMJ). (2010) 8:362–6. doi: 10.3126/kumj.v8i4.6231
87. Friedman DS, Repka MX, Katz J, Giordano L, Ibironke J, Hawse P, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the baltimore pediatric eye disease study. Ophthalmology. (2009) 116:e1–2. doi: 10.1016/j.ophtha.2009.04.034
88. Huynh SC, Samarawickrama C, Wang XY, Rochtchina E, Wong TY, Gole GA, et al. Macular and nerve fiber layer thickness in amblyopia: the Sydney childhood eye study. Ophthalmology. (2009) 116:1604–9. doi: 10.1016/j.ophtha.2009.03.013
89. Lai YH, Hsu HT, Wang HZ, Chang SJ, Wu WC. The visual status of children ages 3 to 6 years in the vision screening program in Taiwan. J AAPOS. (2009) 13:58–62. doi: 10.1016/j.jaapos.2008.07.006
90. Unsal A, Ayranci U, Tozun M. Vision screening among children in primary schools in a district of western turkey: an epidemiological study. Pak J Med Sci. (2009) 25:976–81.
91. Lu P, Chen X, Zhang W, Chen S, Shu L. Prevalence of ocular disease in Tibetan primary school children. Can J Ophthalmol. (2008) 43:95–9. doi: 10.3129/i07-194
92. Robaei D, Kifley A, Rose KA, Mitchell P. Impact of amblyopia on vision at age 12 years: findings from a population-based study. Eye (Lond). (2008) 22:496–502. doi: 10.1038/sj.eye.6702668
93. Salomao SR, Cinoto RW, Berezovsky A, Mendieta L, Nakanami CR, Lipener C, et al. Prevalence and causes of visual impairment in low-middle income school children in Sao Paulo, Brazil. Invest Ophthalmol Vis Sci. (2008) 49:4308–13. doi: 10.1167/iovs.08-2073
94. Sapkota YD, Adhikari BN, Pokharel GP, Poudyal BK, Ellwein LB. The prevalence of visual impairment in school children of upper-middle socioeconomic status in Kathmandu. Ophthalmic Epidemiol. (2008) 15:17–23. doi: 10.1080/09286580701772011
95. He M, Huang W, Zheng Y, Huang L, Ellwein LB. Refractive error and visual impairment in school children in rural Southern China. Ophthalmology. (2007) 114:374–82. doi: 10.1016/j.ophtha.2006.08.020
96. Matsuo T, Matsuo C. Comparison of prevalence rates of strabismus and amblyopia in Japanese elementary school children between the years 2003 and 2005. Acta Med Okayama. (2007) 61:329–34. doi: 10.18926/AMO/32877
97. Matsuo T, Matsuo C, Matsuoka H, Kio K. Detection of strabismus and amblyopia in 1.5- and 3-year-old children by a preschool vision-screening program in Japan. Acta Med Okayama. (2007) 61:9–16. doi: 10.18926/AMO/32910
98. Robaei D, Rose KA, Ojaimi E, Kifley A, Martin FJ, Mitchell P. Causes and associations of amblyopia in a population-based sample of 6-year-old Australian children. Arch Ophthalmol. (2006) 124:878–84. doi: 10.1001/archopht.124.6.878
99. Matsuo T, Matsuo C. The prevalence of strabismus and amblyopia in Japanese elementary school children. Ophthalmic Epidemiol. (2005) 12:31–6. doi: 10.1080/09286580490907805
100. Vision in Preschoolers Study Group. Preschool vision screening tests administered by nurse screeners compared with lay screeners in the vision in preschoolers study. Invest Ophthalmol Vis Sci. (2005) 46:2639–48. doi: 10.1167/iovs.05-0141
101. He M, Zeng J, Liu Y, Xu J, Pokharel GP, Ellwein LB. Refractive error and visual impairment in urban children in Southern China. Invest Ophthalmol Vis Sci. (2004) 45:793–9. doi: 10.1167/iovs.03-1051
102. Lim HT, Yu YS, Park SH, Ahn H, Kim S, Lee M, et al. The seoul metropolitan preschool vision screening programme: results from South Korea. Br J Ophthalmol. (2004) 88:929–33. doi: 10.1136/bjo.2003.029066
103. Tananuvat N, Manassakorn A, Worapong A, Kupat J, Chuwuttayakorn J, Wattananikorn S. Vision screening in schoolchildren: two years results. J Med Assoc Thai. (2004) 87:679–84.
104. Vision in Preschoolers Study Group. Preschool visual acuity screening with hotv and lea symbols: testability and between-test agreement. Optom Vis Sci. (2004) 81:678–83. doi: 10.1097/01.opx.0000144746.80718.67
105. Maaita JF, Sunna LF, Al-Madani MV, Horrani SM. Eye diseases in children in southern Jordan. Saudi Med J. (2003) 24:154–6.
106. Williams C, Northstone K, Harrad RA, Sparrow JM, Harvey I, Team AS. Amblyopia treatment outcomes after preschool screening v school entry screening: observational data from a prospective cohort study. Br J Ophthalmol. (2003) 87:988–93. doi: 10.1136/bjo.87.8.988
107. Murthy GV, Gupta SK, Ellwein LB, Munoz SR, Pokharel GP, Sanga L, et al. Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci. (2002) 43:623–31.
108. Lim HC, Quah BL, Balakrishnan V, Lim HC, Tay V, Emmanuel SC. Vision screening of 4-year-old children in Singapore. Singapore Med J. (2000) 41:271–8.
109. Newman DK, East MM. Prevalence of amblyopia among defaulters of preschool vision screening. Ophthalmic Epidemiol. (2000) 7:67–71. doi: 10.1076/0928-6586(200003)7:1;1-2;ft067
110. Thorburn R, Roland M. The effectiveness of preschool vision screening by health visitors. Br J Community Nurs. (2000) 5:41–4. doi: 10.12968/bjcn.2000.5.1.7434
111. Wedner SH, Ross DA, Balira R, Kaji L, Foster A. Prevalence of eye diseases in primary school children in a rural area of Tanzania. Br J Ophthalmol. (2000) 84:1291–7. doi: 10.1136/bjo.84.11.1291
112. Zhao J, Pan X, Sui R, Munoz SR, Sperduto RD, Ellwein LB. Refractive error study in children: results from Shunyi District, China. Am J Ophthalmol. (2000) 129:427–35. doi: 10.1016/s0002-9394(99)00452-3
113. Kalikivayi V, Naduvilath TJ, Bansal AK, Dandona L. Visual impairment in school children in Southern India. Indian J Ophthalmol. (1997) 45:129–34.
114. Martinez J, Canamares S, Saornil MA, Almaraz A, Pastor JC. Original papers: prevalence of amblyogenic diseases in a preschool population sample of Valladolid, Spain. Strabismus. (1997) 5:73–80. doi: 10.3109/09273979709057390
115. Preslan MW, Novak A. Baltimore vision screening project. Ophthalmology. (1996) 103:105–9. doi: 10.1016/s0161-6420(96)30753-7
116. Rosenberg T, Flage T, Hansen E, Riise R, Rudanko SL, Viggosson G, et al. Incidence of registered visual impairment in the Nordic child population. Br J Ophthalmol. (1996) 80:49–53. doi: 10.1136/bjo.80.1.49
117. Jensen H, Goldschmidt E. Visual acuity in danish school children. Acta Ophthalmol (Copenh). (1986) 64:187–91. doi: 10.1111/j.1755-3768.1986.tb06898.x
Keywords: amblyopia, children, prevalence, worldwide, meta-analysis
Citation: Hu B, Liu Z, Zhao J, Zeng L, Hao G, Shui D and Mao K (2022) The Global Prevalence of Amblyopia in Children: A Systematic Review and Meta-Analysis. Front. Pediatr. 10:819998. doi: 10.3389/fped.2022.819998
Received: 22 November 2021; Accepted: 15 March 2022;
Published: 04 May 2022.
Edited by:
Cheng Shen, Sichuan University, ChinaReviewed by:
Dario Bruzzese, University of Naples Federico II, ItalyNicoletta Berardi, University of Florence, Italy
Copyright © 2022 Hu, Liu, Zhao, Zeng, Hao, Shui and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Budan Hu, NTQzNDk3MTE0QHFxLmNvbQ==; Zongshun Liu, bGl1em9uZ3NodW4wNTA3QDEyNi5jb20=
†These authors have contributed equally to this work
 Budan Hu
Budan Hu Zongshun Liu*†
Zongshun Liu*†