- 1Department of Pediatric Surgery, Children's Hospital of Nanjing Medical University, Nanjing, China
- 2Division of Pediatric Gastroenterology and Nutrition, Shanghai Jiaotong University School of Medicine Xinhua Hospital, Shanghai, China
- 3Department of Neonatal Surgery, Guangzhou Women and Children's Medical Center, Guangzhou, China
- 4Department of Neonatal Surgery, Hunan Children's Hospital, Changsha, China
Objectives: The aim of this study was to identify predictors for enteral autonomy and intestinal failure (IF)-related complications and evaluate the outcomes of a multi-center pediatric cohort in China.
Methods: The medical records of pediatric patients with IF treated at four medical centers in China from January 1, 2012 to November 31, 2020 were retrospectively reviewed. Enteral autonomy was defined as sustained growth and cessation of parenteral nutrition for >90 days. Multivariate logistic regression analysis was used to identify factors predictive of enteral autonomy and the risk factors of complications, such as IF-associated liver disease (IFALD) and catheter-related bloodstream infection (CRBSI).
Results: The study cohort of 92 pediatric patients with IF included 71 (77%) who underwent surgery and 21 (23%) who received non-surgical treatment. Eventually, 63 (68.5%) patients achieved enteral autonomy by the end of the follow-up period. Multivariate logistic regression analysis indicated that longer duration of parenteral nutrition (PN), sepsis, and non-breastfeeding were risk factors for enteral autonomy. When considering the detailed intraoperative data, the presence of an ileocecal valve (ICV) and greater residual small bowel (RSB) length were reaffirmed as predictors of achieving enteral autonomy. Medium/long-chain (MCT/LCT) lipids or sepsis were identified as negative predictors for IFALD. Univariate analysis revealed that the use of MCT/LCT lipids was associated with a greater likelihood of CRBSI.
Conclusion: In this cohort, enteral autonomy was achieved at a percentage of 68.5%, and the risk factors for not achieving enteral autonomy were a longer duration of PN, sepsis, and non-breastfeeding. The presence of an ICV and a greater RSB length were important predictors of achieving enteral autonomy.
Introduction
Intestinal failure (IF) refers to dysfunction of intestinal digestion and absorption, resulting in the inability of the intestine to fully absorb nutrients and liquids to meet the needs for growth and survival. The symptoms of IF include severe diarrhea, loss of water and electrolytes, acid/alkali balance disorder, malnutrition, and weight loss (1). The most common cause of IF in children is short bowel syndrome due to resection or congenital intestinal loss, intestinal motility disorders, and mucosal enteropathy (2–4).
Pediatric IF is a complex disease that was often fatal. However, the establishment of intestinal rehabilitation by multidisciplinary teams and improvements in nursing care and nutritional support have decreased the mortality rate over the past decade from 30% (5) to 10–15% (6–9). However, mortality associated with IF remains relatively high in many countries due to difficulties with treatment.
The main goals of intestinal rehabilitation are to promote intestinal adaptation and enteral autonomy, which is achieved by maintaining sufficient growth after cessation of parenteral nutrition (PN) by oral and/or tube feeding for 3 months (10).
Possible influences of intestinal adaption in pediatric IF patients include diagnostic and anatomical factors, such as necrotizing enterocolitis (NEC), residual small intestine, residual colon length, and ileocecal valve (ICV) dysfunction, as well as biochemical factors, such as plasma citrulline (11–13), timing of colostomy closure, timing and progress of enteral feeding, imbalance in intestinal bacteria, and the incidence of complications, such as IF-associated liver disease (IFALD) and catheter-related bloodstream infections (CRBSIs) (10–15), which can adversely affect intestinal adaption and increase the risk of mortality (16), although sufficient evidence is currently lacking.
The aims of the present study were to identify predictors of survival and enteral autonomy, as well as risk factors of IF-related complications by a multi-center pediatric cohort in China.
Patients and Methods
Study Approval
The study protocol was approved by Ethics Committees of all participating hospitals and conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from the parents/guardians of the children after receiving an explanation of the purpose if the study.
Study Population
The cohort of this retrospective study included 92 children with IF who received treatment at the Children's Hospital of Nanjing Medical University (Nanjing, China), Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China), Guangzhou Women and Children's Medical Center (Guangzhou, China), and Hunan Children's Hospital (Changsha, China) from January 1, 2012 to November 31, 2020.
All patients included in this study met the following inclusion criteria: severe congenital or acquired gastrointestinal diseases; age, <4 years; and PN duration of >42 consecutive days. Enteral autonomy was defined as maintaining sufficient growth after cessation of all PN by oral and/or tube feeding for 3 consecutive months. The exclusion criteria were incomplete clinical data and loss to follow-up after discharge.
Methods
Variables recorded were general information (sex, birth weight, age, family status, occupation of parents, and family history of related diseases), disease-related information (cause of IF, presence of ICV, age at time of surgery, surgical method, percentages of remaining small bowel, and restoration of bowel continuity), enteral nutrition information (enteral nutrition method and formula), parenteral nutrition information (duration of parenteral nutrition time, selection of fat emulsion in parenteral nutrition, and mode of intravenous nutrition), and complications and laboratory indicators during treatment (presence of septicemia or sepsis, maximum leukocyte count, highest level of procalcitonin, presence of IF-related liver disease, and presence of renal failure). Enteral autonomy was defined as sustained growth and cessation of parenteral nutrition for ≥ 90 days. Regular follow-ups were conducted in person at the Outpatient Department or by telephone to February 28, 2021. The primary outcomes of this study were enteral autonomy, persistent dependency on PN, and death. The median follow-up time was 5.2years.
All clinical data were collected and sorted by specially trained personnel assigned by each hospital, and then sent to the Children's Hospital Affiliated to Nanjing Medical University. The personnel were responsible for carefully reviewing and entering the data into the database. For data in question, the project leader of each unit determined whether the data were correct and suitable for interpretation and analysis.
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 24.0. (IBM Corporation, Armonk, NY USA). Continuous non-parametric data are presented as the median and inter quartile range (IQR). The Kruskal–Wallis H test was applied for comparisons of three groups, while the two-tailed Mann–Whitney U test was used for comparisons of two groups. Categorical data were compared using the chi-square test and are summarized as the frequency and percentage. A multivariate logistic regression model was used to identify risk factors for enteral autonomy. A probability (p) value of < 0.05 was considered statistically significant.
Results
Study Population
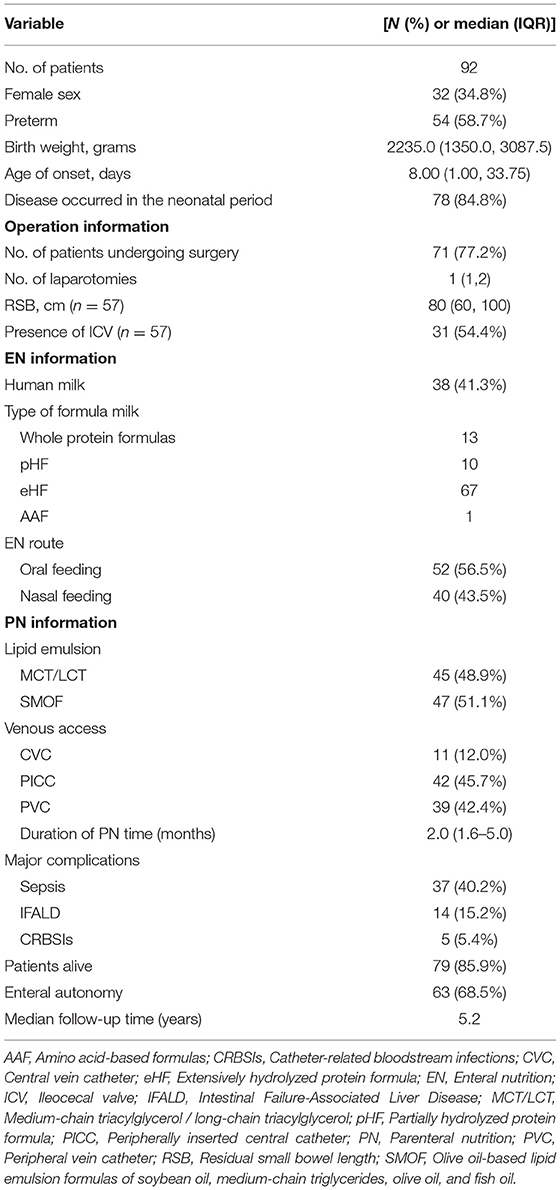
The baseline information of the 92 pediatric IF patients is shown in Table 1. The majority (65.2%) of patients were male and more than half (58.7%) were preterm. The median birth weight was 2235.0 grams (IQR = 1.3500, 3.0875). Most cases (84.8%) occurred during the neonatal period. NEC was the most common cause of IF (n = 43), followed by intestinal atresia (n = 14), volvulus (n = 14), intestinal necrosis (n = 8), and extensive Hirschsprung disease (n = 7). Details of the causes of IF in infants and toddlers are shown in Figure 1.
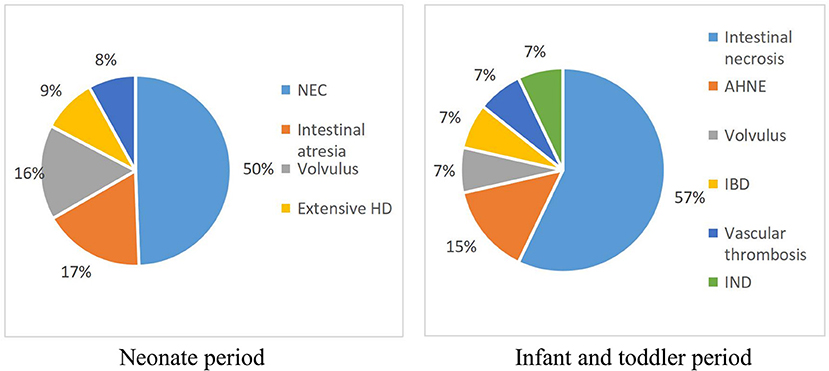
Figure 1. Proportions of the different diagnosis in the study group. AHNE, acute hemorrhagic necrotic enteritis; HD, Hirschsprung disease; IBD, inflammatory bowel disease; IND, intestinal neuronal dysplasia;NEC, necrotizing enterocolitis.
By the end of the follow-up period (February 28, 2021), 63 (68.5%) patients finally achieved enteral autonomy and 13 (14.1%) died. The causes of death included sepsis, severe pneumonia, Takayasu arteritis, chronic hepatic failure, septic shock, multiple organ dysfunction syndrome, and other unknown factors (Some parents gave up treatment and took children home directly. These children eventually died of some unclear reason).
Different Treatment Modalities and Analysis of Outcomes
Of the 92 patients, 71 (77.2%) underwent surgery and 21 (22.8%) received non-surgical treatment (Table 2). The surgical patients underwent at least one laparotomy procedure. The surgical procedures included intestinal resection, intestinal anastomosis, enterostomy, lysis of intestinal adhesions, intestinal repair, intestinal biopsy, and abdominal irrigation and drainage. Of the 71 surgery patients, 45 (63.4%) underwent enterostomy as the initial surgery and 26 (36.6%) received entero-anastomosis treatment. Patients in the non-surgical treatment group had a lower mean birth weight, an earlier age of onset, a higher proportion of premature births, a higher proportion of NEC, a shorter PN duration, and a higher proportion of enteral autonomy, whereas patients in the enterostomy group had a higher rate of breast-feeding.

Table 2. Clinical statistics of the study population in different subgroups of treatment with pediatric intestinal failure.
Specifically, 46 patients underwent only one surgery throughout the whole treatment period, which included 27 who achieved enteral autonomy, while 17 underwent two surgeries (13 cases finally achieved enteral autonomy), which mainly included lysis of intestinal adhesions, intestinal resection, and enterostomy. In addition, five patients underwent three surgeries (two cases finally achieved enteral autonomy) due to intestinal adhesions, intestinal stenosis, and ileum duplication. Three children underwent four surgeries across the duration of the hospital stay.
Enteral Autonomy
By the end of the follow-up period, 63 (68.5%) patients had achieved complete enteral autonomy, 16 (17.4%) were still partially or completely dependent on PN, and 13 (14.1%) had died.
As shown in Table 3, clinical factors associated with enteral autonomy included a relatively short period of PN, feeding of human milk, and use of SMOF-lipids (Fresenius Kabi, Austria). Conversely, sepsis was associated with a lower likelihood of achieving enteral autonomy, while the presence of ICV and greater residual small bowel (RSB) length were significantly associated with enteral autonomy. In addition, 33 children with an underlying diagnosis of NEC eventually achieved enteral autonomy, yet no significant difference was found between NEC group and other disease groups.
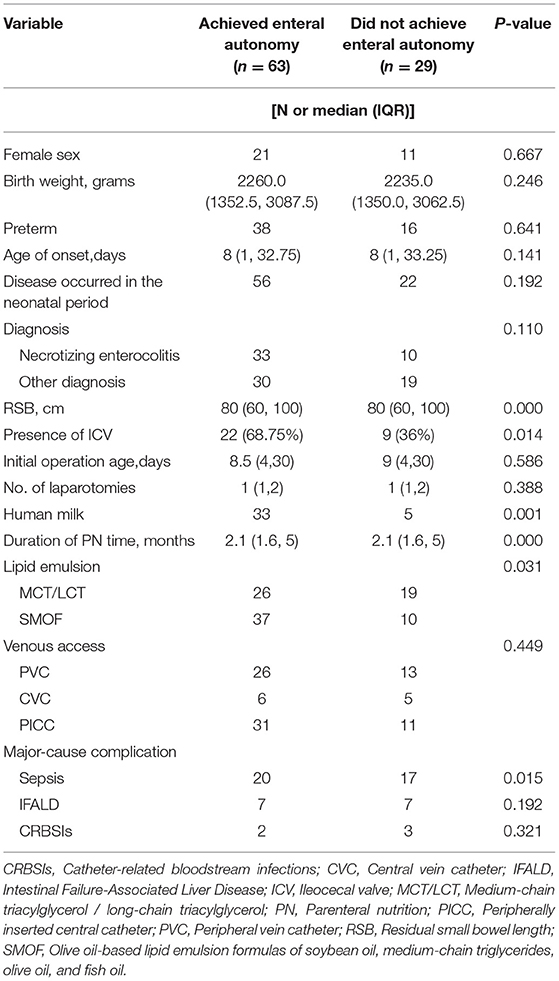
Table 3. Baseline characteristics based on the primary outcome of achievement of enteral autonomy in children with intestinal failure.
Multivariate logistic regression analysis of the whole cohort (n = 92) revealed that longer PN duration (OR = 0.75, 95% CI = 0.565–0.997), and sepsis (OR = 0.277, 95% CI = 0.082–0.938) were risk factors for enteral autonomy, while breastfeeding (OR = 5.295, 95% CI = 1.445–19.404) was a protective factor (Table 4).
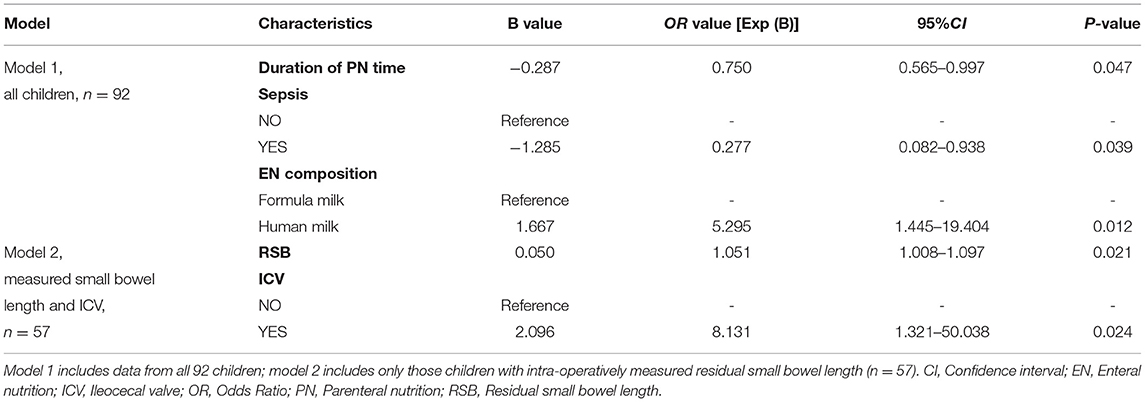
Table 4. Multivariable logistic regression analysis based on the primary outcome of achievement of enteral autonomy in children with intestinal failure.
Further multivariate logistic regression analysis of 57 children with data regarding RSB length and the presence or absence of an ileocecal valve showed that RSB length (OR = 1.051,95% CI = 1.008–1.097) and the presence of an ICV (OR = 8.131,95% CI = 1.321–50.038) were identified as independent factors affecting enteral autonomy. The probability of achieving enteral autonomy increased by 5.1% for each additional 1 cm in RSB length and the presence of ICV was associated with an 8.131-fold greater likelihood of achieving enteral autonomy.
Risk Factors for Development of IFALD
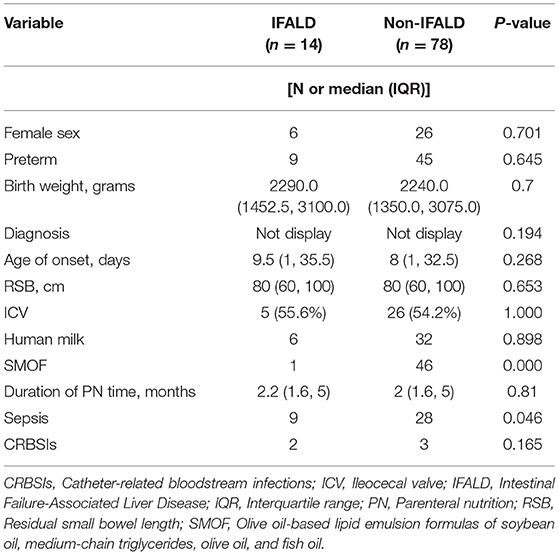
IFALD was defined as cholestasis occurring in the setting of PN for more than 2 weeks upon exclusion of other specific causes of liver disease (18), while cholestasis was defined as an elevated conjugated serum bilirubin level of > 34.2 mmol/L (19). During the study period, 38 (41.3%) patients were complicated with cholestasis and 14 (15.2%) eventually developed IFALD (Table 5). There were significant differences in lipid emulsion and sepsis between the two groups, and the incidence of IFALD was higher in children treated with medium/long-chain (MCT/LCT) lipids or sepsis. MCT/LCT lipids and sepsis were identified as negative predictors of IFALD. Notably, there was no case of renal failure in this cohort.
Risk Factors for Developing CRBSIs
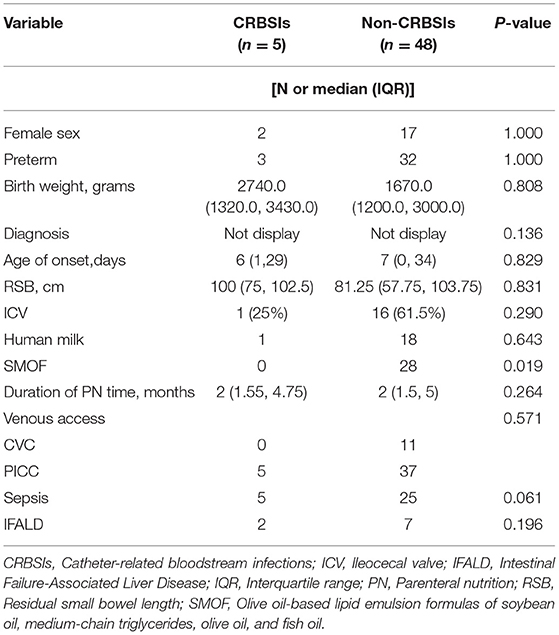
A central venous catheter (CVC) or peripherally inserted central catheter (PICC) is most commonly used for central venous administration in clinical practice, as stable intravenous infusion channels can guarantee long-term intravenous fluid treatment and greatly alleviate pain due to repeated venipuncture. In this study, a CVC was used for infusion in 11 children, while a PICC was used in 42, and the remaining 39 patients received peripheral intravenous infusion (Table 1). Due to prolonged parenteral nutrition, blood stream infections related to the presence of a CVC are among the most common complications in this population. In total, 37 children had concurrent sepsis, which included 21 with positive blood cultures.
CRBSI was defined as a positive blood culture (one sample from the central venous and a second from a peripheral vein) in combination with clinical infectious symptoms (20). Cultures of blood samples collected from five children with CRBSI revealed the presence of Klebsiella pneumoniae (2 cases), Klebsiella oxytoca, Candida guilliermondii, and Candida albicans. Univariate analysis revealed that the use of MCT/LCT lipids was associated with an increased risk of CRBSI (Table 6). More particularly, although a PICC was used for all 5 patients, there was no significant difference of the incidence of CRBSI between the two groups in regard to venous access.
Discussion
The main purpose of managing IF in childhood is to increase intestinal adaptation and achieve enteral autonomy, which is defined as complete cessation of PN for more than 90 days and maintaining acceptable growth parameters. The identification of factors associated with the ability to achieve enteral autonomy is very important for the family and medical team. It is also very important to know which factors may lead to IF-related complications to facilitate prevention and treatment.
The duration of intestinal adaptation varies among patients and the most significant progression usually occurs in the first few years after intestinal surgery. In this study, enteral autonomy was achieved in 63 patients (68.5%) at a follow up of ≥ 1 year, which was slightly higher than in previous reports of 43 to 67% (7, 11, 12, 21, 22). This difference may be explained by the high proportion of non-surgical patients (n = 21, 22.8%) in this study, which included 18 (85.7%) who eventually achieved enteral autonomy. Premature neonates, which comprised the majority of the non-surgical group, are known to have strong compensatory abilities and more likely to obtain intestinal adaptation (23). Additionally, since the integrity of the gut of non-surgical patients is not disrupted, intestinal absorption capacity is less affected, thus enteral nutrition can be carried out as early as possible to reduce dependence on parenteral nutrition and achieve enteral autonomy as soon as possible. Although most children were weaned from PN within the first year, intestinal adaptation may continue throughout childhood.
The mechanism underlying intestinal adaptation after intestinal resection remains unclear. However, some studies have found no difference in the probability of weaning infants with NEC from PN (24), while others suggest that infants with NEC are more likely to achieve enteral autonomy than those without (11). Of the 43 children with NEC enrolled in this study, 33 (76.7%) achieved enteral autonomy, suggesting that children with NEC are more likely to achieve enteral autonomy than those without. However, the difference between the two groups was not significant.
Extensive data have established that the length of the residual small intestine and remaining ileocecal valve have significant effects on enteral autonomy (11, 25, 26). Consistent with the findings of previous studies, the results of the present study showed that a longer RSB or the presence of an ileocecal valve was associated with a greater likelihood of achieving enteral autonomy. For every 1 cm increase in RSB length, the probability of achieving enteral autonomy is increased by 5.1%.
Choosing human milk or deep hydrolyzed formula as a source of enteral nutrition may also play a role in intestinal adaptation. Breast milk has many advantages, including secretory IgA, amino acids, and a variety of growth factors that can enhance the systemic and mucosal immune responses, and many guidelines recommend the use of breast milk. Therefore, in the absence of specific intolerance, breast milk should be strongly considered as the main enteral nutrition source. In the present cohort, almost half (41.3%) of the children were breastfeeding, which can promote enteral autonomy. When unavailable, human breast milk can be partly or fully replaced with special hydrolyzed formula (27). In the present study, 67 patients were fed with deep hydrolyzed formula. However, there was no obvious benefit to recovery.
IFALD is an important factor affecting enteral autonomy in children and may progress to end-stage liver disease, which is negatively correlated with survival (28, 29). Two recent large studies of 279 (30) and 251 (17) pediatric IF patients reported that the prevalence of IFALD was 22 and 20%, respectively. However, the prevalence of IFALD (15%) in the present study was less than that of these previous studies. A possible explanation for this discrepancy may be the high proportion of SMOF-lipid use (51.1%). Importantly, the development of IFALD has a notable effect on clinical outcomes of IF patients (31). In this study, IFALD was not correlated with enteral autonomy, although mortality was higher in children with IFALD than without (χ2 = 6.34, p = 0.0118, detailed data are not included in the table).
The identification of risk factors for IFALD is helpful for the prevention and treatment of IFALD and could benefit prognosis. The results of the present study revealed that the risk factors leading to IFALD included sepsis and intravenous administration of lipids. More specifically, the use of MCT/LCT lipids (IFALD vs. non-IFALD: 13 vs. 32 patients) was associated with a greater risk of IFALD than the use of SMOF-lipids (IFALD vs. non-IFALD: 1 vs. 46 patients) and patients with sepsis were more likely to develop IFALD than those without (IFALD vs. non-IFALD: 64 vs. 36%). Previous studies have reported that the bile ducts and hepatocytes of premature infants are more vulnerable to severe damage (32, 33) and the occurrence of IFALD may be associated with premature birth. In the present study, the proportion of premature infants (59%) was not very high and preterm infants with IF (IFALD vs. non-IFALD: 64 vs. 49%, p = 0.645) were not more susceptible to IFALD.
The exact mechanism underlying PN-induced cholestasis remains unclear. In intestinal stasis or intestinal dysfunction, bacteria are transported through the epithelial barrier and release endotoxins (34), consistent with the finding that sepsis is another factor leading to of aggravating IFALD. Endotoxins bind to CD14 receptors on liver macrophages and induce the release of inflammatory cytokines, such as interleukin-1, interleukin-6, and tumor necrosis factor, leading to liver injury (35). When food passes through the gastrointestinal tract, it can increase the expression of insulin-like growth factor and other growth factors, which may improve liver function (36, 37). The lack of enteral food stimulation may reduce cholecystokinin secretion, decrease gallbladder emptying and bile flow, and increase the risk of cholestasis. Fat emulsion, as an important source of energy, plays an important role in the survival and rehabilitation of children. Recent studies have shown that the medium and long chain fat emulsion derived from soybean fat has pro-inflammatory effects, which have been associated with the occurrence of liver diseases related to IF. Long-term conventional infusion of this kind of fat emulsion can easily lead to cholestasis and cirrhosis (38). The new generation of fish oil fat emulsions, such as SMOF-lipids, are rich in omega-3- polyunsaturated fatty acids, which can help to regulate immunity and anti-inflammatory responses, reduce cholestasis and liver damage, and effectively improve the prognosis of children with IF (39).
A CVC is widely used to obtain intravenous access in pediatric patients with IF requiring long-term parenteral nutrition. However, CVC use has been associated with major complications, such as infection, mechanic, and thrombotic consequences (40). In the present study, the median duration of parenteral nutrition was 2 months and both PICCs (45.7%) and CVCs (12.0%) were widely applied. With the increasingly standardized management of venous catheter use, the incidence of CRBSIs has been very low (5% in our cohort). In this study, lipid emulsion was identified as a risk factor for CRBSI. In addition, the administration of MCT/LCT-lipids was associated with an increased risk of CRBSI. As mentioned before, the anti-inflammatory effect of SMOF-lipids is important for children with IF because this population is especially susceptible to infections caused by intestinal flora disorders, which is potentially beneficial to alleviate the inflammatory response and immunosuppression. In general, the major pathogens isolated in patients with CRBSIs are Gram-positive organisms, particularly coagulase-negative Staphylococcus, Gram-negative Enterobacter sp., Escherichia coli, and Klebsiella sp., and fungi (41, 42). In this study, the pathogens cultured from patients with CRBSIs were similar to those in previous articles. Antibiotic lock therapy has been used to avoid direct removal and rescue the central catheter after CRBSIs. The use of ethanol and standardized nursing care is reported to significantly reduce the incidence of CRBSIs by up to 15% (17, 43, 44).
There were some limitations to this study that should be addressed. First, this was a retrospective study with a relatively small number of patients. Due to the large time span of the study, some important potential factors, such as medication use, remaining colon, etc., could not be completely collected to determine which should be further refined in future studies.
In conclusion, even though patient survival has significantly improved, IF still poses a significant threat to human health. The use of a geographically diverse cohort revealed that a longer PN time and sepsis were risk factors for enteral autonomy, while breastfeeding was a protective factor. When considering the detailed intraoperative data, the presence of ICV and a greater RSB length were reaffirmed as predictors for reaching enteral autonomy. At present, the best strategies to reduce the incidence of IFALD include meticulous care to prevent sepsis and the use of SMOF-lipids. Despite the low incidence of CRBSI, evidence suggests that the use of SMOF-lipids can effectively ameliorate CRBSI. These data are important references for future prospective studies to further improve the outcomes of children with IF.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by IEC of Children's Hospital of Nanjing Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was funded by Key Project of Science and Technology Development of Nanjing health committee, China (ZKX18037); Jiangsu Youth Medical Talent Project, China (QNRC2016081);Top Talents of Jiangsu Provincial Health Committee's Six One Project, China (LGY2020019); the Jiangsu Provincial Key Research and Development Program, China (BE2017609) and General project of Nanjing Health Commission (YKK20121).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IF, Intestinal failure; ICV, Ileocecal valve; EN, Enteral nutrition; PN, Parenteral nutrition; SMOF, Olive oil-based lipid emulsion formulas of soybean oil, medium-chain triglycerides, olive oil, and fish oil; MCT/LCT, Medium-chain triacylglycerol / long-chain triacylglycerol; PVC, Peripheral vein catheter; CVC, Central vein catheter; PICC, Peripherally inserted central catheter; HD, Hirschsprung disease; RSB, Residual small bowel length; OR, Odds Ratio; CI, Confidence interval; CRBSIs, Catheter-related bloodstream infections; IFALD, Intestinal Failure-Associated Liver Disease.
References
1. D'Antiga L, Goulet O. Intestinal failure in children: the European view. J Pediatr Gastroenterol Nutr. (2013) 56:118–26. doi: 10.1097/MPG.0b013e318268a9e3
2. Guarino A, De Marco G, Italian National Network for Pediatric Intestinal Failure. Natural history of intestinal failure, investigated through a national network-based approach. J Pediatr Gastroenterol Nutr. (2003) 37:136–41. doi: 10.1097/00005176-200308000-00010
3. Goulet O, Ruemmele F, Lacaille F, Colomb V. Irreversible intestinal failure. J Pediatr Gastroenterol Nutr. (2004) 38:250–69. doi: 10.1097/00005176-200403000-00006
4. DiBaise JK, Young RJ, Vanderhoof JA. Intestinal rehabilitation and the short bowel syndrome: part 2. Am J Gastroenterol. (2004) 99:1823–32. doi: 10.1111/j.1572-0241.2004.40836.x
5. Schalamon J, Mayr JM, Hollwarth ME. Mortality and economics in short bowel syndrome. Best Pract Res Clin Gastroenterol. (2003) 17:931–42. doi: 10.1016/S1521-6918(03)00079-9
6. Sudan D, DiBaise J, Torres C, Thompson J, Raynor S, Gilroy R, et al. A multidisciplinary approach to the treatment of intestinal failure. J Gastrointest Surg. (2005) 9:165–76. doi: 10.1016/j.gassur.2004.10.014
7. Torres C, Sudan D, Vanderhoof J, Grant W, Botha J, Raynor S, et al. Role of an intestinal rehabilitation program in the treatment of advanced intestinal failure. J Pediatr Gastroenterol Nutr. (2007) 45:204–12. doi: 10.1097/MPG.0b013e31805905f9
8. Hess RA, Welch KB, Brown PI, Teitelbaum DH. Survival outcomes of pediatric intestinal failure patients: analysis of factors contributing to improved survival over the past two decades. J Surg Res. (2011) 170:27–31. doi: 10.1016/j.jss.2011.03.037
9. Modi BP, Langer M, Ching YA, Valim C, Waterford SD, Iglesias J, et al. Improved survival in a multidisciplinary short bowel syndrome program. J Pediatr Surg. (2008) 43:20–4. doi: 10.1016/j.jpedsurg.2007.09.014
10. Belza C, Fitzgerald K, de Silva N, Avitzur Y, Wales PW. Early predictors of enteral autonomy in pediatric intestinal failure resulting from short bowel syndrome: development of a disease severity scoring tool. JPEN J Parenter Enteral Nutr. (2019) 43:961–9. doi: 10.1002/jpen.1691
11. Khan FA, Squires RH, Litman HJ, Balint J, Carter BA, Fisher JG, et al. Predictors of enteral autonomy in children with intestinal failure: a multicenter cohort study. J Pediatr. (2015) 167:29–34. doi: 10.1016/j.jpeds.2015.03.040
12. Demehri FR, Stephens L, Herrman E, West B, Mehringer A, Arnold MA, et al. Enteral autonomy in pediatric short bowel syndrome: predictive factors one year after diagnosis. J Pediatr Surg. (2015) 50:131–5. doi: 10.1016/j.jpedsurg.2014.10.011
13. Belza C, Fitzgerald K, de Silva N, Avitzur Y, Steinberg K, Courtney-Martin G, et al. Predicting intestinal adaptation in pediatric intestinal failure: a retrospective cohort study. Ann Surg. (2019) 269:988–93. doi: 10.1097/SLA.0000000000002602
14. Venick RS. Predictors of intestinal adaptation in children. Gastroenterol Clin North Am. (2019) 48:499–511. doi: 10.1016/j.gtc.2019.08.004
15. Mezoff EA, Cole CR, Cohran VC. Etiology and medical management of pediatric intestinal failure. Gastroenterol Clin North Am. (2019) 48:483–98. doi: 10.1016/j.gtc.2019.08.003
16. Fullerton BS, Hong CR, Jaksic T. Long-term outcomes of pediatric intestinal failure. Semin Pediatr Surg. (2017) 26:328–35. doi: 10.1053/j.sempedsurg.2017.09.006
17. Abi Nader E, Lambe C, Talbotec C, Pigneur B, Lacaille F, Garnier-Lengline H, et al. Outcome of home parenteral nutrition in 251 children over a 14-y period: report of a single center. Am J Clin Nutr. (2016) 103:1327–36. doi: 10.3945/ajcn.115.121756
18. Fredriksson F, Nystrom N, Waldenvik K, Orden H, Lindblom M, Paulsson M, et al. Improved outcome of intestinal failure in preterm infants. J Pediatr Gastroenterol Nutr. (2020) 71:223–31. doi: 10.1097/MPG.0000000000002763
19. Hojsak I, Colomb V, Braegger C, Bronsky J, Campoy C, Domellof M, et al. ESPGHAN Committee on nutrition position paper. intravenous lipid emulsions and risk of hepatotoxicity in infants and children: a systematic review and meta-analysis. J Pediatr Gastroenterol Nutr. (2016) 62:776–92. doi: 10.1097/MPG.0000000000001121
20. Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med. (2004) 140:18–25. doi: 10.7326/0003-4819-140-1-200401060-00007
21. Sigalet D, Boctor D, Brindle M, Lam V, Robertson M. Elements of successful intestinal rehabilitation. J Pediatr Surg. (2011) 46:150–6. doi: 10.1016/j.jpedsurg.2010.09.083
22. Sparks EA, Khan FA, Fisher JG, Fullerton BS, Hall A, Raphael BP, et al. Necrotizing enterocolitis is associated with earlier achievement of enteral autonomy in children with short bowel syndrome. J Pediatr Surg. (2016) 51:92–5. doi: 10.1016/j.jpedsurg.2015.10.023
23. Billiauws L, Thomas M, Le Beyec-Le Bihan J, Joly F. Intestinal adaptation in short bowel syndrome. what is new? Nutr Hosp. (2018) 35:731–7. doi: 10.20960/nh.1952
24. Georgeson KE, Breaux CW. Outcome and intestinal adaptation in neonatal short-bowel syndrome. J Pediatr Surg. (1992) 27:344–8. doi: 10.1016/0022-3468(92)90859-6
25. Enman MA, Wilkinson LT, Meloni KB, Shroyer MC, Jackson TF, Aban I, et al. Key determinants for achieving enteral autonomy and reduced parenteral nutrition exposure in pediatric intestinal failure. JPEN J Parenter Enteral Nutr. (2020) 44:1263–70. doi: 10.1002/jpen.1754
26. Soler WV, Lee AD, D'Albuquerque EMC, Capelozzi V, Albuquerque LC, Capelhuchnick P, et al. The effect of ileocecal valve removal in a model of short bowel syndrome. Arq Bras Cir Dig. (2019) 32:e1417. doi: 10.1590/0102-672020180001e1417
27. Olieman JF, Penning C, Ijsselstijn H, Escher JC, Joosten KF, Hulst JM, et al. Enteral nutrition in children with short-bowel syndrome: current evidence and recommendations for the clinician. J Am Diet Assoc. (2010) 110:420–6. doi: 10.1016/j.jada.2009.12.001
28. Lacaille F, Gupte G, Colomb V, D'Antiga L, Hartman C, Hojsak I, et al. Intestinal failure-associated liver disease: a position paper of the ESPGHAN working group of intestinal failure and intestinal transplantation. J Pediatr Gastroenterol Nutr. (2015) 60:272–83. doi: 10.1097/MPG.0000000000000586
29. Mian SI, Dutta S, Le B, Esquivel CO, Davis K, Castillo RO. Factors affecting survival to intestinal transplantation in the very young pediatric patient. Transplantation. (2008) 85:1287–9. doi: 10.1097/TP.0b013e31816dd236
30. Pichler J, Horn V, Macdonald S, Hill S. Intestinal failure-associated liver disease in hospitalised children. Arch Dis Child. (2012) 97:211–4. doi: 10.1136/archdischild-2011-300274
31. Lee WS, Chew KS, Ng RT, Kasmi KE, Sokol RJ. Intestinal failure-associated liver disease (IFALD): insights into pathogenesis and advances in management. Hepatol Int. (2020) 14:305–16. doi: 10.1007/s12072-020-10048-8
32. Goulet O, Abi Nader E, Pigneur B, Lambe C. Short bowel syndrome as the leading cause of intestinal failure in early life: some insights into the management. Pediatr Gastroenterol Hepatol Nutr. (2019) 22:303–29. doi: 10.5223/pghn.2019.22.4.303
33. Norsa L, Nicastro E, Di Giorgio A, Lacaille F, D'Antiga L. Prevention and treatment of intestinal failure-associated liver disease in children. Nutrients. (2018) 10:664. doi: 10.3390/nu10060664
34. Pappo I, Bercovier H, Berry EM, Haviv Y, Gallily R, Freund HR. Polymyxin B reduces total parenteral nutrition-associated hepatic steatosis by its antibacterial activity and by blocking deleterious effects of lipopolysaccharide. JPEN J Parenter Enteral Nutr. (1992) 16:529–32. doi: 10.1177/0148607192016006529
35. Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. (1990) 249:1431–3. doi: 10.1126/science.1698311
36. Ziegler TR, Almahfouz A, Pedrini MT, Smith RJ. A comparison of rat small intestinal insulin and insulin-like growth factor I receptors during fasting and refeeding. Endocrinology. (1995) 136:5148–54. doi: 10.1210/endo.136.11.7588253
37. Scopa CD, Koureleas S, Tsamandas AC, Spiliopoulou I, Alexandrides T, Filos KS, et al. Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg. (2000) 190:423–31. doi: 10.1016/S1072-7515(99)00285-9
38. El Kasmi KC, Anderson AL, Devereaux MW, Vue PM, Zhang W, Setchell KD, et al. Phytosterols promote liver injury and Kupffer cell activation in parenteral nutrition-associated liver disease. Sci Transl Med. (2013) 5:206ra137. doi: 10.1126/scitranslmed.3006898
39. Gura KM, Calkins KL, Puder M. Use of fish oil intravenous lipid emulsions as monotherapy in the pediatric intestinal failure patient: beyond the package insert. Nutr Clin Pract. (2020) 35:108–18. doi: 10.1002/ncp.10413
40. Anderson KT, Bartz-Kurycki MA, Martin R, Imseis E, Austin MT, Speer AL, et al. Tunneled central venous catheters in pediatric intestinal failure: a single-center experience. J Surg Res. (2018) 231:346–51. doi: 10.1016/j.jss.2018.05.081
41. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: a cohort study. Infect Control Hosp Epidemiol. (2016) 37:939–45. doi: 10.1017/ice.2016.83
42. Hartman C, Shamir R, Simchowitz V, Lohner S, Cai W, Decsi T, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: complications. Clin Nutr. (2018) 37:2418–29. doi: 10.1016/j.clnu.2018.06.956
43. Merras-Salmio L, Mutanen A, Ylinen E, Rintala R, Koivusalo A, Pakarinen MP. Pediatric intestinal failure: the key outcomes for the first 100 patients treated in a national tertiary referral center during 1984-2017. JPEN J Parenter Enteral Nutr. (2018) 42:1304–13. doi: 10.1002/jpen.1164
Keywords: pediatric intestinal failure, enteral autonomy, intestinal failure-associated liver disease, catheter-related bloodstream infections, China
Citation: Jiang W, Chen G, Wang Y, Zhong W, Zhou C, Zhang J, Lv X, Du C, Zhu Z, Geng Q and Tang W (2022) Multi-Center Analysis of Predictive Factors of Enteral Autonomy and Risk Factors of Complications of Pediatric Intestinal Failure in China. Front. Pediatr. 10:813865. doi: 10.3389/fped.2022.813865
Received: 12 November 2021; Accepted: 10 January 2022;
Published: 02 February 2022.
Edited by:
Consolato M. Sergi, Children's Hospital of Eastern Ontario (CHEO), CanadaReviewed by:
Luca Pio, Giannina Gaslini Institute (IRCCS), ItalyNikhil Pai, McMaster University, Canada
Copyright © 2022 Jiang, Chen, Wang, Zhong, Zhou, Zhang, Lv, Du, Zhu, Geng and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weibing Tang, dHdiY24mI3gwMDA0MDtuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Weiwei Jiang1†
Weiwei Jiang1† Ying Wang
Ying Wang Weibing Tang
Weibing Tang