- 1Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University, Changsha, China
- 2National Health Committee (NHC) Key Laboratory of Birth Defect for Research and Prevention, Hunan Provincial Maternal and Child Health Care Hospital, Changsha, China
- 3Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 4Hunan Provincial Key Laboratory of Clinical Epidemiology, Changsha, China
Background: Given that the time lag between cytomegalovirus (CMV) screening and diagnosed testing, a better knowledge of the association between pregnant women with CMV screening test positive and stillbirth in an epidemiological perspective was required to assist people being counseled reframe their pregnancy and birth plans based on the magnitude of the risk.
Methods: This study recruited 44048 eligible pregnant women from March 13, 2013 to December 31, 2019. Serological tests including CMV-specific IgM and IgG, and IgG avidity index were used to screen for maternal CMV infection and were measured by automated chemiluminescence immunoassay. The association was assessed using the inverse probability of group-weighted multivariate-adjusted log-binomial models.
Results: A total of 540 infants ended with a stillbirth (12.3 per 1000 pregnancies), and 2472 pregnancies with maternal CMV infection were screened out (56.1 per 1000 pregnancies) among all eligible pregnancies. In the comparison analysis, 326 infants ended with a stillbirth (86.6 per 1000 pregnancies) in the maternal CMV infection group compared with 214 infants (7.8 per 1000 pregnancies) in the group where mothers were not infected with CMV (RR 12.17; 95% CI 9.43–15.71). After excluding the pregnancies of stillbirth with birth defects, a strong association between the two groups was still observed (RR 9.38; 95% CI 6.92–12.70).
Conclusion: Our findings quantified the risk of a woman having a baby with stillbirth if she had a positive serologic CMV screening test in her first trimester, and supported the value of using CMV serologic tests as part of regular testing in pregnant women.
Trial registration: Registered in Chinese Clinical Trial Registry Center; registration number, ChiCTR1800016635; registration date, 06/14/2018 (Retrospectively registered); URL of trial registry record, https://www.chictr.org.cn/showproj.aspx?proj=28300.
Introduction
Stillbirth was defined as a baby born with no signs of life at 28 weeks’ gestation or more (1). In 2015, an estimated 2.6 million babies (uncertainty range: 2.4–3.0 million) died before birth during the last trimester of pregnancy, meaning a worldwide rate of 18.9 stillbirths per 1000 total births (uncertainty range: 17.4–21.1) (2). Despite improved obstetric and antenatal care, stillbirths have reduced more slowly (Average Annual Rate of Reduction, 1.8%), than either maternal (3.4%) or post-neonatal child mortality (4.5%) since 2000 (2), revealing that stillbirth remained an important public health issue with a large global burden. Although stillbirth had multiple etiologies, there was emerging evidence that viruses cause some stillbirths (3). Human cytomegalovirus (CMV), ubiquitous in nature, was the leading cause of congenital viral infection with an estimated incidence of 0.5–1% of congenital CMV infection in China, and was a known cause of stillbirth (4, 5). It was reported that CMV was detected in fetal tissues and the corresponding placentas from about 16% of stillborn infants, greatly outnumbering other pathogens (6–8), suggesting a strong association between CMV infection in pregnancy and stillbirth. Fetal CMV transmission can occur as a result of either a maternal primary or non-primary infection. The highest rate of congenital CMV infection occurred after primary infections in seronegative mothers (30–40%), while non-primary infections, including CMV reactivations or reinfections, resulted in congenital CMV infection in 0.2–2% of cases, implying that preconceptional immunity may play a role in preventing intrauterine transmission (9).
Maternal CMV screening in early pregnancy proved critical as a secondary preventive strategy in detecting and treating infections in their early stages. In the last decade, major efforts have been made to improve the early laboratory diagnosis of maternal infections (9). Maternal serology was the only reliable screening method in pregnancy that would identify up to 50% of all congenital CMV infections, by performing immunoglobulin M (IgM), immunoglobulin G (IgG), and IgG avidity tests in the population (10, 11). At present, maternal serology was adopted as a regular CMV screening program for pregnant women in China, listed as a crucial item in Chinese Preconception Care Guidelines. If the CMV screening result was positive, a CMV diagnostic test could be performed. The confirming diagnostic test was polymerase chain reaction amplification of CMV-DNA on amniotic fluid that can be collected by amniocentesis from 20 to 22 weeks of gestation or at least 8 weeks after the positive screening test (12, 13). If a woman had a positive serologic CMV screening test in her first trimester, there was a probability of uncovering adverse pregnancy outcomes such as stillbirth and severe fetal anomaly several weeks later, but the probability of this materializing and how it would affect the individual woman who was being counseled remained to be answered. Given that the time lag between screening and diagnosed tests, a better knowledge of the association between pregnant women with CMV screening test positive and stillbirth in an epidemiological perspective was required to assist people being counseled reframe their pregnancy and birth plans based on the magnitude of the risk. Thus, the purpose of this study was to determine the prevalence of pregnant women with a positive serologic CMV screening test in the first trimester, the prevalence of stillbirth, and the risk of women with a positive serologic CMV screening test in their first-trimester pregnancy having a baby with stillbirth. In order to rule out stillbirths caused by factors apart from CMV-mediated impaired placental function, we also explored the association between women who had a positive serologic CMV screening test and stillbirth without those accompanied with major birth defects.
Materials and Methods
Data Sources and Study Design
The study was conducted at the Hunan Provincial Maternal and Child Health Care Hospital (Changsha, Hunan Province, China). The present study was a prospective, hospital-based cohort study. The pregnant women, gestation age between gestational week 8 and week 14 (14), admitted to our hospital for prenatal examination from March 13, 2013 to December 31, 2019 were recruited consecutively, and the follow-up was completed before December 31, 2020. Gestational age was calculated according to the last menstrual period (15). Blood samples and some corresponding information were collected at the antenatal first visit, and subsequently, the samples were tested for serologic tests. The outcome was diagnosed in the inpatient department or outpatient clinic. Thus, a total of 44,673 pregnant women were recruited, and 44,048 were included in our final analysis. The present study has been registered in Chinese Clinical Trial Registry Center (registration number: ChiCTR1800016635); date of registration: June 14, 2018. Besides, this study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Xiangya School of Public Health, Central South University (No. XYGW-2018-36). Informed consent was obtained from all subjects involved in the study.
Outcome Definition
For international comparison WHO used stillbirth to mean the International Classification of Disease 10th revision (ICD 10) definitions of late fetal deaths: fetal deaths ≥ 1000 gms or ≥28 weeks or ≥35 cm (note birth weight was given priority over gestational age) (1). The birth defects were defined as infants diagnosed in the first year of life with major birth defects according to the classification system of the Chinese Surveillance of Congenital Anomalies of subgroups of major congenital anomalies, and the specific ICD 10 codes referred to the previous study (16).
Exposure and Covariate
In China, pregnant women routinely underwent serologic screening tests for CMV in the first trimester pregnancy including IgM, IgG, and IgG avidity testing. Confirmed diagnosis of maternal CMV infection in pregnancy should be based on seroconversion in pregnancy (de novo appearance of CMV-specific IgG in the serum of pregnant women who were previously seronegative). According to the Chinese Guidelines of CMV Infection Screening Procedures in Pregnancy (17), the type of maternal CMV infection was classified as primary infection or non-primary infection (i.e., following reactivation of a previous infection or reinfection with a new strain). Given that CMV screening tests are typically performed in early pregnancy rather than before conception, and that the serologic test was a screening test rather than a diagnostic test, the present study employed presumed maternal CMV infection rather than confirmed maternal CMV infection (18–20). The definition of presumed maternal primary CMV infection was based on the presence of CMV-specific low-avidity IgG and CMV-specific IgM in the first trimester of gestation (19). Furthermore, because IgG appeared later than IgM when primary infection occurred, pregnant women with CMV IgM but no IgG were asked to reexamine CMV-specific IgG 2–3 weeks later, and subsequently those with CMV-specific IgG in the paired samples were also considered primary CMV infection. The definition of presumed maternal non-primary CMV infection was based on the presence of CMV high-avidity IgG and CMV-specific IgM in the first trimester of gestation (9). In the absence of documented recent seroconversion, it was difficult to completely distinguish between primary and non-primary infection as both can be associated with the presence of IgG and IgM antibodies. Therefore, pregnant women were divided into the maternal CMV infection group and the no maternal CMV infection group, and clinical outcome data were collected and evaluated between the two comparative groups.
Specially trained investigators employed a self-designed questionnaire to obtain the corresponding information. The following was a list of the potential confounders, which were selected based on a review of the relevant literature (21–23): fertilization way (artificial fertilization or natural fertilization), age at pregnancy onset (<25, 25–29, 30–34, or≥35), nation (Han nationality or others), areas (urban or rural), education level (<9, 9–11, 12–16, or≥17), pre-pregnancy BMI (<18.5, 18.5–23.9, 24.0–26.9, 27.0–29.9, or ≥ 30), infant sex (male or female), gestation (multiple or single), parity (multipara or primipara), history of stillbirth pregnancy (yes or no), history of sexually transmitted diseases (yes or no), diabetes before conception (yes or no), history of drug abuse (yes or no), history of congenital malformations in family (yes or no), consanguineous marriage (yes or no), active smoking occurred since last menstruation (yes or no), passive smoking occurred since last menstruation (yes or no), drinking occurred since last menstruation (yes or no), folate use (yes or no), dyeing hair or perming since last menstruation (yes or no), decorating housing since last menstruation (yes or no). Having a smoking experience since last menstruation during pregnancy was considered as active smoking exposure. Exposure to secondhand smoke since last menstruation for more than 15 min per day, equal to or more than 4 days a week, for three consecutive months, whether at home and in the workplace was considered as passive smoking. Drinking occurred since last menstruation during pregnancy was defined as drinking exposure. We defined folate use as any use of folic acid in 3 months before pregnancy and/or during the first-trimester pregnancy. Following completion of the questionnaire, the investigator double-checked some of the information by consulting their Maternal and Child Health Manual and medical records. In China, each pregnant woman will be provided with a Maternal and Child Health Manual, which would record their basic demographic characteristics, behavioral habits, illness, and the results of various medical examinations during pregnancy.
We used propensity score estimations and matchings to consider a wide range of baseline characteristics and maternal periconceptional risk factors for stillbirth to isolate the association between pregnant women with CMV screening test positive and having a baby with stillbirth (24, 25). The propensity scores were estimated with a generalized boosted regression model and included all covariates listed in Table 1 as predictors. Notably, these covariates included in model were pre-selected based on literature review and were not, and should not be, selected based on a certain p-value level.
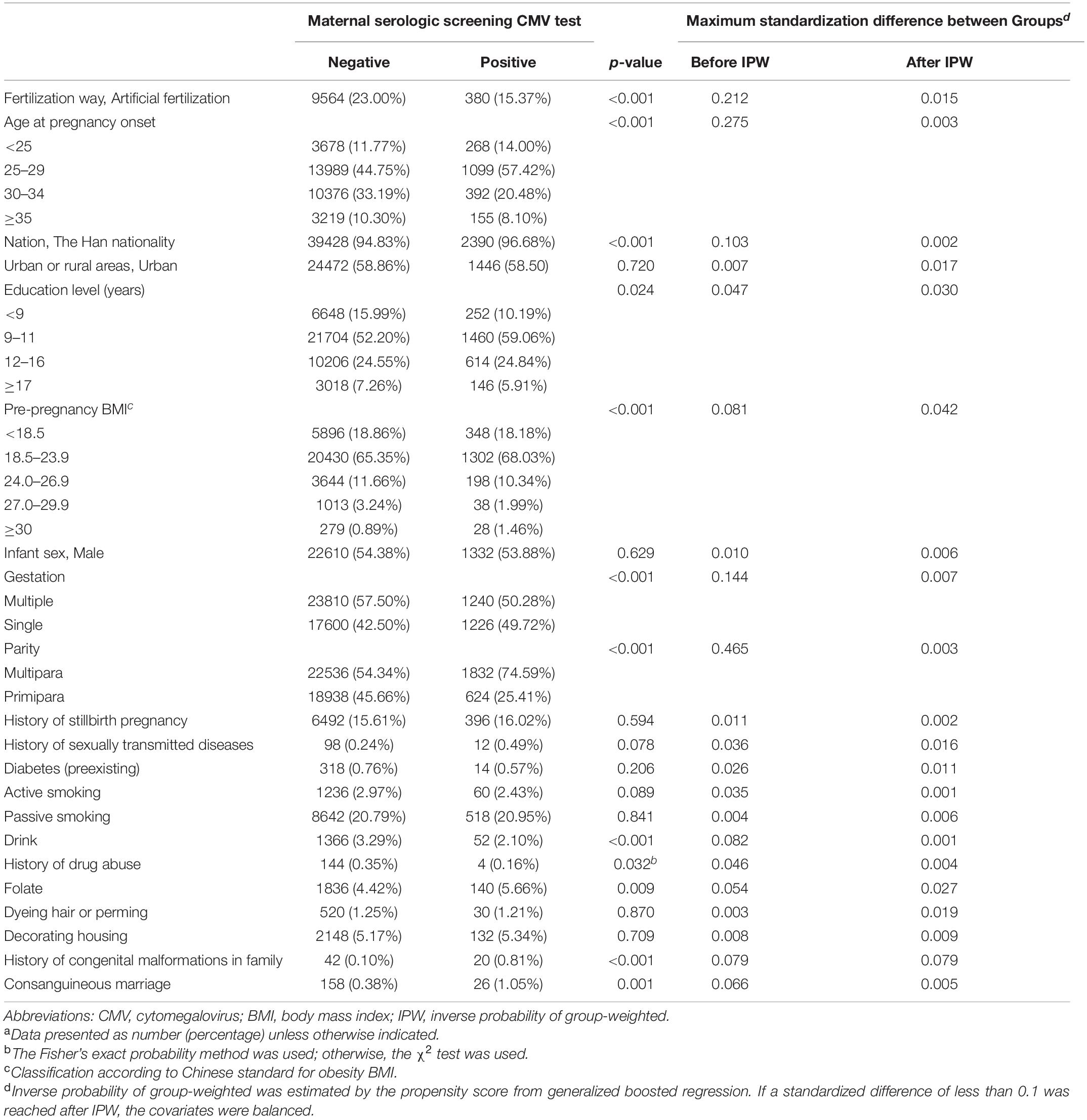
Table 1. The covariates used to define propensity of maternal first-trimester serologic screening CMV testing status.a
Serological Detection
The elbow vein blood of participants (3 mL) was collected for serological tests. CMV-specific IgM antibodies, IgG antibodies and anti-CMV IgG avidity index were measured with an automated chemiluminescence immunoassay (LIAISON XL; DiaSorin, Salugia, Italy). Sera were preabsorbed to avoid false-positive IgM reactions due to rheumatoid factors (26). Samples with concentrations of anti-CMV IgM ≥ 22 U/mL and anti-CMV IgG ≥ 14 U/mL were considered as positive, respectively. Samples with anti-CMV IgG avidity index < 0.20, 0.20 ≤ avidity index ≤ 0.30, and avidity index > 0.30 were considered as low-avidity IgG, moderate-avidity IgG, and high-avidity IgG, respectively.
Statistical Analyses
The distribution of the patient’s baseline characteristics in the study population was presented as a number (proportion) for categorical data and as the mean ± standard deviation for continuous data. Between-group comparisons were done with the Chi-squared tests or Fischer exact tests, as appropriate. The parameters of generalized boosted regression to estimate the propensity scores were summarized in the Supplementary Table 1. The quality of matching was assessed by standardized differences (similar to an effect size, it was defined as the mean difference divided by the common standard deviation). A covariate with a standardized difference of less than 10% between matched groups was considered well balanced. Next, the inverse probability of group-weighted (IPW) study populations was estimated using the calculated propensity scores from generalized boosted regression (27, 28). The association between first-trimester maternal CMV infection and risk of stillbirth was assessed by relative risk (RR) and their corresponding 95% confidence interval (CI), computed with log-binomial models using the IPW-standardized data.
No correction for multiple testing was applied. Statistical analysis was performed using R software, version 3.5.0 (R Foundation for Statistical Computing). All tests were two-tailed and a p-value < 0.05 was considered to indicate a statistically significant difference.
Results
The cohort study included 44,048 (98.6%) pregnant women in the primary analysis. A total of 44,673 pregnancies in the first trimester were recruited, 625 (1.4%) excluded: 178 (0.4%) missing CMV-specific serologic tests data; 447 (1.0%) lacking detailed information on pregnancy outcomes. The mean (standard deviation) age at pregnancy onset was 29.4 (4.2) years old. Among all the 44,048 eligible pregnancies, 540 infants ended with stillbirth presenting an incidence rate of 1.23% (range: 1.12–1.33%); 2,472 pregnancies were screened with a positive CMV serologic test presenting a prevalence rate of 5.61% (range: 5.40–5.83%). The screening procedure for maternal CMV infection during pregnancy among all eligible participants was summarized in Figure 1. Among 2472 women with a positive serologic CMV screening test, 94.01% were considered a non-primary infection and 5.99% had a primary infection. In addition, 38756 CMV seropositive pregnant women were screened out, presenting a mean prevalence of CMV-specific IgG of 94.88% (range: 94.68–95.09%) (Figure 1). The distributions of the estimated propensity scores of the comparative groups were shown in Supplementary Figure 1. The propensity scores showed considerable overlap between the comparative groups in these spreads, which indicated excellent covariate balance can be achieved with weights. The estimated propensity scores of the two comparative groups before and after IPW were presented in Table 1. Before IPW, there were statistically significant differences among the comparative groups across most covariates. After IPW, all the covariates were well balanced with the maximum between group-standardized differences < 10%.
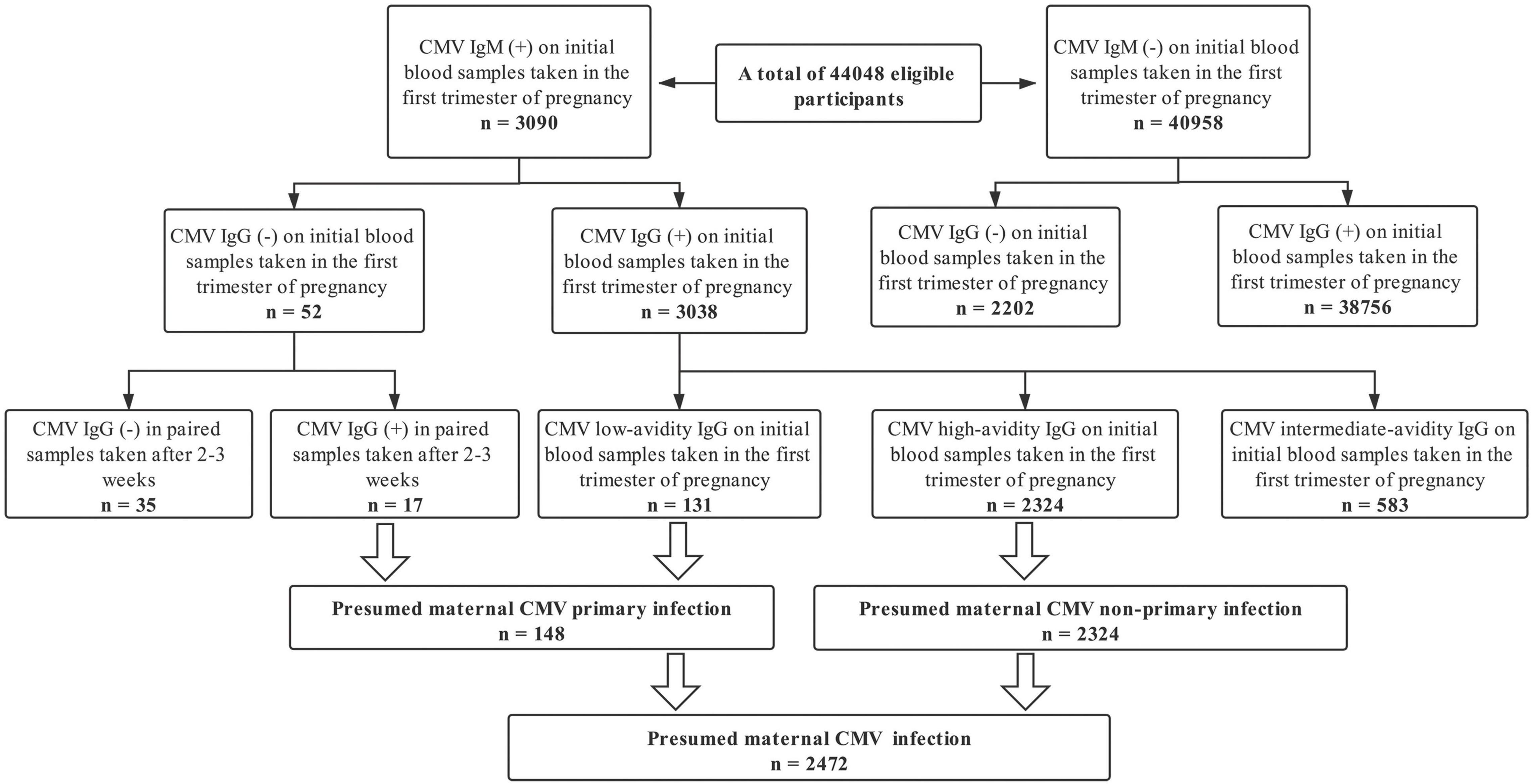
Figure 1. Screening procedure for maternal cytomegalovirus infection during pregnancy among all eligible participants.
The incidence rates of stillbirth in the maternal CMV infection group and the no maternal CMV infection group were presented in Table 2. In the group where women were screened with positive CMV infection results, there were 214 infants ended with stillbirth, with an incidence rate of 8.66% (range: 7.55–9.77%). A total of 326 infants ended with stillbirth in the group where mothers were screened with negative results, presenting an incidence rate of 0.78% (range: 0.70–0.87%). In the comparative analysis of the two groups, the pregnant women with positive CMV screening test in the first trimester of pregnancy showed an 11.17-fold increase in the incidence of stillbirth in newborns (RR 12.17; 95% CI 9.43–15.71) (Table 2). Furthermore, even after excluding the pregnancies of stillbirth accompanied with birth defects, we still observed a 8.38-fold increase in the incidence of stillbirth in newborns (RR 9.38; 95% CI 6.92–12.70).
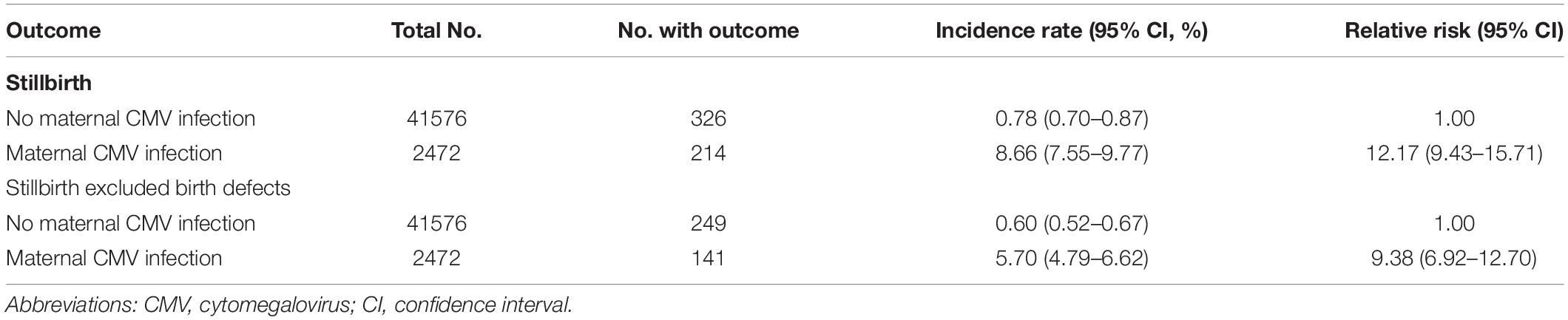
Table 2. The association between maternal first-trimester CMV serologic screening test positive and stillbirth in offspring.
Discussion
Stillbirth has multiple etiologies and several potential pathogenic mechanisms that could result in the death of the fetus. CMV infection is relatively common, with a high rate of transplacental transmission (29), and has been associated with placental damage that is known to result in fetal malformation and intrauterine death (30). Maternal serology including IgM, IgG, and IgG avidity tests is the reliable CMV screening program. Several countries including China de facto offer serologic CMV screening and counseling to advise pregnant women on preventive strategies and eventual laboratory reevaluation (31). Quantifying the risk of women with a positive serologic CMV screening test in their first-trimester pregnancy having a baby with stillbirth is required to assist people being counseled reframe their pregnancy and birth plans based on the magnitude of the risk.
This study observed that the stillbirth rate was estimated to be 12.3 (11.2–13.3) per 1000 births. The stillbirth rate of China in 2015 was higher than our findings, ranging between 5 and 10 per 1000 births (1), which might be due to the growing incidence of stillbirth or the relatively low-quality report data in some less-developed regions of China. The Every Newborn Action Plan aimed for national stillbirth rates of 12 or fewer stillbirths per 1000 births by 2030, but our cohort data revealed that China has not met this target so far. To achieve this target, China should continue to act to reduce preventable stillbirths and strengthen monitoring of stillbirth rates. CMV seroprevalence increased with age and differed by geographic area and socioeconomic status. We also found a very high CMV seroprevalence of about 94.82% among first-trimester pregnancies, which was basically in line with previous literature of 94–98% among Chinese women of child-bearing age (32, 33). The diagnosis of CMV infection in pregnant women based on clinical signs and symptoms is difficult and unreliable since up to 90% of infected mothers are asymptomatic and signs when present are non-specific (rhinitis, pharyngitis, myalgia, arthralgia, headache, fatigue) (34). The gold standard for diagnosis of primary CMV infection is based on serology with evidence of seroconversion documented by the presence of CMV specific IgG in the serum of a pregnant woman who previously tested IgG negative in a serum sample obtained earlier in pregnancy (20). However, this study did not collect the pregestational serum specimens, so the recommended best practice for diagnosing CMV infection is to do a serology test with measurement of IgM, IgG, and IgG avidity (10, 11, 20).
This study employed serologic testing and showed that 5.61% of pregnant women had a positive CMV screening test. Furthermore, pregnant women who had a positive CMV screening test in their first-trimester pregnancy had an 11.17-fold increase in the incidence of newborn stillbirth as compared to those who had a negative CMV screening test (RR 12.17). Our findings were biologically plausible. Congenital CMV infection can cause fetal injury both directly to the fetus and indirectly through placental dysfunction caused by infection or immune-mediated destruction (35). CMV infected and/or bypassed the placenta before it infected the embryo or fetus and was thought to cause adverse pregnancy outcomes that were associated with placental pathology, including intrauterine growth retardation and stillbirth (34, 36). Because fetal growth restriction was related to more than 50% of stillbirths, it was generally considered that both had similar etiologies and risk factors (37). Once there were not enough CMV-specific IgG antibodies to be generated promptly to protect the tissues and organs from infection, CMV would invade the placentas continuously as the gestation age advancing. CMV was able to infect a large spectrum of cells in vivo (34), and it could trigger a constellation of molecular mechanisms that altered placental differentiation (38), leading persistent injury fibrosis in infected placentas (39–41). It was suggested that chronic villitis was more frequently in placentas with CMV detected than in those without CMV (42). Chronic villitis including villous infarction, fibrosis, and avascular villi caused thrombosis in main stem and surface vessels, limiting fetal blood flow (8). As a result, abnormal placental hemodynamics and placental dysfunction might result in reduced oxygen and nutrient transport, eventually leading to stillbirth. Although the in-depth mechanisms were not fully elucidated, several possible mechanisms have been proposed, including impairment of trophoblast progenitor stem cell differentiation and function, impairment of extravillous trophoblast invasiveness, dysregulation of Wnt signaling pathways in cytotrophoblasts, and so on (36). Moreover, stillbirths were mainly caused by severe birth defects and fetal growth restriction (43). To rule out stillbirths caused by factors apart from CMV-mediated impaired placental function, we explored the association between women who had a positive serologic CMV screening test and stillbirth without major birth defects, and observed a robust association (RR 9.38).
The current study quantified the risk of a woman having a baby with stillbirth if she had a positive serologic CMV screening test in her first trimester, which answered an important question during pregnancy counseling. A universal screening program for CMV infection in pregnant women has sparked debate (9, 31). This work did not prove an etiological association between CMV and stillbirth, but supported the value of using serologic tests as part of regular CMV testing in pregnant women. Our findings also emphasized the significance of the continuous monitoring of congenital CMV infection as well as intrauterine growth of the fetus in pregnant women with positive serologic testing in their first-trimester pregnancy. In fact, the 11–14 week’ consultation has become an unmissable one worldwide, and it would represent the most practical compromise if only one sample can be taken. Moreover, valaciclovir, that can be safely used in the early fetal period, decreases vertical transmission by 70% and should be implemented as soon as possible after maternal infection (44). Based on long-term experience, we have reasons to believe that maternal screening provides superior and beneficial results in both the short and long terms (9). The limitations of the study needed to be addressed. First, the determination of CMV DNA in maternal biological fluids such as blood and urine would provide additional information. However, in accordance with Chinese situation, the screening method used in this study was based on the Chinese Guidelines of CMV Infection Screening Procedures in Pregnancy, and it also emphasized the significance of serologic testing as a universal screening program for CMV infection among pregnant women. Second, the causal correlation would be more convincing if the CMV DNA testing was conducted in fetus and the related placentas.
Conclusion
In conclusion, based on the prospective cohort study, we applied the serologic CMV screening test to quantify the risk of women with a positive serologic test in their first trimester pregnancy having a baby with stillbirth, and observed a strong association. Our findings supported the value of using serologic testing as part of regular CMV testing among pregnant women, and also worked in the actual hygiene consultation. When a positive serologic CMV test occurred in the first trimester, continuous monitoring of fetal growth restriction as well as congenital CMV infection of the fetus was supposed to be performed to identify these adverse conditions as early as possible, and thus women would reframe their pregnancies and birth plans according to the magnitude of the risk. To further develop prenatal diagnostic recommendations and optimize prenatal counseling, additional studies evaluating the association between maternal CMV infection and congenital CMV infection, intrauterine growth retardation, and CMV detected in placental and fetal tissue of stillbirths were required in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Xiangya School of Public Health, Central South University (No. XYGW-2018-36). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JD, JL, and YhL performed the experiments. SZ analyzed the data and statistical analyses. JS, YpL, and MS contributed reagents, material, and analysis tools. XlS, TW, and JQ wrote the main manuscript text. LC, JW, and MS collected reference and managed data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Project Funded by National Natural Science Foundation Program of China (82073653 and 81803313), Hunan Provincial Key Research and Development Program (2018SK2063 and 2018SK2062), Hunan Provincial Science and Technology Talent Support Project (2020TJ-N07), Natural Science Foundation of Hunan Province (2018JJ2551), and Open Project from NHC Key Laboratory of Birth Defect for Research and Prevention (KF2020006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the editors and reviewers for their suggestions and all colleagues working in Maternal and Child Health Promotion and Birth Defect Prevention Group.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.803568/full#supplementary-material
References
1. Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Global Health. (2016) 4:E98–108. doi: 10.1016/S2214-109X(15)00275-2
2. Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. (2016) 387:587–603. doi: 10.1016/S0140-6736(15)00837-5
3. Nuovo GJ, Cooper LD, Bartholomew D. Histologic, infectious, and molecular correlates of idiopathic spontaneous abortion and perinatal mortality. Diagn Mol Pathol. (2005) 14:152–8. doi: 10.1097/01.pas.0000176769.18423.37
4. Yamagishi Y, Miyagawa H, Wada K, Matsumoto S, Arahori H, Tamura A, et al. CMV DNA detection in dried blood spots for diagnosing congenital CMV infection in Japan. J Med Virol. (2006) 78:923–5. doi: 10.1002/jmv.20642
5. Gaytant MA, Rours GI, Steegers EA, Galama JM, Semmekrot BA. Congenital cytomegalovirus infection after recurrent infection: case reports and review of the literature. Eur J Pediatr. (2003) 162:248–53. doi: 10.1007/s00431-002-1115-5
6. Pereira L, Maidji E, McDonagh S, Genbacev O, Fisher S. Human cytomegalovirus transmission from the uterus to the placenta correlates with the presence of pathogenic bacteria and maternal immunity. J Virol. (2003) 77:13301–14. doi: 10.1128/jvi.77.24.13301-13314.2003
7. Syridou G, Spanakis N, Konstantinidou A, Piperaki ET, Kafetzis D, Patsouris E, et al. Detection of cytomegalovirus, parvovirus B19 and herpes simplex viruses in cases of intrauterine fetal death: association with pathological findings. J Med Virol. (2008) 80:1776–82. doi: 10.1002/jmv.21293
8. Iwasenko JM, Howard J, Arbuckle S, Graf N, Hall B, Craig ME, et al. Human cytomegalovirus infection is detected frequently in stillbirths and is associated with fetal thrombotic vasculopathy. J Infect Dis. (2011) 203:1526–33. doi: 10.1093/infdis/jir121
9. Saldan A, Forner G, Mengoli C, Gussetti N, Palù G, Abate D. Testing for cytomegalovirus in pregnancy. J Clin Microbiol. (2017) 55:693–702. doi: 10.1128/jcm.01868-16
10. Lagrou K, Bodeus M, Van Ranst M, Goubau P. Evaluation of the new architect cytomegalovirus immunoglobulin M (IgM), IgG, and IgG avidity assays. J Clin Microbiol. (2009) 47:1695–9. doi: 10.1128/JCM.02172-08
11. Delforge ML, Desomberg L, Montesinos I. Evaluation of the new LIAISON(®) CMV IgG, IgM and IgG avidity II assays. J Clin Virol. (2015) 72:42–5. doi: 10.1016/j.jcv.2015.09.002
12. Enders M, Daiminger A, Exler S, Ertan K, Enders G, Bald R. Prenatal diagnosis of congenital cytomegalovirus infection in 115 cases: a 5 years’ single center experience. Prenat Diagn. (2017) 37:389–98. doi: 10.1002/pd.5025
13. Leruez-Ville M, Foulon I, Pass R, Ville Y. Cytomegalovirus infection during pregnancy: state of the science. Am J Obstet Gynecol. (2020) 223:330–49. doi: 10.1016/j.ajog.2020.02.018
14. Leruez-Ville M, Magny JF, Couderc S, Pichon C, Parodi M, Bussières L, et al. Risk factors for congenital cytomegalovirus infection following primary and nonprimary maternal infection: a prospective neonatal screening study using polymerase chain reaction in saliva. Clin Infect Dis. (2017) 65:398–404. doi: 10.1093/cid/cix337
15. Alaee E, Gharib MJ, Fouladinejad M. Penile length and anogenital distance in male newborns from different Iranian ethnicities in Golestan province. Iran Red Crescent Med J. (2014) 16:e16729. doi: 10.5812/ircmj.16729
16. Andersson NW, Olsen RH, Andersen JT. Association between use of macrolides in pregnancy and risk of major birth defects: nationwide, register based cohort study. BMJ. (2021) 372:n107. doi: 10.1136/bmj.n107
17. Voordouw B, Rockx B, Jaenisch T, Fraaij P, Mayaud P, Vossen A, et al. Performance of zika assays in the context of Toxoplasma gondii, parvovirus B19, rubella virus, and cytomegalovirus (TORCH) diagnostic assays. Clin Microbiol Rev. (2020) 33:e00130-18. doi: 10.1128/CMR.00130-18
18. Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. (2013) 56:1232–9. doi: 10.1093/cid/cit018
19. Maltezou PG, Kourlaba G, Blázquez-Gamero D, Ville Y, Lilleri D, Dimopoulou D, et al. Maternal type of CMV infection and sequelae in infants with congenital CMV: systematic review and meta-analysis. J Clin Virol. (2020) 129:104518. doi: 10.1016/j.jcv.2020.104518
20. Navti OB, Al-Belushi M, Konje JC. Cytomegalovirus infection in pregnancy – an update. Eur J Obstet Gynecol Reprod Biol. (2021) 258:216–22.
21. Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet. (2011) 377:1331–40. doi: 10.1016/S0140-6736(10)62233-7
22. Selvaratnam RJ, Rolnik DL. Stillbirth: are we making more progress than we think? A retrospective cohort study. BJOG. (2021) 128:1304–12. doi: 10.1111/1471-0528.16665
23. Escañuela Sánchez T, Meaney S, O’Donoghue K. Modifiable risk factors for stillbirth: a literature review. Midwifery. (2019) 79:102539. doi: 10.1016/j.midw.2019.102539
24. Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. (2009) 51:171–84. doi: 10.1002/bimj.200810488
25. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
26. Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand J Infect Dis. (1999) 31:443–57. doi: 10.1080/00365549950163969
27. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. (2013) 32:3388–414. doi: 10.1002/sim.5753
28. Raghavan S, Liu WG, Saxon DR, Grunwald GK, Maddox TM, Reusch JEB, et al. Oral diabetes medication monotherapy and short-term mortality in individuals with type 2 diabetes and coronary artery disease. BMJ Open Diabetes Res Care. (2018) 6:e000516. doi: 10.1136/bmjdrc-2018-000516
29. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. (2007) 17:253–76. doi: 10.1002/rmv.535
30. Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. (2003) 289:1008–11. doi: 10.1001/jama.289.8.1008
31. Adler SP. Screening for cytomegalovirus during pregnancy. Infect Dis Obstet Gynecol. (2011) 2011:1–9. doi: 10.1155/2011/942937
32. Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. (1986) 256:1904–8. doi: 10.1001/jama.1986.03380140074025
33. Wang Y, Li S, Ma N, Zhang Q, Wang H, Cui J, et al. The association of ToRCH infection and congenital malformations: a prospective study in China. Eur J Obstet Gynecol Reprod Biol. (2019) 240:336–40. doi: 10.1016/j.ejogrb.2019.04.042
34. Burny W, Liesnard C, Donner C, Marchant A. Epidemiology, pathogenesis and prevention of congenital cytomegalovirus infection. Expert Rev Anti Infect Ther. (2004) 2:881–94. doi: 10.1586/14789072.2.6.881
35. Njue A, Coyne C, Margulis AV. The role of congenital cytomegalovirus infection in adverse birth outcomes: a review of the potential mechanisms. Viruses. (2020) 13:20. doi: 10.3390/v13010020
36. Njue A, Coyne C, Margulis AV, Wang D, Marks MA, Russell K, et al. The role of congenital cytomegalovirus infection in adverse birth outcomes: a review of the potential mechanisms. Viruses. (2021) 13:20. doi: 10.3390/v13010020
37. Leite DFB, Cecatti JG. Fetal growth restriction prediction: how to move beyond. ScientificWorldJournal. (2019) 2019:1519048. doi: 10.1155/2019/1519048
38. Pereira L, Maidji E, McDonagh S, Tabata T. Insights into viral transmission at the uterine-placental interface. Trends Microbiol. (2005) 13:164–74. doi: 10.1016/j.tim.2005.02.009
39. Benirschke K, Mendoza GR, Bazeley PL. Placental and fetal manifestations of cytomegalovirus infection. Virchows Arch B Cell Pathol. (1974) 16:121–39. doi: 10.1007/BF02894070
40. Monif GR, Dische RM. Viral placentitis in congenital cytomegalovirus infection. Am J Clin Pathol. (1972) 58:445–9. doi: 10.1093/ajcp/58.5.445
41. Garcia AG, Fonseca EF, Marques RL, Lobato YY. Placental morphology in cytomegalovirus infection. Placenta. (1989) 10:1–18. doi: 10.1016/0143-4004(89)90002-7
42. Tsuge M, Hida AI, Minematsu T, Honda N, Oshiro Y, Yokoyama M, et al. Prospective cohort study of congenital cytomegalovirus infection during pregnancy with fetal growth restriction: serologic analysis and placental pathology. J Pediatr. (2019) 206:42–8.e42. doi: 10.1016/j.jpeds.2018.10.003
43. Pereira L. Have we overlooked congenital cytomegalovirus infection as a cause of stillbirth? J Infect Dis. (2011) 203:1510–2. doi: 10.1093/infdis/jir126
Keywords: cytomegalovirus, stillbirth, prospective cohort study, antenatal care, TORCH
Citation: Song X, Li Q, Diao J, Li J, Li Y, Zhang S, Chen L, Wei J, Shu J, Liu Y, Sun M, Sheng X, Wang T and Qin J (2022) Association Between First-Trimester Maternal Cytomegalovirus Infection and Stillbirth: A Prospective Cohort Study. Front. Pediatr. 10:803568. doi: 10.3389/fped.2022.803568
Received: 28 October 2021; Accepted: 23 February 2022;
Published: 17 March 2022.
Edited by:
Ilknur Aydin Avci, Ondokuz Mayıs University, TurkeyReviewed by:
Cinzia Auriti, Bambino Gesù Children’s Hospital (IRCCS), ItalyLorena Elena Melit, George Emil Palade University of Medicine, Pharmacy, Science and Technology of Târgu Mureş, Romania
Copyright © 2022 Song, Li, Diao, Li, Li, Zhang, Chen, Wei, Shu, Liu, Sun, Sheng, Wang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiabi Qin, cWluamlhYmkxMjNAMTYzLmNvbQ==; Tingting Wang, d2FuZ3Rpbmc5MTEyM0AxMjYuY29t
 Xinli Song
Xinli Song Qiongxuan Li1
Qiongxuan Li1 Jiabi Qin
Jiabi Qin