- 1Haematology Research, Murdoch Children’s Research Institute, Melbourne, VIC, Australia
- 2Department of Paediatrics, The University of Melbourne, Melbourne, VIC, Australia
- 3Department of Anatomy and Physiology, The University of Melbourne, Melbourne, VIC, Australia
- 4Kids Cancer Centre, Sydney Children’s Hospital, Randwick, NSW, Australia
- 5Department of Haematology, Royal Children’s Hospital, Melbourne, VIC, Australia
The Fontan circulation introduces an increased risk of thromboembolism which is associated with substantial mortality and morbidity. Adverse outcomes of thromboembolic complications post-Fontan surgery vary in both nature and severity, ranging from local tissue infarction and pulmonary embolism to Fontan failure and ischemic stroke. Furthermore, recent studies have identified that subclinical stroke is common yet underdiagnosed in Fontan patients. Fontan patients are commonly treated with antiplatelet agents and/or anticoagulants as primary thromboprophylaxis. Optimal thromboprophylaxis management in the Fontan population is still unclear, and clinical consensus remains elusive despite the growing literature on the subject. This perspective will describe the nature of thromboembolism post-Fontan surgery and provide evidence for the use of both current and emerging thromboprophylaxis options for children and adults living with Fontan circulation.
Introduction
The Fontan procedure has enabled increasing numbers of pediatric patients with an array of single-ventricle physiologies to survive into adulthood. However, the Fontan circulation introduces an increased risk of thromboembolism (TE), which is in turn associated with substantial mortality and morbidity (1–3). Adverse outcomes of thromboembolic complications post-Fontan surgery vary in both nature and severity, ranging from local tissue infarction and pulmonary embolism, to Fontan failure and ischemic stroke (2, 4). Fontan patients are therefore commonly treated with antiplatelet agents or various anticoagulants, as prophylaxis to attenuate this increased thrombotic risk. However, the optimal management in the Fontan population remains unclear, and clinical consensus regarding thromboprophylaxis remains elusive (5).
This perspective aims to provide a brief outline of the nature of TE post-Fontan surgery, as well as insight into the growing body of literature evaluating the outcomes of conventional, as well as emerging thromboprophylactic regimes in the pediatric and adult Fontan populations.
TE Post-Fontan Surgery
Post-Fontan TE in the short- and long-term following surgery remains a significant complication of the procedure, and an enduring cause of morbidity and mortality (6, 7). Thromboembolic complications arising from the Fontan circuit commonly present in the arterial circulation as intracardiac thrombosis, ischemic stroke or arterial embolism, and in the venous circulation as central venous thrombosis or pulmonary embolism (2–4, 8, 9).
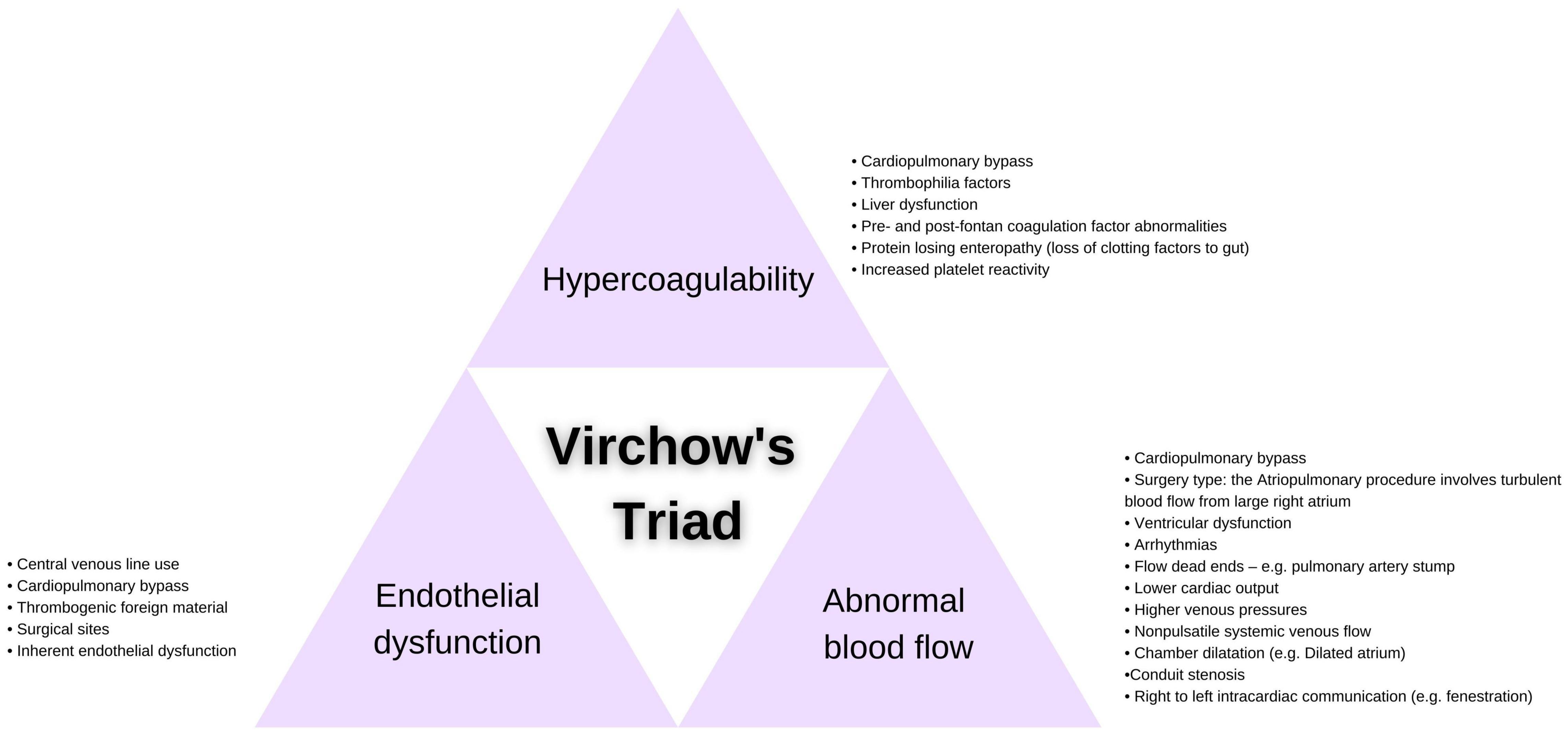
The Fontan circulation itself presents a highly thrombogenic environment and an accompanying complex pathophysiology, impacting all three elements of Virchow’s triad: endothelial cell dysfunction, abnormal blood flow and a hypercoagulable state (10) (Figure 1).
Endothelial injury results directly from surgical disruption of the endothelium and the introduction of thrombogenic prosthetic material in the Fontan circuit (11, 12). As a consequence of endothelial cell activation, release of anti-thrombotic factors is impaired, and secretion of pro-thrombotic mediators is enhanced, priming the Fontan vasculature for thrombus formation (13–15).
Abnormal blood flow in the Fontan circulation is caused by turbulence, stasis and a reduced pulmonary artery flow rate dictated by the low returning venous pressure (16), all of which contribute to local initiation of coagulation and an increased thrombotic risk (17).
Hypercoagulability caused by coagulation factor abnormalities has been outlined in a number of studies both prior to and following the Fontan procedure (11, 18–20). In particular, decreased levels of circulating anticoagulants Protein C, Protein S and antithrombin have been reported (17, 19–21), though a lack of adequate pediatric reference ranges has limited meaningful interpretation of these reports (2, 10). An increased level of coagulation factor VIII and decreased levels of factors II, V, VII and X in Fontan patients have also been described when compared to age-matched controls (22). Coagulation abnormalities being compounded by the development of liver dysfunction and/or protein-losing enteropathy post-Fontan surgery (and its associated serum protein imbalances) presents an additional mechanism for the dysregulation of hemostasis (23–25). There is a paucity of pediatric cohort studies using age-appropriate reference intervals to investigate thromboembolic phenomena in the Fontan circulation, limiting insight into coagulopathy for the youngest post-Fontan patients.
Cardiac surgery increases the likelihood of post-operative thromboembolic complications in both children and adults (4, 26–28). The reported increased risk of thrombosis following Fontan surgery is likely multi-factorial, owing to the insertion of central venous lines, the use of cardiopulmonary bypass and the variable surgical manipulation of tissue (29–31). Recent studies have also suggested that the altered hemodynamics resulting from stenosis and thrombosis may be related to the graft material selected and the Fontan procedure performed (12), with Deshaies et al. specifying a possible lower thromboembolic risk in the case of extracardiac conduit Fontan surgery (32). However, a number studies have found no correlation between Fontan circuit type and thrombosis (7, 25), and further research is needed to investigate these conflicting findings.
Despite TE being recognized as a common complication post-Fontan surgery, there is considerable variation in the reported incidence. For example, the incidence of venous thrombosis varies from 4 to 19% and the incidence of stroke ranges from 3 to 19% (33–37). Additionally, the incidence of intracardiac thrombosis has been reported to be between 17 and 33% (38–40), with the thrombi most commonly observed in the systemic venous atrium (48%) and pulmonary venous chamber (44%)(25).
A prospective, multi-center, randomized controlled trial conducted by Monagle et al. assessed thromboprophylaxis post-Fontan surgery (41) and demonstrated a clinically detected incidence of thrombosis of 7% and an overall incidence of thrombosis within the first two years post-Fontan surgery of 22%. Interestingly, all TEs observed in this study were venous and there was no record of arterial thrombosis. A post hoc analysis of Monagle et al.’s trial by McCrindle et al. demonstrated a time-related freedom from thrombosis of 69% at 2.5 years post-randomization (31). The highest time-related risk of thrombosis was observed during the immediate post-operative period up until six months post-surgery which was followed by a lower but increasing risk of thrombosis in the next two years. These results are supported by multiple studies which have also observed an immediate high risk of thrombosis in the first year post-Fontan (37, 39, 42–44) with thrombosis risk leveling off at 3.5 years before being followed by a second peak at 10 years post-Fontan (34, 45). Regarding long term incidence of TE, an Australian national registry including 1006 survivors of the Fontan operation demonstrated freedom from thromboembolic events was 82% at 25 years post-Fontan (95% CI, 74–87%) (46).
Interestingly there is emerging evidence that subclinical thrombosis is highly prevalent following Fontan surgery. A recent cross-sectional study that utilized magnetic resonance imaging (MRI), demonstrated that stroke was highly prevalent with an incidence of 39% at > 5 years post Fontan (3). Of these, only 6% of patients were diagnosed clinically. Similarly, Monagle et al. determined that the overall thrombosis rate was 22%, with a clinically detected thrombosis rate of 7%, suggesting that silent thrombosis is highly prevalent in this population (41). Whether silent thrombosis is clinically important remains unknown, however, given the widespread nature, certainly warrants further investigation.
Discrepancies in reports of thromboembolic complications can likely be attributed to variability in outcomes measured (e.g., intracardiac thrombus, pulmonary embolism, clinical or silent thrombosis), diagnostic imaging technique used, patient age, thromboprophylactic regimen, and inconsistencies in follow-up duration (1, 5, 7, 9, 47–49). Additional prospective studies are therefore necessary to reconcile these differences.
Thromboprophylaxis in the Fontan Population
The current clinical guidelines and consensus denote that thromboprophylaxis post-Fontan surgery is appropriate to reduce the risk of thrombosis in the Fontan circulation. Guidelines from the American Heart Association (AHA), American College of Cardiology (ACC) and the American College of Chest Physicians (ACCP) all nevertheless highlight that additional studies are needed to provide sufficient evidence for defining an optimal post-Fontan antithrombotic regime (50–53). The latest AHA recommendations state:
1. “Given the increased risk of the Fontan population for thromboembolic events, some form of thromboprophylaxis is warranted.
2. It is reasonable for all patients with a Fontan-class circulation to receive aspirin as thromboprophylaxis, whereas anticoagulation with warfarin should be reserved for patients with presumed risk factors, previous thrombotic events, or for older Fontan populations.
3. Both antiplatelet and anticoagulant agents retain a significant residual risk of post-Fontan surgery thrombosis.
4. The comparative safety and efficacy of DOACs for thromboprophylaxis in the Fontan patient remains to be determined.”
For children post Fontan surgery, the ACCP recommends aspirin or therapeutic UFH followed by vitamin K antagonists such as warfarin. The ACCP guidelines state that aspirin use for pediatric antiplatelet therapy should be administered in doses of 1–5 mg/kg per day, and pediatric patients receiving VKAs should be monitored to achieve a target INR of 2.5 (range 2.0–3.0) (51). The optimal duration of thromboprophylaxis remains to be determined however many patients continue therapy for life.
The emerging use of direct oral anticoagulant (DOAC) therapies post-Fontan surgery may present a promising alternate course of thromboprophylaxis in both children and adults. Studies of emerging anticoagulants must be carefully designed to ensure comparable safety and efficacy of these antithrombotic agents, particularly in complex cardiac populations (50, 54).
Currently, there are insufficient outcome data to recommend DOACs in a patient with a Fontan circulation (50, 55). Important considerations regarding the prescription of a suitable thromboprophylactic agent are outlined in Table 1.

Table 1. Comparison of considerations when prescribing warfarin, aspirin, and direct oral anticoagulants.
Aspirin and Warfarin
Fontan patients are generally prescribed lifelong thromboprophylactic treatment with aspirin or warfarin to mitigate the risk of thrombosis post-surgery. However, the superiority of aspirin or warfarin as a thromboprophylactic agent following Fontan palliation remains to be determined. Selection of a suitable thromboprophylactic regime must therefore consider mechanistic differences, drug interactions and monitoring requirement, as well as patient-related factors such as diet, concomitant medications and pre-existing conditions or history.
Warfarin is an oral anticoagulant that disrupts the vitamin K cycle, inhibiting the synthesis and activation of vitamin K-dependent clotting factors II, VII, IX, and X, as well as the anticoagulant proteins C, S, and Z. As a vitamin k antagonist (VKA), warfarin reduces the overall activation and efficacy of the coagulation cascade, permitting an antithrombotic state in the Fontan circulation. Warfarin use has a number of limitations, including a narrow therapeutic range, significant drug and food interactions and necessitating regular monitoring to ensure safe, controlled and effective anticoagulation (56).
Aspirin, or acetylsalicylic acid (ASA), is an oral antiplatelet drug that prevents the generation of thromboxane A2, irreversibly inhibiting platelet activation and aggregation to impair downstream platelet plug and thrombus formation (57). Although, monitoring of aspirin is not routinely performed in most clinical settings, the possible development of aspirin resistance and a consequential increased risk of thrombosis in a subset of the Fontan population must be considered. Preliminary estimates from small sample cohort studies of postoperative aspirin resistance in the Fontan population have ranged from 50 to 73% (58–60). However, future studies are needed to comprehensively assess the incidence of aspirin resistance in the Fontan patients, as well as its significance as a risk factor for thromboembolism.
With differing modes of action and considerations presented by each drug, several studies have aimed to compare outcomes of aspirin and warfarin after Fontan surgery. The study by Monagle et al. remains the only randomized trial to directly compare outcomes of warfarin and aspirin as thromboprophylaxis post-Fontan surgery (41). The initial results from a cohort of 111 Fontan patients, show that there was no significant difference in thrombotic outcomes between those receiving aspirin and warfarin over the two years post-Fontan surgery (41). However, a secondary analysis of this RCT revealed a 3.5-fold increased risk of thrombosis associated with poorly controlled warfarin therapy (≤30% of INR values within target range when compared to patients who consistently achieved target INR levels or received aspirin (31). This finding is consistent with a retrospective study from Faircloth et al. where high time within therapeutic INR for warfarin was associated with significantly lower rates of thrombotic and bleeding events in the Fontan population (61), indicating the importance of maintaining INR within a target range for effective warfarin thromboprophylaxis in the Fontan population.
A recent multicenter study by Attard et al. (3) utilized MRI, bone densitometry (dual-energy x-ray absorptiometry, DXA), bleeding and Quality of Life (QoL) tools to assess outcomes of aspirin and warfarin use after more than five years after Fontan surgery. This cross-sectional study provides some of the most comprehensive evidence of the long-term outcomes of thromboprophylaxis post Fontan surgery. The key findings of the study were the widespread prevalence of asymptomatic stroke (39%) and cerebral micro-hemorrhage (96%) in the Fontan population, irrespective of thromboprophylaxis type. In addition, high bleeding rates were reported in both groups, with bleeding being more frequent in the warfarin group. Bone mineral density (BMD) was reduced across the cohort compared with the general population; however, BMD was poorer in those receiving warfarin. In the warfarinized patient a reduction in BMD is most likely caused via warfarin’s inhibition of the vitamin K-dependent protein osteocalcin, impairing its key role in bone mineralization. Given the young age of the study cohort, inadequate bone mass accumulation is of particular concern as it is associated with increased fracture risk and osteoporosis in later life (62). Given the widespread levels of reduced BMD that were observed, BMD screening in the Fontan population, particularly those receiving long-term warfarin therapy may be warranted. Furthermore, selection of aspirin as primary thromboprophylaxis in the absence of contraindications may therefore improve bone health outcomes and in turn decrease a possible increased risk of fracture and osteoporosis in the Fontan population.
QoL has also become an increasingly significant metric used when assessing the wider impact of different clinical interventions in pediatric anticoagulant populations (63). Interestingly, in the Attard study, quality of life was similar between the warfarin and aspirin groups (3). Perhaps not surprisingly, in the warfarin group, home INR monitoring was associated with improved QoL scores compared to those receiving community monitoring. Previous studies have also demonstrated an improved QoL score when patients on warfarin commenced Home INR monitoring (64). Furthermore, a reduction in financial burden were demonstrated to both the family and the broader healthcare system when home INR monitoring was employed (65).
These findings, alongside the persistent debate regarding the relative efficacy of aspirin and warfarin, highlight that more favorable thromboprophylactic therapies are likely needed to resolve the significant residual risk of thrombosis and cerebrovascular injury in the aging Fontan population.
Emerging Antithrombotic Therapies
Given the limitations of VKAs as thromboprophylaxis, there has been extensive research into novel therapies in the broader population indicated for anticoagulation, though studies specific to safety and efficacy in patients with a Fontan-class circulation remain in their infancy (50, 66). Among the new classes of oral anticoagulants that have been developed are direct thrombin inhibitors (dabigatran) and direct factor Xa inhibitors (apixaban, endoxaban, rivaroxaban). These novel antithrombotic agents are collectively referred to as direct oral anticoagulants, or DOACs.
Rivaroxaban, like apixaban and endoxaban, is a highly selective direct inhibitor of factor Xa (67). By acting primarily to inhibit FXa, rivaroxaban hinders the flux through the intrinsic and extrinsic pathways of the coagulation cascade, ultimately impairing downstream thrombin generation and thrombus formation (68). Dabigatran instead inhibits both fibrin-bound and unbound (free) thrombin directly, restricting its powerful thrombogenic function as the final effector in the coagulation cascade (69).
DOACs offer attractive benefits when compared to VKA anticoagulants such as warfarin, namely their rapid onset of action, minimal drug and food interactions and predictable pharmacokinetics, avoiding the need for regular patient monitoring (70). However, adequate renal function is crucial to avoid an increased risk of hemorrhage during DOAC use in the adult congenital heart disease (ACHD) population (71).
While evidence-based analysis of DOACs for primary thromboprophylaxis in the Fontan population is rare across the published literature, there is a growing body of research (66). In a single-center study, Georgekutty et al. reported the first retrospective data on the safety and efficacy of DOACs as either primary or secondary thromboprophylaxis in 21 adult patients with a Fontan-type circulation (72). Over a cumulative 316 patient-months, one thrombotic event occurred in a patient with a failing Fontan physiology and Protein-losing enteropathy, and no major bleeding events were observed (median follow-up 13 months).
A larger multicenter prospective study by Yang et al. assessed DOAC use in 530 ACHD patients, with 14% (n = 74) having a Fontan-class circulation (73). All major bleeds and 50% of thromboembolic events occurred in Fontan patients (n = 3, 4.1%), emphasizing the susceptibility of the Fontan population to hemostatic complications. Further analysis of this Fontan cohort nevertheless determined comparable rates of thromboembolism and major bleeding to VKAs as short-term thromboprophylaxis (74). A recent retrospective multicenter study by Kawamatsu et al. consisting of 139 adult Fontan patients similarly proposed that DOACs may present benefits over VKAs in safety and efficacy, reducing the risk of thrombosis and hemorrhage (75).
Although the safety and efficacy of DOACs compared to VKAs has primarily been evaluated in adults, a recent trial investigated the safety and efficacy of rivaroxaban compared to aspirin in children post-Fontan procedure (81). The UNIVERSE study compared thromboprophylaxis with rivaroxaban or aspirin in 112 children post-Fontan surgery and revealed that patients who received rivaroxaban had a similar safety profile to those who received aspirin. A lower thrombotic event rate was also observed in the rivaroxaban group (2% vs. 9%) however this difference was statistically insignificant (76).
However, recent literature has also suggested that DOACs may incur significant risks when used for thromboprophylaxis in circulations that are intercalated with artificial surfaces, as is the case for the Fontan population. Three independent trials assessing dabigatran or rivaroxaban against standard of care in cardiac surgery patients were terminated prematurely due to safety concerns, as there was an excess of thromboembolic and hemorrhagic events experienced by patients in the DOAC treatment groups (77–79).
These adverse trial events occurred in patients whose circulations were exposed to significant amounts of synthetic material—namely mechanical heart valves (77), Left Ventricular Assist Devices (78) and transcatheter aortic-valve replacements (79). Given the similar reliance on thrombogenic artificial surfaces in the two dominant current Fontan procedures, the lateral tunnel and extracardiac conduit (12, 32), these studies indicate the need for careful consideration of possible complications when assessing the use of DOACs in the Fontan circulation.
There are currently three ongoing studies examining the safety and efficacy of DOACs as thromboprophylaxis for adult congenital heart disease (ACHD). A prospective multi-center observational study is aiming to assess routine apixaban use in ACHD, including Fontan-class circulations, and atrial arrythmias (NCT03854149) (80). There is also one ongoing interventional randomized control trial evaluating the safety and efficacy of DOACs in the pediatric Fontan population. The SAXOPHONE study (Safety of ApiXaban On Pediatric Heart disease On the preventioN of Embolism), entails a comparison of antithrombotic viability and QoL between apixaban and existing standard of care anticoagulants—VKAs and low molecular weight heparin in acquired and congenital heart disease pediatric patients (NCT02981472) (54). The SAXOPHONE study will stratify patients based on diagnosis into three groups: single ventricle, acquired and all other congenital heart disease. The single ventricle group will include Fontan patients and therefore this group can be analyzed to determine the efficacy of apixaban in the pediatric Fontan population. The outcomes of these trials will be extremely important to the field.
Conclusion
Recent literature has determined that cerebrovascular injury is a frequent occurrence after Fontan surgery, regardless of thromboprophylaxis type. In addition to TE incidence, several important secondary clinical outcomes have been identified that should be considered when determining an individual’s thromboprophylaxis regime.
Hence, the following recommendations should be considered for managing post-Fontan TE risk:
1. Where no further clinical complexities exist, it is reasonable to offer aspirin to Fontan patients as thromboprophylaxis. However, before one could be definitive about optimal thromboprophylaxis, consideration must bex given to important clinical features such as cardiac and lung function.
2. Routine BMD screening should be considered all Fontan patients, particularly those on long-term warfarin therapy. As with all populations that are particularly vulnerable to osteopenia/osteoporosis, vitamin D sufficiency and adequate dietary calcium intake are important factors to ensure bone mass accrual and maintenance. Furthermore, patients should be encouraged to engage in weight bearing exercise, in line with their physical capabilities.
3. When warfarin is indicated, a comprehensive anticoagulation service that facilitates home INR monitoring can be of great benefit to the patient in terms of both QoL and financially.
4. The routine use of DOACs as primary thromboprophylaxis after Fontan surgery is not currently recommended given the current limited evidence of efficacy in this cohort.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SV and CS: literature search. CA, SV, CS, VI, and PM: drafting of the manuscript. CA, VI, and PM: editing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kverneland LS, Kramer P, Ovroutski S. Five decades of the Fontan operation: a systematic review of international reports on outcomes after univentricular palliation. Congenit Heart Dis. (2018) 13:181–93. doi: 10.1111/chd.12570
2. Monagle P, Karl TR. Thromboembolic problems after the Fontan operation. Pediatr Card Surg Annu Semin Thorac Cardiovasc Surg. (2001) 5:36–47. doi: 10.1053/pcsu.2002.29716
3. Attard C, Monagle PT, d’Udekem Y, Mackay MT, Briody J, Cordina R, et al. Long-term outcomes of warfarin versus aspirin after Fontan surgery. J Thorac Cardiovasc Surg. (2021) 162:1218–28.e3. doi: 10.1016/j.jtcvs.2020.12.102
4. Egbe AC, Connolly HM, Niaz T, Yogeswaran V, Taggart NW, Qureshi MY, et al. Prevalence and outcome of thrombotic and embolic complications in adults after Fontan operation. Am Heart J. (2017) 183:8. doi: 10.1016/j.ahj.2016.09.014
5. Agarwal A, Firdouse M, Brar N, Yang A, Lambiris P, Chan AK, et al. Incidence and management of thrombotic and thromboembolic complications following the superior cavopulmonary anastomosis procedure: a literature review. Clin Appl Thromb. (2018) 24:405–15. doi: 10.1177/1076029617739702
6. Al-Shawk M, Banjoko A, Axiaq A, Amin K, Harky A. Perioperative and long-term management of Fontan patients. Cardiol Young. (2021) 31:775–85. doi: 10.1017/S1047951120004618
7. Faircloth JM, Roe O, Alsaied T, Palumbo JS, Vinks A, Veldtman GR. Intermediate term thrombotic risk in contemporary total cavo-pulmonary connection for single ventricle circulations. J Thromb Thrombolysis. (2017) 44:275–80. doi: 10.1007/s11239-017-1530-0
8. Monagle P, Cochrane A, McCrindle B, Benson L, Williams W, Andrew M. Thromboembolic complications after Fontan procedures-the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg. (1998) 115:493–8. doi: 10.1016/s0022-5223(98)70310-1
9. Tsang W, Johansson B, Salehian O, Holm J, Webb G, Gatzoulis MA, et al. Intracardiac thrombus in adults with the Fontan circulation. Cardiol Young. (2007) 17:7. doi: 10.1017/S104795110700145X
10. Attard C, Huang J, Monagle P, Ignjatovic V. Pathophysiology of thrombosis and anticoagulation post Fontan surgery. Thromb Res. (2018) 172:204–13. doi: 10.1016/j.thromres.2018.04.011
11. Tomkiewicz-Pajak L, Hoffman P, Trojnarska O, Lipczyńska M, Podolec P, Undas A. Abnormalities in blood coagulation, fibrinolysis, and platelet activation in adult patients after the Fontan procedure. J Thorac Cardiovasc Surg. (2014) 147:1284–90. doi: 10.1016/j.jtcvs.2013.06.011
12. Kelly JM, Mirhaidari GJM, Chang Y-C, Shinoka T, Breuer CK, Yates AR, et al. Evaluating the longevity of the Fontan pathway. Pediatr Cardiol. (2020) 41:1539–47. doi: 10.1007/s00246-020-02452-6
13. Binotto MA, Maeda NY, Lopes AA. Altered endothelial function following the Fontan procedure. Cardiol Young. (2008) 18:70–4. doi: 10.1017/S1047951107001680
14. Mahle WT, Todd K, Fyfe DA. Endothelial function following the Fontan operation. Am J Cardiol. (2003) 91:1286–8. doi: 10.1016/s0002-9149(03)00289-3
15. Lambert E, d’Udekem Y, Cheung M, Sari CI, Inman J, Ahimastos A, et al. Sympathetic and vascular dysfunction in adult patients with Fontan circulation. Int J Cardiol. (2013) 167:1333–8. doi: 10.1016/j.ijcard.2012.04.015
16. Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. (2016) 102:1081–6. doi: 10.1136/heartjnl-2015-307467
17. Jahangiri M, Kreutzer J, Zurakowski D, Bacha E, Jonas RA. Evaluation of hemostatic and coagulation factor abnormalities in patients undergoing the Fontan operation. J Thorac Cardiovasc Surg. (2000) 120:778–82. doi: 10.1067/mtc.2000.108903
18. Cheung EWY, Chay GW, Ma ESK, Cheung Y. Systemic oxygen saturation and coagulation factor abnormalities before and after the Fontan procedure. Am J Cardiol. (2005) 96:1571–5. doi: 10.1016/j.amjcard.2005.07.074
19. Odegard KC, McGowan FX, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ, et al. Coagulation factor abnormalities in patients with single-ventricle physiology immediately prior to the Fontan procedure. Ann Thorac Surg. (2002) 73:1770–7. doi: 10.1016/s0003-4975(02)03580-4
20. Callegari A, Christmann M, Albisetti M, Kretschmar O, Quandt D. Single ventricle physiology patients and coagulation abnormalities: is there a relationship with hemodynamic data and postoperative course? A pilot study. Clin Appl Thromb. (2019) 25:1–8. doi: 10.1177/1076029619888695
21. Jahangiri M, Shore D, Kakkar V, Lincoln C, Shinebourne E. Coagulation factor abnormalities after the Fontan procedure and its modifications. J Thorac Cardiovasc Surg. (1997) 113:989–93. doi: 10.1016/s0022-5223(97)70283-6
22. Odegard KC, McGowan FX, Zurakowski D, DiNardo JA, Castro RA, del Nido PJ, et al. Procoagulant and anticoagulant factor abnormalities following the Fontan procedure: increased factor VIII may predispose to thrombosis. J Thorac Cardiovasc Surg. (2003) 125:1260–7. doi: 10.1016/s0022-5223(02)73605-2
23. Rychik J, Dodds KM, Goldberg D, Glatz AC, Fogel M, Rossano J, et al. Protein losing enteropathy after Fontan operation: glimpses of clarity through the lifting fog. World J Pediatr Congenit Heart Surg. (2020) 11:92–6. doi: 10.1177/2150135119890555
24. Al Balushi A, Mackie AS. Protein-losing enteropathy following Fontan palliation. Can J Cardiol. (2019) 35:1857–60. doi: 10.1016/j.cjca.2019.07.625
25. Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL. Thrombus formation after the Fontan operation. Ann Thorac Surg. (2001) 71:1990–4. doi: 10.1016/s0003-4975(01)02472-9
26. Aziz F, Patel M, Ortenzi G, Reed AB. Incidence of postoperative deep venous thrombosis is higher among cardiac and vascular surgery patients as compared with general surgery patients. Ann Vasc Surg. (2015) 29:661–9. doi: 10.1016/j.avsg.2014.11.025
27. Silvey M, Hall M, Bilynsky E, Carpenter SL. Increasing rates of thrombosis in children with congenital heart disease undergoing cardiac surgery. Thromb Res. (2018) 162:15–21. doi: 10.1016/j.thromres.2017.12.009
28. Khoury H, Lyons R, Sanaiha Y, Rudasill S, Shemin RJ, Benharash P. Deep venous thrombosis and pulmonary embolism in cardiac surgical patients. Ann Thorac Surg. (2020) 109:1804–10. doi: 10.1016/j.athoracsur.2019.09.055
29. Heying R, van Oeveren W, Wilhelm S, Schumacher K, Grabitz RG, Messmer BJ, et al. Children undergoing cardiac surgery for complex cardiac defects show imbalance between pro- and anti-thrombotic activity. Crit Care. (2006) 10:11. doi: 10.1186/cc5108
30. Manlhiot C, Menjak IB, Brandao LR, Gruenwald CE, Schwartz SM, Sivarajan VB, et al. Risk, clinical features, and outcomes of thrombosis associated with pediatric cardiac surgery. Circulation. (2011) 124:1511–9. doi: 10.1161/CIRCULATIONAHA.110.006304
31. McCrindle BW, Manlhiot C, Cochrane A, Roberts R, Hughes M, Szechtman B, et al. Factors associated with thrombotic complications after the Fontan procedure: a secondary analysis of a multicenter, randomized trial of primary thromboprophylaxis for 2 years after the Fontan procedure. J Am Coll Cardiol. (2013) 61:346–53. doi: 10.1016/j.jacc.2012.08.1023
32. Deshaies C, Hamilton RM, Shohoudi A, Trottier H, Poirier N, Aboulhosn J, et al. Thromboembolic risk after atriopulmonary, lateral tunnel, and extracardiac conduit Fontan surgery. J Am Coll Cardiol. (2019) 74:1071–81. doi: 10.1016/j.jacc.2019.06.051
33. Rosenthal David N, Friedman Alan H, Kleinman Charles S, Kopf Gary S, Rosenfeld Lynda E, Hellenbrand William E. Thromboembolic complications after Fontan operations. Circulation. (1995) 92:287–93. doi: 10.1161/01.cir.92.9.287
34. Jahangiri M, Ross DB, Redington AN, Lincoln C, Shinebourne EA. Thromboembolism after the Fontan procedure and its modifications. Ann Thorac Surg. (1994) 58:1409–13. doi: 10.1016/0003-4975(94)91924-0
35. du Plessis AJ, Chang AC, Wessel DL, Lock JE, Wernovsky G, Newburger JW, et al. Cerebrovascular accidents following the Fontan operation. Pediatr Neurol. (1995) 12:230–6. doi: 10.1016/0887-8994(95)00027-d
36. Day RW, Boyer RS, Tait VF, Ruttenberg HD. Factors associated with stroke following the Fontan procedure. Pediatr Cardiol. (1995) 16:270–5. doi: 10.1007/bf00798060
37. Mathews K, Bale JF, Clark EB, Marvin WJ, Doty DB. Cerebral infarction complicating Fontan surgery for cyanotic congenital heart disease. Pediatr Cardiol. (1986) 7:161–6. doi: 10.1007/BF02424991
38. Balling G, Vogt M, Kaemmerer H, Eicken A, Meisner H, Hess J. Intracardiac thrombus formation after the Fontan operation. J Thorac Cardiovasc Surg. (2000) 119:745–52. doi: 10.1016/S0022-5223(00)70010-9
39. Fyfe DA, Kline CH, Sade RM, Gillette PC. Transesophageal echocardiography detects thrombus formation not identified by transthoracic echocardiography after the Fontan operation. J Am Coll Cardiol. (1991) 18:1733–7. doi: 10.1016/0735-1097(91)90512-8
40. Stümper O, Sutherland GR, Geuskens R, Roelandt JRTC, Bos E, Hess J. Transesophageal echocardiography in evaluation and management after a Fontan procedure. J Am Coll Cardiol. (1991) 17:1152–60. doi: 10.1016/0735-1097(91)90847-3
41. Monagle P, Cochrane A, Roberts R, Manlhiot C, Weintraub R, Szechtman B, et al. A multicenter, randomized trial comparing heparin/warfarin and acetylsalicylic acid as primary thromboprophylaxis for 2 years after the Fontan procedure in children. J Am Coll Cardiol. (2011) 58:645–51. doi: 10.1016/j.jacc.2011.01.061
42. Cromme-Dijkhuis AH, Bijleveld CMA, Henkens CMA, Hillege HL, Bom VJJ, vd Meer J. Coagulation factor abnormalities as possible thrombotic risk factors after Fontan operations. Lancet. (1990) 336:1087–90. doi: 10.1016/0140-6736(90)92568-3
43. Driscoll DJ. Long-term results of the Fontan operation. Pediatr Cardiol. (2007) 28:438–42. doi: 10.1007/s00246-007-9003-4
44. Putnam JB, Lemmer JH, Rocchini AP, Bove EL. Embolectomy for acute pulmonary artery occlusion following Fontan procedure. Ann Thorac Surg. (1988) 45:335–6. doi: 10.1016/s0003-4975(10)62478-2
45. Hanséus K, Björkhem G, Jögi P, Sonesson S-E. Formation of thrombus and thromboembolism after the bidirectional Glenn anastomosis, total cavopulmonary connection and the Fontan operation. Cardiol Young. (1998) 8:211–6. doi: 10.1017/s1047951100006107
46. d’Udekem Y, Iyengar AJ, Galati JC, Forsdick V, Weintraub RG, Wheaton GR, et al. Redefining expectations of long-term survival after the Fontan procedure. Circulation. (2014) 130(11 Suppl. 1):S32–8. doi: 10.1161/CIRCULATIONAHA.113.007764
47. Anderson PAW, Breitbart RE, McCrindle BW, Sleeper LA, Atz AM, Hsu DT, et al. The Fontan patient: inconsistencies in medication therapy across seven pediatric heart network centers. Pediatr Cardiol. (2010) 31:1219–28. doi: 10.1007/s00246-010-9807-5
48. Small AJ, Aboulhosn JA, Lluri G. Thromboprophylaxis in adults with atrio-pulmonary Fontan. World J Pediatr Congenit Heart Surg. (2018) 9:504–8. doi: 10.1177/2150135118772837
49. Firdouse M, Agarwal A, Chan AK, Mondal T. Thrombosis and thromboembolic complications in Fontan patients: a literature review. Clin Appl Thromb. (2014) 20:484–92. doi: 10.1177/1076029613520464
50. Rychik J, Atz AM, Celermajer DS, Deal BJ, Gatzoulis MA, Gewillig MH, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American heart association. Circulation [Internet]. (2019) 140:e234–84. doi: 10.1161/CIR.0000000000000696
51. Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. (2012) 141:e737S–e801. doi: 10.1378/chest.11-2308
52. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 139:e698–800.
53. Giglia TM, Massicotte MP, Tweddell JS, Barst RJ, Bauman M, Erickson CC, et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: a scientific statement from the American heart association. Circulation. (2013) 128:2622–703. doi: 10.1161/01.cir.0000436140.77832.7a
54. Payne RM, Burns KM, Glatz AC, Li D, Li X, Monagle P, et al. A multi-national trial of a direct oral anticoagulant in children with cardiac disease: design and rationale of the Safety of ApiXaban On Pediatric Heart disease On the preventioN of Embolism (SAXOPHONE) study. Am Heart J. (2019) 217:52–63. doi: 10.1016/j.ahj.2019.08.002
55. Mongeon F-P, Macle L, Beauchesne LM, Bouma BJ, Schwerzmann M, Mulder BJM, et al. Non-Vitamin K antagonist oral anticoagulants in adult congenital heart disease. Can J Cardiol. (2019) 35:1686–97. doi: 10.1016/j.cjca.2019.06.022
56. Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin C, et al. Guidelines on oral anticoagulation with warfarin - fourth edition: guideline. Br J Haematol. (2011) 154:311–24. doi: 10.1111/j.1365-2141.2011.08753.x
57. Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. (2003) 110:255–8. doi: 10.1016/s0049-3848(03)00379-7
58. Berganza FM, Gonzalez de Alba C, Egbe AC, Bartakian S, Brownlee J. Prevalence of aspirin resistance by thromboelastography plus platelet mapping in children with CHD: a single-centre experience. Cardiol Young. (2019) 29:24–9. doi: 10.1017/S1047951118000021
59. Tomkiewicz-Pajak L, Wojcik T, Chłopicki S, Olszowska M, Pajak J, Podolec J, et al. Aspirin resistance in adult patients after Fontan surgery. Int J Cardiol. (2015) 181:19–26. doi: 10.1016/j.ijcard.2014.11.219
60. Patregnani J, Klugman D, Zurakowski D, Sinha P, Freishtat R, Berger J, et al. High on aspirin platelet reactivity in pediatric patients undergoing the Fontan procedure. Circulation. (2016) 134:1303–5. doi: 10.1161/CIRCULATIONAHA.116.023457
61. Faircloth JM, Miner KM, Alsaied T, Nelson N, Ciambarella J, Mizuno T, et al. Time in therapeutic range as a marker for thrombotic and bleeding outcomes in Fontan patients. J Thromb Thrombolysis. (2017) 44:38–47. doi: 10.1007/s11239-017-1499-8
62. Flynn J, Foley S, Jones G. Can BMD assessed by DXA at age 8 predict fracture risk in boys and girls during puberty: an eight-year prospective study. J Bone Miner Res Off J Am Soc Bone Miner Res. (2007) 22:1463–7. doi: 10.1359/jbmr.070509
63. Jones S, Newall F, Manias E, Monagle P. Assessing outcome measures of oral anticoagulation management in children. Thromb Res. (2011) 127:75–80. doi: 10.1016/j.thromres.2010.09.001
64. Jones S, Monagle P, Manias E, Bruce AAK, Newall F. Quality of life assessment in children commencing home INR self-testing. Thromb Res. (2013) 132:37–43. doi: 10.1016/j.thromres.2013.05.011
65. Gaw JR, Crowley S, Monagle P, Jones S, Newall F. The economic costs of routine INR monitoring in infants and children–examining point-of-care devices used within the home setting compared to traditional anticoagulation clinic monitoring. Thromb Res. (2013) 132:26–31. doi: 10.1016/j.thromres.2013.04.028
66. Stalikas N, Doundoulakis I, Karagiannidis E, Bouras E, Kartas A, Frogoudaki A, et al. Non-vitamin K oral anticoagulants in adults with congenital heart disease: a systematic review. J Clin Med. (2020) 9:1794. doi: 10.3390/jcm9061794
67. Thomas TF, Ganetsky V, Spinler SA. Rivaroxaban: an oral factor Xa inhibitor. Clin Ther. (2013) 35:4–27. doi: 10.1016/j.clinthera.2012.12.005
68. Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer K-H, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939-an oral, direct Factor Xa inhibitor. J Thromb Haemost. (2005) 3:514–21. doi: 10.1111/j.1538-7836.2005.01166.x
69. Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation. (2011) 123:1436–50. doi: 10.1161/circulationaha.110.004424
70. Bauer KA. Pros and cons of new oral anticoagulants. Hematology. (2013) 2013:464–70. doi: 10.1182/asheducation-2013.1.464
71. Pujol C, Müssigmann M, Schiele S, Nagdyman N, Niesert A-C, Kaemmerer H, et al. Direct oral anticoagulants in adults with congenital heart disease – a single centre study. Int J Cardiol. (2020) 300:127–31. doi: 10.1016/j.ijcard.2019.09.077
72. Georgekutty J, Kazerouninia A, Wang Y, Ermis PR, Parekh DR, Franklin WJ, et al. Novel oral anticoagulant use in adult Fontan patients: a single center experience. Congenit Heart Dis. (2018) 13:541–7. doi: 10.1111/chd.12603
73. Yang H, Bouma BJ, Dimopoulos K, Khairy P, Ladouceur M, Niwa K, et al. Non-vitamin K antagonist oral anticoagulants (NOACs) for thromboembolic prevention, are they safe in congenital heart disease? Results of a worldwide study. Int J Cardiol. (2020) 299:123–30. doi: 10.1016/j.ijcard.2019.06.014
74. Yang H, Veldtman GR, Bouma BJ, Budts W, Niwa K, Meijboom F, et al. Non-vitamin K antagonist oral anticoagulants in adults with a Fontan circulation: are they safe. Open Heart. (2019) 6:e000985. doi: 10.1136/openhrt-2018-000985
75. Kawamatsu N, Ishizu T, Machino-Ohtsuka T, Masuda K, Horigome H, Takechi F, et al. Direct oral anticoagulant use and outcomes in adult patients with Fontan circulation: a multicenter retrospective cohort study. Int J Cardiol. (2021) 327:74–9. doi: 10.1016/j.ijcard.2020.11.024
76. McCrindle BW, Michelson AD, Van Bergen AH, Suzana Horowitz E, Pablo Sandoval J, Justino H, et al. Thromboprophylaxis for children post-Fontan procedure: insights from the UNIVERSE study. J Am Heart Assoc. (2021) 10:e021765.
77. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. (2013) 369:1206–14.
78. Andreas M, Moayedifar R, Wieselthaler G, Wolzt M, Riebandt J, Haberl T, et al. Increased Thromboembolic events with dabigatran compared with vitamin K antagonism in left ventricular assist device patients: a randomized controlled pilot trial. Circ Heart Fail. (2017) 10:e003709.
79. Dangas GD, Tijssen JGP, Wöhrle J, Søndergaard L, Gilard M, Möllmann H, et al. A controlled trial of rivaroxaban after transcatheter aortic-valve replacement. N Engl J Med. (2020) 382:120–9.
80. Kartas A, Doundoulakis I, Ntiloudi D, Koutsakis A, Kosmidis D, Rampidis G, et al. Rationale and design of a prospective, observational, multicentre study on the safety and efficacy of apixaban for the prevention of thromboembolism in adults with congenital heart disease and atrial arrhythmias: the PROTECT-AR study. BMJ Open. (2020) 10:e038012. doi: 10.1136/bmjopen-2020-038012
81. Pina LM, Dong X, Zhang L, Samtani MN, Michelson AD, Justino H, et al. Rivaroxaban, a direct Factor Xa inhibitor, versus acetylsalicylic acid as thromboprophylaxis in children post–Fontan procedure: rationale and design of a prospective, randomized trial (the UNIVERSE study). Am Heart J. (2019) 213:97–104. doi: 10.1016/j.ahj.2019.04.009
Keywords: Fontan, anticoagualtion, direct-acting oral anticoagulant (DOAC), warfarin, aspririn, stroke, thromboemboilc disease
Citation: Van Den Helm S, Sparks CN, Ignjatovic V, Monagle P and Attard C (2022) Increased Risk for Thromboembolism After Fontan Surgery: Considerations for Thromboprophylaxis. Front. Pediatr. 10:803408. doi: 10.3389/fped.2022.803408
Received: 27 October 2021; Accepted: 28 February 2022;
Published: 28 March 2022.
Edited by:
Jack Rychik, Children’s Hospital of Philadelphia, United StatesReviewed by:
Peter Kramer, Deutsches Herzzentrum Berlin, GermanyLeslie Raffini, Children’s Hospital of Philadelphia, United States
Copyright © 2022 Van Den Helm, Sparks, Ignjatovic, Monagle and Attard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chantal Attard, Y2hhbnRhbC5hdHRhcmRAbWNyaS5lZHUuYXU=
 Suelyn Van Den Helm
Suelyn Van Den Helm Christopher Noel Sparks1,3
Christopher Noel Sparks1,3 Vera Ignjatovic
Vera Ignjatovic Paul Monagle
Paul Monagle Chantal Attard
Chantal Attard