
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 23 March 2022
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.799524
 Xueqiang Yan1†
Xueqiang Yan1† Nannan Zheng2†
Nannan Zheng2† Jinfu Jia3†
Jinfu Jia3† Houfang Kuang1
Houfang Kuang1 Haiyan Lei1
Haiyan Lei1 Hongqiang Bian1
Hongqiang Bian1 Xinke Qin1
Xinke Qin1 Xuan Sun1
Xuan Sun1 Xufei Duan1*
Xufei Duan1* Jianghua Zhan4*
Jianghua Zhan4*Objective: This study aimed to explore the etiology, clinical features, diagnosis, and treatment of spontaneous bile duct perforation (SBDP) in children.
Methods: The clinical data of children with SBDP who were admitted to Wuhan Children's Hospital between January 2014 and January 2020 were retrospectively analyzed.
Results: In all, 28 cases of children with SBDP (male, 28.6%; female, 71.4%; male-to-female ratio, 1:2.5; average age, 2.15 years) were analyzed. The most common symptoms were fever (85.7%), nausea and vomiting (78.6%), and abdominal distension (67.9%). Among the 28 patients, 26 (92.9%) had elevated hypersensitive C-reactive protein, 24 (85.7%) had an increased neutrophil percentage, and 22 (78.6%) had raised peripheral blood leukocyte counts. Moreover, 19 patients (67.9%) showed increased serum total bilirubin levels, and 5 (17.9%) showed an elevated conjugated bilirubin level. Abdominal CT examination revealed that the gallbladder wall of patients was thickened with edema, accompanied by gallbladder stenosis and gallbladder mucosa enhancement; furthermore, ascites was found in the abdominal cavity and lesser omental bursa. Twenty-two patients underwent abdominal paracentesis, and 20 (90.9%) of them were exposed to bile-based ascites. Among the 28 patients, four recovered with conservative treatment, whereas the others (85.7%) were surgically treated. Of the twenty-four patients undergoing surgery, the perforation site was found at the union of the hepatic and cystic ducts in 12 patients (50%), no perforation site was observed in 9 patients (37.5%), and a common hepatic duct was observed in 3 patients (12.5%). All 24 patients underwent stage I surgery, and temporary biliary drainage was performed because of severe abdominal inflammation. Cholangiography and enhanced CT revealed an abnormal location of the pancreatic duct joining the bile duct in 64.3% patients. Following surgery, 15 patients underwent hepaticojejunostomy. Subsequently, 3-month to 6-year follow-up (median, 30 months) indicated that the patients recovered well with no serious complications.
Conclusion: SBDP in children may be associated with pancreaticobiliary malunion (PBM) and congenital weakness of the bile duct wall. However, the clinical manifestations of this condition lack specificity; this limitation can be assisted through diagnosis via abdominal CT and by performing abdominal paracentesis. Once SBDP diagnosis is confirmed, the patient should follow the principles of individualized treatment.
Spontaneous bile duct perforation (SBDP) is a rare disease, mostly affecting children about 6 months of age, with the age at onset ranging from 25 weeks of gestation to 7 years after birth (1–3). The most common presentation of SBDP is abdominal distension, ascites, and jaundice; other symptoms may include localized or generalized peritonitis, pyrexia, and septic shock with or without signs and symptoms of a biliary tract disease (2, 4, 5).
The exact etiopathogenesis of SBDP is yet to be elucidated, although various theories have been proposed such as congenital weakness of the bile duct (1), distal biliary obstruction (6), and pancreaticobiliary duct anomalies (pancreaticobiliary malunion (PBM), etc.) (7).
Management strategies for SBDP are variable, ranging from non-operative management techniques [such as the use of broad-spectrum antibiotics, endoscopic retrograde cholangiopancreatography (ERCP), and percutaneous drainage] to complex surgical procedures (such as Roux-en-Y anastomosis) (8–14).
Given the infrequent nature of SBDP, the diverse management strategies, and the lack of defined outcomes, here, we conducted a study in 28 SBDP patients admitted to Wuhan Children's Hospital from January 2014 to January 2020 and reported the findings as follows.
In all, 28 patients with SBDP were admitted to Wuhan Children's Hospital from January 2014 to January 2020, 4 of whom were clinically diagnosed and 24 were confirmed by surgical exploration (male, 8; female, 20; male-to-female ratio, 1:2.5). The age at onset ranged from 6.5 months to 7.5 years, with the average age of 2.15 years. Informed consent forms were signed by the parents/guardians of all patients, and the study was approved by the Hospital Ethics Committee.
In reference to the symptoms and signs, laboratory examinations, gallbladder ultrasound, and abdominal CT examinations were performed in the patients suspected with SBDP. In cases with difficulty in clinical diagnosis, patients underwent further abdominal paracentesis examination, and an SBDP diagnosis was clinically confirmed if abdominal paracentesis yielded biliary ascites. According to the caliber size of bile duct perforation during surgery, an appropriate surgical drainage scheme was determined. For patients with large perforated calibers (sufficiently large to place T-tubes), an extra-tube drainage of the bile ducts was administered. In patients with small (difficult to place T-tubes) or those without perforated calibers, a cholecystostomy tube was inserted instead. The subhepatic and pouch of Douglas drainage were added as necessary, and they were removed within 1 week if there was no drainage liquid and the abdominal ultrasound showed no ascites. When the T-tube drainage volume decreased, cholangiography was performed 2 weeks after surgery to observe the biliary tract, and the tube was clamped for 48 h. Both the biliary stent and external drain were removed only when the distal common bile duct showed no obstruction symptoms (e.g., abdominal pain, fever, and bile overflow). The patients were followed up regularly after surgery. If symptoms such as abdominal pain, fever, biliary dilatation, obstruction, and PBM (7, 15, 16) were confirmed, hepaticojejunostomy was performed.
Most SBDP cases analyzed in this study were acute or subacute, and the time from onset to treatment was between 4 h and 15 days. The common symptoms were fever (24 cases, 85.7%), nausea and vomiting (22 cases, 78.6%), abdominal distension (19 cases, 67.9%), abdominal pain (15 cases, 53.6%), and clay-like stool (3 cases, 10.7%) (Table 1).
Of the 28 patients, 26 (92.9%) had elevated high-sensitivity C-reactive protein ranging from 3.86 to 155 mg/L, 24 (85.7%) had increased neutrophil count ranging from 53 to 87.5, 22 (78.6%) had exceptional peripheral blood leukocyte count ranging from 11.2 × 109/L to 33.1 × 109/L, 19 (67.9%) had increased serum total bilirubin level ranging from 24.4 to 731 μmol/L, 5 (17.9%) had raised conjugated bilirubin level ranging from 8.4 to 72.3 μmol/L, 8 (28.6%) had both raised alanine aminotransferase and aspartate aminotransferase levels, and 15 (53.6%) had high blood amylase levels (Table 1).
The common imaging features of SBDP patients, such as thickened gallbladder wall with edema, void gallbladder cavity, and reinforced gallbladder mucosa (red arrow), were observed on abdominal CT examination before surgery. Ascites were found in the abdominal cavity and lesser omental bursa (black arrow) (Figure 1A). Furthermore, common bile duct rupture was observed in large perforations (Figure 1B). After surgical exploration, cholangiography revealed that the pancreatic duct merged with the common bile duct in advance in 18 patients (64.3%) with PBM (Figures 1C,D).
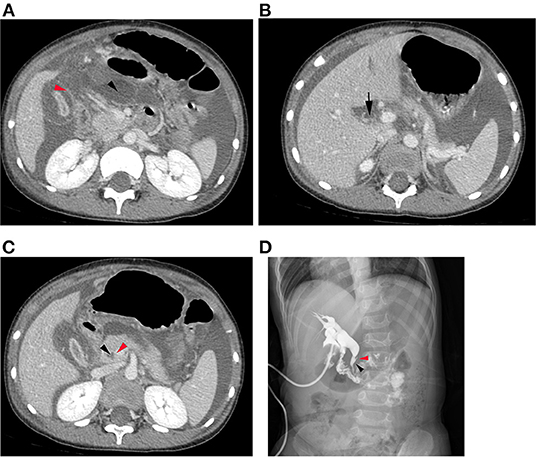
Figure 1. (A) Abdominal CT examination before surgery revealed thickened gallbladder wall with edema, narrow gallbladder cavity, and reinforced gallbladder mucosa (red arrow). Hydrops were found in the abdominal cavity and lesser omental bursa (black arrow). (B) A common bile duct rupture was observed in large perforations (black arrow). (C) Abdominal CT examination before surgery revealed that the pancreatic duct (red arrow) merged with the common bile duct (black arrow) in advance in cases with abnormal bile pancreatic confluence. (D) Cholangiography revealed that the pancreatic duct merged with the common bile duct in advance in cases with PBM.
Overall, 22 patients underwent abdominal paracentesis, and biliary ascites were discharged from the puncture point in 20 patients (Table 1). Furthermore, 24 patients underwent surgical exploration, and all of them were confirmed to have SBDP; their abdominal bile volumes ranged from 50 to 600 ml. Among these 24 cases, 12 had perforation sites at the confluence of the hepatic and cystic ducts, 9 had no visible perforation site, and 3 had a perforation site in the common hepatic duct. In the nine cases with no visible perforation site, eight underwent routine laparotomy or laparoscopic cholecystostomy. One patient (Case 14) underwent abdominal drainage. Furthermore, one patient (Case 1) was re-operated because of the presence of postoperative ascites; in this case, drainage tubes were placed in the omental foramen, subhepatic area, and splenic recess. One patient underwent laparoscopic common bile duct external T-tube drainage. Three patients had common hepatic duct perforations, one of whom opted for laparoscopic external T-tube drainage of the common bile duct, whereas the other two chose laparoscopy or laparoscopic cholecystostomy. Of these two patients, one (Case 21) underwent laparoscopic external choledochal T-tube drainage for the second time because of insufficient bile drainage on the third day after surgery. Among the 12 patients with perforation sites at the confluence of the common hepatic and cystic ducts, 8 opted for laparoscopy or laparoscopic cholecystostomy, whereas the other 4 underwent laparotomy or laparoscopic common bile duct external drainage (Table 2). Overall, 24 patients underwent cholangiography through T-tube or cholecystostomy tube for 1–3 months after surgery, and no obvious signs of obstruction were observed. After clamping the drainage tube for 48 h, drainage was removed only when the patient did not present with abdominal pain, fever, bile spillage, and other obstructive symptoms of the common bile duct.
In 28 patients with SBDP, 4 were cured by conservative treatment and reported no discomfort and no obvious bile duct stenosis or dilatation via MRCP during follow-up period. After removal of the biliary drainage tube in 15 patients, the diameter of their common bile duct was 1.1–6.0 cm (confirmed by MRCP) after 1.5–9 months of observation. Among these patients, 14 underwent laparoscopic common bile duct cystectomy and common hepatic duct jejunum Roux-en-Y anastomosis, and 1 underwent open choledochal duct cystectomy and common hepatic duct jejunum Roux-en-Y anastomosis (Table 2). These 15 patients who underwent hepaticojejunostomy had presented with PBM, and common bile duct dilatation confirmed via imaging examination. After 6–66 months of follow-up, no patient reported any discomfort. Nine patients underwent simple external biliary drainage; the indwelling time of the drainage tube was 1–3 months (mean = 2 months). After 3–74 months of follow-up, the diameter of the patients' common bile duct was 0.5–2.2 cm, and they did not report any discomfort. PBM was observed in three out of these nine cases. Among these three cases, one had no common bile duct dilatation while the others had. Case 23 was lost to contact with a short-term follow-up time of only 3 months (Table 2).
Freedland reported the first case of SBDP in 1882 through an autopsy (17). SBDP usually affects children below the age of 4 years and is particularly reported in 6-month-old children. Studies have shown that 85% patients with SBDP are aged ≤2 years (3). In this study, more female than male patients were diagnosed with SBDP, with a ratio of 2.5:1. The minimum age at onset was 6.5 months, and the oldest patient was 7.5 years old. The average age was 2.15 years.
At present, the exact pathogenesis of SBDP remains unclear, and it may be associated with various factors. SBDP is extremely rare in adults. Furthermore, acute pancreatitis, acalculous cholecystitis, human immunodeficiency virus infection, Hodgkin's lymphoma, tuberculosis, and severe necrotizing enterocolitis are considered important causative factors of SBDP in adults (18, 19). In contrast, health professionals believe that in the pediatric population, common bile duct dilation and SBDP may share a common etiology, i.e., PBM (7, 15, 16). In such cases, the convergence of pancreatic and bile ducts is abnormally located outside the duodenal wall (7). The abnormal confluence of pancreatic juice and bile leads to the formation of insoluble protein plugs in the distal end of the bile duct, in turn blocking the bile and pancreatic ducts and triggering abdominal pain (20, 21). In this study, 78.6% patients showed gastrointestinal symptoms, such as nausea and vomiting, and 53.6% showed clinical manifestations such as abdominal pain. Moreover, 53.6% patients underwent hepaticojejunostomy because of PBM and bile duct dilatation. Therefore, this study supports the clinical hypothesis that PBM is associated with SBDP. Distal choledochal stenosis, distal choledochal atresia, biliary calculi, and biliary parasites may cause cholestasis, which will then lead to inflammation, and increase the pressure on the bile duct, thereby increasing the possibility of SBDP. In this study, 24 patients with a higher number of biliary tract protein plugs during operation can support this view. Congenital weakness of the bile duct wall is another important factor observed among children with SBDP (1). Approximately, 60% of biliary blood supply comes from the posterior superior pancreaticoduodenal artery, whereas 38% originates from the hepatic artery. These blood vessels run along the course of the common bile duct. The union of the common bile duct and the cystic duct is seen at the end of the blood supply, which is the lowest point of blood supply. The union of the cystic duct and the common bile duct is therefore a congenital weak area of the biliary tract, and this is the main reason why SBDP usually develops here (22). In this study, intraoperative observation confirmed that the perforation site was at the union of the common hepatic and cystic ducts in half of the cases. This observation provided strong evidence for the theory of congenital weakness of the bile duct. In cases of abnormal union of pancreatic and bile ducts, pancreatic juice enters the biliary tract and causes local tissue damage. The pressure of the biliary tract suddenly rises because of biliary obstruction (cause by protein plugs), aggravating ischemia in the case of poor blood flow. These abovementioned factors work together to eventually cause SBDP (23) (Figure 2).
Pediatric SBDP lacks specific clinical manifestations. In this study, no evident peritoneal irritation sign was found in patients. The main reason is probably because bile leakage arising from biliary tract perforation was sterile. At an early stage, perforation causes sterile biliary peritonitis with inconspicuous peritoneal irritation. As the disease progresses and is combined with bacterial infection, non-specific symptoms such as fever, nausea, and vomiting are observed as the most common manifestations (24).
Laboratory and imaging examinations are required to assist SBDP diagnosis. Among the 28 patients in this study, 26 (92.9%) had elevated hypersensitive C-reactive protein, which was considerably higher than the proportion of patients with a high peripheral leukocyte count (78.6%). Thus, an increase in hypersensitive C-reactive protein can be used as an indicator for the early recognition of inflammation. In this study, 67.9% patients showed increased serum total bilirubin levels, but only 17.9% showed elevated conjugated bilirubin level; 28.6% patients had increased alanine aminotransferase and aspartate aminotransferase levels at the same time. Given that the perforation of the biliary tract can partially relieve cholestasis, no typical obstructive jaundice was observed. Therefore, the cases of SBDP should not be clinically measured using liver function indicators, such as increase in total bilirubin and conjugated bilirubin levels. In addition, 53.6% patients had elevated blood amylase levels; cholangiography and enhanced CT revealed the abnormal location of the joining of the pancreatic duct and the bile duct in 64.3% patients (Figures 1C,D). This result provided a basis for the clinical hypothesis that PBM is the leading point of SBDP.
Ultrasound is an important diagnostic tool in SBDP diagnostics. If abdominal ultrasound shows dilatation of the extrahepatic bile duct with ascites, then SBDP and bile peritonitis should be considered. The formation of limited fluid aggregation or pseudocysts (25) in and around the porta hepatis is also an important sign. However, SBDP is not easy to diagnose with only ascites and no bile duct expansion (21). In addition, ultrasound examination is limited by intestinal gases and restricted in the case of a non-expanding biliary system.
Enhanced CT examination is as well a key diagnostic modality for SBDP diagnosis. SBDP often tends to manifest as cholecystitis and/or cholangitis, and their characteristics can be clearly distinguished on enhanced CT. The following two signs are highly suggestive of SBDP: (1) remarkably reduced gallbladder tension and wrinkled gallbladder wall accompanied by ascites, mainly around the gallbladder and (2) highly edematous gallbladder wall (1–2 cm water-like density band around the gallbladder), shrunken gallbladder cavity, and enhanced wrinkles in the inner mucous membrane (26). In addition, the breach can directly be observed when the perforation is large enough, providing strong evidence for SBDP diagnosis (Figure 3).
In resource-limited conditions, abdominal paracentesis is a practical alternative. In this study, 92.9% of patients successfully yielded yellow-brown ascites via abdominal paracentesis. These ascites had a higher bilirubin level (>6 mg/dl) than the normal range (0.7–0.8 mg/dl), suggesting the presence of bile ascites (27).
The therapeutic strategy for SBDP must be tailored to the situation. We reviewed 65 case reports or series published on SBDP, and the treatments were variable (Table 3 and Supplementary Table 1). In our study, four patients barely had symptoms of abdominal distension and vomiting and recovered successfully through fasting, antibiotic treatment, and nutritional support. In cases with large ascites, external drainage can be performed via ultrasound-guided abdominal paracentesis. Furthermore, abdominal distension is often a sign of paralytic intestinal obstruction, and a progressive increase in abdominal distension requires prompt surgery. The purpose of surgical treatment is to prevent persistent contamination by controlling bile leakage, draining the peritoneal cavity, and restoring biliary tract patency. The pathological causes of the biliary tract leading to perforation must be resolved as early as possible through laparotomy, laparoscopy, or robotic technology (28). Among the 24 patients who underwent surgery in our study, the choice between intraoperative cholecystostomy and common bile duct external T-tube drainage mainly depended on the intraoperative status of porta hepatis. When the perforation of the bile duct was large, common bile duct external T-tube drainage was performed. Bile leaks often cause severe inflammation in porta hepatis. Cholecystostomy is usually preferred because of its simplicity and for preventing damage to the surrounding blood vessels and porta hepatis. This procedure can be given priority at non-specialized hospitals or units with weaker hepatobiliary surgery technology. Successful SBDP treatment with the endoscopic placement of biliary stents has been recently reported in adult cases (1, 19, 29, 30), but not in children. In all cases, the abdominal cavity, especially subhepatic space (31), must be adequately flushed during surgery. However, after cholecystostomy, various problems may occur, such as insufficient bile drainage and ascites. To resolve this issue, multiple drainage tubes can be placed under the liver and pelvis to reduce the probability of a re-operation (7). Intraoperative repair of the perforation is unnecessary, and direct repair during surgery can easily lead to secondary injuries and postoperative stenosis (29). Under extreme conditions where the anatomical structure is unrecognizable because of severe abdominal inflammation, a simple extra-abdominal drainage can also be an option. In general, in this procedure, three drainage tubes are required to be placed under the liver, spleen fossa, and the pouch of Douglas for 3–4 weeks (31, 32).
Some researchers have reported distal obstruction caused by stones or stenosis in the distal bile duct to be a cause of perforation. However, others believe that obstruction may be a consequence of perforation, which results in slow bile transport and cholestasis, rather than being the cause of perforation. Obstruction is usually resolved by proper external biliary drainage (29, 33). Therefore, in the absence of intraoperative cholangiography, the best treatment is still cholecystostomy or T-tube drainage even if there is an obstruction in the distal biliary tract (34, 35).
Furthermore, patients with SBDP should be followed up regularly after surgery. In cases of recurrent abdominal pain, biliary dilatation, obstruction, and abnormal pancreaticobiliary duct confluence, biliary tract hepaticojejunostomy should be performed (17). Upadhyaya et al. (36) believed that if cholangiography (via T-tube or magnetic resonance cholangiopancreatography) suggests dilation of the common bile duct, hepaticojejunostomy should be performed to avoid biliary cirrhosis, portal hypertension, recurrent pancreatitis, and cholangiocarcinoma. However, hepaticojejunostomy must be performed after inflammation control. In this study, 24 patients underwent stage I surgery, and temporary external biliary drainage was performed because of severe abdominal inflammation. After operation, cases 5 and 6 exhibited persistent abdominal pain and discomfort. Abdominal CT revealed that their biliary tract was dilated to varying degrees (1.1 and 1.2 cm, respectively). Furthermore, hepaticojejunostomy in cases 1–4 and 7–15 was performed when the dilation of the common bile duct was over 2.0 cm, and good recovery was observed in these cases. The dilation of the common bile duct in cases 16, 18–22, and 24 was within 1.0 cm. Surprisingly, although biliary dilatation was observed in cases 17 and 23 (especially case 23, where the diameter of the common bile duct reached 2.2 cm) after the surgery, they did not have any postoperative complications, which indicated that hepaticojejunostomy was unnecessary.
Existing studies have indicated that SBDP and congenital biliary dilatation (CBD) share the same etiology (8, 37). Thus, we suggest that PBM is the key in the determination of whether to perform hepaticojejunostomy. In our experience, in SBDP patients with biliary dilatation after surgery, hepaticojejunostomy may temporarily not be performed if the patient does not experience persistent or recurrent abdominal pain, fever, and other symptoms. The patients can simply be instructed to visit for regular follow-ups, and hepaticojejunostomy can be actively performed if the aforementioned symptoms occur. Besides, if hepaticojejunostomy is performed in asymptomatic patients with a common bile duct diameter of ≤1.0 cm, the difficulty of biliary and intestinal anastomosis will be increased. We reckon that hepaticojejunostomy should be performed if the following conditions exist (Figure 3): (1) common bile duct dilation (diameter ≥2.0 cm); (2) presentation of clinical symptoms and mild dilation of the common bile duct (1.0 cm < diameter <2.0 cm) combined with PBM; and (3) no peritonitis 2–8 weeks after SBDP external drainage.
There are some limitations to the current study. The current study reports the experience of a single pediatric tertiary center. Although our study is the largest study of SDBP to date, a total of 28 cases are still insufficient. Further analysis of follow-up information was limited by insufficient follow-up time, especially for patients with a common bile duct diameter of ≤1.0 and >2.0 cm but no hepaticojejunostomy. Furthermore, it is currently unclear whether the bile ducts will continue to expand or increase the risk of carcinogenesis. Further studies need to be conducted to evaluate these factors in detail.
Finally, based on our experience, three treatments should be followed for SBDP treatment (Figure 3): (1) nonsurgical treatment, such as antibiotic treatment, and B-ultrasound-guided external drainage by abdominal paracentesis; (2) laparotomy or laparoscopy and drainage through cholecystostomy tube or biliary T-tube; and (3) biliary tract reconstruction by laparoscopic or open hepaticojejunostomy in phase II. Conservative treatment is given priority for SBDP in clinical practice. Furthermore, external biliary drainage (temporary cholecystostomy or external biliary drainage through T-tube) with or without subhepatic drainage can be performed to promote spontaneous closure of the biliary tract perforation. Finally, hepaticojejunostomy should only be considered for patients with malformations of pancreaticobiliary duct or common bile duct dilatation (30).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Wuhan Children's Hospital Ethics Committee. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.799524/full#supplementary-material
1. Evans K, Marsden N, Desai A. Spontaneous perforation of the bile duct in infancy and childhood: a systematic review. J Pediatr Gastroenterol Nutr. (2010) 50:677–81. doi: 10.1097/MPG.0b013e3181d5eed3
2. Huda F, Naithani M, Singh SK, Saha S. Ascitic fluid/serum bilirubin ratio as an aid in preoperative diagnosis of choleperitoneum in a neglected case of spontaneous common bile duct perforation. Euroasian J Hepato Gastroenterol. (2017) 7:185–6. doi: 10.5005/jp-journals-10018-1246
3. Cai W, Pan K, Li Q, Miao X, Shu C. Biliary peritonitis due to spontaneous perforation of the left intrahepatic bile duct in an adult: a case report and review of literature. Int Surg. (2019) 103:339–43. doi: 10.9738/INTSURG-D-14-00264.1
4. Masroor M, Sarwari MA. Spontaneous common bile duct perforation in full term pregnancy: a rare case report and review of literature. BMC Surg. (2021) 21:239. doi: 10.1186/s12893-021-01230-2
5. Leung LJ, Vecchio MJH, Rana A, Behrle-Yardley A, Brewer N, McBride W. Total laparoscopic management of spontaneous biliary perforation. Clin J Gastroenterology. (2020) 13:818–22. doi: 10.1007/s12328-020-01122-7
6. Davenport M, Heaton ND, Howard ER. Spontaneous perforation of the bile duct in infants. Br J Surg. (1991) 78:1068–70. doi: 10.1002/bjs.1800780912
7. Fukuzawa H, Urushihara N, Miyakoshi C, Kajihara K, Kawahara I, Isono K, et al. Clinical features and risk factors of bile duct perforation associated with pediatric congenital biliary dilatation. Pediatr Surg Int. (2018) 34:1079–86. doi: 10.1007/s00383-018-4321-6
8. Kasat LS, Borwankar SS, Jain M, Naregal A. Spontaneous perforation of the extrahepatic bile duct in an infant. Pediatr Surg Int. (2001) 17:463–4. doi: 10.1007/s003830000477
9. Hasegawa T, Udatsu Y, Kamiyama M, Kimura T, Sasaki T, Okada A, et al. Does pancreatico-biliary maljunction play a role in spontaneous perforation of the bile duct in children. Pediatr Surg Int. (2000) 16:550–3. doi: 10.1007/s003830000433
10. Kanojia RP, Sinha SK, Rawat J, Wakhlu A, Kureel S, Tandon R. Spontaneous biliary perforation in infancy and childhood: clues to diagnosis. Indian J Pediatr. (2007) 74:509–10. doi: 10.1007/s12098-007-0091-1
11. Hirigoyen MB, Geoghegan JG, Owen ER, Singh MP. Spontaneous perforation of the bile duct in infancy. Eur J Pediatr Surg. (1995) 5:375–6. doi: 10.1055/s-2008-1066248
12. Sahnoun L, Belghith M, Jouini R, Maazoun K, Mekki M, Ben Brahim M, et al. Spontaneous perforation of the extrahepatic bile duct in infancy: report of two cases and literature review. Eur J Pediatr Surg. (2007) 17:132–5. doi: 10.1055/s-2007-965123
13. Goldberg D, Rosenfeld D, Underberg-Davis S. Spontaneous biliary perforation: biloma resembling a small bowel duplication cyst. J Pediatr Gastroent Nutr. (2000) 31:201–3. doi: 10.1097/00005176-200008000-00024
14. Barnes BH, Narkewicz R, Sokol RJ. Spontaneous perforation of the bile duct in a toddler: the role of endoscopic retrograde cholangiopancrea-tography in diagnosis and therapy. J Pediatr Gastroent and Nutr. (2006) 43:695–7. doi: 10.1097/01.mpg.0000233162.43409.ec
15. Todani T, Watanabe Y, Fujii T, Uemura S. Anomalous arrangement of the pancreatobiliary ductal system in patients with a choledochal cyst. Am J Surg. (1984) 147:672–6. doi: 10.1016/0002-9610(84)90139-9
16. Tashiro S, Imaizumi T, Ohkawa H, Okada A, Katoh T, Kawaharada Y, et al. Pancreaticobiliary maljunction: retrospective and nationwide survey in Japan. J Hepatobiliary Pancreat Surg. (2003) 10:345–51. doi: 10.1007/s00534-002-0741-7
17. Malik HS, Cheema HA, Fayyaz Z, Hashmi MA, Parkash A, Waheed N, et al. Spontaneous perforation of bile duct, clinical presentation, laboratory work up, treatment and outcome. J Ayub Med Coll Abbottabad. (2016) 28:518–22.
18. Yasar NF, Yasar B, Kebapci M. Spontaneous common bile duct perforation due to chronic pancreatitis, presenting as a huge cystic retroperitoneal mass: a case report. Cases J. (2009) 2:6273. doi: 10.4076/1757-1626-2-6273
19. Gerrard AD, Thind G, Date R. Primary bile duct perforation associated with pancreatitis. ACG Case Rep J. (2018) 5:e45. doi: 10.14309/crj.2018.45
20. Pei W, Gao M, Liu D, Wu L. Domestic pediatric spontaneous biliary perforation in 320 cases of clinical meta-analysis. J Clin Pediatr Surg. (2013) 12:113–16. doi: 10.3969/j.issn.1671-6353.2013.02.011
21. Lee MJ, Kim MJ, Yoon CS. MR cholangiopancreatography findings in children with spontaneous bile duct perforation. Pediatr Radiol. (2010) 40:687–92. doi: 10.1007/s00247-009-1447-7
22. Jiang H, Liu B, Shi W. Spontaneous perforation of choledochal cyst in children: CT manifestations. J Pract Radiol. (2013) 29:1646–8. doi: 10.3969/j.issn.1002-1671.2013.10.025
23. Chen Y, Zhang J, Wang Y, Wei Q, Li X. Spontaneous perforation of choledochal cyst in children: report of 16 cases. Chin J Gen Surg. (2002) 17:102–3.
24. Chiang L, Chui CH, Low Y, Jacobsen AS. Perforation: a rare complication of choledochal cysts in children. Pediatr Surg Int. (2011) 27:823–7. doi: 10.1007/s00383-011-2882-8
25. Lloyd DA, Mickel RE. Spontaneous perforation of the extra-hepatic bile ducts in neonates and infants. Br J Surg. (1980) 67:621–3. doi: 10.1002/bjs.1800670905
26. Wu X. CT diagnosis of acute biliary tract perforation (analysis in 15 cases). J Med Imaging. (2005) 7:89. doi: 10.3969/j.issn.1008-7664.2005.01.081
27. Logedi V, Hansen EN. Spontaneous biliary perforation in a 3-month old. J Pediatr Surg Case Rep. (2018) 39:42–4. doi: 10.1016/j.epsc.2018.07.027
28. Atwez A, Augustine M, Nottingham JM. Non-traumatic perforation of common hepatic duct: case report and review of literature HP. Int J Surg Case Rep. (2017) 41:188–90. doi: 10.1016/j.ijscr.2017.10.023
29. Jeanty C, Derderian SC, Hirose S, Lee H, Padilla BE. Spontaneous biliary perforation in infancy: management strategies and outcomes. J Pediatr Surg. (2015) 50:1137–41. doi: 10.1016/j.jpedsurg.2014.07.012
30. Pereira E Cotta MV, Yan J, Asaid M, Ferguson P, Clarnette T. Conservative management of spontaneous bile duct perforation in infancy. J Pediatr Surg. (2012) 47:1757–9. doi: 10.1016/j.jpedsurg.2012.06.023
31. Mohanty SK, Mahapatra T, Behera BK, Acharya B, Kumar S, Dash JR, et al. Spontaneous perforation of common bile duct in a young female: an intra-operative surprise. Int J Surg Case Rep. (2017) 35:17–20. doi: 10.1016/j.ijscr.2017.04.002
32. Subasinghe D, Udayakumara EAD, Somathilaka U, Huruggamuwa M. Spontaneous perforation of common bile duct: a rare presentation of gall stones disease. Case Rep Gastrointestinal Med. (2016) 2016:1–3. doi: 10.1155/2016/5321304
33. Zhou C, Wei B, Gao K, Zhai R. Biliary tract perforation following percutaneous endobiliary radiofrequency ablation: a report of two cases. Oncol Lett. (2016) 11:3813–16. doi: 10.3892/ol.2016.4436
34. Zimmer V. Cholangioscopic finding of common bile duct perforation. Gastrointest Endosc. (2019) 90:525–6. doi: 10.1016/j.gie.2019.05.003
35. Upadhyaya VD, Kumar B, Singh M, Rudramani, Jaiswal S, Lal R, et al. Spontaneous biliary peritonitis: is bed side diagnosis possible? Afr J Paediatr Surg. (2013) 10:112–16. doi: 10.4103/0189-6725.115034
36. Hopper L, Hao SB, Rodeberg D, Longshore S. Spontaneous bile duct perforation in a neonate. J Pediatr Surg Case Rep. (2018) 38:57–60. doi: 10.1016/j.epsc.2018.08.003
Keywords: biliary tract, perforation, common bile duct dilatation, pediatric surgery, spontaneous bile duct perforation, pancreaticobiliary malunion
Citation: Yan X, Zheng N, Jia J, Kuang H, Lei H, Bian H, Qin X, Sun X, Duan X and Zhan J (2022) Analysis of the Clinical Characteristics of Spontaneous Bile Duct Perforation in Children. Front. Pediatr. 10:799524. doi: 10.3389/fped.2022.799524
Received: 21 October 2021; Accepted: 25 January 2022;
Published: 23 March 2022.
Edited by:
Anshu Srivastava, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), IndiaReviewed by:
Vikrant Sood, The Institute of Liver and Biliary Sciences (ILBS), IndiaCopyright © 2022 Yan, Zheng, Jia, Kuang, Lei, Bian, Qin, Sun, Duan and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xufei Duan, YWxlbmR4ZjZAaG90bWFpbC5jb20=; Jianghua Zhan, emhhbmppYW5naHVhdGpAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.