
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 05 April 2022
Sec. Children and Health
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.797208
This article is part of the Research Topic Evidenced Based Medical Care of Hospitalized Children with Local Adaptations in Low-Resource Settings View all 10 articles
 Nour Abdallah Ba-alwi1†
Nour Abdallah Ba-alwi1† John Ogooluwa Aremu2†
John Ogooluwa Aremu2† Michael Ntim3
Michael Ntim3 Russel Takam4
Russel Takam4 Mwanaidi Amiri Msuya5
Mwanaidi Amiri Msuya5 Hamid Nassor6
Hamid Nassor6 Hong Ji1*
Hong Ji1*Background: Neonatal sepsis is still a major cause of death and morbidity in newborns all over the world. Despite substantial developments in diagnosis, treatments, and prevention strategies, sepsis remains a common problem in clinical practice, particularly in low-resource countries.
Methods: A retrospective cohort study of 238 neonates with positive blood culture-proven sepsis (in Muhimbili National Hospital) was conducted from January 2019 to December 2020. The outcomes of hospitalization were survival and death.
Results: In total, 45.4% mortality resulted from 238 neonates who had sepsis exclusively based on blood culture positivity. A significant association was found between very low birth weight (VLBW), hyperglycemia, mechanical ventilation, and high neonatal mortality. Among the different clinical presentations of neonatal sepsis, lethargy, vomiting, and respiratory distress were found to be frequently associated with neonatal mortality. Furthermore, sepsis with Gram-negative bacteria and early-onset sepsis were also associated with high neonatal mortality. Of the 108 neonatal deaths, the largest proportion (40%) was observed with Staphylococcus aureus, and the remaining 38% was caused by Klebsiella, 14% by Escherichia coli, 5% by Pseudomonas, 4% by Acinetobacter, and 2% by Streptococcus. No neonatal deaths from Serratia infection were observed. The overall resistance of isolated organisms to the recommended first-line antibiotics was 84% for ampicillin and 71.3% for gentamicin. The resistance pattern for the recommended second-line antibiotics was 76.2% for ceftriaxone, 35.9% for vancomycin, and 17.5% for amikacin.
Conclusion: VLBW, early-onset sepsis, clinical and laboratory parameters like lethargy, vomiting, and hyperglycemia, sepsis with Gram-negative bacteria, and being on mechanical ventilation are strong predictors of death in neonatal sepsis. In addition, this study discovered extraordinarily high resistance to conventional antibiotics. These findings give light on the crucial aspects to consider in preventing this disease and poor outcomes.
Neonatal sepsis is a clinical condition defined as an infection in newborns accompanied by or caused by an infection of the blood, typically bacterial and rarely fungal. Its symptoms and clinical features include temperature instability, respiratory difficulties, and refusal to eat (1). Neonatal sepsis can be categorized as early-onset sepsis (EOS) and late-onset sepsis (LOS). EOS is defined as the onset of signs and symptoms of infection within 72 h of life and may be associated with pathogen isolation or not. In LOS, signs and symptoms are present after 72 h of life (2). Sepsis is the most common reason for infant mortality, with around 2.4 million fatalities each year or 6,700 per day (3). Neonatal deaths that occur around the world each year total to ~4 million. About 30–50% of the total newborn fatalities in underdeveloped nations are caused by neonatal sepsis (4). Early detection, appropriate antibiotic medication, and aggressive supportive care are all elements of prompt diagnosis (4). The risk of newborn death is greatest during the first 28 days of life, and children below 5 years are most vulnerable to sepsis. According to the findings of a new study, more than three-quarters of neonatal deaths occur in the first week of life, with one-third of all neonatal deaths occurring on the first day after birth (5).
Antimicrobial resistance is a major factor in the development of sepsis and septic shock. Every year, over 214,000 newborns die from sepsis caused by resistant infections (6). Neonatal sepsis is a serious global health issue, resulting in significant health burdens due to medical expenses and productivity loss as well as negative consequences on health and quality of life. Africa experiences the slowest decrease in mortality globally, where neonatal mortality decrease has been slower compared to maternal and child mortality reductions—with stillbirth rates being the slowest (7). Neonatal fatalities occur quickly, necessitating rapid medical interventions. According to a study conducted in Tanzania by Mangu et al. newborn fatalities accounted for 11.3% of all in-hospital deaths (8). Most neonatal deaths occur in the first week of life.
To ensure the efficient and long-term detection of newborn sepsis, there is a need to understand the diagnosis, etiology, and treatment of neonatal sepsis at all levels of the health system. The response to antimicrobial drugs may vary substantially over time and across regions, undermining the efficacy of empirical therapy (9).
This study was conducted to understand the bacteriological profile and predictors of death among neonates with blood culture-proven sepsis in Muhimbili National Hospital, Tanzania. This hospital is the national and tertiary referral hospital that receives sick neonates from different health facilities, whether private or public, across the country and is thus a critical spot to determine retrospectively the efficacy of medications provided in combating neonatal infections in the last 2 years.
A retrospective cohort study of recorded data from hospital pediatric database and files was used to study neonates who were admitted to the neonatal department, diagnosed with sepsis, and had positive blood cultures. This study was conducted from January 2019 to December 2020 in the neonatal department of a tertiary referral and teaching hospital—Muhimbili National Hospital in Dar es Salaam, Tanzania. Patient medical records including clinical symptoms, hematological parameters, pathogen types, and antimicrobial susceptibility were reviewed. A data collection sheet was designed and used to obtain socio-demographic data and other relevant factors related to neonatal sepsis, like maternal fever, prolonged rupture of the membrane (PROM), mode of delivery, birth weight of the baby, gestational age (<37 completed weeks was considered as premature), the temperature of the infant, vomiting, respiratory rate, jaundice, umbilical redness, convulsions, and inability to breastfeed.
A total of 238 neonates with positive blood cultures were included in the study. The sample size was determined using the single-population proportion formula based on the prevalence of positive blood cultures of 19.2% as found by Mkony et al. (10) in the same hospital. Allowing 5% margin of error (MOE) with 95% confidence interval (CI), (z/2 = 1.96) using a single-population proportion (p) formula, 1 - p being the proportion of the population that does not possess the character of interest, the sample size was calculated as follows:
n = (Zα/2)2 *p*(1-p)/MOE2
All neonates who were admitted with blood culture positives within the specified time were included in this study. However, clinically suspected cases with negative blood cultures as well as neonates with positive blood cultures but with incomplete or missing records were excluded from this study. All neonates who died <3 days from birth were not included in the study. Blood cultures with Staphylococcus coagulase-negative as well as yeast infections were excluded from the study.
The primary researcher collected information from the hospital's pediatric database and files on all positive blood culture-proved sepsis cases admitted between January 2019 and December 2020. The medical files were traced using the patients' card number on the registry. Data collection sheets were employed; the data sheets were filled after reading through the manually filled files containing written histories and investigations performed by patients during their stay. Age, sex, birth weight, gestational age at birth (term or preterm), Apgar score at 5 min, place of delivery, mode of delivery, and specific clinical features, such as jaundice, temperature instability (hypothermia, hyperthermia), respiratory distress, poor feeding, vomiting, convulsions, poor reflexes, pallor, jaundice, and umbilical redness, are among the neonatal data collected. Maternal obstetric history of PROM that lasted more than 24 h, maternal urinary tract infection, antibiotic usage, and presence of chorioamnionitis were all noted. Blood culture profile, hemoglobin levels, random blood glucose (RGB) levels, white blood cells, platelets, and C-reactive protein (CRP) levels were among the laboratory features investigated. Anemia was defined as hemoglobin level <10 g/dl, while leucopenia was defined as total white blood count <5,000/mm3, leukocytosis was defined as total white blood count >20,000/mm, and thrombocytopenia was defined as a platelet count of <150,000/mm2. According to the Muhimbili National Hospital, CRP was considered positive when it rises above 5 mg/L, while in other studies CRP is considered positive when it is elevated above 10 mg/L (11). Hospitalization outcomes were documented as survival at discharge and at death. Any neonate who died 3 days or more from birth at the neonatal unit was considered deceased (mortality). The total sample size of neonates determined was then divided into two groups. Group A consisted of neonates who lived until discharge, while Group B consisted of neonates who died while in the hospital.
Under aseptic conditions, each study patient has two blood specimens that were taken for the blood cultures from different peripheral venipuncture sites at 1/2- to 1-h intervals by the hospital protocol; however, the times were not documented. Approximately, 2–5 ml of blood was drawn and kept in aerobic culture bottles, and the sample was immediately transported to the Central Pathology Laboratory at Muhimbili National Hospital (MNH) for processing. Antimicrobial sensitivity testing was carried out at MNH for ampicillin, cloxacillin, and gentamicin, which are used as first-line antibiotics, as well as ceftriaxone, vancomycin, and amikacin, which are used as second-line medications for the treatment of newborn sepsis. The results were classified as resistant, intermediate, or sensitive. During data analysis, isolates with intermediate resistance were labeled as resistant.
The extracted data was processed using SPSS 20.0 software by performing descriptive and inferential statistics. Student's t-test was used to compare the means. Statistical significance was established if the p-value was <0.05 at 95% CI. The parameters that had significant correlations with death were considered potential risk factors for a poor outcome in newborn sepsis. These variables were used to get the odds ratio by using risk estimate analysis.
The study was approved by the ethics committee of Muhimbili National Hospital, Tanzania.
Out of a total of 238 newborns with positive blood cultures (128 male and 110 female), 108 died (57 male, 51 female), accounting for a mortality rate of 45.4% (44.5% male, 46.4% female) of the neonates with blood culture-proven sepsis. Table 1 shows that the case fatality rate for early-onset sepsis was 55.9% (61 out of 109), which was higher than the 36.4% rate for late-onset sepsis (47 out of 129). The case fatality rate was 66.7% in infants whose mothers had chorioamnionitis (10 out of 15 neonates). Furthermore, neonates with birth weights of <1.5 kg (54.8%), preterm infants (49.0%), and those delivered at home (63.6%) had a higher case fatality rate than those with birth weights >1.5 kg (43.6%), term infants (39.1%), and those delivered at a health facility (44.5%), respectively. In addition, newborns whose mothers had PROM for more than 24 h had a 60% case fatality rate (30 out of 50 neonates). There was a reduction in case fatalities in neonates whose mothers utilized antibiotics during pregnancy (31.2%).
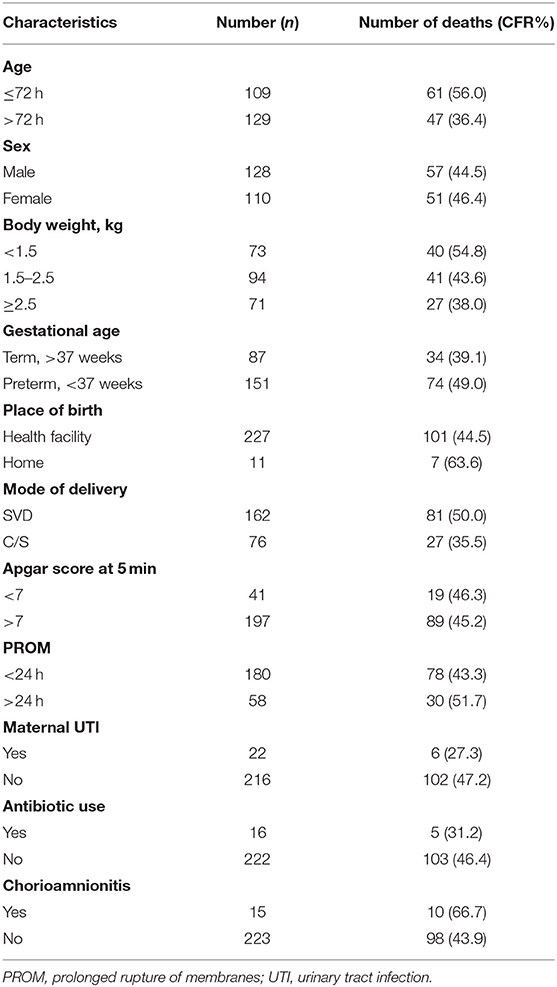
Table 1. Distribution of case fatality rate (CFR) of 238 study neonates with culture-proven sepsis based on infant and maternal factors.
Table 2 shows that infants with vomiting (63.2%), lethargy (59.1%), convulsions (62.5%), and respiratory distress (53.5%) died at a higher rate than those with other clinical characteristics. Random blood glucose (RBG) levels (65.5%) as well as leukocytosis (62.5%) were the laboratory indicators related to a higher frequency of newborn deaths due to neonatal sepsis. There was no significant difference between neonates with positive CRP, thrombocytopenia, and leukopenia who survived or died due to neonatal sepsis.
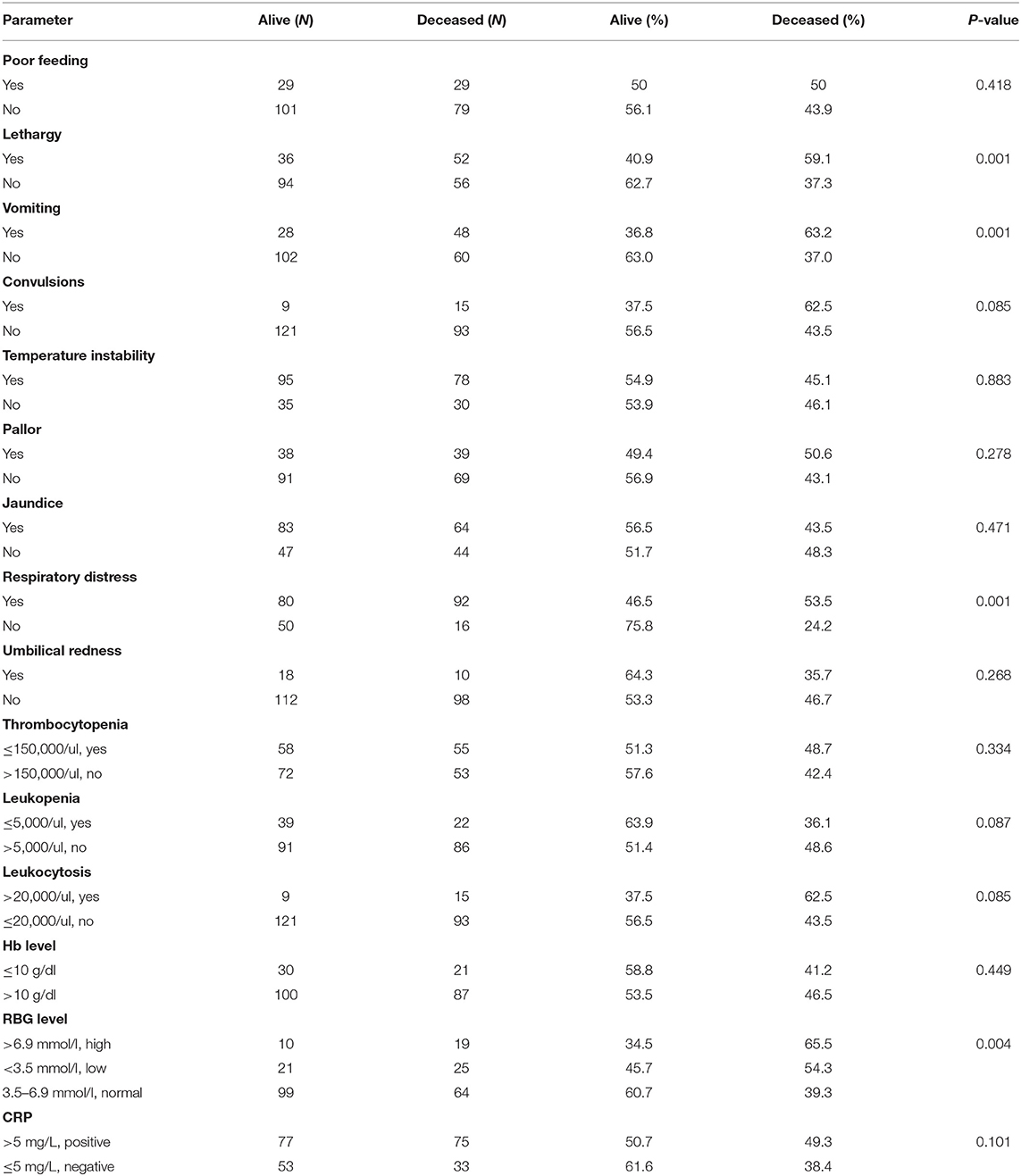
Table 2. Comparison of neonates with sepsis between those who survived and those who died according to clinical features and laboratory parameters.
All the variables in the study were subjected to odds ratio analysis to determine whether variables had a positive correlation with neonatal death or poor prognosis among neonates with sepsis. The results of the analysis are provided in Table 3. It was observed that the following factors were all significant in determining their association to death in neonates with sepsis: very low birth weight (OR, 1.441), early-onset sepsis (OR, 1.536), vomiting (OR, 1.705), lethargy (OR, 1.583), respiratory distress (OR, 2.206), high RBG level (OR, 1.669), positive blood culture for Gram-negative bacteria (OR, 1.424), and being on mechanical ventilation (OR, 2.206).
According to the results in Table 4, Gram-positive bacteria (120 out of 238 neonates) predominated in causing newborn sepsis as compared to Gram-negative bacteria (118 out of 238 neonates). Gram-negative bacteria (53.8%) outnumbered Gram-positive bacteria in early-onset sepsis. In late-onset sepsis, Gram-positive bacteria predominated (60.0%) over Gram-negative bacteria. Klebsiella species (56.2%), Escherichia coli, Staphylococcus aureus, and Pseudomonas species were shown to predominate in early-onset newborn sepsis. S. aureus is the most common cause of late-onset sepsis. Figure 1 shows that sepsis caused by Gram-negative bacteria was related to a higher proportion of neonate mortality than Gram-positive bacteria. Of the 108 neonatal deaths, the largest proportion (40%) was observed with S. aureus, and the remaining 38% was caused by Klebsiella species, 14% by E. coli, 5% by Pseudomonas species, 4% by Acinetobacter species, and 2% by Streptococcus species. No neonatal deaths from Serratia species infection were observed.
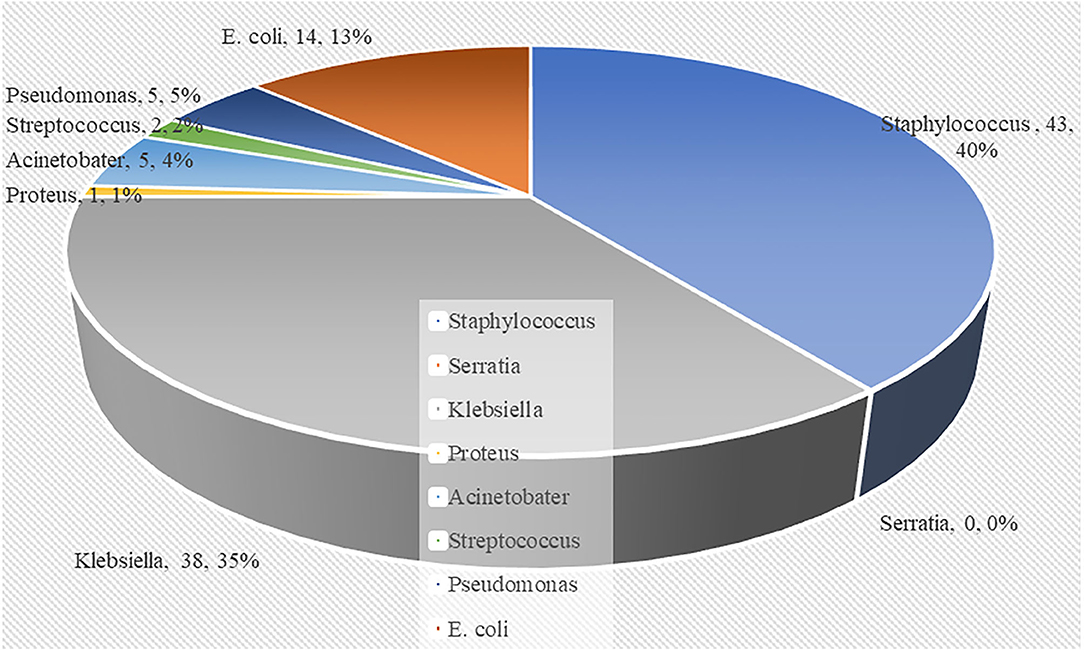
Figure 1. Pie chart showing the distribution of deceased neonates according to the causative bacteria.
A greater proportion of neonates with Gram-positive infections were neonates with low birth weight. Majority of the neonates were Staphylococcus-infected and were mostly <2.5 kg at birth. A similar distribution was observed in the Gram-negative-infected neonates. Klebsiella infection was dominant among the neonates <2.5 kg (low birth weight) (Table 5). Gram-negative sepsis predominates in the preterm group (about 37.8%), while Gram-positive sepsis was at about 25.6%. However, when it comes to specific causative bacteria, we can see that S. aureus and Klebsiella are equally prevalent as a cause of neonatal sepsis in preterm infants with 24.4%. Gram-positive bacteria (24.8%) outnumber the Gram-negative bacteria (11.8%) in the term neonates. Furthermore, S. aureus had a higher percentage (24.8%) than Klebsiella spp. (6.3%). Overall, S. aureus was nearly equally responsible for sepsis in the preterm (24.4%) and term neonates (24.8%) (Table 6).
Table 7 displays the antibiotic sensitivity and resistance trends of the isolated microorganisms. Overall, 84% of the isolated organisms were resistant to the recommended first-line antibiotics for ampicillin and 71.3% for gentamicin. Cefotaxime had a resistance pattern of 78.1%, ceftriaxone had a resistance pattern of 76.2%, vancomycin had a resistance pattern of 35.9%, and amikacin had a resistance pattern of 17.5%. The Staphylococcus infections were shown to be 95.7% resistant to penicillin and 80.43% resistant to ampicillin. Klebsiella was resistant to ampicillin in 92.3% of cases, cefotaxime in 82.5% of cases, and gentamicin in 69.2% of cases. E. coli was resistant to gentamicin in 86.7% of cases, ampicillin in 75% of cases, and ceftriaxone in 93.7% of cases. Acinetobacter was completely resistant to ampicillin and 80% resistant to gentamicin. Streptococcus was completely resistant to gentamicin, completely resistant to penicillin G, and completely resistant to imipenem. Proteus was found to be completely resistant to ceftriaxone, completely resistant to ciprofloxacin, and completely resistant to aztreonam.
Prompt diagnosis and treatment are crucial in decreasing infant sepsis mortality and sequelae. As a result, it is crucial to identify neonates who are at risk of developing sepsis and, if sepsis develops, to identify the characteristics linked with a bad prognosis as soon as possible. The mortality rate of newborn sepsis varies in institutions and countries. According to this study, the mortality rate is 45.4%. This was significantly higher than a study conducted in the same hospital in 2012, which found 13.9% (12). This demonstrates that the death rate owing to neonatal sepsis has increased significantly at Muhimbili National Hospital. This increase could be attributed to rising antibiotic resistance. Kayange et al. found a death rate of 28.5% of infants with positive blood cultures in a 2010 study at Bugando Medical Center in Tanzania (13). Different and some similar rates have been found in other studies conducted in South China (9.5%) (14), Uganda (15.2%) (15), India (36%), Bhutan (20.5%) (16), Nigeria (32.2%) (14), Niger (38.24%) (17), Zambia (43%) (18), and Congo (21%) (19). The discrepancies in death rates between studies can be attributed to a variety of factors, including socioeconomic factors, geographical factors, equipment levels, and the efficacy of each hospital's preventative and therapeutic strategies (19).
Additionally, this study evaluated the major predictors of death in patients with culture-proven sepsis. The significant predicting factors of death for culture-proven sepsis were found to include a birth weight of <1.5 kg, early-onset sepsis, lethargy, vomiting, respiratory distress, hyperglycemia, sepsis with Gram-negative bacteria, and mechanical ventilation. This study indicates that low birth weight (<1.5 kg) is a risk factor for death (OR, 1.441) in neonates with sepsis. A similar finding has been reported in studies from different countries (17, 19, 20). Newborns with weight <1.5 kg had increased mortality, possibly due to impairments in humoral and cellular immunity as well as prolonged hospitalization, which raises the risk of nosocomial infection. Infection is a big concern, and it compounds the already bad outcome for a baby born prematurely (17). Similarly, an Indian study (14) revealed that lethargy (OR, 1.583) and hyperglycemia (OR, 1.669) are significant predictors of neonatal death. Respiratory distress (OR, 2.207), vomiting (OR, 1.705), and mechanical ventilation (OR, 2.206) were also associated with high neonatal mortality in this present study. TNF, IL-1, IL-6, and IL-8 have been shown to be elevated in individuals with acute respiratory distress syndrome and septic shock. These cytokines' levels in the blood may help determine sepsis severity and prognosis (21, 22).
According to this study, Gram-positive bacteria (50.4%) predominated in causing newborn sepsis as compared to Gram-negative bacteria (49.6%). In contrast, a 2012–2016 study in South China reported that Gram-negative bacteria (n = 371) outnumbered Gram-positive bacteria (n = 218, 35.2%) and fungi (n = 30, 4.8%) (23). However, Gram-negative sepsis (OR, 1.424) was found to be a significant predictor of neonatal sepsis mortality when compared to Gram-positive sepsis in this present study. Other research yielded similar outcomes (12, 24). A greater mortality rate could be related to significantly higher CRP and IL-6 levels in Gram-negative bacteremia than in Gram-positive bacteremia (24). There are evidences for two separate mechanisms by which Gram-negative bacteria generate systemic reactions. Bacteria enter the circulation via a normal or damaged epithelium, triggering systemic immunological responses (such as enhanced vascular permeability, leukocyte–endothelial adhesion, complement, and clotting pathways) that lead to multiorgan failure. Toxins or circulating microorganisms are not necessary as direct stimuli for intravascular inflammation, according to a second idea (25). In our study, S. aureus was the most common cause in both early-onset sepsis and late-onset sepsis, but the percentage was higher in LOS at 58.97%. It must be noted that this percentage included all neonates, either alive or dead, with positive blood cultures. In a study by Said et al. Staphylococcus capitis-related sepsis was identified as an independent risk factor for severe morbidity in low-birth-weight infants with late-onset sepsis (26). Staphylococcus epidermidis has also been reported as the most prevalent pathogenic bacterium species in the LOS group (27). In this study, it can be observed in Table 1 that the majority of these neonates with sepsis have an extremely low birth weight (<1.5 kg; 73 out of 238) and low birth weight (1.5–2.5 kg; 94 out of 238), for a total proportion of 70.2% neonates. Furthermore, it was discovered that preterm neonates predominated (151 out of 238), accounting for ~63.4% of the overall sample. The presence of S. aureus in LOS may also be because very-late-onset sepsis is frequently diagnosed in newborns with extremely low birth weight who are usually hospitalized for several weeks after birth. The intravascular catheters required for their care, prolonged antimicrobial drug exposure, the persistence of immature host defensive mechanisms, prematurity, and a prolonged stay in the neonatal intensive care unit (NICU) are the most prominent risk factors for their predisposition (28). These findings imply that differences in host responses and pathogenicity mechanisms of different pathogenic microbes should be considered in the treatment of bacteremia patients and that new antimicrobial counter-measures beyond standard antimicrobial drugs are urgently needed (24). The routine reporting of Gram stain reaction laboratory data could significantly improve newborn sepsis.
The EOS group had a death rate 55.9% greater than the LOS group (36.4%), indicating that factors such as maternal genitourinary tract infections directly affect the occurrence of infant sepsis. Ogunlesi and his colleague found comparable results (14). Nevertheless, LOS has also been linked to an elevated mortality rate in some studies (29, 30).
Overall, the isolated organisms were resistant to 84% of ampicillin and 71.3% of gentamicin. Ceftriaxone resistance was 76.2%, cefotaxime resistance was 78.1%, vancomycin resistance was 35.9%, and amikacin resistance was 17.5%. Inappropriate antibiotic use may be a contributing factor to the hospital's extremely high levels of antibiotic resistance documented in this and previous research (9, 31). Between 1999 and 2012, investigations conducted at MNH and Bugando in Tanzania (1, 10, 12–14, 32) showed a progressive increase in resistance not just to first-line antibiotics but also to alternative drugs such as cefotaxime, vancomycin, and amikacin. Surprisingly, in this investigation, we observed an alarming increase in resistance to ceftriaxone, gentamicin, and amikacin compared to previous studies conducted in the same setting in 2012 (10, 12) showing an unprecedented increase in antibiotic resistance. In our study, we discovered an alarmingly high level of vancomycin resistance. Out of 62 isolates tested for S. aureus sensitivity and resistance, 22 (35.5%) were resistant to vancomycin. Vancomycin has been the first-line treatment for methicillin-resistant S. aureus since its discovery (33). The emergence of vancomycin resistance poses a serious threat to public health around the world. A recent study (34) found that vancomycin-resistant S. aureus was recently discovered in Egyptian slaughterhouses. According to recent reports from Michigan, USA, S. aureus has decreased susceptibility and resistance to vancomycin in July 2021 (33). As a result, it is critical to monitor these infections and conduct additional research to determine why antimicrobial resistance is increasingly becoming a problem.
The strengths of this study include a single center for all investigations, ensuring that records and common practice were consistent; the laboratory analyses were done in one microbiology laboratory; and a relatively large and recent cohort of neonates with proven sepsis were included, making the obtained information for antimicrobial resistance and bacterial profiles relevant to the local population. However, because this is a retrospective study, it has some limitations. Because of the lack of intra-observer reliability, variable degrees of clinical skills/awareness in NICU settings, and incomplete documentation, retrospective chart reviews that attempt to capture the clinical features of neonatal sepsis are frequently incomplete. The procedure for collecting blood samples for cultures was also not documented in the files, and therefore we had to rely on information from the microbiology department about what the standard procedure should be. We were likewise unable to determine the precise timing of random blood glucose levels. It should be noted that there was no consistency in testing specific antibiotics against bacterial isolates at Muhimbili in this study, thus resulting in different susceptibility results.
Infant demography research has found a significant connection between VLBW and early-onset sepsis as well as neonatal mortality in the newborn population. High blood sugar levels and mechanical ventilation were also identified as risk factors for neonatal mortality. As a result of neonatal sepsis' clinical manifestations, such as lethargy, vomiting, and respiratory distress, newborn mortality was found to be associated with these symptoms. Gram-negative bacteria are typically found in septicemic newborns who have died. Early detection and treatment of these risk factors would dramatically lower the likelihood of serious and life-threatening problems in newborns as well as the likelihood of mortality in these babies. In addition, this study discovered an abnormally high level of resistance to common antibiotics, which is of great concern. Antibiotic resistance may be due to overuse and misuse of antibiotics. We advocate for a change of currently prescribed antibiotics in our setting, the need to do a blood culture as soon as sepsis is suspected, and the need for a prospective study which we are currently undertaking to bridge the gaps found in this study and also provide updated data for policy.
The data analyzed in this study is subject to the following licenses/restrictions: These are retrospective datasets analyzed for the purposes of this study and can only be shared upon reasonable request and subsequent approval from the Ethics Committee. Requests to access these datasets should be directed to YXJjaGFuZ2VsbW50aW1AeWFob28uY28udWs=; bnVyYV9hYmRAeWFob28uY29t.
The studies involving human participants were reviewed and approved by Ethics Committee of Muhimbili National Hospital, Tanzania. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
NB-a contributed to the data collection, data analysis, and manuscript preparation. JA contributed to the manuscript preparation. MN contributed to the manuscript preparation and revision. RT contributed to the data analysis and manuscript preparation. MM and HN contributed to the data collection and data analysis. HJ contributed to the conceptualization, design, and manuscript preparation. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to acknowledge the management and staff of the Muhimbili National Hospital, Tanzania.
1. Dessu S, Habte A, Melis T, Gebremedhin M. Survival status and predictors of mortality among newborns admitted with neonatal sepsis at public hospitals in Ethiopia. International Journal of Pediatrics. (2020) 2020:10. doi: 10.1155/2020/8327028
2. Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. Am J Perinatol. (2013) 30:131–42. doi: 10.1055/s-0032-1333413
3. Alkema L, Chao F, You D, Pedersen J, Sawyer CC. National, regional, and global sex ratios of infant, child, and under-5 mortality and identification of countries with outlying ratios: a systematic assessment. Lancet Global Health. (2014) 2:e521–30. doi: 10.1016/S2214-109X(14)70280-3
4. Verma P, Berwal PK, Nagaraj N, Swami S, Jivaji P, Narayan S. Neonatal sepsis: epidemiology, clinical spectrum, recent antimicrobial agents and their antibiotic susceptibility pattern. Int J Contemp Pediatr. (2015) 2:176–80. doi: 10.18203/2349-3291.ijcp20150523
5. Moxon S. Service readiness for inpatient care of small and sick newborns: Improving measurement in low-and middle-income settings. Lond Scl Hyg Trop Med. (2020). doi: 10.17037/PUBS.04658448
6. Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. (2016) 387:168–75. doi: 10.1016/S0140-6736(15)00474-2
7. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet. (2014) 384:189–205. doi: 10.1016/S0140-6736(14)60496-7
8. Mangu CD, Rumisha SF, Lyimo EP, Mremi IR, Massawe IS, Bwana VM, et al. Trends, patterns and cause-specific neonatal mortality in Tanzania: a hospital-based retrospective survey. International Health. (2021) 13:334–43. doi: 10.1093/inthealth/ihaa070
9. Blomberg B, Manji KP, Urassa WK, Tamim BS, Mwakagile DS, Jureen R, et al. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infect Dis. (2007) 7:1–14. doi: 10.1186/1471-2334-7-43
10. Mkony MF, Mizinduko MM, Massawe A, Matee M. Management of neonatal sepsis at Muhimbili National Hospital in Dar es Salaam: diagnostic accuracy of C–reactive protein and newborn scale of sepsis and antimicrobial resistance pattern of etiological bacteria. BMC Pediatr. (2014) 14:1–7. doi: 10.1186/s12887-014-0293-4
11. Hengst JM. The role of C-reactive protein in the evaluation and management of infants with suspected sepsis. Adv Neonatal Care. (2003) 3:3–13. doi: 10.1053/adnc.2003.50010
12. Mhada TV, Fredrick F, Matee MI, Massawe A. Neonatal sepsis at Muhimbili National Hospital, Dar es Salaam, Tanzania; aetiology, antimicrobial sensitivity pattern and clinical outcome. BMC Public Health. (2012) 12:1–6. doi: 10.1186/1471-2458-12-904
13. Kayange N, Kamugisha E, Mwizamholya DL, Jeremiah S, Mshana SE. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. (2010) 10:1–9. doi: 10.1186/1471-2431-10-39
14. Ogunlesi TA, Ogunfowora OB. Predictors of mortality in neonatal septicemia in an underresourced setting. J Natl Med Assoc. (2010) 102:915–22. doi: 10.1016/S0027-9684(15)30710-0
15. Tumuhamye J, Sommerfelt H, Bwanga F, Ndeezi G, Mukunya D, Napyo A, et al. Neonatal sepsis at Mulago national referral hospital in Uganda: Etiology, antimicrobial resistance, associated factors and case fatality risk. PLoS ONE. (2020) 15:e0237085. doi: 10.1371/journal.pone.0237085
16. Jatsho J, Nishizawa Y, Pelzom D, Sharma R. Clinical and bacteriological profile of neonatal sepsis: a prospective hospital-based study. Int J Pediatrics. (2020) 2020:1835945. doi: 10.1155/2020/1835945
17. Meshram RM, Gajimwar VS, Bhongade SD. Predictors of mortality in outborns with neonatal sepsis: A prospective observational study. Niger Postgrad Med J. (2019) 26:216. doi: 10.4103/npmj.npmj_91_19
18. Kabwe M, Tembo J, Chilukutu L, Chilufya M, Ngulube F, Lukwesa C, et al. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in Zambia. Pediatr Infect Dis J. (2016) 35:e191–8. doi: 10.1097/INF.0000000000001154
19. Nyenga AM, Mukuku O, Kabamba A, Mutombo CWM, Numbi O. Predictors of mortality in neonatal sepsis in a resource-limited setting. Health. (2021) 4:057–61. doi: 10.29328/journal.japch.1001034
20. Trotman H, Bell Y, Thame M, Nicholson A, Barton M. Predictors of poor outcome in neonates with bacterial sepsis admitted to the University Hospital of the West Indies. West Indian Med J. (2006) 55:80–4. doi: 10.1590/S0043-31442006000200003
21. Damas P, Canivet JL, De Groote D, Vrindts Y, Albert A, Franchimont P, et al. Sepsis and serum cytokine concentrations. Crit Care Med. (1997) 25:405–12. doi: 10.1097/00003246-199703000-00006
22. Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS: plasma IL-1β and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. (1995) 107:1062–73. doi: 10.1378/chest.107.4.1062
23. Gao K, Fu J, Guan X, Zhu S, Zeng L, Xu X, et al. Incidence, bacterial profiles, and antimicrobial resistance of culture-proven neonatal sepsis in south China. Infect Drug Resist. (2019) 12:3797. doi: 10.2147/IDR.S223597
24. Abe R, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Tateishi Y, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care. (2010) 14:1–7. doi: 10.1186/cc8898
25. Alexandraki I. Palacio C. Gram-negative versus Gram-positive bacteremia: what is more alarmin (g)? Crit Care. (2010) 14:1–2. doi: 10.1186/cc9013
26. Said MB, Hays S, Bonfils M, Jourdes E, Rasigade JP, Laurent F, et al. Late-onset sepsis due to Staphylococcus capitis ‘neonatalis' in low-birthweight infants: a new entity? J Hosp Infect. (2016) 94:95–8. doi: 10.1016/j.jhin.2016.06.008
27. Li X, Ding X, Shi P, Zhu Y, Huang Y, Li Q, et al. Clinical features and antimicrobial susceptibility profiles of culture-proven neonatal sepsis in a tertiary children's hospital, 2013 to 2017. Medicine. (2019) 98:e14686. doi: 10.1097/MD.0000000000014686
28. Ozkan H, Cetinkaya M, Koksal N, Celebi S, Hacimustafaoglu M. Culture-proven neonatal sepsis in preterm infants in a neonatal intensive care unit over a 7 year period: Coagulase-negative S taphylococcus as the predominant pathogen. Pediatr Int. (2014) 56:60–6. doi: 10.1111/ped.12218
29. Dawodu A, Al Umran K, Twum-Danso K. A case control study of neonatal sepsis: experience from Saudi Arabia. J Trop Pediatr. (1997) 43:84–8. doi: 10.1093/tropej/43.2.84
30. Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. (2002) 110:285–91. doi: 10.1542/peds.110.2.285
31. Blomberg B, Jureen R, Manji KP, Tamim BS, Mwakagile DS, Urassa WK, et al. High rate of fatal cases of pediatric septicemia caused by gram-negative bacteria with extended-spectrum beta-lactamases in Dar es Salaam, Tanzania. J Clin Microbiol. (2005) 43:745–9. doi: 10.1128/JCM.43.2.745-749.2005
32. Blomberg B, Mwakagile DS, Urassa WK, Maselle SY, Mashurano M, Digranes A, et al. Surveillance of antimicrobial resistance at a tertiary hospital in Tanzania. BMC Public Health. (2004) 4:1–11. doi: 10.1186/1471-2458-4-45
33. Unni S, Siddiqui TJ, Bidaisee S. Reduced Susceptibility and Resistance to Vancomycin of Staphylococcus aureus: a review of global incidence patterns and related genetic mechanisms. Cureus. (2021) 13:e18925. doi: 10.7759/cureus.18925
Keywords: neonatal sepsis, sepsis, bacteriological profile, antibiotic susceptibility, Gram-negative bacteria, Gram-positive bacteria
Citation: Ba-alwi NA, Aremu JO, Ntim M, Takam R, Msuya MA, Nassor H and Ji H (2022) Bacteriological Profile and Predictors of Death Among Neonates With Blood Culture-Proven Sepsis in a National Hospital in Tanzania—A Retrospective Cohort Study. Front. Pediatr. 10:797208. doi: 10.3389/fped.2022.797208
Received: 18 October 2021; Accepted: 07 February 2022;
Published: 05 April 2022.
Edited by:
Cynthia Howard, University of Minnesota Health Twin Cities, United StatesReviewed by:
Guoping Lu, Fudan University, ChinaCopyright © 2022 Ba-alwi, Aremu, Ntim, Takam, Msuya, Nassor and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ji, amlob25nc2RxQDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.