
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 07 April 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.760029
This article is part of the Research Topic Extremely Premature Infant Care: Advances in Prevention and Treatment Strategies View all 9 articles
Background: Prophylactic indomethacin has been widely used as an effective intervention for reducing mortalities and morbidities in preterm infants including the cardiopulmonary and neurodevelopmental morbidities such as intraventricular hemorrhage (IVH), but many studies have reported contradictory outcomes regarding its significance. Therefore, we aim to systematically review and meta-analyze the data of prophylactic indomethacin on preterm infants.
Methods: Our systematic search included the following databases: Pubmed, Google Scholar, Scopus, Web of Science, The New York Academy of Medicine (NYAM), Virtual health library (VHL), and the System for Information on Grey Literature in Europe (SIGLE) to include studies that assessed the use of prophylactic indomethacin in preterm infants until 12 August 2021.
Results: The final list of our included studies is comprised of 23 randomized trials and cohort studies. Among all the studies outcomes, significant favorable outcome was lowering the rate of PDA, surgical PDA ligation (P < 0.001) and severe IVH (P = 0.008) while no significance was recorded with BPD, pulmonary hemorrhage, intraventricular hemorrhage, necrotizing enterocolitis, intestinal perforation, mortality, and length of hospital stay.
Conclusion: Since the meta-analysis results regarding effectiveness of prophylactic indomethacin varied based on the study design particularly with regard to outcomes such as surgical PDA ligation and severe IVH, this warrants the need for more evidence regarding the effectiveness of prophylactic indomethacin in very low birth weight infants.
Many cardiopulmonary and neurologic disabilities have been associated with preterm labor including patent ductus arteriosus (PDA), pulmonary hemorrhage, intracranial hemorrhage, and developmental delay (1–4). Although advances in modern medicine have improved the survival rates of very low birth weight (VLBW) infants, many neurodevelopmental complications are still present due to preterm birth such as blindness, deafness, and cerebral palsy. VLBW infants are at risk of developing intraventricular hemorrhage (IVH) which is usually associated with neurodevelopmental decays when related to the brain parenchyma. IVH grade 3–4 is a major risk factor for the occurrence of these complications in preterm infants (5–8). Although the incidence rate of IVH has been markedly reduced since the 1980s (9, 10), as no or minimal reductions have been recorded recently (11, 12).
Many pre- and postnatal interventions have been reported to effectively treat IVH and reduce its incidence in preterm infants (13). One of these is indomethacin prophylaxis which is better administered within the first 6 h after birth (14–17). Besides, it helps in the closure of ductus arteriosus and therefore, can prevent the complications of PDA such as pulmonary hypertension (14, 15, 18). Its mechanisms of action include prostaglandin synthesis inhibition by inhibiting the cyclooxygenase pathways, reduction of hyperemic responses resulting from cerebrovascular hypoxia and hypercapnia, increasing the blood–brain barrier permeability, and prevention of cerebral perfusion-induced ischemia (19–23). Moreover, it enhances microvascular development in the germinal matrix (24). Perfusion-related factors such as hypoxia, hypercapnia, and hypotension usually develop after birth in VLBW infants (25). Most cases of preterm infants develop IVH within 6–8 h after birth regardless of the gestational age (26). It happens probably due to the increased levels of angiopoietin 2 and vascular endothelial growth factor in the germinal matrix that normally decreases within hours after birth (13).
The results of previously published randomized controlled trials (RCTs) have shown that early administration of indomethacin after birth lowers the incidence of symptomatic PDA and severe IVH as a prophylactic measure (16, 27–29). Although indomethacin administration showed favorable outcomes in reducing IVH incidence, many concerns have arised regarding its effect on cerebral perfusion (30, 31). The rates of mortalities, bronchopulmonary dysplasia (BPD), or long-term neurodevelopmental decays reportedly seem to have been not affected. A previously published large RCT advised against using indomethacin as a prophylactic agent (15). Although the study showed favorable outcomes in terms of reducing incidence rates of PDA, PDA ligation, IVH, and pulmonary hemorrhage, no improvement regarding the incidence of death and neurodevelopmental disorders rates has been found. Therefore, in this systematic review, we aim to analyze the data of previously published investigations on the use of prophylactic indomethacin in preterm infants.
In accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses statement (PRISMA) recommendations, we performed this systematic review and meta-analysis (32). A systematic electronic database search was conducted for relevant studies published, from inception until 12 August 2021, in seven databases: Pubmed, Google Scholar, Scopus, Web of Science, The New York Academy of Medicine (NYAM), Virtual health library (VHL), and the System for Information on Grey Literature in Europe (SIGLE). The search process was conducted using keywords, medical subject (MeSH) terms, and publication types based on the PICO framework (participants, comparison, intervention, and outcomes). Participants were any preterm infants, the intervention was the prophylactic indomethacin, the comparison was placebo or no treatment groups, and all possible outcomes were included. The systematic search was followed by a manual search in references of the included papers to include missed papers (33).
We included all original studies that assessed the use of prophylactic indomethacin in preterm infants. Papers were excluded if there were one of the following exclusion criteria: (i) non-original studies; (ii) articles in non-English language; (iii) in vitro or animal studies; (iv) data duplication, overlapping or unreliably extracted or incomplete data; and (v) abstract only articles, reviews, thesis, books, conference papers, or articles without available full texts (conferences, editorials, author response, letters, and comments). The title and abstract screening were performed by four independent reviewers. Furthermore, three independent reviewers performed full-text screening to ensure the inclusion of relevant papers in our systematic review. Any disagreement was done by discussion and consulting the senior member when necessary.
Two authors made the pilot extraction of a few papers for building the data extraction sheet. The data extraction sheet included: patient’s characteristics, and outcomes. Two authors extracted the data and was reviewed by a third reviewer when necessary. If a disagreement occurred, a senior author was consulted.
All data were analyzed using Comprehensive Meta-analysis Software Version 3.0, odds ratios (OR) and Standardized mean difference (SDM) outcomes were calculated. The corresponding 95% confidence intervals (CI) of pooled effect size were calculated using a fixed-effects or random-effects, according to heterogeneity level. Heterogeneity was assessed with Q statistics and I2 test.
The publication bias was assessed using Egger’s regression test (34, 35) and represented graphically by Begg’s funnel plot (36). when there were 10 or more studies/effect sizes. Egger’s regression test P-value <0.10 was considered significant. Whenever publication bias was found, the trim and fill method of Duvall and Tweedie was applied (37) to add studies that appeared to be missing to enhance the symmetry.
We identified 3,801 records after excluding of 506 duplicates by using Endnote software version X9. Title and abstract screening resulted in 36 records for further full-text screening. The later yielded 20 eligible papers for inclusion in our study. Three papers were added after performing manual search trials. Finally, we included 23 studies for this systematic review and meta-analysis (Figure 1).
Out of the 23 included studies; 15 were randomized controlled trials and the remaining eight were cohort in design. The sample size of the included studies was highly variable ranging from 19 and as high as 34,602 pre-term infants. The average mean age in all reported treatment group and control group was 27 weeks (ranging from 26 to 28 weeks). Table 1 shows the main characteristics of the included studies (15, 17, 27–29, 38–55).
With regard to articles with a cohort study design, no publication bias was found in the studies relating to the outcome of death (P = 0.852) using Begg’s adjusted rank correlation test. Publication bias related to bronchopulmonary dysplasia, severe intraventricular hemorrhage, necrotizing enterocolitis and surgical PDA ligation was not assessed owing to few number of studies.
Regarding publication bias among RCT studies, overall no publication bias was found in the studies. Regarding PDA, no publication bias was found in the studies (P = 0.524) using Egger’s test (Figure 2A). No publication bias was found in studies (P = 0.458) using Begg’s adjusted rank correlation test with regard to severe interventricular hemorrhage. Regarding necrotizing enterocolitis, no publication bias was found in studies (P = 0.652) using Begg’s adjusted rank correlation test. With regard to death, no publication bias was found in the studies (P = 0.394) using Egger’s test (Figure 2B). Publication bias related to bronchopulmonary dysplasia, intraventricular hemorrhage, pulmonary hemorrhage, intestinal perforation, surgical PDA ligation and hospitalization days were not assessed owing to few studies.

Figure 2. Publication bias among randomized controlled trial studies for the outcome (A) patent ductus arteriosus (B) death.
With regard to the publication bias in both cohort and RCT studies, in general no publication bias was seen with the exception of patent ductus arteriosus where publication bias was found in the studies (P = 0.083) using Egger’s test (Figure 3A). No publication bias was found related to bronchopulmonary dysplasia in studies (P = 0.543) using Begg’s adjusted rank correlation test. With regard to intraventricular hemorrhage, no publication bias was found in studies (P = 0.348) using Begg’s adjusted rank correlation test. For severe interventricular hemorrhage as well, no publication bias was found in studies (P = 0.217) using Egger’s test (Figure 3B). Regarding necrotizing enterocolitis, no publication bias was found in studies (P = 0.364) using Egger’s test (Figure 3C). With regard to death, no publication bias was found in studies (P = 0.449) using Egger’s test (Figure 3D). Using Begg’s adjusted rank correlation test, no publication bias was found in studies (P = 0.176) with regard to surgical PDA ligation. Publication bias related to pulmonary hemorrhage, intestinal perforation and hospitalization days was not assessed owing to few studies.
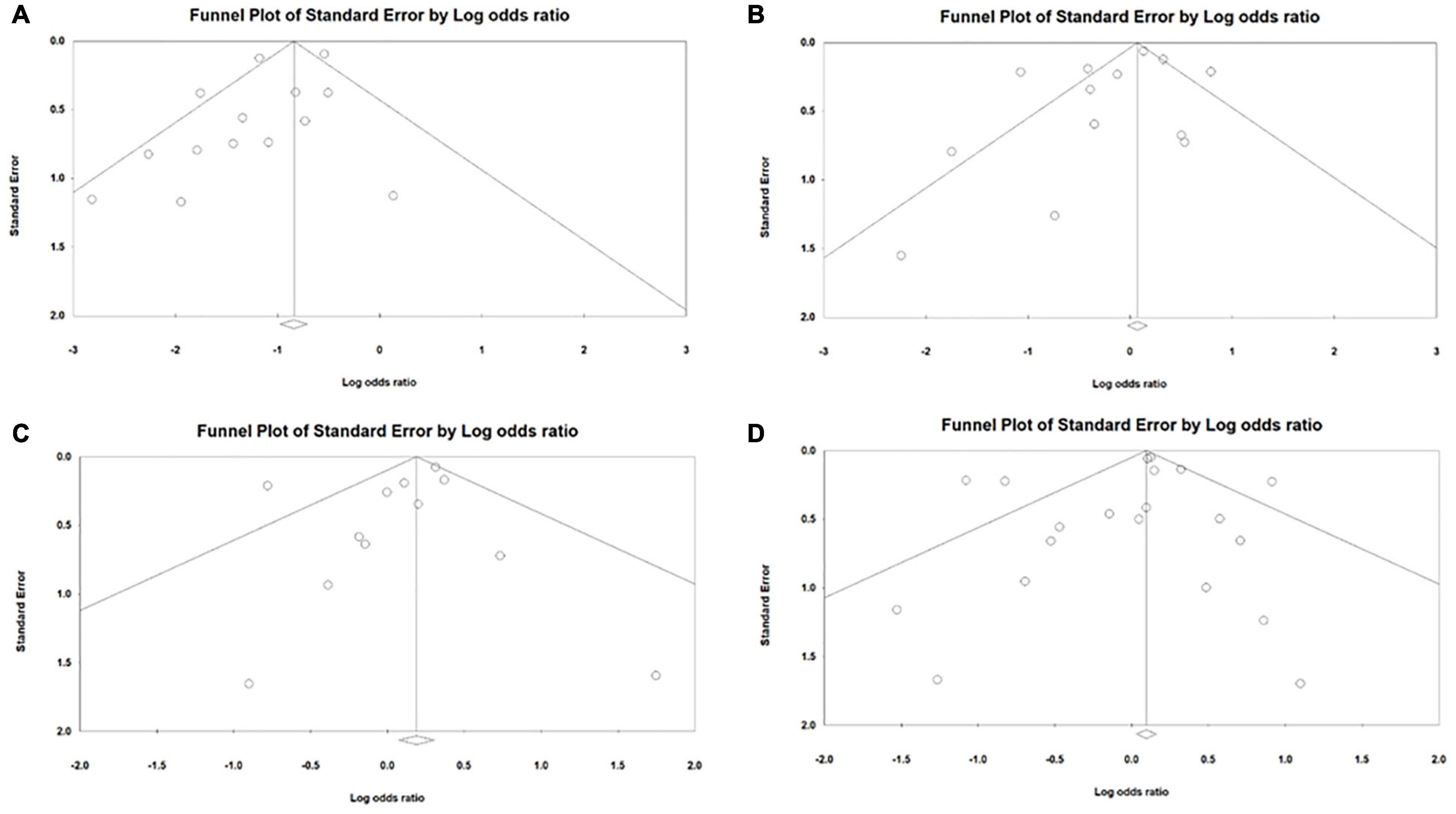
Figure 3. Publication bias among cohort and randomized controlled trial study designs for the outcome (A) patent ductus arteriosus (B) interventricular hemorrhage (C) necrotizing enterocolitis (D) death.
In the meta-analysis of cohort studies, no significant difference was seen between the group of infants given prophylactic doses of indomethacin and the placebo or no treatment group with regard to the rates of bronchopulmonary dysplasia (OR = 0.88; 95% CI = 0.53–1.46; P-value = 0.628). There was high significant heterogeneity among the included studies (I2 = 91%; P-value < 0.001) (Figure 4A).
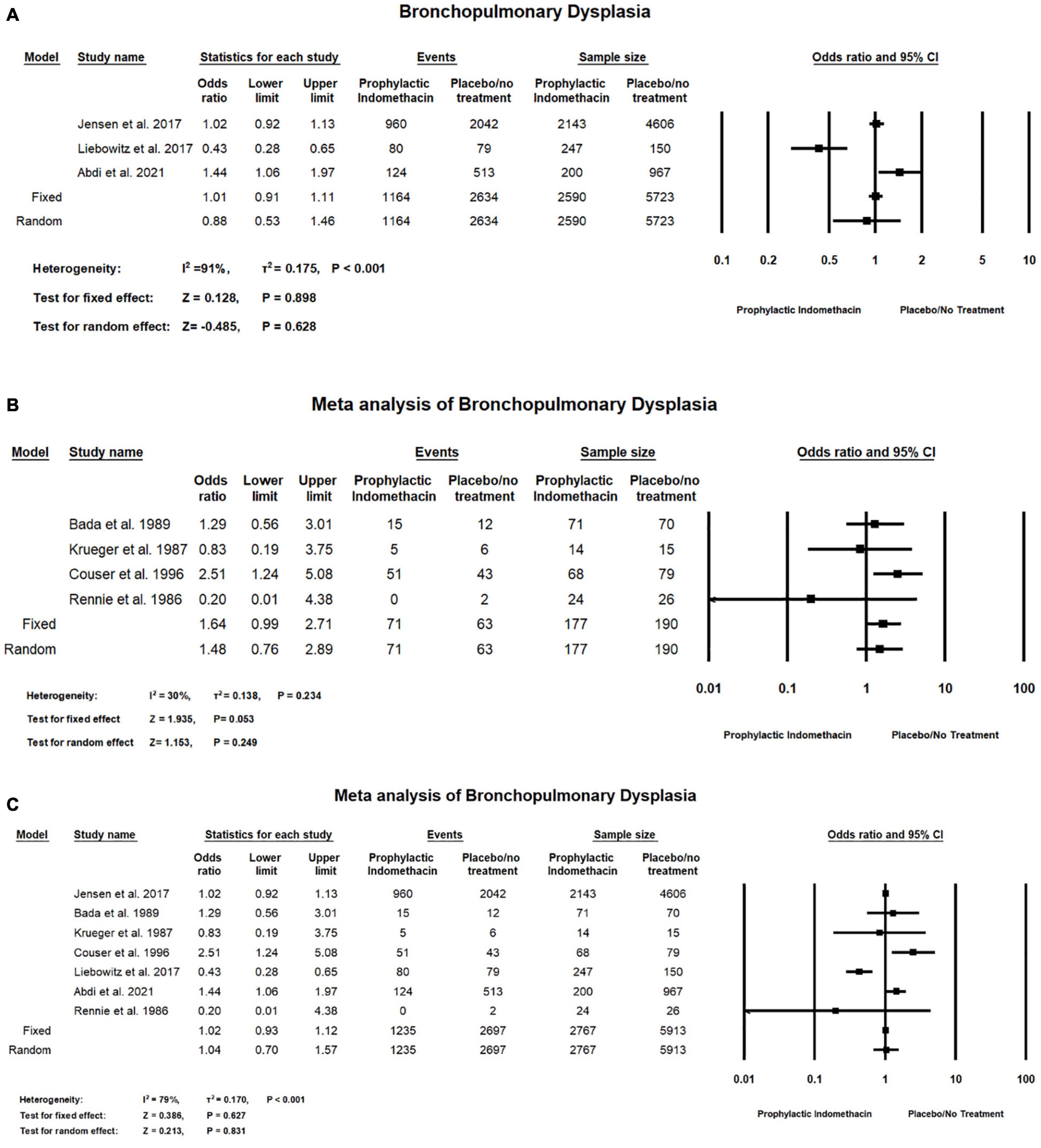
Figure 4. Meta-analysis of bronchopulmonary dysplasia from (A) cohort studies, (B) RCT studies, (C) combination of cohort and RCT studies.
Meta-analysis of RCT studies shows there was no significant difference between the group of infants with prophylactic doses of indomethacin and the group of placebo or no treatment with regard to the rates of bronchopulmonary dysplasia (OR = 1.64; 95% CI = 0.99–2.71; P-value = 0.053). There was no significant heterogeneity among the included studies (I2 = 30%; P-value = 0.234) (Figure 4B).
In the combined meta-analysis of cohort and RCT studies, there was no significant difference between the prophylactic indomethacin group and the placebo or no treatment group regarding the rates of bronchopulmonary dysplasia (OR = 1.04; 95% CI = 0.70–1.57; P-value = 0.831). There was high significant heterogeneity among the included studies (I2 = 79%; P-value < 0.001) (Figure 4C).
Meta-analysis of RCT studies shows infants given prophylactic doses of indomethacin have significantly lower rates of PDA compared to those who did not (OR = 0.31; 95% CI = 0.25–0.38; P-value < 0.001). There was no significant heterogeneity among the included studies (I2 = 10%; P-value = 0.341) (Figure 5A).
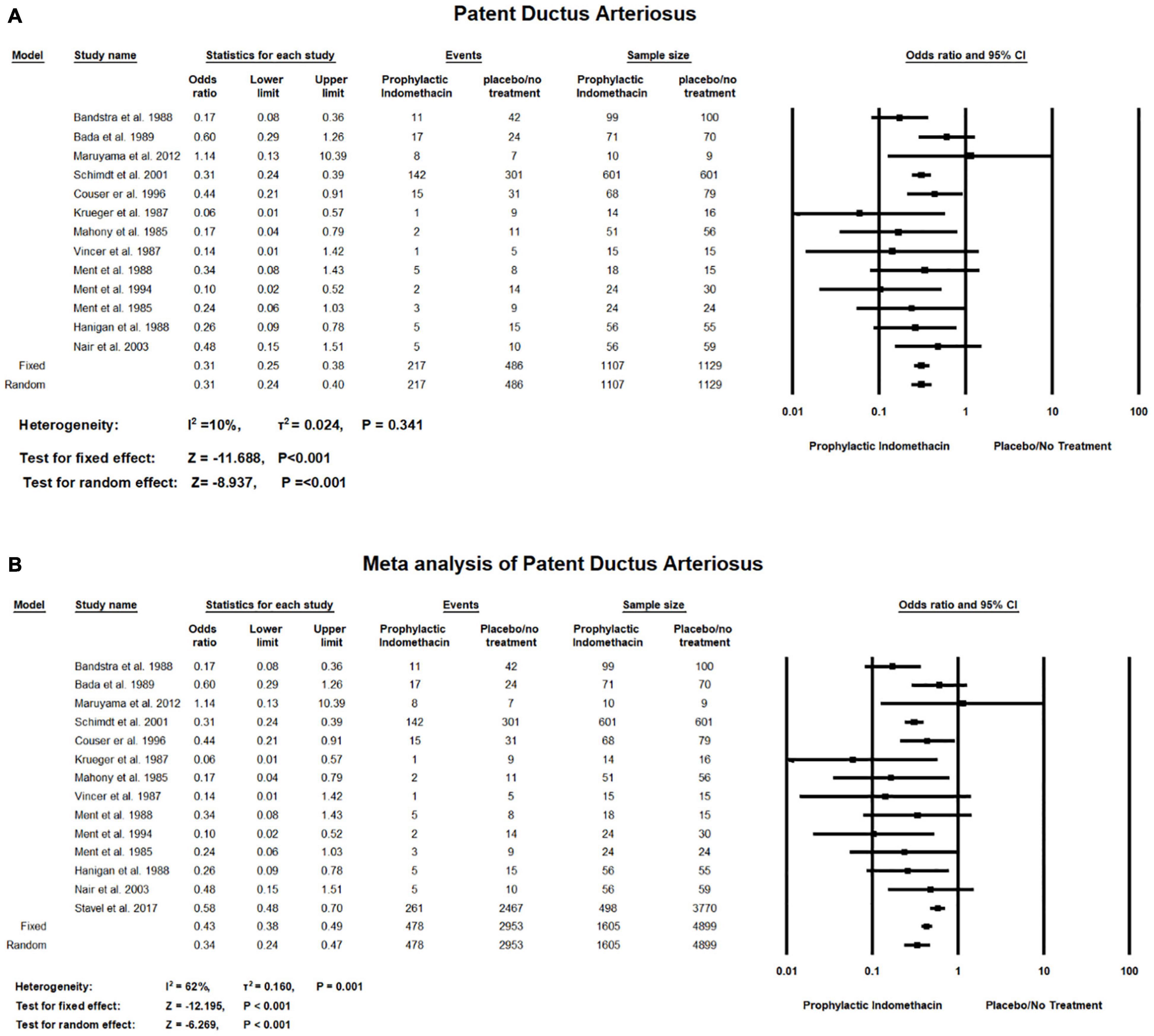
Figure 5. Meta-analysis of patent ductus arteriosus from (A) RCT studies, (B) combination of cohort and RCT studies.
Combined meta-analysis of cohort and RCT studies shows infants given prophylactic doses of indomethacin have significantly lower rates of Patent Ductus Arteriosus compared to those who did not (OR = 0.34; 95% CI = 0.24–0.47; P-value < 0.001). However, there was medium significant heterogeneity among the included studies (I2 = 62%; P-value = 0.001) (Figure 5B).
In the meta-analysis of cohort studies, there was no significant differences between the group of infants given prophylactic doses of indomethacin and placebo or no treatment group with regard to the rates of surgical PDA ligation (OR = 3.84; 95% CI = 0.78–14.67; P-value = 0.104). There was high significant heterogeneity among the included studies (I2 = 95%; P-value < 0.001) (Figure 6A).
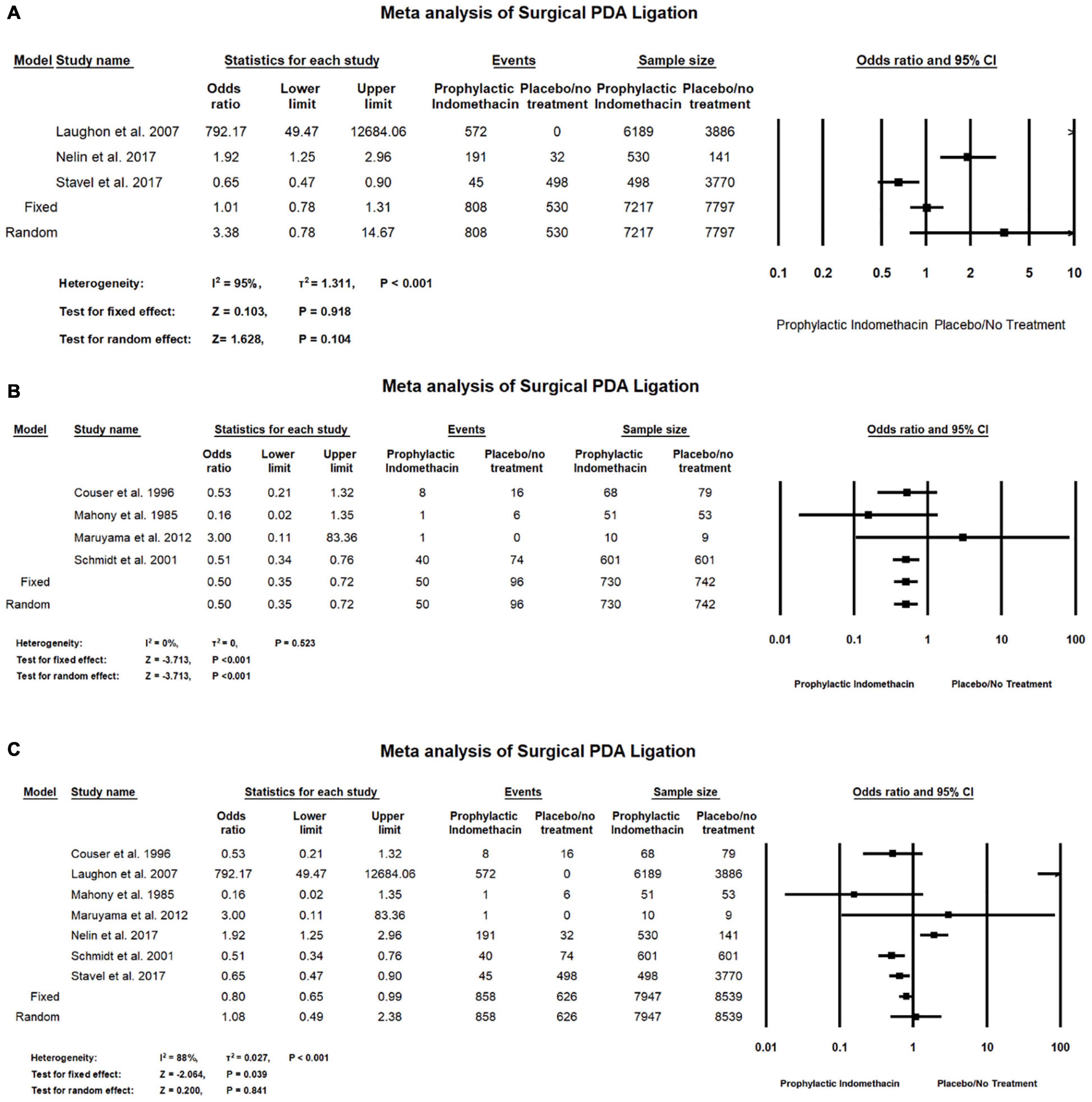
Figure 6. Meta-analysis of surgical PDA ligation from (A) cohort studies, (B) RCT studies, (C) combination of cohort and RCT studies.
Meta-analysis of RCT studies shows infants with prophylactic doses of indomethacin have significantly lower rates of surgical PDA ligation compared to those who did not (OR = 0.50; 95% CI = 0.35–0.72; P-value < 0.001). There was no significant heterogeneity among the included studies (I2 = 0%; P-value = 0.523) (Figure 6B).
Combined meta-analysis of cohort and RCT studies shows there was no significant differences between the group of infants given prophylactic doses of indomethacin and the infants in the placebo or no treatment group with regard to the rates of necrotizing enterocolitis (OR = 1.10; 95% CI = 0.79–1.52; P-value = 0.571). There was no significant heterogeneity among the included studies (I2 = 0%; P-value = 0.825) (Figure 6C).
Meta-analysis of RCT studies shows there was no significant differences between the group of infants given prophylactic doses of indomethacin and placebo or no treatment group regarding the rates of pulmonary hemorrhage (OR = 0.86; 95% CI = 0.64–1.15; P-value = 0.303). There was no significant heterogeneity among the included studies (I2 = 0%; P-value = 0.606) (Figure 7).
Meta-analysis of RCT studies shows there was no significant differences between the group of infants given prophylactic doses of indomethacin and the placebo or no treatment group with regard to the rates of intraventricular hemorrhage (OR = 0.88; 95% CI = 0.58–1.32; P-value = 0.532). There was low heterogeneity among the included studies (I2 = 35%; P-value = 0.186) (Figure 8A).
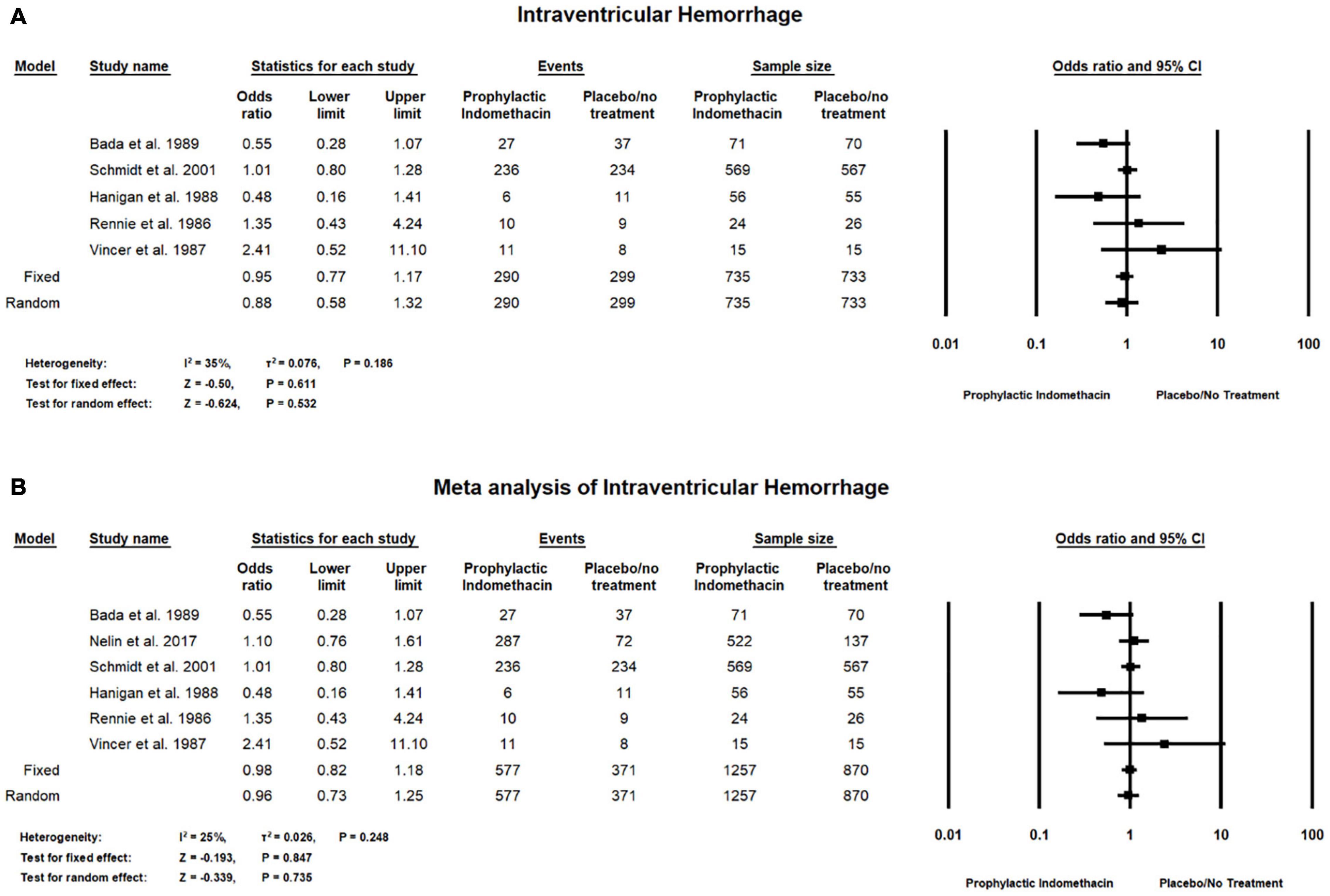
Figure 8. Meta-analysis of intraventricular hemorrhage from (A) RCT studies, (B) combination of cohort and RCT studies.
Combined meta-analysis of cohort and RCT studies shows there was no significant differences between the group with infants given prophylactic doses of indomethacin and placebo or no treatment group with regard to the rates of intraventricular hemorrhage (OR = 0.96; 95% CI = 0.73–1.25; P-value = 0.735). There was low heterogeneity among the included studies (I2 = 25%; P-value = 0.248) (Figure 8B).
In the meta-analysis of cohort studies, no significant difference was found between the group of infants given prophylactic doses of indomethacin and the placebo or no treatment group regarding the rates of severe intraventricular hemorrhage (OR = 1.03; 95% CI = 0.67–1.57; P-value = 0.607). There was high significant heterogeneity among the included studies (I2 = 91%; P-value < 0.001) (Figure 9A).
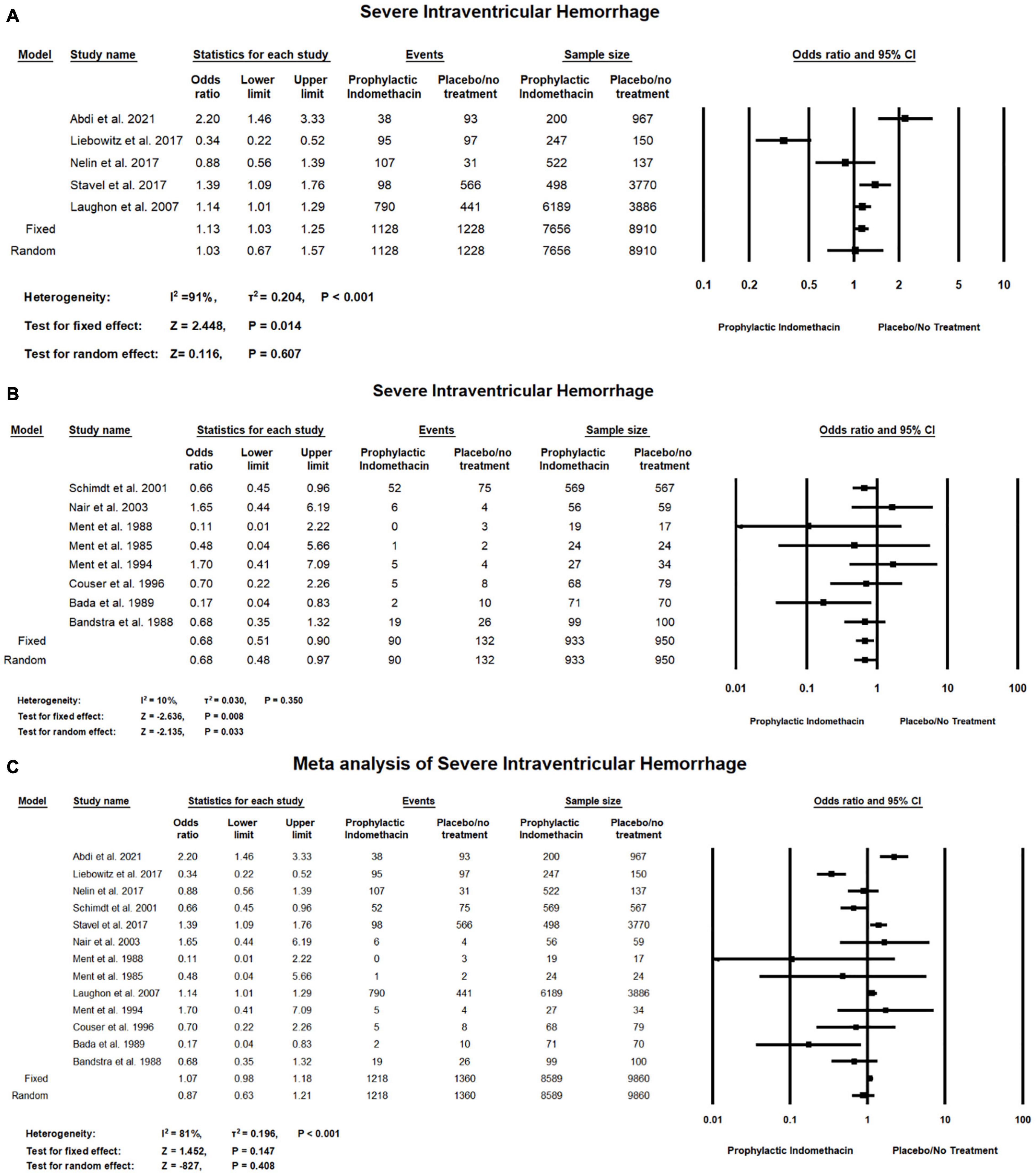
Figure 9. Meta-analysis of severe intraventricular hemorrhage from (A) cohort studies, (B) RCT studies, (C) combination of cohort and RCT studies.
For meta-analysis of RCT studies, as seen in Figure 9B, infants with prophylactic doses of indomethacin have significantly lower rates of severe intraventricular hemorrhage compared to those who did not (OR = 0.68; 95% CI = 0.51–0.90; P-value = 0.008). There was no significant heterogeneity among the included studies (I2 = 10%; P-value = 0.350).
In the combined meta-analysis of cohort and RCT studies, no significant differences between the group of infants given prophylactic doses of indomethacin and the placebo or no treatment group regarding the rates of severe intraventricular hemorrhage (OR = 0.87; 95% CI = 0.63–1.21; P-value = 0.408). However, there was high significant heterogeneity among the included studies (I2 = 81%; P-value < 0.001) (Figure 9C).
In the meta-analysis of cohort studies, regarding the rate of necrotizing enterocolitis, there was no significant differences between the group of infants with prophylactic doses of indomethacin and the infants in the placebo or no treatment group (OR = 1.03; 95% CI = 0.69–1.54; P-value = 0.884). There was high significant heterogeneity among the included studies (I2 = 84%; P-value < 0.001) (Figure 10A).
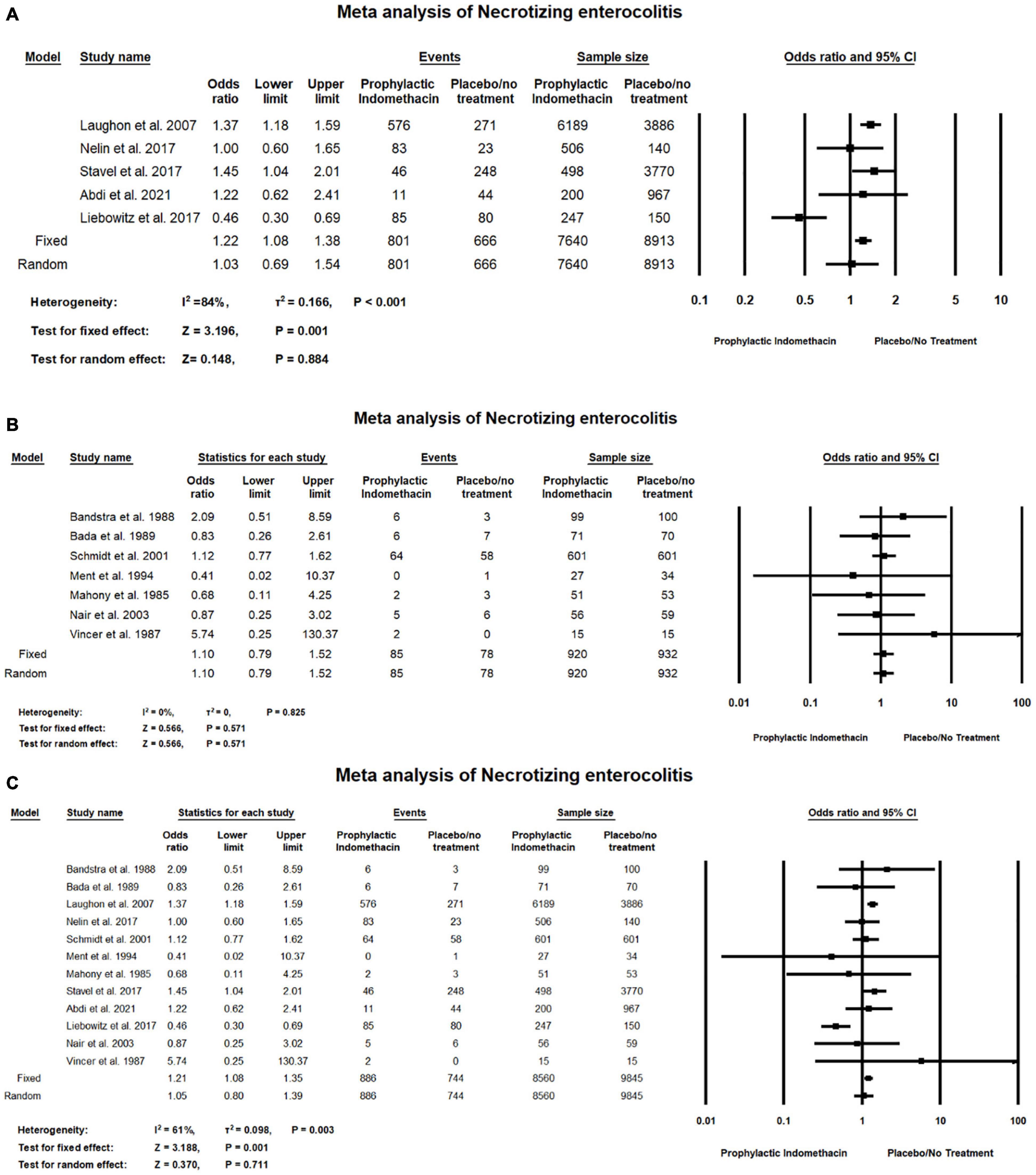
Figure 10. Meta-analysis of necrotizing enterocolitis from (A) cohort studies (B) RCT studies (C) combination of cohort and RCT studies.
Meta-analysis of RCTs shows there was no significant difference between the group of infants given prophylactic doses of indomethacin and the infants in the placebo or no treatment group with regard to the rates of necrotizing enterocolitis (OR = 1.10; 95% CI = 0.79–1.52; P-value = 0.571). There was no significant heterogeneity among the included studies (I2 = 0%; P-value = 0.825) (Figure 10B).
Combined meta-analysis of cohort and RCT studies shows there was no significant differences between the group of infants with prophylactic doses of indomethacin and the infants in the placebo or no treatment group regarding the rates of necrotizing enterocolitis (OR = 1.05; 95% CI = 0.80–1.39; P-value = 0.711). However, there was medium significant heterogeneity among the included studies (I2 = 61%; P-value = 0.003) (Figure 10C).
Combined meta-analysis of cohort and RCT studies, shows there was no significant differences between the group of infants given prophylactic doses of indomethacin and the infants in the placebo or no treatment group with regard to the rates of intestinal perforation (OR = 1.58; 95% CI = 0.89–2.82; P-value = 0.121). However, there was high significant heterogeneity among the included studies (I2 = 77%; P-value = 0.039) (Figure 11).
Meta-analysis of RCT studies shows that two studies with 340 patients were included in the analyses of hospitalization days. On comparing this outcome among the prophylactic indomethacin and placebo/no treatment groups, there was no statistically significant difference for hospitalization days (SMD = 0.08; 95% CI = -0.26: 42; P-value = 0.631). There was a medium significant heterogeneity in the analysis of hospitalization days (I2 = 60%; P-value = 0.116) (Figure 12).
In the meta-analysis of cohort studies, Figure 13A shows there was no significant differences between the group of infants given prophylactic doses of indomethacin and the placebo or no treatment group with regard to the rates of death (OR = 0.96; 95% CI = 0.71–1.29; P-value = 0.884). There was high significant heterogeneity among the included studies (I2 = 92%; P-value < 0.001).
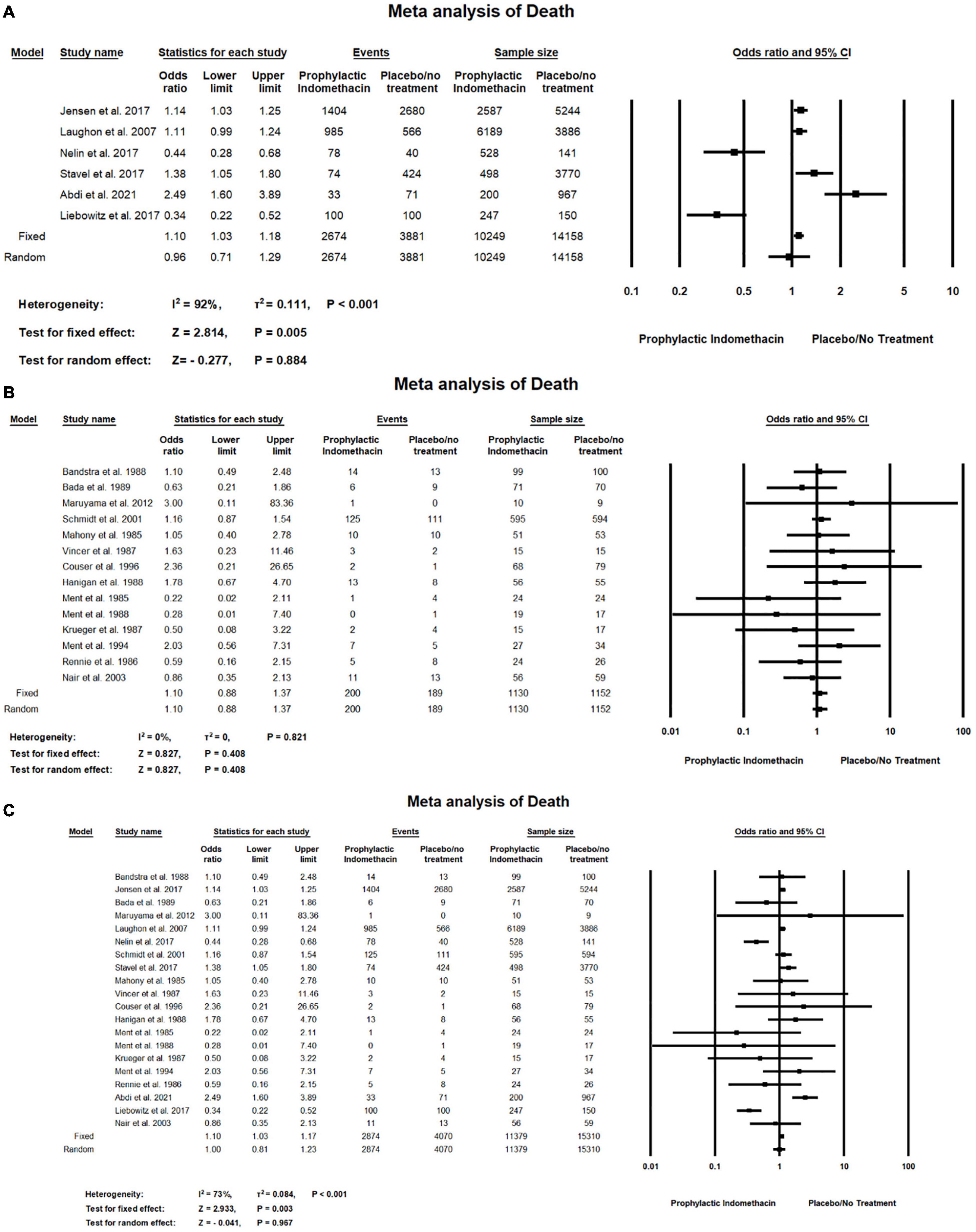
Figure 13. Meta-analysis of death from (A) cohort studies, (B) RCT studies, (C) combination of cohort and RCT studies.
Meta-analysis of RCT studies shows there was no significant differences between the group of infants given prophylactic doses of indomethacin and the placebo or no treatment group regarding the rates of death (OR = 1.10; 95% CI = 0.88–1.37; P-value = 0.408). There was no significant heterogeneity among the included studies (I2 = 0%; P-value = 0.821) (Figure 13B).
Combined meta-analysis cohort and RCT studies shows there was no significant differences between the group of infants given prophylactic doses of indomethacin and the group of placebo or no treatment regarding the rates of death (OR = 1.00; 95% CI = 0.81–1.23; P-value = 0.967). However, there was high significant heterogeneity among the included studies (I2 = 73%; P-value < 0.001) (Figure 13C).
In this study, we have included 23 studies from the systematic and manual search to be analyzed to study indomethacin as a prophylactic measure in pre-term infants from many aspects including bronchopulmonary dysplasia, patent ductus arteriosus, pulmonary hemorrhage, intraventricular hemorrhage, severe intraventricular hemorrhage, necrotizing enterocolitis, intestinal perforation, death, hospitalization days, and surgical ligation of PDA.
The analyzed data showed a varied heterogeneity in some outcomes which is probably due to the difference in study designs, the different dosages of indomethacin injection, and outcome definition between studies. Moreover, it is important to note that this meta-analysis is fundamentally different from prior ones, in that data from both randomized trials and retrospective cohort studies are included in the present analyses and is likely to be the dominant factor for differences in results.
As for the cardiopulmonary outcomes, our meta-analysis of RCT studies and combined meta-analysis of RCT and cohort studies showed that prophylactic indomethacin administration in infants significantly lowers the rates of PDA formation (P-value < 0.001) and no significant heterogeneity was estimated (I2 = 10%; P-value = 0.341) in case of the included RCT studies while medium significant heterogeneity was found in the combined analysis of RCT and cohort studies. (I2 = 62%; P-value = 0.001) which could be due to the different study designs that were included in the analysis similar to previously published studies (56, 57). Regarding the outcome of PDA surgical ligation, meta-anaylsis of RCT studies revealed significantly lower rates of surgical PDA ligation among the infants given prophylactic doses of indomethacin (P-value < 0.001) which is similar to the findings of Fowlie et al. who reported a significant lower incidence of surgical PDA ligation among the indomethacin prophylactic group (typical RR 0.51, 95% CI 0.37,0.71) (14).
On the other hand, in the present study, no significant difference was reported between indomethacin prophylactic group and the placebo/no treatment group with regard to the outcome of BPD and pulmonary hemorrhage rates in the meta-analysis of cohort and RCT studies and combined analysis. Jensen et al. (57) in their analysis of observational data found that prophylactic indomethacin did not increase or decrease the risk of developing BPD. Moreover, the authors compared these results with another analysis of RCTs, however, the analysis indicated the same information that prophylactic indomethacin had no beneficial effects on BPD.
With regard to the risk of intraventricular hemorrhage, our analysis showed no significant difference between the group of infants given prophylactic indomethacin when compared to the placebo group. However, with regard to severe IVH, meta-analysis of RCT studies showed significantly lower rates of severe IVH in the prophylactic indomethacin group (P-value = 0.008). Similarly, Fowlie et al. found a significant reduction in severe IVH incidence in infants that were prophylactically injected with indomethacin (typical RR 0.66, 95% CI 0.53–0.82) (14). However, significant heterogeneity in this study was estimated due to the inconsistency of treatment efficacy among their included studies (56). None of the studies, however, measured the long-term outcomes, they have only focused on the short ones. Schmidt et al. (15) in their large trial on 18-month infants reported statistical insignificance on long term neurodevelopmental outcomes although IVH grade 3 and 4 were significantly reduced. Therefore, concerns should be made to assess the overall quality of the effect of indomethacin on the long-term neurodevelopmental outcomes and the rate of adverse events incidence due to the vasoconstrictive nature of the drug which may alter the cerebral blood flow.
Furthermore, we found no significance between the use of prophylactic indomethacin on infants in reducing the time of hospital stay. The findings reported by Fowlie et al. favored the control groups in terms of time spent in the hospital with no significance (P = 0.087) (14). With regard to the outcome of death, no significant effect of prophylactic indomethacin was reported in the current study in both cohort and RCT studies. Jensen et al. reported a weak association between indomethacin prophylaxis and decreased risk-adjusted odds of mortality (0.81, 95% CI 0.66–0.98), however, the authors included observational data only (57).
Limitations to our study include variable heterogeneity in the analysis of some outcomes due to the different study designs that were included in this study. However, we estimated the publication bias in most cases no publication bias was found.
Prophylactic indomethacin in VLBW infants has proven efficient in preventing short-term events such as PDA, surgical PDA ligation, and severe IVH. On the other hand, it showed no significance with regard to outcomes such as IVH, BPD, pulmonary hemorrhage, necrotizing enterocolitis, intestinal performation, death and hospital stays. Since the meta-analysis results regarding effectiveness of prophylactic indomethacin varied based on the study design particularly with regard to outcomes such as surgical PDA ligation and severe IVH, this warrants the need for long term studies with larger sample size to determine the effectiveness of prophylactic indomethacin.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AAl proposed, conceptualized, and designed the study and wrote the manuscript. SA and AAS helped in the pilot extraction of a few manuscript for building the data extraction sheet and assessed the risk of bias among different included studies. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. Jama. (2003) 289:1124–9. doi: 10.1001/jama.289.9.1124
2. Laptook AR, O’Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. (2005) 115:673–80. doi: 10.1542/peds.2004-0667
3. Vohr B, Garcia Coll C, Flanagan P, Oh W. Effects of intraventricular hemorrhage and socioeconomic status on perceptual, cognitive, and neurologic status of low birth weight infants at 5 years of age. J Pediatr. (1992) 121:280–5. doi: 10.1016/s0022-3476(05)81204-1
4. Piecuch RE, Leonard CH, Cooper BA, Kilpatrick SJ, Schlueter MA, Sola A. Outcome of infants born at 24-26 weeks’ gestation: II. Neurodevelopmental outcome. Obstet Gynecol. (1997) 90:809–14. doi: 10.1016/S0029-7844(97)00429-8
5. Shaver DC, Bada HS, Korones SB, Anderson GD, Wong SP, Arheart KL. Early and late intraventricular hemorrhage: the role of obstetric factors. Obstet Gynecol. (1992) 80:831–7.
6. Ment LR, Duncan CC, Ehrenkranz RA, Lange RC, Taylor KJ, Kleinman CS, et al. Intraventricular hemorrhage in the preterm neonate: timing and cerebral blood flow changes. J Pediatr. (1984) 104:419–25. doi: 10.1016/s0022-3476(84)81109-9
7. Vohr B, Allan WC, Scott DT, Katz KH, Schneider KC, Makuch RW, et al. Early-onset intraventricular hemorrhage in preterm neonates: incidence of neurodevelopmental handicap. Semin Perinatol. (1999) 23:212–7. doi: 10.1016/s0146-0005(99)80065-2
8. Dolfin T, Skidmore MB, Fong KW, Hoskins EM, Shennan AT. Incidence, severity, and timing of subependymal and intraventricular hemorrhages in preterm infants born in a perinatal unit as detected by serial real-time ultrasound. Pediatrics. (1983) 71:541–6. doi: 10.1542/peds.71.4.541
9. Philip AG, Allan WC, Tito AM, Wheeler LR. Intraventricular hemorrhage in preterm infants: declining incidence in the 1980s. Pediatrics. (1989) 84:797–801. doi: 10.1542/peds.84.5.797
10. Marba ST, Caldas JP, Vinagre LE, Pessoto MA. Incidence of periventricular/intraventricular hemorrhage in very low birth weight infants: a 15-year cohort study. J Pediatr. (2011) 87:505–11. doi: 10.2223/JPED.2137
11. Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern-Fetal Neonatal Med. (2009) 22:491–500. doi: 10.1080/14767050902769982
12. McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. (2008) 35:777–92,vii. doi: 10.1016/j.clp.2008.07.014
13. Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. (2010) 67:1–8. doi: 10.1203/PDR.0b013e3181c1b176
14. Fowlie PW, Davis PG, McGuire W. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. (2010) 2010:Cd000174.
15. Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. (2001) 344:1966–72. doi: 10.1056/NEJM200106283442602
16. Ment LR, Oh W, Ehrenkranz RA, Philip AG, Vohr B, Allan W, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. (1994) 93:543–50. doi: 10.1542/peds.93.4.543
17. Mirza H, Oh W, Laptook A, Vohr B, Tucker R, Stonestreet BS. Indomethacin prophylaxis to prevent intraventricular hemorrhage: association between incidence and timing of drug administration. J Pediatr. (2013) 163:706–10.e1. doi: 10.1016/j.jpeds.2013.02.030
18. Alfaleh K, Smyth JA, Roberts RS, Solimano A, Asztalos EV, Schmidt B. Prevention and 18-month outcomes of serious pulmonary hemorrhage in extremely low birth weight infants: results from the trial of indomethacin prophylaxis in preterms. Pediatrics. (2008) 121:e233–8. doi: 10.1542/peds.2007-0028
19. Coyle MG, Oh W, Petersson KH, Stonestreet BS. Effects of indomethacin on brain blood flow, cerebral metabolism, and sagittal sinus prostanoids after hypoxia. Am J Physiol. (1995) 269(4 Pt 2):H1450–9. doi: 10.1152/ajpheart.1995.269.4.H1450
20. Coyle MG, Oh W, Stonestreet BS. Effects of indomethacin on brain blood flow and cerebral metabolism in hypoxic newborn piglets. Am J Physiol. (1993) 264(1 Pt 2):H141–9. doi: 10.1152/ajpheart.1993.264.1.H141
21. Leffler CW, Mirro R, Shibata M, Parfenova H, Armstead WM, Zuckerman S. Effects of indomethacin on cerebral vasodilator responses to arachidonic acid and hypercapnia in newborn pigs. Pediatr Res. (1993) 33:609–14. doi: 10.1203/00006450-199306000-00016
22. McCalden TA, Nath RG, Thiele K. The role of prostacyclin in the hypercapnic and hypoxic cerebrovascular dilations. Life Sci. (1984) 34:1801–7. doi: 10.1016/0024-3205(84)90672-6
23. Zuckerman SL, Mirro R, Armstead WM, Shibata M, Leffler CW. Indomethacin reduces ischemia-induced alteration of blood-brain barrier transport in piglets. Am J Physiol. (1994) 266(6 Pt 2):H2198–203. doi: 10.1152/ajpheart.1994.266.6.H2198
24. Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA. Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke. (1992) 23:1132–7. doi: 10.1161/01.str.23.8.1132
25. Ando Y, Takashima S, Takeshita K. Cerebral blood flow velocity in preterm neonates. Brain Dev. (1985) 7:385–91. doi: 10.1016/s0387-7604(85)80135-2
26. Ment LR, Oh W, Ehrenkranz RA, Philip AG, Schneider K, Katz KH, et al. Risk period for intraventricular hemorrhage of the preterm neonate is independent of gestational age. Semin Perinatol. (1993) 17:338–41.
27. Bandstra ES, Montalvo BM, Goldberg RN, Pacheco I, Ferrer PL, Flynn J, et al. Prophylactic indomethacin for prevention of intraventricular hemorrhage in premature infants. Pediatrics. (1988) 82:533–42. doi: 10.1542/peds.82.4.533
28. Bada HS, Green RS, Pourcyrous M, Leffler CW, Korones SB, Magill HL, et al. Indomethacin reduces the risks of severe intraventricular hemorrhage. J Pediatr. (1989) 115:631–7. doi: 10.1016/s0022-3476(89)80300-2
29. Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr B, Allan W, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. (1994) 124:951–5. doi: 10.1016/s0022-3476(05)83191-9
30. Edwards AD, Wyatt JS, Richardson C, Potter A, Cope M, Delpy DT, et al. Effects of indomethacin on cerebral haemodynamics in very preterm infants. Lancet (Lond Engl). (1990) 335:1491–5. doi: 10.1016/0140-6736(90)93030-s
31. Saliba E, Chantepie A, Autret E, Gold F, Pourcelot L, Laugier J. Effects of indomethacin on cerebral hemodynamics at rest and during endotracheal suctioning in preterm neonates. Acta Paediatr Scand. (1991) 80:611–5. doi: 10.1111/j.1651-2227.1991.tb11918.x
32. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.1371/journal.pmed.1000100
33. Vassar M, Atakpo P, Kash MJ. Manual search approaches used by systematic reviewers in dermatology. J Med Libr Assoc JMLA. (2016) 104:302. doi: 10.3163/1536-5050.104.4.009
34. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Research Ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
35. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. Jama. (2006) 295:676–80. doi: 10.1001/jama.295.6.676
36. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
37. Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x
38. Jensen EA, Dysart KC, Gantz MG, Carper B, Higgins RD, Keszler M, et al. Association between use of prophylactic indomethacin and the risk for bronchopulmonary dysplasia in extremely preterm infants. J Pediatr. (2017) 186:34–40.e2. doi: 10.1016/j.jpeds.2017.02.003
39. Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. (2007) 27:164–70. doi: 10.1038/sj.jp.7211662
40. Liebowitz M, Clyman RI. Prophylactic indomethacin compared with delayed conservative management of the patent ductus arteriosus in extremely preterm infants: effects on neonatal outcomes. J Pediatr. (2017) 187:119–26.e1. doi: 10.1016/j.jpeds.2017.03.021
41. Maruyama K, Fujiu T. Effects of prophylactic indomethacin on renal and intestinal blood flows in premature infants. Pediatr Int. (2012) 54:480–5. doi: 10.1111/j.1442-200X.2012.03583.x
42. Narayanan M, Cooper B, Weiss H, Clyman RI. Prophylactic indomethacin: factors determining permanent ductus arteriosus closure. J Pediatr. (2000) 136:330–7. doi: 10.1067/mpd.2000.103414
43. Nelin TD, Pena E, Giacomazzi T, Lee S, Logan JW, Moallem M, et al. Outcomes following indomethacin prophylaxis in extremely preterm infants in an all-referral NICU. J Perinatol. (2017) 37:932–7. doi: 10.1038/jp.2017.71
44. Stavel M, Wong J, Cieslak Z, Sherlock R, Claveau M, Shah PS. Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. J Perinatol. (2017) 37:188–93. doi: 10.1038/jp.2016.196
45. Couser RJ, Ferrara TB, Wright GB, Cabalka AK, Schilling CG, Hoekstra RE, et al. Prophylactic indomethacin therapy in the first twenty-four hours of life for the prevention of patent ductus arteriosus in preterm infants treated prophylactically with surfactant in the delivery room. J Pediatr. (1996) 128(5 Pt 1):631–7. doi: 10.1016/s0022-3476(96)80127-2
46. Hanigan WC, Kennedy G, Roemisch F, Anderson R, Cusack T, Powers W. Administration of indomethacin for the prevention of periventricular-intraventricular hemorrhage in high-risk neonates. J Pediatr. (1988) 112:941–7. doi: 10.1016/s0022-3476(88)80224-5
47. Krueger E, Mellander M, Bratton D, Cotton R. Prevention of symptomatic patent ductus arteriosus with a single dose of indomethacin. J Pediatr. (1987) 111:749–54. doi: 10.1016/s0022-3476(87)80262-7
48. Yaseen H, Al Umran K, Ali H, Rustum M, Darwich M, Al-Faraidy A. Effects of early indomethacin administration on oxygenation and surfactant requirement in low birth weight infants. J Trop Pediatr. (1997) 43:42–6. doi: 10.1093/tropej/43.1.42
49. Vincer M, Allen A, Evans J, Nwaesei C, Stinson D, Rees E, et al. Early intravenous indomethacin prolongs respiratory support in very low birth weight infants. Acta Paediatr Scand. (1987) 76:894–7. doi: 10.1111/j.1651-2227.1987.tb17260.x
50. Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Pitt BR, Taylor KJ, et al. Randomized indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight infants. J Pediatr. (1985) 107:937–43. doi: 10.1016/s0022-3476(85)80197-9
51. Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Taylor KJ, Scott DT, et al. Randomized low-dose indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight neonates. J Pediatr. (1988) 112:948–55. doi: 10.1016/s0022-3476(88)80225-7
52. Kumar Nair PA, Pai MG, Gazal HA, Da Costa DE, Al Khusaiby SM. Indomethacin prophylaxis for intraventricular hemorrhage in very low birth weight babies. Indian Pediatr. (2004) 41:551–8.
53. Rennie JM, Doyle J, Cooke RW. Early administration of indomethacin to preterm infants. Arch Dis Child. (1986) 61:233–8. doi: 10.1136/adc.61.3.233
54. Mahony L, Caldwell RL, Girod DA, Hurwitz RA, Jansen RD, Lemons JA, et al. Indomethacin therapy on the first day of life in infants with very low birth weight. J Pediatr. (1985) 106:801–5. doi: 10.1016/s0022-3476(85)80361-9
55. Abdi HH, Backes CH, Ball MK, Talavera-Barber MM, Klebanoff MA, Jadcherla SR, et al. Prophylactic Indomethacin in extremely preterm infants: association with death or BPD and observed early serum creatinine levels. J Perinatol. (2021) 41:749–55. doi: 10.1038/s41372-021-00995-x
56. Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2003) 88:F464–6. doi: 10.1136/fn.88.6.f464
Keywords: patent ductus arteriosus, intraventricular hemorrhage, prophylactic indomethacin, preterm infants, neonatal outcome
Citation: Al-matary A, Abu Shaheen A and Abozaid S (2022) Use of Prophylactic Indomethacin in Preterm Infants: A Systematic Review and Meta-Analysis. Front. Pediatr. 10:760029. doi: 10.3389/fped.2022.760029
Received: 17 August 2021; Accepted: 23 February 2022;
Published: 07 April 2022.
Edited by:
Giovanni Vento, Catholic University of the Sacred Heart, ItalyReviewed by:
Yogen Singh, Cambridge University Hospitals NHS Foundation Trust, United KingdomCopyright © 2022 Al-matary, Abu Shaheen and Abozaid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulrahman Al-matary, YW1hdGFyeUB5YWhvby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.