
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Pediatr., 16 March 2022
Sec. Pediatric Critical Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.756643
This article is part of the Research TopicPediatric Critical Care in Resource-Limited Settings, Volume IIView all 13 articles
 Teresa B. Kortz1,2*
Teresa B. Kortz1,2* Katie R. Nielsen3,4
Katie R. Nielsen3,4 Rishi P. Mediratta5
Rishi P. Mediratta5 Hailey Reeves2
Hailey Reeves2 Nicole F. O'Brien6
Nicole F. O'Brien6 Jan Hau Lee7,8
Jan Hau Lee7,8 Jonah E. Attebery9
Jonah E. Attebery9 Emaan G. Bhutta10
Emaan G. Bhutta10 Carter Biewen1
Carter Biewen1 Alvaro Coronado Munoz11
Alvaro Coronado Munoz11 Mary L. deAlmeida12
Mary L. deAlmeida12 Yudy Fonseca13
Yudy Fonseca13 Shubhada Hooli14
Shubhada Hooli14 Hunter Johnson6
Hunter Johnson6 Niranjan Kissoon15,16
Niranjan Kissoon15,16 Mara L. Leimanis-Laurens17,18
Mara L. Leimanis-Laurens17,18 Amanda M. McCarthy11
Amanda M. McCarthy11 Carol Pineda19
Carol Pineda19 Kenneth E. Remy20,21
Kenneth E. Remy20,21 Sara C. Sanders6
Sara C. Sanders6 Yemisi Takwoingi22,23
Yemisi Takwoingi22,23 Matthew O. Wiens24,25†
Matthew O. Wiens24,25† Adnan T. Bhutta13† The Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network on Behalf of the PALISI Global Health Subgroup
Adnan T. Bhutta13† The Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network on Behalf of the PALISI Global Health SubgroupBackground: The majority of childhood deaths occur in low- and middle-income countries (LMICs). Many of these deaths are avoidable with basic critical care interventions. Quantifying the burden of pediatric critical illness in LMICs is essential for targeting interventions to reduce childhood mortality.
Objective: To determine the burden of hospitalization and mortality associated with acute pediatric critical illness in LMICs through a systematic review and meta-analysis of the literature.
Data Sources and Search Strategy: We will identify eligible studies by searching MEDLINE, EMBASE, CINAHL, and LILACS using MeSH terms and keywords. Results will be limited to infants or children (ages >28 days to 12 years) hospitalized in LMICs and publications in English, Spanish, or French. Publications with non-original data (e.g., comments, editorials, letters, notes, conference materials) will be excluded.
Study Selection: We will include observational studies published since January 1, 2005, that meet all eligibility criteria and for which a full text can be located.
Data Extraction: Data extraction will include information related to study characteristics, hospital characteristics, underlying population characteristics, patient population characteristics, and outcomes.
Data Synthesis: We will extract and report data on study, hospital, and patient characteristics; outcomes; and risk of bias. We will report the causes of admission and mortality by region, country income level, and age. We will report or calculate the case fatality rate (CFR) for each diagnosis when data allow.
Conclusions: By understanding the burden of pediatric critical illness in LMICs, we can advocate for resources and inform resource allocation and investment decisions to improve the management and outcomes of children with acute pediatric critical illness in LMICs.
Greater than 80% of the global 6.64 million annual deaths in children and adolescents in 2017 occurred in low- and middle-income countries (LMICs) (1). Acute pediatric illnesses (e.g., sepsis, pneumonia, diarrheal disease, trauma) are the leading causes of death and disability outside of the neonatal period (1–5). The World Health Organization defines acute pediatric critical illness as “any severe problem with the airway, breathing, or circulation, or acute deterioration of conscious state; [which] includes apnea, upper airway obstruction, hypoxemia, central cyanosis, severe respiratory distress, total inability to feed, shock, severe dehydration, active bleeding requiring transfusion, unconsciousness, or seizures” (6). A significant number of children's lives could be saved with supportive critical care interventions, such as fluid resuscitation, high-flow oxygen therapy, non-invasive and invasive mechanical ventilation, and vasoactive support (7–11). Unfortunately, critical care services, defined as hospital care for children with sudden, serious reversible disease, are not universally available and are frequently lacking in LMIC settings, where disease burden, both in terms of hospitalization and mortality, is the highest (7). Furthermore, it is difficult to assess the burden of critical illness in settings without formal critical care services, where critical illness is frequently managed in emergency departments and inpatient wards.
Several recent global point prevalence studies have described the prevalence of key, individual acute pediatric critical illnesses. The Pediatric Acute Lung Injury Ventilation (PALIVE) study, conducted in 59 pediatric intensive care units (PICUs), found that 10.8% of children were diagnosed with acute lung injury (12). The Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) study reported a prevalence of pediatric acute respiratory distress syndrome of 3.2% and an associated mortality of 17% mortality in children admitted to 145 PICUs from 27 countries (13). The International Survey of Critically Ill Children with Acute Neurological Insults (PANGEA) study conducted in 107 PICUs across 23 countries found an overall prevalence of acute neurologic insult to be 16.2% and all-cause hospital mortality was 12% (14). Finally, the Sepsis Prevalence, Outcomes, and Therapies (SPROUT) study was conducted in 128 PICUs across 26 countries and demonstrated a prevalence of pediatric severe sepsis of 8.2% with a hospital mortality of 25% (15).
While each of these studies contributed significant knowledge about specific acute pediatric critical illnesses, there are limitations to the available data. The first limitation stems from the focus on a single, critical illness or insult as opposed to all pediatric critical illnesses. There is substantial overlap between illnesses (e.g., pneumonia is a frequent cause of sepsis). In addition, critical care resources support patients with many diagnoses (e.g., mechanical ventilation supports children with pneumonia, shock, or trauma), and resource availability, or lack thereof, greatly impacts patient outcomes. A narrow, illness-specific view fails to capture the burden of pediatric critical illness, which makes it difficult to prioritize resources and achieve the greatest potential impact on child mortality. The most significant limitation, however, is that current global pediatric critical illness point prevalence studies do not reflect the prevalence of disease is LMICs. The PALIVE study was conducted exclusively in North American and European countries; (12) no low-income countries were included in the PARDIE study; (13) approximately 80% of PANGEA study sites were in North America and Europe; (14) and the SPROUT study, while it included several LMICs, did not include any countries from sub-Saharan Africa outside of South Africa (15). Each of these global point prevalence studies required PICU admission as an inclusion criterion. This drastically limited which centers and settings could participate and may have resulted in a gross underestimation of pediatric critical illness in LMICs where critical illness may be managed in sites without a formal PICU (7).
In this systematic review, we will describe the burden of hospitalizations and mortality associated with acute pediatric critical illness in LMICs including in settings that may not have a PICU or formal intensive care services. This review will contribute to our knowledge of the etiologies and prevalence of acute pediatric critical illness in settings with the highest burden of disease. This information will help guide decisions justifying resource allocation and investment as well as inform educational, policy, and research priorities to improve outcomes following acute pediatric critical illness globally.
The objectives of this study are to (1) determine common causes of pediatric hospital admissions (critical and non-critical) and mortality in LMICs; (2) determine the prevalence of and mortality associated with acute pediatric critical illness in LMICs; and (3) analyze the differences in common causes of critical illness by age and region.
This protocol follows the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines (PRISMA), is registered in the international prospective register of systematic reviews (PROSPERO #230228) and was organized and reviewed by the Pediatric Acute Lung Injury and Sepsis Investigator (PALISI) Network Global Health subgroup and PALISI Network Scientific committee (16, 17). The multinational and multidisciplinary scientific Working Group responsible for development of the systematic review protocol includes subject matter and/or methodology experts from across the globe who are members in good standing of the PALISI Global Health subgroup. The inclusion criteria are presented according to published guidelines for prevalence systematic reviews of observational studies (CoCoPop or condition, context, and population) (18).
The population of interest is a general pediatric admission population admitted to a hospital in a low-, lower-middle, or middle-income country (LMIC), defined below in Section Context. The age range of interest includes post-neonatal (>28 days of age) and pre-adolescent (<13 years of age) children; studies that do not include some portion of this age range (>28 days to <13 years) will be excluded. However, studies that include this age range (>28 days to <13 years) plus either neonates and/or adolescents will be included, if it is a pediatric study population (e.g., includes study participants <18 years of age). All hospital admissions, regardless of admission disposition (high-dependency unit, PICU, ward, etc.) will be included. Studies where the available denominator represents a specific patient population and not all hospital admissions, such as emergency department patients, neonatal intensive care admissions, pediatric intensive care admissions, and neonatal populations, will be excluded. In situations when the denominator of interest does not represent the entire general pediatric admission population due to study-imposed exclusions, Working Group members will assess these texts individually and decide whether the study exclusion criteria likely resulted in a significantly different case mix (i.e., highly prevalent condition, condition highly relevant to critical illness) compared to the overall, general pediatric admission population. If so, then the text will be excluded. If not, then it will be included and assessed for bias during quality assessment.
The burden of critical illness is hospitalization or mortality due to a critical illness. Critical illness is defined as a state of ill health with vital organ system dysfunction and/or a high risk of imminent death. Studies must report the proportion of children with a specific admission diagnosis or cause of death (the numerator), such as pneumonia, human immunodeficiency virus (HIV), malaria, etc., relative to the number of general pediatric hospital admissions (the denominator) over that same period to be included. Both the numerator and denominator must represent the same patient population.
Observational studies (prospective or retrospective cohorts, surveillance studies, hospital database publications, cross-sectional studies, before data from before-and-after studies, registry data, etc.) must be published since January 1, 2005, in Spanish, French, or English to be included. For studies including data collected before the year of 2000, only data from 2000 to present will be included; however, if it is not possible to extract only data after the year 2000, the study will be excluded in its entirety. Exclusion of data before the year 2000 and the publication date of January 2005 were chosen to reflect recent trends in pediatric hospitalization and mortality.
Only studies conducted in LMICs will be included. Low- and middle-income country status will be determined by the Global Burden of Disease (GBD) 2017 Socio-Demographic Index (SDI) (19). The SDI is a composite indicator that includes indices of total fertility rate for women under age 25 years, mean education for people 15 years and older, and a lag-distributed income per capita. Socio-Demographic Index represents a country's overall development status and strongly correlates with health outcomes. Studies that present aggregated data representing multiple countries (e.g., multi-center study) will be included, and we will report regional data. Publications conducted in LMICs but not representative of the setting (e.g., medical mission, foreign military hospital, disaster response efforts) will be excluded.
Abstract only publications, case studies, narrative reviews, surveys, study protocols, comments, editorials, letters, notes, conference materials, interventional trials, and texts for which we cannot locate the full text will be excluded. The search may be updated prior to publication to include more recent publications.
A search strategy was developed among co-investigators and an academic librarian and tested for feasibility. The final search results are shown in Figure 1. We identified eligible studies by searching Ovid MEDLINE (1946 to February 26, 2021, with Epub Ahead of Print, In-Process, and Other Non-Indexed Citations), EMBASE.com (1974 to March 2021), CINAHL (1981 to March 2021), and LILACS (1982 to March 2021) (Table 1). The MEDLINE search was performed using MeSH and key words for “hospitalization,” “patient admission,” “patient readmission,” “hospital units,” “critical care,” “intensive care,” “mortality,” and “developing countries.” Countries determined to be LMICs by SDI criteria were listed individually to increase the specificity of the search. The MEDLINE strategy was adapted to search EMBASE, CINAHL, and LILACS. All results were limited to infants or children (ages 29 days to 12 years) and publication years 2005 to present. There were no language restrictions; texts in languages other than English, Spanish, or French will be manually excluded during screening. Specified publication types were excluded in MEDLINE and EMBASE (e.g., comments, editorials, letters, notes, conference materials) (Supplementary Table 1).
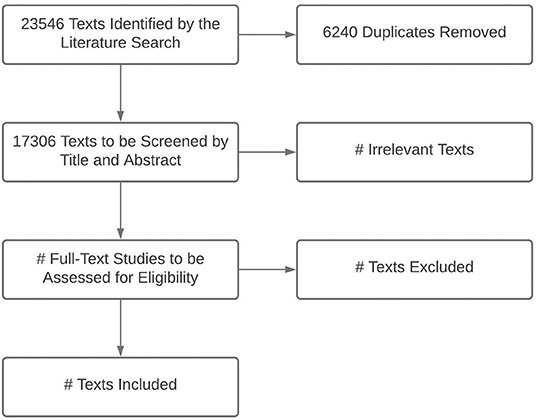
Figure 1. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA) flowchart for title and abstract screening and text selection from the final search (conducted March 1, 2021).
The titles from the search will be uploaded to and screened using Covidence (Veritas Health Innovation, Melbourne, Australia) (20). Covidence is a web-based systematic review platform designed to facilitate citation screening, full-text upload, and conflict resolution. Citations will be screened for eligibility based on title and abstract, and full text using a study-specific flowchart (Figure 2).
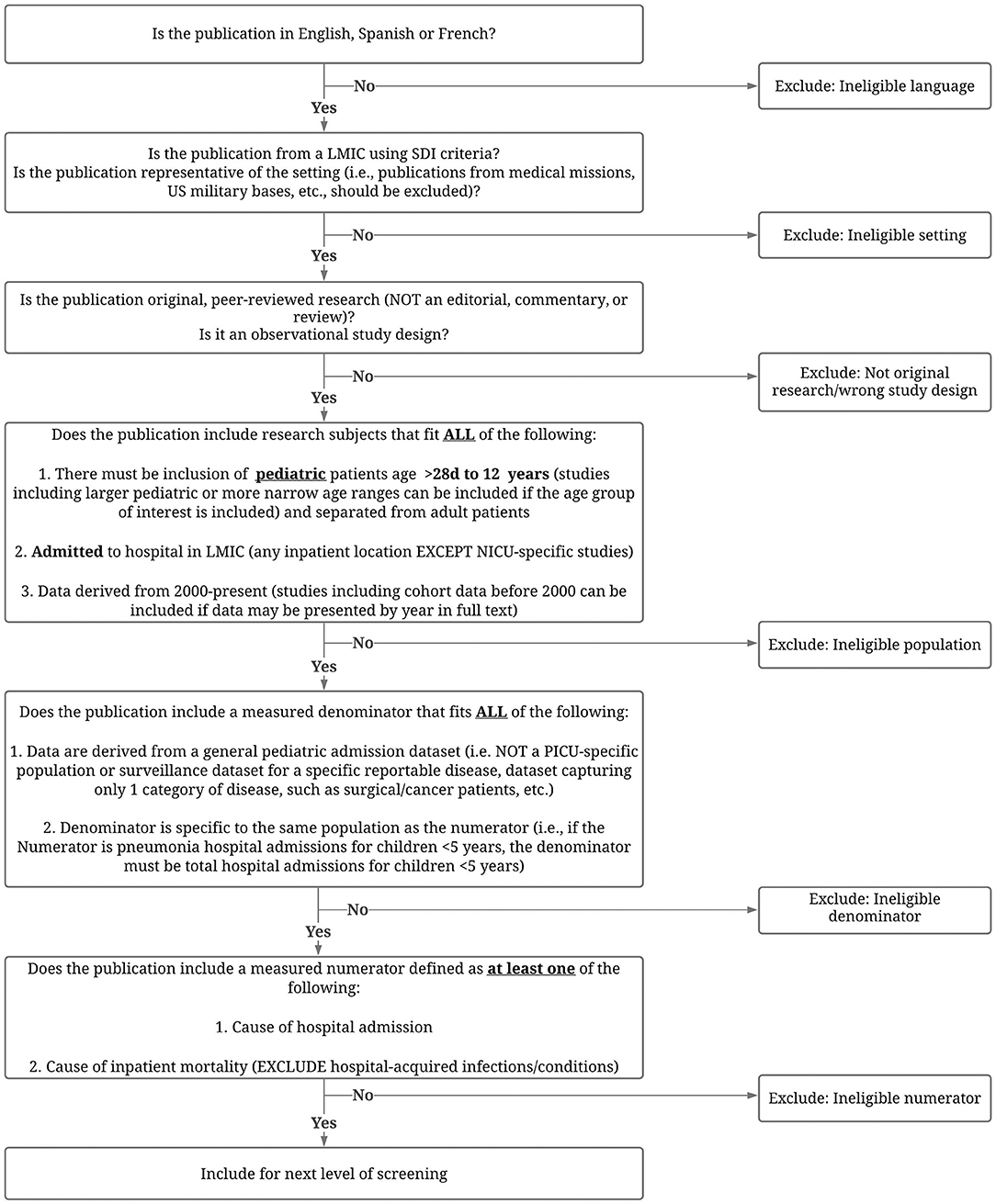
Figure 2. Approach to screening abstracts, titles and texts for eligibility. LMIC, low- and middle-income country; SDI, Socio-Demographic Index; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
Working Group members will complete a training set of 25 citations (titles and abstracts) before initiating screening for the project. At least 5–10 true positives will be purposely included in the training set. The training set will be created by the study investigator (TK). Members of the Working Group will independently screen the titles and abstracts and then discuss and align on the final decision during a Working Group meeting.
Each title will be screened by two reviewers using the predetermined eligibility criteria. Specific Working Group Members fluent in non-English languages will be designated to review citations in Spanish (ACM, KN, TK, YF, CP) and French (ACM, HR, NO, CP). Titles that are eliminated by both reviewers will be rejected; titles accepted by both reviewers will advance to full-text screening; and titles in which a consensus is not reached will be resolved by a third member. Each full-text article will be assessed by two members of the Working Group for inclusion in the final set of articles for data extraction. At each screening and assessment phase, conflicts will be resolved by a third member of the Working Group using the conflict resolution function in Covidence.
For full texts with exclusion criteria, a reason for exclusion will be recorded (e.g., ineligible language; ineligible setting; not original research/wrong study design; ineligible population; ineligible denominator; ineligible numerator; full text not found; duplicate article). Texts identified by title and abstract screening will be excluded if the full text cannot be found after the following stepwise process is completed: search of available journal article subscriptions at two or more academic institutions; a general web-based search using Google; an Interlibrary Loan request from at least two academic institutions; an article request via direct email to the corresponding author or editor. For multiple publications from one dataset, we will only include the data once (e.g., the most recent or most relevant publication). For publications with multiple years of data presented by year or groups of years (e.g., vaccine surveillance studies), we will include the most recent year(s) as this is more likely to reflect the current epidemiology of disease. Publications with data from more than one country (e.g., global prevalence studies) will be considered for inclusion if either (a) all included countries meet LMIC criteria, or (b) data from LMICs can be extracted separately from non-LMIC data. We may contact authors to stratify data by age for already published texts.
Data from all included full-text articles will be extracted by two, independent Working Group members and managed using REDCap, a secure, web-based application and electronic data capture tool hosted at the University of California, San Francisco (21). Data extraction conflicts will be resolved by a third member of the Working Group using the data comparison functionality in REDCap.
Data extraction will include information related to study characteristics (i.e., title, authors, year of publication, date of enrollment, urban/rural, country, language, journal, study design, sample size, inclusion/exclusion criteria, data source); hospital characteristics (public/private/faith-based, referral/district, community/academic, children's hospital, intensive care resources available, number of beds); underlying population characteristics (population served, proportion living in poverty, malaria rate, HIV rate, malnutrition rate); patient population characteristics (age, sex, presence of malnutrition, and other comorbidities); and outcomes (cause of admission, cause of death, length of stay).
Two members of the Working Group will independently assess the quality of each selected article and risk of bias using an adapted version of the Quality in Prognosis Studies (QUIPS) tool (22). While this review will not assess prognostic factors for admission and/or mortality, biases relevant to prognostic factors are similar to those relevant to the assessment of causes of these outcomes. The QUIPS tool includes six domains of bias, of which the three deemed appropriate for this review are: (1) study participation; (2) study attrition; and (3) prognostic factor (i.e., cause) measurement. The fourth, outcome measurement, is not relevant as this systematic review assesses causes of admission (and where relevant, death), and the cross-sectional nature precludes a temporally linked outcome to the cause. The fifth and sixth domains, study confounding and statistical analysis, respectively, were also deemed not relevant as the data to be extracted are counts. Issues around improper analyses will be adequately captured in domains 2 (attrition) and 3 (measurement of cause). The adapted domains, key issues, and items for consideration relevant to this review are shown in Table 2. Risk of bias will be classified as high, moderate, or low when the relationship between the listed causes and outcome is very likely to be, may be, or unlikely to be, respectively, different for participants and eligible non-participants. Conflicts in the risk of bias assessment will be resolved by discussion or by a member of the Working Group if consensus cannot be reached. We will produce one or more summary of findings tables that will provide an overview of the evidence to make the findings accessible to readers. The tables will include summaries of the methodological quality (risk of bias), precision of summary estimates (imprecision), concerns about heterogeneity (inconsistency), applicability of the findings to our review question (indirectness), and issues with publication bias. The tables will also include any additional limitations of the evidence. We will explore the impact of the risk of bias domains in sensitivity analyses.

Table 2. Risk of bias domains and questions adapted from the Quality in Prognosis Studies (QUIPS) criteria.
We will summarize data on study (author, publication year, study country, study design, sample size, ages included, data source), hospital [catchment population, type of hospital (level, affiliation, pediatric, etc.), number of health facility and pediatric beds, and available intensive care resources] and patient (median age, prevalence of comorbidities such as malnutrition, congenital heart disease, prematurity, malignancy, malaria, and anemia) characteristics; outcomes; and risk of bias assessment using tables, graphs, and narrative summaries. Continuous outcomes will be summarized using mean and standard deviations (SDs) or medians with interquartile ranges as appropriate. Binary outcomes will be summarized using frequencies and percentages.
The primary outcomes of interest are (1) cause of hospital admission and (2) cause of in-hospital mortality. Causes of hospital admissions will be further categorized as critical (potentially life-threatening) and non-critical (unlikely to be life threatening) based on group consensus and a review of the literature. If available, data for secondary outcomes will be collected including in-hospital mortality, case fatality rate (CFR), and length of hospital stay.
We will report the causes of admission and mortality (categorized by GBD grouping) by region (Central Europe, Eastern Europe, and Central Asia; Latin America and Caribbean; North Africa and Middle East; South Asia; Southeast Asia, East Asia, Oceania; Sub-Saharan Africa), SDI country income level (low-, lower-middle, or middle-income), and age (<5 years, 5–12 years).
When possible, we will report the CFR for each cause of admission and/or cause of death. This may require calculating these estimates from individual studies when not reported directly, provided that the necessary data to perform these calculations are reported. Causes of hospital admissions will be categorized as non-critical or critical (potentially life-threatening) by the same multinational, multidisciplinary scientific Working Group compiled of experts described above. The Working Group will reach consensus as to whether the reason for admission is consistent with vital organ system dysfunction and/or a high risk of imminent death based on a review of region-specific literature. We will explore different definitions and cut-offs for critical illness (proportion of total admissions, proportion of total mortality, CFR).
As the data allow, we will perform a meta-analysis on the proportions of causes of admission and causes of death, as well as the CFRs using random-effects models. We will conduct meta-regression, where possible, to explore predictors for all-cause and cause-specific mortality (pneumonia, sepsis, and diarrhea). Possible predictors will include SDI, facility type, and geographic region. Additionally, we will explore temporal trends in admission and mortality by age and region. We will consider subgroup analyses if we have adequate numbers of studies and/or patients within the included studies.
We will examine sources of heterogeneity, including differences in methodology, setting (urban vs. rural), region, income level, and patient populations (e.g., age, sex, prevalence of comorbidities, etc.). Statistical heterogeneity will be assessed using the variance estimates from the random effects model. It is likely that there will be significant heterogeneity between studies, and we will therefore pool results when studies are comparable. All analyses will be performed using STATA (version 16).
Through this systematic review, we expect to identify the most common causes of acute pediatric critical illness resulting in hospital admission and mortality in LMICs by age and region. If data are available, we will also show temporal trends in admission and mortality by age and region. We will classify causes of admission as critical or non-critical and illustrate the global prevalence of critical illness with a map. Furthermore, we anticipate identifying diagnoses with the highest CFR for each age and region and illustrating these results through a series of forest plots for all-cause mortality, cause-specific mortality (pneumonia, sepsis, diarrhea, malaria), critical illness, and hospital length of stay (data permitting).
There are several advantages to the proposed approach. First, with broad inclusion criteria, we expect to capture most if not all relevant texts. Second, by not restricting the search to exclusively pediatric intensive care populations, we will be able to calculate the prevalence of critical illness across settings, including those without a formal PICU. Third, by including both individual LMICs by name and terms such as “resource-limited,” “low income,” and “developing” in the search strategy, we will likely identify more texts from LMICs, which will provide a more complete assessment of the burden of critical and non-critical disease in these countries.
There are potential limitations to the proposed protocol. First, neonatal and adolescent populations are included in some pediatric studies, and the search was not designed to capture these populations. We will intentionally exclude exclusively neonatal and adolescent populations from data analyses and will not be able to draw conclusions about children <28 days or >12 years of age. Second, we will exclude disease-specific studies that do not report overall pediatric hospital admissions, which may result in an underestimation of disease prevalence. Additionally, estimates will not include disease prevalence during outbreaks, potentially underestimating the true prevalence of disease and overall required critical care capacity. Third, we will restrict study inclusion to publications in Spanish, French, or English, and may not identify all potentially relevant texts. Fourth, we may underestimate the true burden of critical illness in LMICs by excluding emergency department or PICU population studies that lack the denominator of interest (general hospital admissions). However, without a common denominator, we cannot draw comparisons across studies. Sixth, it is possible that critical, but rare illnesses, will not be adequately represented in this systematic review as they are often categorized in the “other” category in texts. This systematic review will, however, describe the most common causes of pediatric critical illness, which is of greatest importance when the objective is to improve overall child health outcomes and inform resource allocation. Finally, we expect to include a small number of studies where the denominator does not represent the entire general pediatric admission population due to original study-imposed exclusions. The degree of bias from these texts should be minimal because only those with a similar case mix to the overall, general pediatric admission population will be included.
There is intense competition for limited resources in many LMICs and children are frequently overlooked as the global focus shifts away from infectious diseases toward non-communicable diseases, which are far more common in adult populations (23). To decrease childhood morbidity and mortality, health systems require capacity to deliver both preventative medicine and treatment, such as proven, effective therapies, like critical care (23). While dedicated PICUs are being developed in LMICs, clinician, and staff education is sub-optimal due to a lack of appreciation of the common pediatric critical illnesses.
The objective of this systematic review is to describe the most common causes of critical illnesses causing hospitalization and death in children in LMICs. This will provide much needed insight into the burden, etiology, and distribution of pediatric critical illness in LMICs, especially in settings where formal critical care services may not be currently available. Region-specific data that capture the burden of disease and outcomes for children in LMICs are essential to inform educational initiatives and training, shape advocacy and policy objectives, allocate limited resources appropriately, and implement context-appropriate, evidence-based critical care interventions for children in need. This systematic review is a crucial first step in setting future educational, advocacy, policy, research, and health delivery priorities for children with acute critical illness in LMICs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
TK: substantial contributions to the conception, design of the work, drafting, and revising the work. KN, RM, HR, NOB, JL, JA, EB, CB, YF, HJ, ML-L, ACM, AMM, MLd, SH, SS, NK, KR, CP, YT, MW, and AB: substantial contributions to the conception, design of the work, and revising the work critically for important intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work.
Research effort to create this publication was supported by the National Institute of Allergy and Infectious Diseases (award number K23AI144029, TK; award number 5U01AI126610, AB) of the National Institutes of Health (NIH), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (award number 1R21HD106252-01, NO) of the NIH, the National Institute of General Medical Sciences (award number 5K08GM129763, KR) of the NIH, the National Medical Research Council, Singapore (MOH-000446-00, JL), the National Institute for Health Research (NIHR) Birmingham Biomedical Research Center of the National Health Services (YT), and Grand challenges Canada (NK). The views expressed are those of the author(s) and not necessarily those of the NIH, Singapore MOH, NHS, NIHR, or the Department of Health and Social Care.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YHM declared a shared affiliation with one of the authors JL to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Sue Groshong from Seattle Children's Hospital for help developing and executing the search strategy.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.756643/full#supplementary-material
1. Reiner RC Jr., Olsen HE, Ikeda CT, Echko MM, Ballestreros KE, Manguerra H, et al. Diseases, injuries, and risk factors in child and adolescent health, 1990 to 2017: findings from the global burden of diseases, injuries, and risk factors 2017 study. JAMA pediatr. (2019). 173:e190337. doi: 10.1001/jamapediatrics.2019.0337
2. Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, Rudan I, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. (2013) 381:1417–29. doi: 10.1016/S0140-6736(13)60648-0
3. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388:3027–35. doi: 10.1016/S0140-6736(16)31593-8
4. Collaborators GBDCM. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1725–74. doi: 10.1016/S0140-6736(16)31575-6
5. Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017. Seattle, WA: IHME (2018).
6. WHO. Updated Guideline: Paediatric Emergency Triage, Assessment and Treatment. Geneva: World Health Organization (2016).
7. Baker T. Pediatric emergency and critical care in low-income countries. Paediatr Anaesth. (2009) 19:23–7. doi: 10.1111/j.1460-9592.2008.02868.x
8. Cheah IG, Soosai AP, Wong SL, Lim TO, Cost-effectiveness NSG. Cost-effectiveness analysis of Malaysian neonatal intensive care units. J Perinatol. (2005) 25:47–53. doi: 10.1038/sj.jp.7211196
9. Cubro H, Somun-Kapetanovic R, Thiery G, Talmor D, Gajic O. Cost effectiveness of intensive care in a low resource setting: a prospective cohort of medical critically ill patients. World J Cri Care Med. (2016) 5:150–64. doi: 10.5492/wjccm.v5.i2.150
10. Profit J, Lee D, Zupancic JA, Papile L, Gutierrez C, Goldie SJ, et al. Clinical benefits, costs, and cost-effectiveness of neonatal intensive care in Mexico. PLoS Med. (2010) 7:e1000379. doi: 10.1371/journal.pmed.1000379
11. Tripathi S, Kaur H, Kashyap R, Dong Y, Gajic O, Murthy S, et al. survey on the resources and practices in pediatric critical care of resource-rich and resource-limited countries. J Intens Care. (2015) 3:40. doi: 10.1186/s40560-015-0106-3
12. Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJ, Carroll CL, et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. (2010) 11:681–9. doi: 10.1097/PCC.0b013e3181d904c0
13. Khemani RG, Smith L, Lopez-Fernandez YM, Kwok J, Morzov R, Klein MJ, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med. (2019) 7:115–28. doi: 10.1016/S2213-2600(18)30344-8
14. Fink EL, Kochanek PM, Tasker RC, Beca J, Bell MJ, Clark RS, et al. international survey of critically ill children with acute neurologic insults: the prevalence of acute critical neurological disease in children: a global epidemiological assessment study. Pediatr Crit Care Med. (2017) 18:330–42. doi: 10.1097/PCC.0000000000001093
15. Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. (2015) 191:1147–57. doi: 10.1164/rccm.201412-2323OC
16. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
17. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
18. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
19. Network, GBoDC. Global Burden of Disease Study 2017 (GBD 2017) Socio-Demographic Index (SDI) 1950–2017. Seattle, WA: Institute for Health Metrics and Evaluation. (updated Mar 30, 2019). Available online at: http://ghdx.healthdata.org/record/ihme-data/gbd-2017-socio-demographic-index-sdi-1950%E2%80%932017 (accessed August 3, 2021).
20. Covidence. Melbourne, Australia (2019). Available online at: https://www.covidence.org/
21. harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
22. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
Keywords: critical illness, resource limited setting, pediatrics—children, child health, global health, hospitalization, low- and middle-income countries (LMIC)
Citation: Kortz TB, Nielsen KR, Mediratta RP, Reeves H, O'Brien NF, Lee JH, Attebery JE, Bhutta EG, Biewen C, Coronado Munoz A, deAlmeida ML, Fonseca Y, Hooli S, Johnson H, Kissoon N, Leimanis-Laurens ML, McCarthy AM, Pineda C, Remy KE, Sanders SC, Takwoingi Y, Wiens MO and Bhutta AT (2022) The Burden of Critical Illness in Hospitalized Children in Low- and Middle-Income Countries: Protocol for a Systematic Review and Meta-Analysis. Front. Pediatr. 10:756643. doi: 10.3389/fped.2022.756643
Received: 10 August 2021; Accepted: 31 January 2022;
Published: 16 March 2022.
Edited by:
Yves Ouellette, Mayo Clinic, United StatesReviewed by:
A. M. Iqbal O'Meara, Virginia Commonwealth University, United StatesCopyright © 2022 Kortz, Nielsen, Mediratta, Reeves, O'Brien, Lee, Attebery, Bhutta, Biewen, Coronado Munoz, deAlmeida, Fonseca, Hooli, Johnson, Kissoon, Leimanis-Laurens, McCarthy, Pineda, Remy, Sanders, Takwoingi, Wiens, Bhutta and The Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network on Behalf of the PALISI Global Health Subgroup. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teresa B. Kortz, dGVyZXNhLmtvcnR6QHVjc2YuZWR1
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.