
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr., 09 January 2023
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1093371
This article is part of the Research TopicSARS-CoV-2: Implications for Maternal-Fetal-Infant and Perinatal Mortality, Morbidity, Pregnancy Outcomes and Well-BeingView all 15 articles
 Kayla Rodriguez1,2*
Kayla Rodriguez1,2* Matthew J. Nudelman1,3
Matthew J. Nudelman1,3 Priya Jegatheesan3
Priya Jegatheesan3 Angela Huang3
Angela Huang3 Kamakshi Devarajan4,5
Kamakshi Devarajan4,5 Jessica E. Haas1
Jessica E. Haas1 Rosemarie Cervantes4
Rosemarie Cervantes4 Kelle Falbo4
Kelle Falbo4 Sudha Rani Narasimhan3
Sudha Rani Narasimhan3 Machelnil Cormier3
Machelnil Cormier3 Mary Beth Stewart1
Mary Beth Stewart1 Rupalee Patel3
Rupalee Patel3 Balaji Govindaswami1,3,6
Balaji Govindaswami1,3,6
Objective: We evaluated the prevalence of preterm birth (PTB) and very low birth weight (VLBW) during Jan-Dec 2,020 (early COVID era) at 5 hospitals (2 in West Virginia, 3 in California) compared to Jan 2017–Dec 2019 (pre-COVID) inclusive of 2 regional perinatal centers (1 in Huntington, WV and 1 in San Jose, CA) and 3 community hospitals (1 each in Cabell, Los Angeles and Santa Clara counties).
Design/methods: We examined PTB and VLBW rates of live births at 5 US hospitals from Jan 2017–Dec 2020. We compared PTB and VLBW rates in 2020 to 2017–2019 using Poisson regression and rate ratio with a 95% confidence interval. We stratified live births by gestational age (GA) (<37, 33–36, and <33 weeks) and birth weight (≤1,500 g, >1,001 g to ≤1,500 g, ≤1,000 g). We examined PTB rates at 4 of the hospitals during Jan-Dec 2020 and compared them to the prior period of Jan 2017–Dec 2019 using Statistical Process Control (SPC) for quarterly data.
Results: We examined PTB and VLBW rates in 34,599 consecutive live births born Jan 2017–Dec 2019 to rates of 9,691 consecutive live births in 2020. There was no significant change in PTB (<37 weeks GA) rate, 10.6% in 2017–2019 vs. 11.0% in 2020 (p = 0.222). Additionally, there was no significant change when comparing VLBW rates in 2017–2019 to 2020, 1.4% in 2017–2019 vs. 1.5% in 2020 (p = 0.832).
Conclusion: We found no significant change in the rates of PTB or VLBW when combining the live birth data of 5 US hospitals in 3 different counties.
Early in the SARS-CoV-2 pandemic, regional preterm birth (PTB) prevalence of very low birth weight (VLBW) infants in a designated Irish region reported temporal reduction from ∼8 to ∼2/1,000 live births (1). A similar reduction in extremely PTB from 2.19/1,000 to 0.19/1,000 during the nationwide lockdown was shown in a Danish study of live born singletons (2). Additionally, preterm birth rates of liveborn singletons born at 32–36 week GA were notably decreased following the lockdown implementation in China during Feb-May 2020 (3). In contrast, a pregnancy cohort of all births in two hospitals in Philadelphia did not show significant changes in singleton preterm or stillbirth rates (4). Stillbirths were no different in England either (5). Ten of the first twenty SARS-CoV-2 U.S. cases were reported in California (6) while West Virginia was the last US state to report the virus, several weeks after the first report on the West Coast. Our aim was to assess the PTB and VLBW rates of all live births at five US hospitals during the COVID-19 pandemic, including centers in California and West Virginia, to be representative of temporal variation in spread of the virus and all major racial, ethnic, and sociodemographic groups. Of note, all 3 US regions had undergone hospital bankruptcies, mergers and acquisitions during our study period.
We examined PTB and VLBW rates of live births at five US hospitals, two in West Virginia and three in California, from Jan 2017 to Dec 2020. We compared PTB and VLBW rates in 2020 to 2017–2019 using Poisson regression and rate ratio with a 95% confidence interval. We stratified live births by gestational age (GA) (<37, 33–36, and <33 weeks) and birth weight (≤1,500 g, >1,001g to ≤1,500 g, ≤1,000 g). We examined PTB rates at four of the hospitals during Jan-Dec 2020 and compared them to prior period of Jan 2017–Dec 2019 using Statistical Process Control (SPC) for quarterly data.
We examined PTB and VLBW rates in 34,599 consecutive live births born Jan 2017–Dec 2019 to the rates of 9,691 consecutive live births in 2020. Table 1 compares PTB rates of 34,599 consecutive live births in 2017–2019 to 9,691 live births in 2020. There was no significant change in PTB (<37 weeks GA) rate, 10.6% in 2017–2019 vs. 11.0% in 2020 (p = 0.222). There was no difference in the subcategories of gestational age <33 or 33–36 weeks in any of the centers. Table 2 compares VLBW rates in 2017–2019 to 2020 and shows no significant change, 1.4% in 2017–2019 vs. 1.5% in 2020 (p = 0.832). There was no difference in the subcategories of birth weight 1,000–1,500 or ≤1,000 g in any of the centers except the regional NICU in San Jose, California showed an increase in ELBW (≤1,000 g) from 0.4% in 2017–2019 to 0.8% in 2020 (p = 0.013). Figures 1, 2 illustrate the quarterly birth rates in the birth weight and gestational age categories. The preterm birth rate in 2020 first quarter was an outlier due to an increase in the 33–36 weeks gestational age subcategory, although the subsequent rates are stable within the control limits. There are no outliers or significant shift in the overall birth rates in the gestational age or birth weight categories. The racial and ethnic distributions of all live births examined are as follows: 53% Hispanic, 30% Caucasian, 8% African American, 8% Asian, and 1% Other (data not presented).
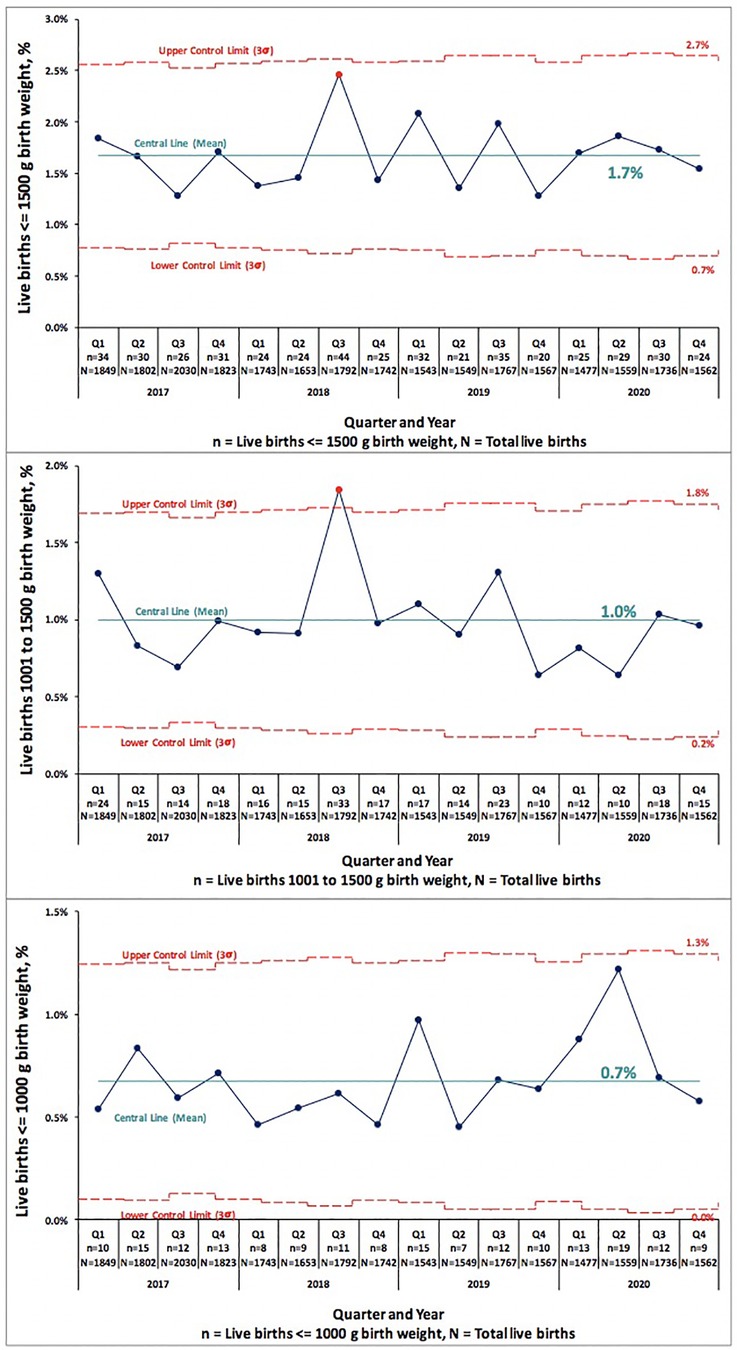
Figure 1. Pre-COVID-19 era (2017–2019) and COVID-19 era (2020) birth rates across five medical centers stratified by gestational age shown as statistical process control “p” chart. The central line represents mean and the upper and lower control limit lines are three standard deviations above and below mean.
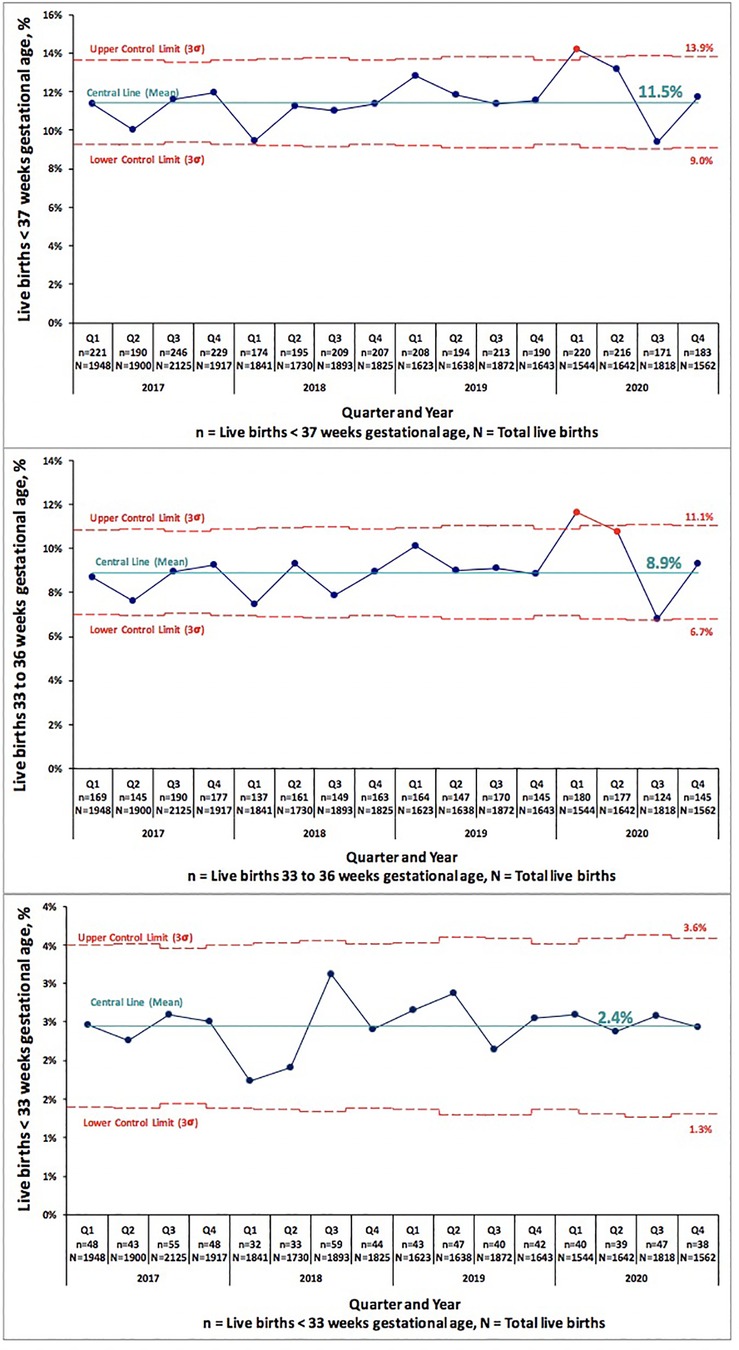
Figure 2. Pre-COVID-19 era (2017–2019) and COVID-19 era (2020) birth rates across five medical centers stratified by birth weight shown as statistical process control “p” chart. The central line represents mean and the upper and lower control limit lines are three standard deviations above and below mean.
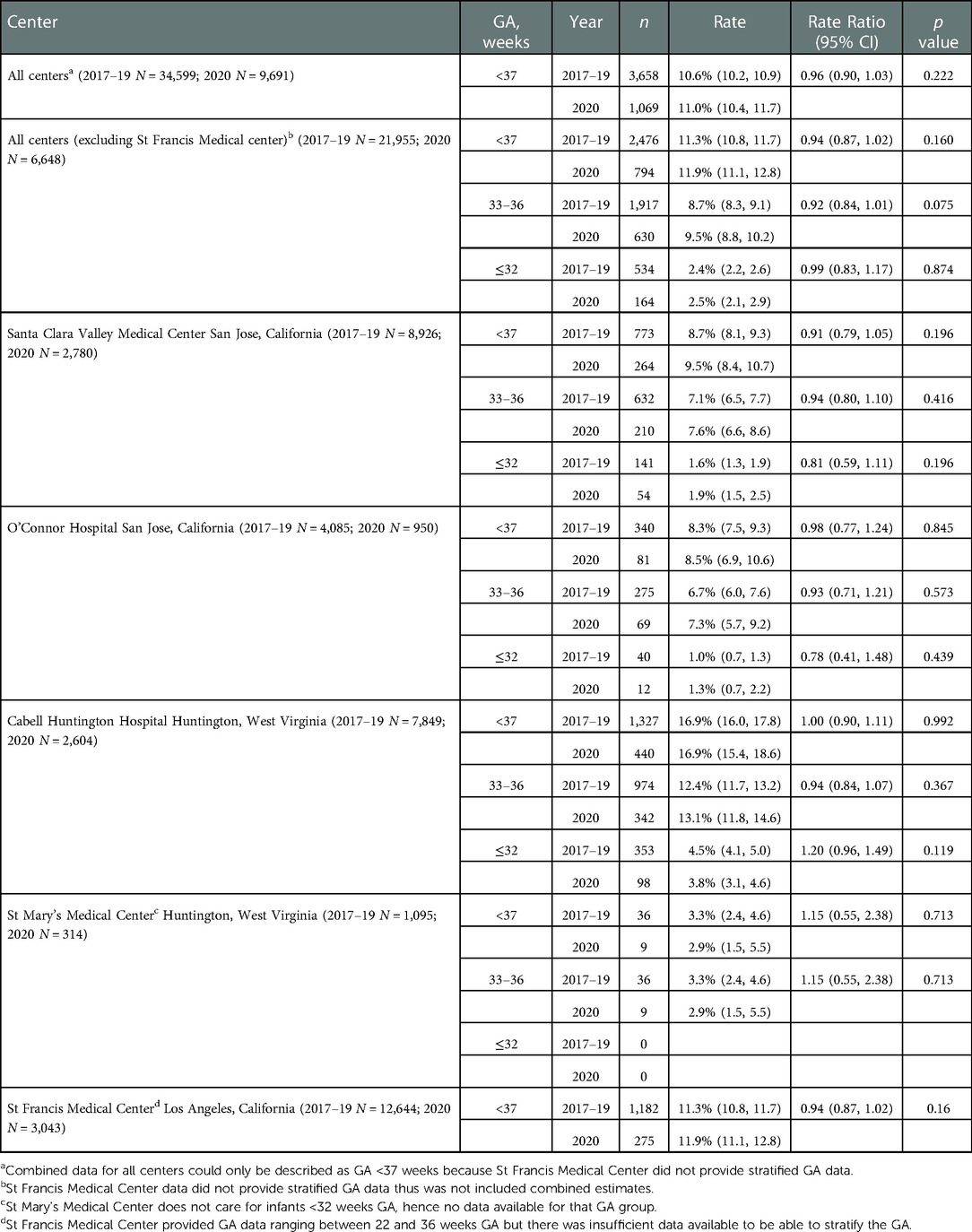
Table 1. Comparison of pre-COVID era (2017–2019) vs. COVID era (2020) preterm birth rates across five medical centers stratified by gestational age (GA).
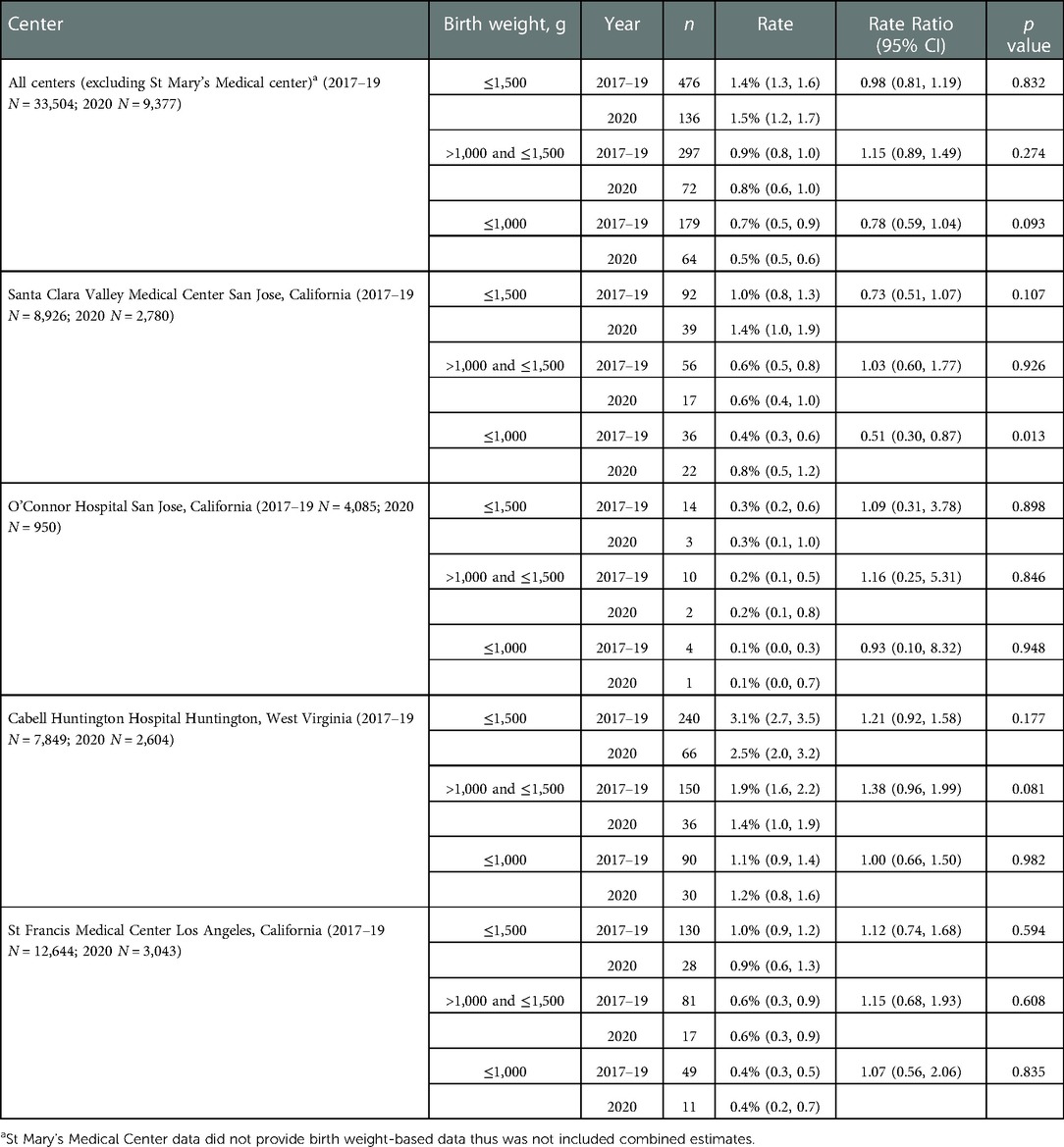
Table 2. Comparison of pre-COVID era (2017–2019) vs. COVID era (2020) low birth weight rates across five medical centers stratified by birth weight.
In the first year of the pandemic, 2020, we did not find a significant change in the rates of PTB or VLBW when combining the live birth data of five US hospitals in two different states and three different regions. No decrease in PTB or VLBW rates were noted at any of the five hospitals examined.
Similar results are noted in a study from France seeking to examine effect of lockdowns in perinatal outcomes in 2020, showing neither differences in preterm, nor stillbirth rates, nor LBW and adjusted VLBW rates in their cohort (7). In addition, preterm birth rates were reported unchanged in a large hospital system in Boston, MA in the USA during the first peak of the pandemic era (April-July 2020), secondary findings included no difference in spontaneous verse iatrogenic preterm birth rates (8). A more recent study from Australia showed decline in births <34 weeks GA during 2020–21 lockdowns but without significant change for births <28 weeks GA (9). A consortium of European nations, report decline in live birth rates, some with a subsequent rebound in early 2021 and excess COVID mortality as a putative reason for declining live births (10, 11). Recent (2020–21) Canadian surveillance data suggest increased preterm birth risk among 6,012 SARS-CoV-2–affected pregnancies (11.05% vs. 6.76%; relative risk, 1.63 [95% CI, 1.52–1.76]), inclusive of milder disease not requiring hospitalization, compared to unaffected contemporaneous pregnancies (12). A study from Bronx, New York, found preterm birth rates to be altered by the SARS-CoV-2 variant in women testing positive for SARS-CoV-2 during pregnancy (13). The rates of PTB were found to be lower during the Omicron variant surge when compared to the PTB rates during the original strain surge (13). These findings were attributed to differing variant virulence, increased vaccination availability, and improved SARS-CoV-2 management guidelines amongst other factors (13).
There was a significant increase in ELBWs in the San Jose regional hospital which was likely related to the change in referral patterns of high risk deliveries across the local county rather than the pandemic. The county acquired two community hospitals in early 2019 and the referral pattern for high-risk mothers were streamlined to deliver at the regional center. In addition, there was a local closure of the Labor and Delivery service at another community center that also redirected high risk mothers to the regional center.
As detailed above, multiple studies have been performed to analyze the effects of the COVID19 pandemic on perinatal outcomes and have shown conflicting results. A large rapid review and meta-analysis by Vaccaro et. al., was performed to examine the impact of the COVID-19 lockdown on the incidence of preterm birth, low birth weight, and stillbirth during the lockdown measures (14). When combining the data of 14 previous studies, the meta-analysis showed a significant risk of stillbirth during the COVID-19 lockdown when compared to the prepandemic period (14). However, PTB, LBW, and VLBW were not associated with a significant risk during the lockdown period (14).
Strengths of our study include a longer period of observation of all pregnancies into the early COVID era, a robust sample size with geographic, racial and ethnic heterogeneity. Limitations of our observations include lack of stillbirth data and inability to distinguish spontaneous vs. medically indicated preterm birth. Further limitations of our study include completing our study in 2020 thus lacking accurate SARS-CoV-2 infection rates and possibly largely excluding impact of vaccines and differing SARS-CoV-2 variants in pregnant women. In addition, local factors such as changes in referral patterns related to bankruptcies, mergers and acquisitions cannot be excluded.
We conclude that in the first COVID-era year studied, 2020, SARS-CoV-2 did not reduce preterm or low birth weight rates in three different regions in the United States when combining the birth data of five US hospitals, compared to live births 2017–2019 at these same institutions. Local, regional and population-wide studies show variation in impact in SARS-CoV-2 on preterm birth rates, due to multitude of dynamic factors. These will have implications for planning and delivery of regionalized perinatal health care systems.
The original contributions presented in the study are included in the article/further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements.
KR, MN, PJ, KD, SN, RP and BG conceptualized and designed the study. Data collection by AH, JH, RC, KF, MC, MS. MN, AH and JH responsible for SPC and data analysis. All authors contributed to data interpretation and manuscript writing and approval. All authors contributed to the article and approved the submitted version.
We thank First Five of Santa Clara Valley and VMC Foundation for funding support in the publication of this article. The content of this manuscript has been presented by Kayla Rodriguez, in part at the 2021 Pediatric Academic Societies meeting as a Highlighted e-poster within the Global Neonatal & Children's health section. We thank the Obstetric, Family Medicine, Labor and delivery and Pediatric medical and house staff, nursing, patients and families, for their selfless dedication and contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Philip RK, Purtill H, Reidy E, Daly M, Imcha M, McGrath D, et al. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: a ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob Health. (2020) 5(9). doi: 10.1136/bmjgh-2020-003075
2. Hedermann G, Hedley PL, Bækvad-Hansen M, Hjalgrim H, Rostgaard K, Poorisrisak P, et al. Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. (2021) 106:93–5. doi: 10.1136/archdischild-2020-319990
3. Bian Z, Qu X, Ying H, Liu X. Are COVID-19 mitigation measures reducing preterm birth rate in China? BMJ Glob Health. (2021) 6(8):e006359. doi: 10.1136/bmjgh-2021-006359
4. Handley SC, Mullin AM, Elovitz MA, Gerson KD, Montoya-Williams D, Lorch SA, et al. Changes in preterm birth phenotypes and stillbirth at 2 Philadelphia hospitals during the SARS-CoV-2 pandemic, march-June 2020. JAMA. (2021) 325(1):87–9. doi: 10.1001/jama.2020.20991
5. Stowe J, Smith H, Thurland K, Ramsay ME, Andrews N, Ladhani SN. Stillbirths during the COVID-19 pandemic in England, April-June 2020. JAMA. (2021) 325(1):86–7. doi: 10.1001/jama.2020.21369
6. Worldometer: United States. Available at: https://www.worldometers.info/coronavirus/country/us/#ref-17 (Accessed March 11, 2021).
7. Garabedian C, Dupuis N, Vayssière C, Bussières L, Ville Y, Renaudin B, et al. Impact of COVID-19 lockdown on preterm births, low birthweights and stillbirths: a retrospective cohort study. J Clin Med. (2021) 10(23):5649. doi: 10.3390/jcm10235649
8. Wood R, Sinnott C, Goldfarb I, Clapp M, McElrath T, Little S. Preterm birth during the coronavirus disease 2019 (COVID-19) pandemic in a large hospital system in the United States. Obstet Gynecol. (2021) 137(3):403–4. doi: 10.1097/AOG.0000000000004237
9. Stansfield S, Rattan A, Mol BW, Rolnik DL, Malhotra A. Impact of the COVID-19 pandemic and multiple community lockdowns on total live birth rates and preterm births in Melbourne, Australia. Aust N Z J Obstet Gynaecol. (2022) 62:786–9. doi: 10.1111/ajo.13527
10. Pomar L, Favre G, de Labrusse C, Contier A, Boulvain M, Baud D. Impact of the first wave of the COVID-19 pandemic on birth rates in Europe: a time series analysis in 24 countries. Hum Reprod. (2022) 37(12):deac215. doi: 10.1093/humrep/deac215
11. De Geyter C, Masciocchi M, Gobrecht-Keller U. Excess mortality caused by the COVID-19 pandemic negatively impacts birth numbers in European countries. Hum Reprod. (2022) 37(4):822–7. doi: 10.1093/humrep/deac031
12. McClymont E, Albert AY, Alton GD, Boucoiran I, Castillo E, Fell DB, et al. Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. JAMA. (2022) 327(20):1983–91. doi: 10.1001/jama.2022.5906
13. Seaton CL, Cohen A, Henninger EM, Gendlina I, Hou W, Bernstein PS, et al. Coronavirus disease 2019 (COVID-19) perinatal outcomes across the pandemic at an academic medical center in New York city. Obstet Gynecol. (2022) 141(1):144–151. doi: 10.1097/AOG.0000000000004978
Keywords: SARS – CoV – 2, preterm, birth, very low birth weight, extremely low birth weight
Citation: Rodriguez K, Nudelman MJ, Jegatheesan P, Huang A, Devarajan K, Haas JE, Cervantes R, Falbo K, Narasimhan SR, Cormier M, Stewart MB, Patel R and Govindaswami B (2023) Are preterm birth and very low birth weight rates altered in the early COVID (2020) SARS-CoV-2 era?. Front. Pediatr. 10:1093371. doi: 10.3389/fped.2022.1093371
Received: 8 November 2022; Accepted: 5 December 2022;
Published: 9 January 2023.
Edited by:
Suksham Jain, Government Medical College and Hospital, IndiaReviewed by:
Lizelle Van Wyk, Tygerberg Hospital, South Africa© 2023 Rodriguez, Nudelman, Jegatheesan, Huang, Devarajan, Haas, Cervantes, Falbo, Narasimhan, Cormier, Stewart, Patel and Govindaswami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kayla Rodriguez a2F5cm9kcmlndUBtb250ZWZpb3JlLm9yZw==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.