
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 22 December 2022
Sec. Pediatric Hematology and Hematological Malignancies
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1073624
This article is part of the Research TopicCase Reports in Pediatric Hematology and Hematological Malignancies 2022View all 14 articles
 Mariam Thalji1,†
Mariam Thalji1,† Asil Yagmour1*†
Asil Yagmour1*† Dania Alameh1
Dania Alameh1 Hanin Shatrit1
Hanin Shatrit1 Mais Inerat2
Mais Inerat2 Sami Issa Bannoura3
Sami Issa Bannoura3 Amir Atawneh2
Amir Atawneh2 Motee Abuawaad1,2
Motee Abuawaad1,2
Congenital cutaneous Langerhans cell histiocytosis-(LCH), named Hashimoto Pritzker disease, is a rare subtype among the clinical spectrum of LCH that often presents at birth or through the neonatal term and spontaneously resolve within a few months. In rare instances, infants with congenital cutaneous LCH may present with a blueberry-muffin rash. We reported a case of a male newborn who presented with blueberry muffin rash and was diagnosed with congenital cutaneous LCH later on. The diagnosis was confirmed by excluding other possible systemic causes of blueberry muffin rash, followed by a skin biopsy. Skin biopsy showed reticular dermis-hypodermis infiltration by medium-sized cells which had a pale eosinophilic cytoplasm and irregular nuclei. The lesional cells were positive for Langerin, CD1a, S100, and CD68 immunostains, consistent with congenital cutaneous LCH. Investigations were performed and revealed no systematic disease involvement. After a discussion with the pediatric Hemato-Oncologist, the decision was to keep track of a “wait-and-see” approach. Long-term follow-up revealed no recurrence of the cutaneous lesions or any systemic involvement, which further leads to congenital cutaneous LCH diagnosis. Even though it is very rare, blueberry muffin rash differential diagnosis should include congenital cutaneous LCH. Early recognition of this condition protects patients from unnecessary and possibly unsafe systemic treatment.
Langerhans cell Histiocytosis (LCH) is a rare condition described initially by Lichtenstein in 1953. It is defined by clonal proliferation and accretion of Langerhans cells in different tissues resulting in organ damage or malignancy formation. Although the mechanism behind this accumulation remains uncertain, LCH is supposed to have a neoplastic or inflammatory process (1–4). LCH mainly affects the pediatric population, with wide heterogeneity in clinical presentations and consequences. The incidence of LCH is about 5 per million children and cutaneous involvement presents in 40% of these cases (5).
Previously, LCH was classified into four subcategories: Letterer-Siwe disease, eosinophilic granuloma, Hand-Schüller-Christian disease, and Hashimoto-Pritzker disease. However, recently it has been specified as a clinical spectrum with a new classification based on involving one or more organ systems, one or more locations within a single organ, and involvement of high-risk organs, particularly the liver, spleen, and bone marrow (4–9). Congenital cutaneous LCH is generally a benign condition involving one-system, distinguished by generalized red-brown macules, papules, and nodules. The disease rarely involves the skeleton, lungs, eyes, and abdomen (10, 11).
The term “Blueberry muffin baby” (BMB) was initially used for the description of congenital rubella cutaneous manifestations in the 1960s (12). They are characterized by multiple, non-blanching, purple to dark blue macules, papules, or nodules. Rather than rubella, various diseases have been related to BMB, including congenital infections (e.g., Toxoplasmosis, Rubella, Cytomegalovirus, Herpes and other agents (TORCH), Parvovirus), hematologic disorders, metabolic disorders, neoplastic and other diseases (i.e., Langerhans cell histiocytosis, neonatal lupus). In rare cases, Congenital cutaneous LCH manifests clinically as BMB (13, 14). Herein, we describe an unusual Congenital cutaneous LCH case, who presented with blueberry muffin rash and had an excellent clinical outcome.
Our patient is a male newborn who was referred to our center at the age of 4 days with a blueberry muffin rash. He was born via normal vaginal delivery after an uneventful full-term pregnancy for a healthy 21-year-old primigravida woman. Physical examination revealed several scattered dark purple papules-like spots over the face, neck, and limbs of variable sizes (2–4 mm) (Figure 1). The rash was palpable with a smooth surface. There was no hepatosplenomegaly, jaundice, or ecchymosis. He had normal physical neuroexam. Transfontanelle ultrasound for brain was done and normal. Otherwise, the baby was active and healthy. No history of bruises, bleeding, fever, or abnormality in movement. His growth parameters were between the mean and −1 Standard Deviation (SD).
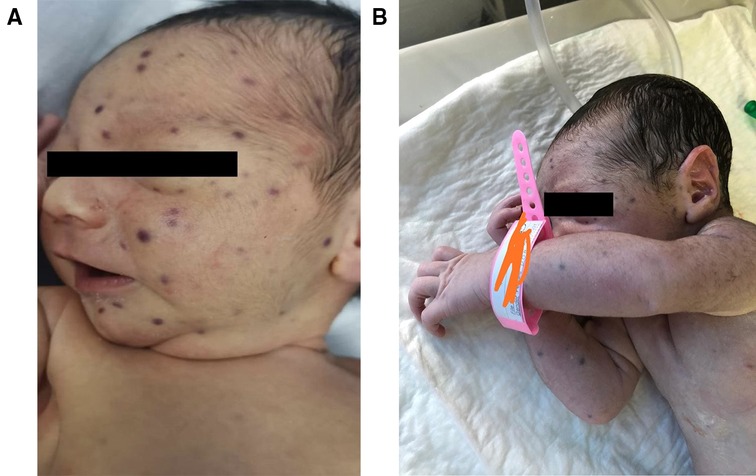
Figure 1. Blueberry muffin baby. (A) & (B) Disseminated dark red to purplish macules and papules on the face, neck, and upper limbs.
Multiple Investigations were performed, including complete blood count (CBC), blood serum chemistry, coagulation profile, liver function tests, and blood film, all of which were normal. Serology for congenital infections showed the following results; VDRL: non-reactive, TORCH: negative except for high cytomegalovirus (CMV) IgG antibody, and Qualitative PCR testing for urine and blood were negative. A urine sample analysis to rule out neuroblastoma did not show homovanillic acid (HVA) or Vanillylmandelic acid (VMA). Skin biopsy revealed a reticular dermis-hypodermis infiltration by medium-sized cells, which had a pale eosinophilic cytoplasm and irregular nuclei. The cells were positive for Langerin, CD1a, S100, and CD68 immunostains, consistent with cutaneous Langerhans cell histiocytosis (Figures 2,3). Further investigations were performed to exclude systemic involvement, including a skeletal survey with a skull view, and abdominal ultrasound, all of which were normal, and supported the diagnosis of congenital cutaneous LCH.
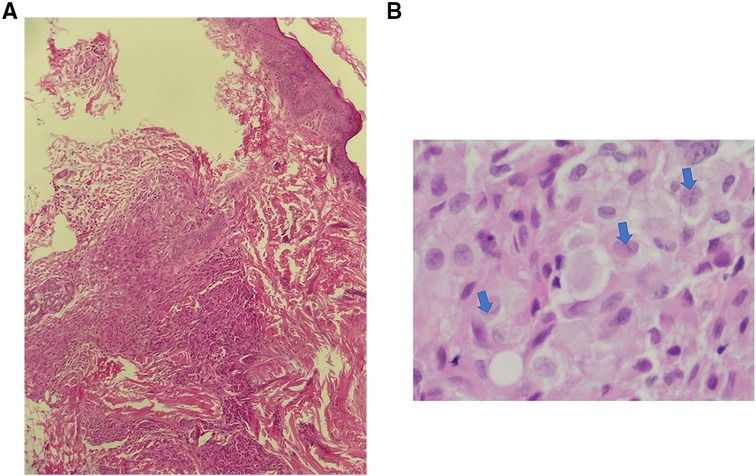
Figure 2. Congenital cutaneous langerhans cell histiocytosis. (A). Dermal- hypodermal infiltration by histocytes (H & E. 20X), (B). The lesional cells have abundant, light eosinophilic cytoplasm and irregular nuclei with frequent nuclear folds and grooves (arrows), obscure nucleoli and fine chromatin.

Figure 3. Immunohistochemistry. The lesional histocytes are diffusely positive for CD1a (A), CD68 (B), and S100 (C).
After a discussion with the pediatric Hemato-Oncologist, a “wait-and-see” approach was preferred. The infant remained stable and active during his stay with good general condition. Labs were followed up and showed good results; the rash faded with time. He was discharged home with recommendations to follow up with a pediatric hemato-oncologist. At the age of two months, almost the whole lesions on his skin have resolved. His last follow up was at the age of two years, there was no evidence of recurrence or any extracutaneous manifestations. No continuity of follow up beyond this age.
Blueberry muffin baby (BMB) is a clinical manifestation caused by extramedullary hematopoiesis within the dermis. Clinically, it is characterized by diffuse dark bluish to purple papules or nodules. BMB appearance had a broad spectrum of differential diagnoses, including congenital infections (TORCH and Epstein Barr virus), malignancies/proliferative conditions (neuroblastoma, congenital leukemia, congenital rhabdomyosarcoma, LCH), hematologic disorders (rhesus hemolytic anemia, hereditary spherocytosis, ABO blood groups incompatibility, and twin-twin transfusion syndrome) (14–16). Congenital Cutaneous LCH is an infrequent presentation among the BMB differentials (17).
Congenital cutaneous LCH is considered a rare variant of single-site LCH, first reported by Hashimoto and Pritzker in 1973 (10, 11). Most cases are present at birth or later in the neonatal term with cutaneous manifestations represented by reddish-brown or violaceous papules or nodular lesions. The disease clinical course is often benign with a spontaneous –resolution tendency. However, in a few patients, the disease may progress to multi-systemic LCH affecting other organs, especially the spleen, liver, bone, and lymph node (11, 15, 16, 18).
Besides the disease's clinical course, skin biopsy demonstrates histologic and immunohistochemical findings are crucial in the diagnosis. The typical histopathological appearance of cutaneous LCH shows infiltration of the dermis by cells that have abundant, light eosinophilic cytoplasm and irregular nuclei with frequent nuclear folds and grooves, obscure nucleoli, and fine chromatin. Some inflammatory cells, such as lymphocytes, eosinophils, neutrophils, and mast cells, may coexist. Immunohistochemical stains, including S100, CD207 (langerin), and CD1a, are essential to confirm the diagnosis. The gold standard is evidence of Birbeck granules on electron microscopy (15, 16, 18).
Regarding our case, a step-wise plan was followed to investigate the cause of the patient's BMB presentation. Congenital infection was our first concern; therefore, extensive infectious workup was done, which revealed negative results except for high levels of CMV IgG anti-body. Hematological malignancies were unlikely, with a normal CBC and blood film. Also, neuroblastoma was excluded by a normal urine level of VMA and HMA. A skin biopsy confirmed the diagnosis of LCH. To look for other body systems involvement, a skeletal survey was done, including skull views as it is the most common site for LCH and it was free of any lesions. Additionally, the abdominal ultrasound was normal. The rash gradually faded and it disappeared entirely by the age of two months. His last follow-up at the age of two years has revealed no evidence of recurrence or any extracutaneous manifestations.
A comprehensive literature review on PubMed, Google Scholar, and Embase revealed 11 cases of congenital cutaneous LCH present with BMB, including the current case. All cases had spontaneous resolution of the disease during the first few weeks of life, ranging from 15 days up to 18 months without requiring any treatment. In our case, the rash disappeared at the age of two months. Follow up period in the reported cases ranging from 11 months till 2 years and none of those cases showed evidence of recurrence (2, 17, 19–22).
Since most cases of isolated cutaneous LCH has a self-limited course within a few months, the treatment is usually conservative. A careful watchful waiting approach is approved in many cases which is comparable to our case. Topical steroids may also be used in non-resolving cases. Furthermore, severe symptomatic cutaneous lesions necessitate the use of other treatment options such as topical nitrogen mustard or psoralen with ultraviolet light therapy (17, 18). Close monitoring for any signs of recurrence or progression to multisystem disease is crucial. Following the early thorough workup, all newborns should be monitored by a careful clinical examination, CBC, and possibly ultrasounds. A potentially serious multisystem disease progression might happen within several weeks to months, for which systemic therapy is indicated (16).
Our case has some limitations, lack of follow up information at time of writing the report, and parents didn't adhere to follow up in our hospital.
To summarize, our case highlights the importance of raising the index of suspicion toward considering congenital cutaneous LCH as a cause of BMB. A comprehensive diagnostic approach should be implemented to exclude infectious and systemic causes as well as skin biopsy findings, which are critical for the diagnosis and thus allow the wait-and-observe approach. Regular follow-up is mandatory in these patients for the detection of any possible relapses or disease progression.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
AY and MT contributed equally in writing the bulk of the article and made a significant contribution to the work. DA and HS assist in writing and communication with the patient's parents. SB and AA participated in the coordination and acquisition of data, MA and MI conceived the study. All authors contributed to manuscript revisions, and all authors approved the final version of the manuscript and agreed to be held accountable for its content. All authors contributed to the article and approved the submitted version.
The completion of this case could not have been possible without the participation and assistance of many people whose names may not be enumerated like our patient's parents, their contribution are sincerely appreciated and gratefully acknowledged.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in langerhans cell histiocytosis. Blood. (2010) 116(11):1919–23. doi: 10.1182/blood-2010-04-279083
2. Cyr J, Langley A, Demellawy DE, Ramien M. A neonate with langerhans cell histiocytosis presenting as blueberry muffin rash: case report and review of the literature. SAGE Open Med Case Reports. (2020) 8:2050313X20919616. doi: 10.1177/2050313X20919616
3. Singh A, Mandal A, Singh L, Mishra S, Patel A. Delayed treatment response in a neonate with multisystem langerhans cell histiocytosis -case report and review of literature. Sultan Qaboos Univ Med J. (2017) 17:e225–28. doi: 10.18295/squmj.2016.17.02.016
4. Singh A. Writing group of the histiocyte society.histiocytosis syndromes in children. Lancet. (1987) 1(8526):208–9. doi: 10.1016/S0140-6736(87)90016-X
5. Fronek LF, Grubbs H, Dorton DW, Miller R. Isolated cutaneous langerhans cell histiocytosis presenting in an adult Male. Cureus. (2020) 12(8):e9861. doi: 10.7759/cureus.9861
6. El Demellawy D, Young JL, de Nanassy J, Chernetsova E, Nasr A. Langerhans cell histiocytosis: a comprehensive review. Pathol. (2015) 47(4):294–301. doi: 10.1097/PAT.0000000000000256
7. Grois N, Pötschger U, Prosch H, Minkov M, Arico M, Braier J, et al. Risk factors for diabetes insipidus in langerhans cell histiocytosis. Pediatr Blood Cancer. (2006) 46(2):228–33. doi: 10.1002/pbc.20425
8. Querings K, Starz H, Balda BR. Clinical spectrum of cutaneous Langerhans’ cell histiocytosis mimicking various diseases. Acta Derm Venereol. (2006) 86(1):39–43. doi: 10.2340/00015555-0003
9. Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: history, classification, pathobiology, clinical manifestations, and prognosis. J Am Acad Dermatol. (2018) 78(6):1035–44. doi: 10.1016/j.jaad.2017.05.059
10. Allen CE, Merad M, McClain KL. Langerhans-Cell histiocytosis. N Engl J Med. (2018) 379(9):856–68. doi: 10.1056/NEJMra1607548
11. Hashimoto K, Bale GF, Hawkins HK, Langston C, Pritzker MS. Congenital self-healing reticulohistiocytosis (hashimoto-pritzker type). Int J Dermatol. (1986) 25(8):516–23. doi: 10.1111/j.1365-4362.1986.tb00867.x
12. Hendricks WM, Hu CH. Blueberry muffin syndrome: cutaneous erythropoiesis and possible intrauterine viral infection. Cutis. (1984) 34(6):549–51. Corpus ID: 19268962
13. Gottesfeld E, Silverman RA, Coccia PF, Jacobs G, Zaim MT. Transient blueberry muffin appearance of a newborn with congenital monoblastic leukemia. J Am Acad Dermatol. (1989) 21(2 Pt 2):347–51. doi: 10.1016/S0190-9622(89)80032-5
14. Mehta V, Balachandran C, Lonikar V. Blueberry muffin baby: a pictoral differential diagnosis. Dermatol Online J. (2008) 14(2):8. doi: 10.5070/D353q852nc
15. Chu AC. Histiocytoses. In: Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Rook's textbook of dermatology. London: Blackwell Science (1998). p. 2311–36.
16. Sankilampi U, Huikko-Tarvainen S, Kärjä V, Pirinen E, Naukkarinen A, Hollmén A. Congenital langerhans cell histiocytosis mimicking a “blueberry muffin baby”. J Pediatr Hematol Oncol. (2008) 30(3):245–8. doi: 10.1097/MPH.0b013e318161a9cb
17. Popadic S, Brasanac D, Arsov B, Nikolic M. Congenital self-healing histiocytosis presenting as blueberry muffin baby: a case report and literature review. Indian J Dermatol Venereol Leprol. (2012) 78:407. doi: 10.4103/0378-6323.98100
18. Ameer A, Salman A, Marzoq Y, Hajri A, Omran I, Dossari S, et al. Congenital self-healing reticulohistiocytosis (hashimoto–pritzker syndrome). J Dermatol & Dermatol Surg. (2015) 19:55–7. doi: 10.1016/j.jdds.2014.03.002
19. Lasek-Duriez A, Charkaluk ML, Gosset P, Modiano P. [Blueberry muffin baby and Langerhans’ congenital cell histiocytosis]. Ann Dermatol Venereol. (2014) 141(2):130–3. doi: 10.1016/j.annder.2013.10.057
20. Schmitt AR, Wetter DA, Camilleri MJ, Khan SP, Tollefson MM. Langerhans cell histiocytosis presenting as a blueberry muffin rash. Lancet. (2017) 390(10090):155. doi: 10.1016/S0140-6736(17)30564-0
21. Kallesh A, Kumar VS. Congenital langerhans-cell histiocytosis. N Engl J Med. (2021) 385(24):e86. doi: 10.1056/NEJMicm2112460
Keywords: langerhans cell histiocytosis (LCH), muffin rash, hashimoto pritzker disease, pediatrics, hematology, dermatology, pediatric hematological diseases
Citation: Thalji M, Yagmour A, Alameh D, Shatrit H, Inerat M, Bannoura SI, Atawneh A and Abuawaad M (2022) Case report: “Congenital cutaneous langerhans cell histiocytosis presenting with blueberry Muffin Rash”. Front. Pediatr. 10:1073624. doi: 10.3389/fped.2022.1073624
Received: 18 October 2022; Accepted: 23 November 2022;
Published: 22 December 2022.
Edited by:
Paulo Sérgio da Silva Santos, University of São Paulo, BrazilReviewed by:
Verônica Caroline Brito Reia, University of São Paulo, Brazil© 2022 Thalji, Yagmour, Alameh, Shatrit, Inerat, Bannoura, Atawneh and Abuawwad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asil Yagmour YXNpbC55YWdtb3VyQHN0dWRlbnRzLmFscXVkcy5lZHU=, eWFnaG1vdXJhc2VlbDI2QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Hematology and Hematological Malignancies, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.