- 1Surgical Oncology Unit, Department of Surgery, Bambino Gesù Children’s Hospital – IRCCS, Rome, Italy
- 2General Surgery Unit, Department of Surgery, Bambino Gesù Children’s Hospital – IRCCS, Rome, Italy
- 3Department of Pediatric Hematology/Oncology and Cell and Gene Therapy, Bambino Gesù Children’s Hospital – IRCCS, Rome, Italy
- 4Department of Imaging, Bambino Gesù Children’s Hospital – IRCCS, Rome, Italy
- 5Department of Pathology, Bambino Gesù Children’s Hospital – IRCCS, Rome, Italy
Burkitt's lymphoma (BL) is defined as a highly invasive B-cell lymphoma, usually characterized by an excellent prognosis, more than 90% of children and adolescents being cured with highly dose-intensive multiagent chemotherapy. Primary ovarian localization without involvement of other organs is a rare manifestation of BL, especially in pediatric population. Symptoms at diagnosis are similar to other ovarian lesions and differential diagnosis may be challenging for clinicians. A 12-year-old girl was referred to our institution for abdominal pain and palpable mass observed by the pediatrician. Diagnostic work-up demonstrated a large mass arising from the right ovary, causing compression on abdominal aorta, inferior vena cava, ureters and bowel, with a second smaller lesion on the left ovary. At surgery, a 15 cm-large, ruptured mass arising from the right ovary was found, associated with a second lesion originating from the left ovary (8 cm) and multiple nodules of the greater omentum. Right salpingo-oophorectomy was performed, incisional biopsies were taken from the left ovary and omental nodules and peritoneal fluid samples were collected for cytology. Pathology revealed a Burkitt lymphoma and the patient underwent chemotherapy according to AIEOP LNH-97 Protocol, group R3 with Rituximab. Preoperative diagnosis of primary ovarian lymphoma is extremely difficult. Surgical exploration is often necessary in patients presenting with acute abdominal or pelvic pain; when the suspicion of primary ovarian lymphoma arises intraoperatively, every effort should be made to minimize invasive procedure in order to enhance post-operative recovery.
Introduction
Ovarian neoplasms are rare in pediatric population, accounting for approximately 1% of all childhood malignancies; among these patients, more than half are affected by germ cell tumors, followed by epithelial neoplasms and sex cord stromal tumors (1, 2). Rarely, patients presenting with isolated ovarian masses are affected by primary ovarian lymphomas (3).
Clinical presentations include abdominal or pelvic pain, dysuria and evidence of a palpable mass and, considering the overlap among the possible histotypes (3–5), differential diagnosis may be challenging for clinicians.
In the present paper, the authors report a case of an adolescent affected by primary ovarian lymphoma and discuss the problems of differential diagnosis in girls affected by ovarian neoplasms.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements, as well as written informed consent from the patients or participants' legal Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements, as well as written informed consent from the patients or participants' legal guardian.
Case description
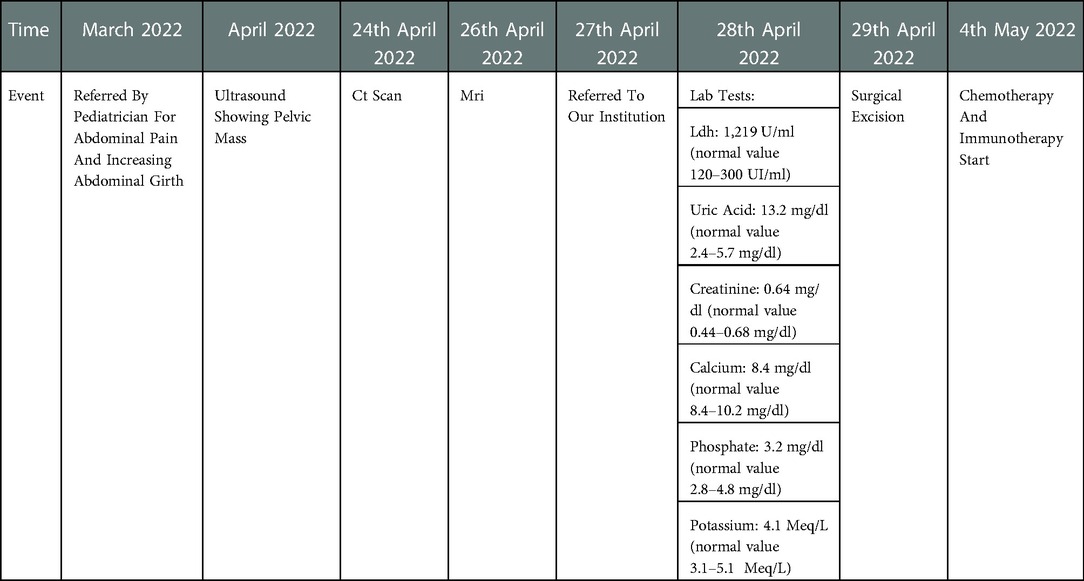
A 12-year-old girl was referred to our institution for abdominal pain and palpable mass observed by the pediatrician. After admission, a complete blood count (CBC) test was performed, showing normocytic anemia (Hb 10.3 g/dl, RBC 3.600.000/ul, mean globular volume 88.3 fl). High serum level of lactate dehydrogenase (1,219 U/ml; normal value 120–300 UI/ml) and uric acid (13.2 mg/dl; normal value 2.4–5.7 mg/dl) were reported, while the results of liver and kidney function tests were normal. Tumor markers such as Beta-human chorionic gonadotropin (β-HCG), Alfa-fetoprotein (α-FP), CA 19-9 and CEA were negative; only CA-125 was higher than the reference value (patient's value 314 UI/ml; normal value 0–35 UI/ml).
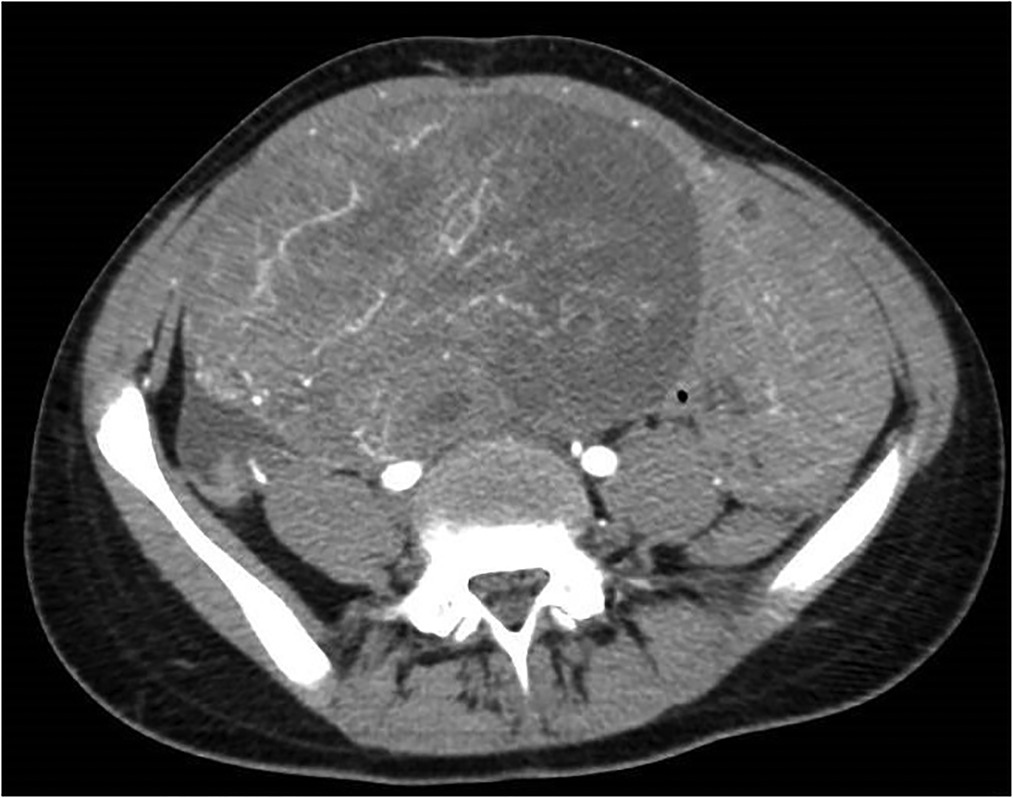
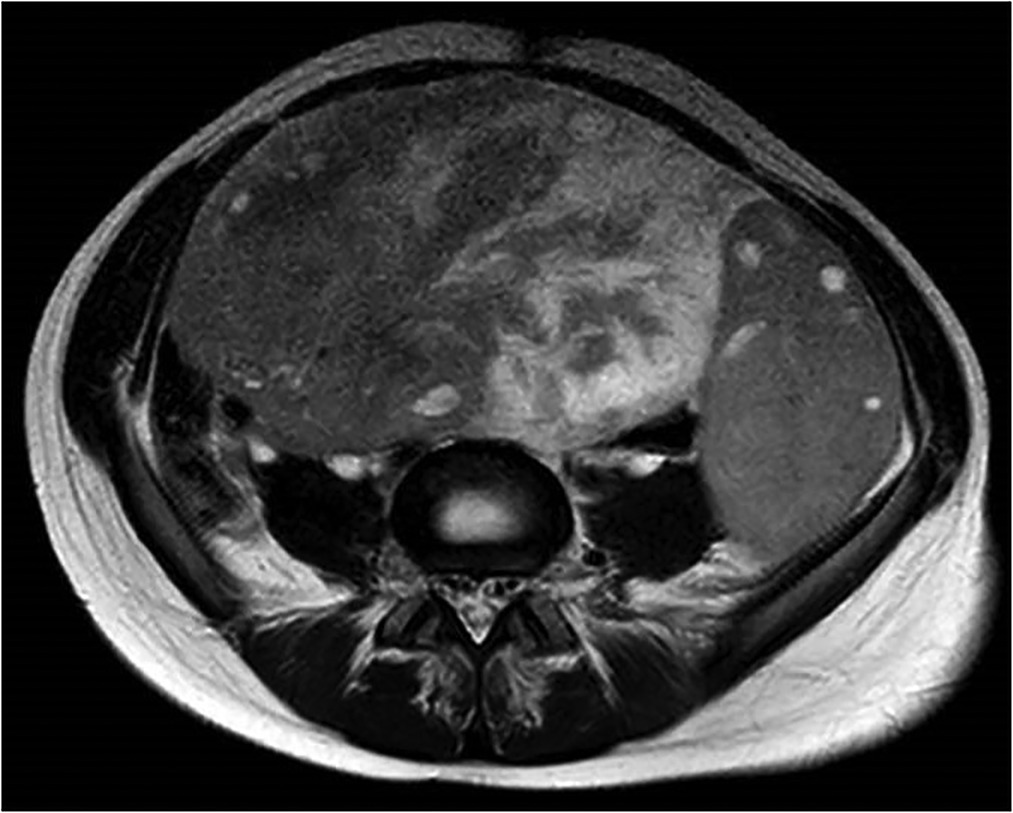
After initial ultrasound, the patient underwent Computed Tomography (CT) scan: the results showed a voluminous hypogenic mass (size 20 cm × 13 cm × 10 cm) arising from the right ovary, causing compression on the contiguous structures, especially abdominal aorta, inferior vena cava, ureters and bowel (Figure 1); another smaller mass (8 cm × 8 cm × 5 cm) was detected on the left ovary and abundant ascites was described. No metastasis or pathological lymph nodes were identified. Magnetic Resonance Imaging (MRI) was then performed, defining more specifically the characteristics of the neoplasm and confirming its origin from the right ovary (Figure 2).
The case was discussed in a multidisciplinary meeting with radiologists, oncologists and pathologists. Images were judged suggestive of primary right ovarian tumor with ischemic changes due to vascular compression in the contralateral ovary; surgical exploration was indicated in order to resect what was thought to be a right ovarian neoplasm and relieve compression on the left ovary.
Pfannenstiel laparotomy was performed; intraoperatively, a 15 cm-large, ruptured mass arising from the right ovary was found, associated with a second lesion originating from the left ovary (8 cm) and multiple nodules of the greater omentum. Right ovary was completely distorted by the mass and was judged non-salvageable; right salpingo-oophorectomy was then performed and incisional biopsies were taken from the left ovary and omental nodules and peritoneal fluid samples were collected for cytology. No surgical complications were reported.
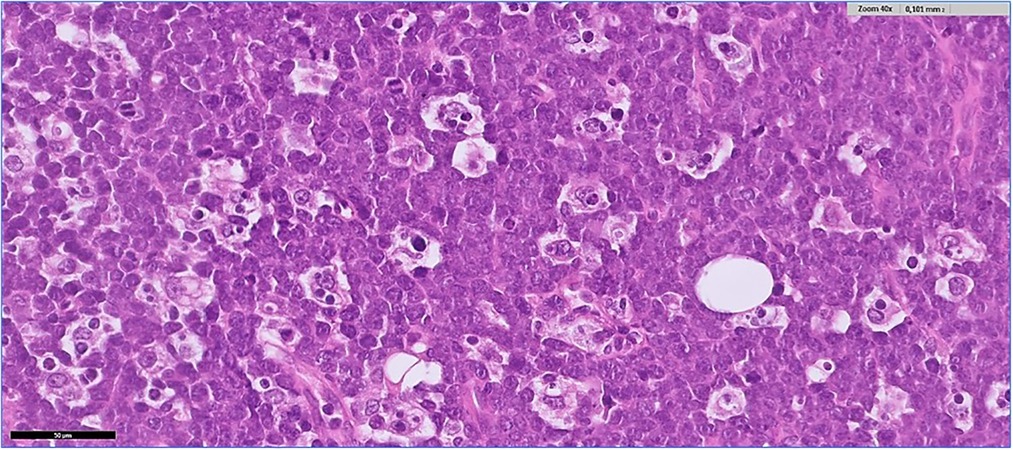
Histological examination revealed a diffuse proliferation of monomorphic, medium size lymphoid cells, with high nucleus/cytoplasmic ratio, finely dispersed chromatin and multiple small nucleoli. There were abundant mitoses and apoptosis and many macrophages containing tingible-bodies (“starry sky” pattern). There were also areas of necrosis (Figure 3). The same neoplastic infiltration was seen in all specimens, from both ovaries and from the omental nodules. Immunohistochemistry showed positivity of the neoplastic cells for B-cell markers (CD20, CD19, CD22, CD79a) and for germinal center B-cell markers (CD10, BCL6), and negativity for BCL2, TdT, CD138 and CD3. Proliferative index (Ki67) was positive in more than 95% of the neoplastic cells. MYC immunostain showed strong positivity in more than 90% of the neoplastic cells, suggesting a c-myc gene rearrangement. Fluorescence in situ hybridization (FISH) confirmed the presence of the MYC(8q24)-IGH(14q32) rearrangement. In situ hybridization for Epstein-Barr virus (EBER) was negative. A final diagnosis of Burkitt lymphoma was given.
The patient underwent complete staging of the disease according to the International Pediatric Non-Hodgkin Lymphoma Staging System (6) by total-body CT scan and PET-CT scan, lumbar puncture and bone marrow biopsy (both negative for neoplastic cells). At the end of the work-up the patient was classified as stage III Burkitt's lymphoma and chemotherapy according to AIEOP LNH 97 protocol, risk group R4 (7), that includes intravenous cyclophosphamide, vincristine, methotrexate, iphosphamide, cytarabine, etoposide, daunorubicin and dexamethasone and intrathecal therapy with methotrexate, cytarabine and methylprednisolone, was started five days after surgery, associated with intravenous Rituximab.
At the time of writing, the patient is still under induction chemotherapy (V course), which has been, so far, well tolerated.
Follow-up imaging shows reduction of the mass in the left ovary (Figure 4).
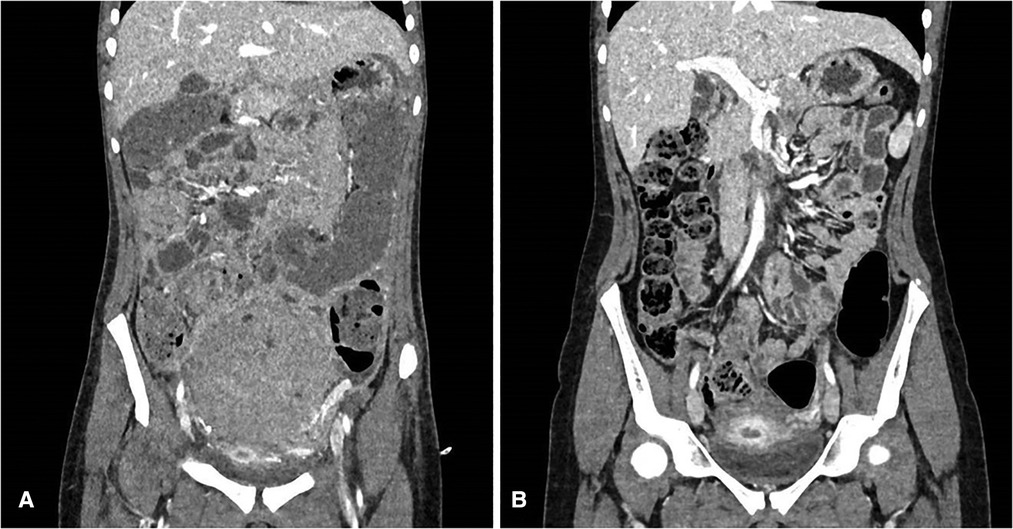
Figure 4. (A) CT scan after surgery (before chemotherapy); (B) CT scan after four cycles of chemotherapy.
Discussion
The incidence of ovarian tumors in girls and adolescents increases with age, starting from 0.4 per 100,000 during infancy to 25–30 per 100,000 at the age of 18 (8); they are mainly represented by benign lesions (80% of cases) (4). Primary lymphomas of the ovary are rare findings, accounting for less than 1% (9), and may appear as a localized ovarian lesion or as aggressive and metastatic form, frequently involving the central nervous system (CNS) and bone marrow (3). The sporadic type is frequently seen in patients under the age of 35, with a higher incidence among Caucasians and Central Americans (10).
Symptoms at presentation are very similar across different histologic types and include abdominal distension, palpable pelvic masses or acute pain due to torsion or rupture (3–5, 11). Abnormal vaginal bleeding, irregular menses, urination, bowel obstruction, ascites, fever, nights sweats, fatigue and weight loss are possible additional symptoms. Meningeal infiltration, headaches, visual impartment, and paraplegia may occur if the CNS is affected (3). When systemic symptoms are absent, differential diagnosis is impossible on clinical presentation alone.
The most prevalent ovarian lymphoma imaging characteristics on CT scan, ultrasonography, and MRI are described by Ferrozzi et al. (12) When compared to ultrasound, which has homogeneous, hypoechogenic structures and a non-specific appearance, CT scans show hypodense lesions with modest contrast enhancement. Hypointense T1-weighted scans and slightly hyperintense T2-weighted images on an MRI reveal homogenous masses with areas of contrast enhancement, irregular margins, extracapsular tumor growth and ascites (13); however, different malignant histotypes might have similar appearance on MRI (14). It's interesting to note that bilateral ovarian involvement was seen in 67% of primary ovarian lymphoma cases reported (3); this feature is highly suggestive but not exclusive for ovarian lymphoma, since bilateral lesions can occasionally be observed both in ovarian germ cell tumors (15) and in epithelial neoplasms (5). Ovarian lymphoma is the most likely diagnosis if ascites is absent and a homogenous bilateral tumor occurs in the ovaries.
Serum levels of carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), alpha-fetoprotein (AFP), and β-HCG biomarkers usually remain normal in patients with ovarian lymphoma but may be helpful for the differential diagnosis of other ovarian tumors. Serum levels of LDH and uric acid are usually elevated in patients affected by Non-Hodgkin lymphomas (16) and high levels of CA-125 have been associated with primary ovarian lymphomas (3). These serum markers may help differentiate between benign and malignant lesions, although they are not specific; high levels of serum LDH can be observed in advanced-stage germ cell tumors or in epithelial tumors (5, 17), while high level of CA-125 might be suggestive of epithelial tumors (5) or endometriosis (18).
Staging is crucial in assessing the extent of the disease and enables the application of a standardized treatment strategy; in the pediatric population, the St. Jude staging system has largely been replaced with the International Pediatric Non-Hodgkin Lymphoma Staging System (6).
Treatment of primary lymphoma of the ovary consists in multiagent chemotherapy (3, 7). Surgical procedures in ovarian lymphoma play a role in the diagnostic process, providing samples for diagnosis and staging(3); extensive debulking or bilateral oophorectomy is not beneficial and tumor resection should be avoided since recovery from surgery may delay the initiation of systemic therapy, which is needed urgently in this fast-growing tumor (19, 20).
Differentiating ovarian lymphoma from other ovarian neoplasms is essential, since ovarian lymphoma is treated by systemic chemotherapy (3, 6) while in germ cell tumors and epithelial neoplasms surgical resection is indicated (5, 11), followed by chemotherapy only in advanced stage disease (4, 15).
Despite the limited role of surgery in ovarian lymphoma, the majority of reported patients underwent laparotomic or laparoscopic exploration on the basis of the acute clinical presentation and the suspicion of adnexal torsion (3, 21, 22); in the present case, the patient complained of lower abdominal pain that was interpreted as a sign of vascular compression, and surgical exploration was therefore warranted.
Although lymphomas have a rapid growth rate, the estimated survival rate in pediatric patients is greater than 90%, while lower values are reported in prospective clinical trials for adults, ranging 75%–85% (23–25). Young patients with lymphomas who receive aggressive therapies have excellent outcomes, but treatment-related toxicity is still a considerable challenge in the treatment of lymphoma, especially in older patients. In adult patients, the five-year survival rate in advanced-stage disease has been reported as 60%–85% (26); in pediatric and adolescent population there is little information available regarding long-term prognosis of lymphomas involving the ovary.
In conclusion, preoperative diagnosis of primary ovarian lymphoma is extremely difficult. Laboratory tests including a complete blood count, comprehensive metabolic panels, measurement of LDH and uric acid levels can be helpful in the differential diagnosis of ovarian neoplasms.
Surgical exploration is often necessary in patients presenting with acute abdominal or pelvic pain; when the suspicion of primary ovarian lymphoma arises intraoperatively, every effort should be made to minimize invasive procedure in order to enhance post-operative recovery.
Timeframe of the relevant events
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
GP, AI, CM and AC conceived the case report and wrote the first draft of the manuscript. GP and CM reviewed the literature. AI revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brookfield KF, Cheung MC, Koniaris LG, Sola JE, Fischer AC. A population-based analysis of 1037 malignant ovarian tumors in the pediatric population. J Surg Res. (2009) 156(1):45–9. doi: 10.1016/j.jss.2009.03.069
2. Hazard FK, Longacre TA. Ovarian surface epithelial neoplasms in the pediatric population: incidence, histologic subtype, and natural history. Am J Surg Pathol. (2013) 37(4):548–53. doi: 10.1097/PAS.0b013e318273a9ff
3. Stepniak A, Czuczwar P, Szkodziak P, Wozniakowska E, Wozniak S, Paszkowski T. Primary ovarian Burkitt’s lymphoma: a rare oncological problem in gynaecology: a review of literature. Arch Gynecol Obstet. (2017) 296(4):653–60. doi: 10.1007/s00404-017-4478-6
4. Cecchetto G. Gonadal germ cell tumors in children and adolescents. J Indian Assoc Pediatr Surg. (2014) 19(4):189–94. doi: 10.4103/0971-9261.141995
5. Virgone C, Alaggio R, Dall'Igna P, Buffa P, Tonegatti L, Ferrari A, et al. Epithelial tumors of the ovary in children and teenagers: a prospective study from the Italian TREP project. J Pediatr Adolesc Gynecol. (2015) 28(6):441–6. doi: 10.1016/j.jpag.2014.12.010
6. Rosolen A, Perkins SL, Pinkerton CR, Guillerman RP, Sandlund JT, Patte C, et al. Revised international pediatric non-hodgkin lymphoma staging system. J Clin Oncol. (2015) 33(18):2112–8. doi: 10.1200/JCO.2014.59.7203
7. Pillon M, Mussolin L, Carraro E, Conter V, Aricò M, Vinti L, et al. Detection of prognostic factors in children and adolescents with Burkitt and diffuse large B-cell lymphoma treated with the AIEOP LNH-97 protocol. Br J Haematol. (2016) 175(3):467–75. doi: 10.1111/bjh.14240
8. de Campos Vieira Abib S, Chui CH, Cox S, Abdelhafeez AH, Fernandez-Pineda I, Elgendy A, et al. Non-Hodgkin’s lymphoma involving the gynecologic tract: a review of 88 cases. Adv Anat Pathol. (2001) 8(4):200–17. doi: 10.1097/00125480-200107000-00002
9. Vang R, Medeiros LJ, Fuller GN, Sarris AH, Deavers M. International society of paediatric surgical oncology (IPSO) surgical practice guidelines. Ecancermedicalscience. (2022) 16:1356. doi: 10.3332/ecancer.2022.1356
10. Dozzo M, Carobolante F, Donisi PM, Scattolin A, Maino E, Sancetta R, et al. Burkitt lymphoma in adolescents and young adults: management challenges. Adolesc Health Med Ther. (2017) 8:11–29. doi: 10.2147/AHMT.S9417028096698
11. Shaikh F, Murray MJ, Amatruda JF, Coleman N, Nicholson JC, Hale JP, et al. Paediatric extracranial germ-cell tumours. Lancet Oncol. (2016) 17(4):e149–162. doi: 10.1016/S1470-2045(15)00545-8
12. Ferrozzi F, Catanese C, Uccelli M, Bassi P. Ovarian lymphoma. Findings with ultrasonography, computerized tomography and magnetic resonance. Radiol Med. (1998) 95:493–7.9687927
13. Janssen CL, Littooij AS, Fiocco M, Huige JCB, de Krijger RR, Hulsker CCC, et al. The diagnostic value of magnetic resonance imaging in differentiating benign and malignant pediatric ovarian tumors. Pediatr Radiol. (2021) 51(3):427–34. doi: 10.1007/s00247-020-04871-2
14. Heo SH, Kim JW, Shin SS, Jeong SI, Lim HS, Choi YD, et al. Review of ovarian tumors in children and adolescents: radiologic-pathologic correlation. Radiographics. (2014) 34(7):2039–55. doi: 10.1148/rg.347130144
15. Billmire DF. Malignant germ cell tumors in childhood. Semin Pediatr Surg. (2006) 15(1):30–6. doi: 10.1053/j.sempedsurg.2005.11.006
16. Cairo MS, Coiffier B, Reiter A, Younes A, TLS Expert Panel, TLS Expert Panel, et al. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. (2010) 149(4):578–86. doi: 10.1111/j.1365-2141.2010.08143.x
17. International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. (1997) 15(2):594–603. doi: 10.1200/JCO.1997.15.2.594
18. Magalhães JS, Jammal MP, Crispim PCA, Murta EFC, Nomelini RS. Role of biomarkers CA-125, CA-15.3 and CA-19.9 in the distinction between endometriomas and ovarian neoplasms. Biomarkers. (2021) 26(3):268–74. doi: 10.1080/1354750X.2021.1885490
19. Shacham-Abulafia A, Nagar R, Eitan R, Levavi H, Sabah G, Vidal L, et al. Burkitt’s lymphoma of the ovary: case report and review of the literature. Acta Haematol. (2013) 129(3):169–74. doi: 10.1159/000345248
20. Roschewski M, Staudt LM, Wilson WH. Burkitt’s lymphoma. N Engl J Med. (2022) 387(12):1111–22. doi: 10.1056/NEJMra2025746
21. Sergi W, Marchese TRL, Botrugno I, Baglivo A, Spampinato M. Primary ovarian Burkitt’s lymphoma presentation in a young woman: a case report. Int J Surg Case Rep. (2021) 83:105904. doi: 10.1016/j.ijscr.2021.105904
22. Pourghasemian M, Danandeh Mehr A, Alavizadeh E, Behzadi F, Roosta Y. Primary bilateral ovarian involvement in Burkitt’s lymphoma with an adnexal torsion-like manifestation: a case report. Clin Case Rep. (2021) 9(11):e05058. doi: 10.1002/ccr3.5058
23. Haralambieva E, Boerma EJ, van Imhoff GW, Rosati S, Schuuring E, Müller-Hermelink HK, et al. Clinical, immunophenotypic, and genetic analysis of adult lymphomas with morphologic features of Burkitt lymphoma. Am J Surg Pathol. (2005) 29:1086–109. doi: 10.1097/01.pas.0000168176.71405.e5
24. Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. (2013) 369(20):1915–25. doi: 10.1056/NEJMoa1308392
25. Roschewski M, Dunleavy K, Abramson JS, Powell BL, Link BK, Patel P, et al. Multicenter study of risk-adapted therapy with dose-adjusted EPOCH-R in adults with untreated Burkitt lymphoma. J Clin Oncol. (2020) 38(22):2519–29. doi: 10.1200/JCO.20.00303
Keywords: lymphoma, ovarian cancer, surgery, pediatrics, burkitt’s lymphoma
Citation: Persano G, Crocoli A, Martucci C, Vinti L, Cassanelli G, Stracuzzi A, Cardoni A and Inserra A (2023) Case report: Primary ovarian Burkitt's lymphoma: A puzzling scenario in pediatric population. Front. Pediatr. 10:1072567. doi: 10.3389/fped.2022.1072567
Received: 17 October 2022; Accepted: 15 December 2022;
Published: 11 January 2023.
Edited by:
Luca Pio, St. Jude Children's Research Hospital, United StatesReviewed by:
Joanna Stefanowicz, Medical University of Gdansk, PolandFatih Akbiyik, Yuksek Ihtisas University, Turkey
© 2023 Persano, Crocoli, Martucci, Vinti, Cassanelli, Stracuzzi, Cardoni and Inserra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgio Persano Z2lvcmdpby5wZXJzYW5vQG9wYmcubmV0
†ORCID Giorgio Persano orcid.org/0000-0002-8093-2726 Alessandro Crocoli orcid.org/0000-0002-8093-2726 Cristina Martucci orcid.org/0000-0002-8093-2726 Luciana Vinti orcid.org/0000-0002-8093-2726 Giulia Cassanelli orcid.org/0000-0002-8093-2726 Alessandra Stracuzzi orcid.org/0000-0002-8093-2726 Antonello Cardoni orcid.org/0000-0002-8093-2726 Alessandro Inserra orcid.org/0000-0002-8093-2726
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 Giorgio Persano
Giorgio Persano Alessandro Crocoli
Alessandro Crocoli Cristina Martucci
Cristina Martucci Luciana Vinti
Luciana Vinti Giulia Cassanelli4,†
Giulia Cassanelli4,† Alessandra Stracuzzi
Alessandra Stracuzzi Alessandro Inserra
Alessandro Inserra