- 1Adult and Pediatric Dermatology, Private Practice, Huntington, WV, United States
- 2Department of Internal Medicine, Joan C. Edwards School of Medicine, Huntington, WV, United States
Early identification of the dermatologic manifestations of SARS-CoV-2 in perinatal and maternal-fetal-infant populations is essential for early intervention in the diagnosis, treatment, and prevention of short and long term sequelae. Although cutaneous signs of SARS-CoV-2 are less common in pregnant women, neonates, and infants, the recognition of related skin lesions with regard to timing, location, duration, and pattern can lead to determining disease severity. While many pediatric patients may be asymptomatic with negative SARS-CoV-2 testing, skin lesions may be the only clue of infection. SARS-CoV-2 infection in pregnancy can lead to severe life threatening illness and by understanding the cutaneous manifestations associated with SARS-CoV-2 infection, early diagnosis can be made with improved maternal-fetal outcomes. A wide array of dermatologic presentations associated with SARS-CoV-2 are reported in the literature. This review explores the expanding reports in the literature of the dermatologic presentations of skin lesions related to SARS-CoV-2 specifically in perinatal and maternal-fetal-infant health and the implications for management. The collaboration of the specialties of dermatology, pediatrics, obstetrics/gynecology, and infectious disease in the approach to SARS-CoV-2 disease can lead to a better understanding of the scope and presentation of this disease.
Introduction
Since the first report and isolation of SARS-CoV-2 infection in December 2019 in Wuhan, China, more than 600 million people have been infected globally causing over 6.4 million deaths (1). The ability to predict SARS-CoV-2 disease course and prevent transmission remains challenging but identifying dermatologic manifestations may have diagnostic and prognostic implications. Early reports of adverse effects associated with pregnancy were scarce but recent comparison studies present evidence that pregnant women with SARS-CoV2 have an increased susceptibility to hospitalization and severe illness (2, 3). The incidence of neonatal and infant SARS-CoV-2 infection is less common than adults but when infected have the potential for serious complications (3–5).
Dermatologic manifestations of SARS-CoV-2 were first reported in March 2020 by Recalcati (6) with the description of infected patients presenting with an erythematous vesicular and urticarial eruption. Subsequently, multiple varying presentations in infected patients were eventually categorized into distinct patterns. Certain types of skin patterns are associated with more severe SARS-CoV-2 infections and can help establish the timeline of the disease process. Skin manifestations of SARS-CoV2 must be differentiated from diseases that normally be seen or exacerbated in pregnant women, neonates, and infants. A team approach of dermatologists, obstetricians, neonatologists, pediatricians and infectious disease specialists is ideal to optimize patient care.
Immunology
There is a complex interplay of physiologic immunological responses in healthy pregnant women, neonates, and infants that may affect SARS-CoV-2 susceptibility and skin disease presentation (7). Natural immunological shifts in pregnancy to protect the fetus result in down regulation of cell mediated immunity and upregulation of humoral immunity responses. The results are decreased T helper 1 cell (Th1) cytokine production (interleukin-12 (IL-12), interferon-gamma (IFN γ) and increased T helper 2 cell cytokines (IL-4, IL-10) (7, 8). Cytokines are needed for cell signaling and development of healthy neonates and infants especially with respect to adaptive immune responses. Innate decreased expression of IFN γ and tumor necrosis factor-alpha (TNF-α) in neonates and infants is postulated to be associated with increased susceptibility to infection (8–11). Physiologic cytokine alterations may lead to exacerbation of skin diseases in pregnancy (10).
Serious SARS-CoV-2 complications are attributed to a viral stimulated hyperinflammatory state leading to immunological responses and an exaggerated release of cytokines (“Cytokine Storm”) (12). Among the main inflammatory mediators associated in this process are IFN γ, IL-6, and TNF-α, prime mediators involved in the physiological immune shifts in pregnant women, neonates and infants and in the pathogenesis of certain SARS-CoV-2 skin manifestations (13, 14). Tanacan et al. (13) reported in a study of 90 SARS-CoV-2 infected pregnant women that severity of illness correlated with elevation of IFN γ, IL-6 and D-Dimer and lower levels of IL-2, IL-10, and IL-17.
The combination of immunologic responses in pregnant women, neonates and infants with SARS-CoV-2 and skin disease results in a challenging complicated clinical picture created by the interactions of cytokines and pathophysiologic mechanisms (15).
Dermatologic patterns
Several main dermatological patterns associated with SARS-CoV-2 have been categorized and should be recognized in pregnant women, neonates and infants (16–20). Although patients with SARS-CoV-2 can present with polymorphic skin lesions, six common patterns have been described in the literature as (1) maculopapular (2) urticarial (3) vesicular (4) chilblain-like (5) livedo and (6) purpuric-vasculitic patterns (19, 20). The first 3 groups comprise lesions that are inflammatory and exanthematous and the latter 3 categories including cutaneous vasculitic disorders and vasculopathies (16, 17). These patterns have been noted in other skin diseases in pregnancy, neonatology and infancy recognizing the importance of a keen differential dermatologic diagnosis. Table 1 summarizes SARS CoV-2 dermatologic patterns and relevant clinical aspects to pregnant women, neonates, and infants.
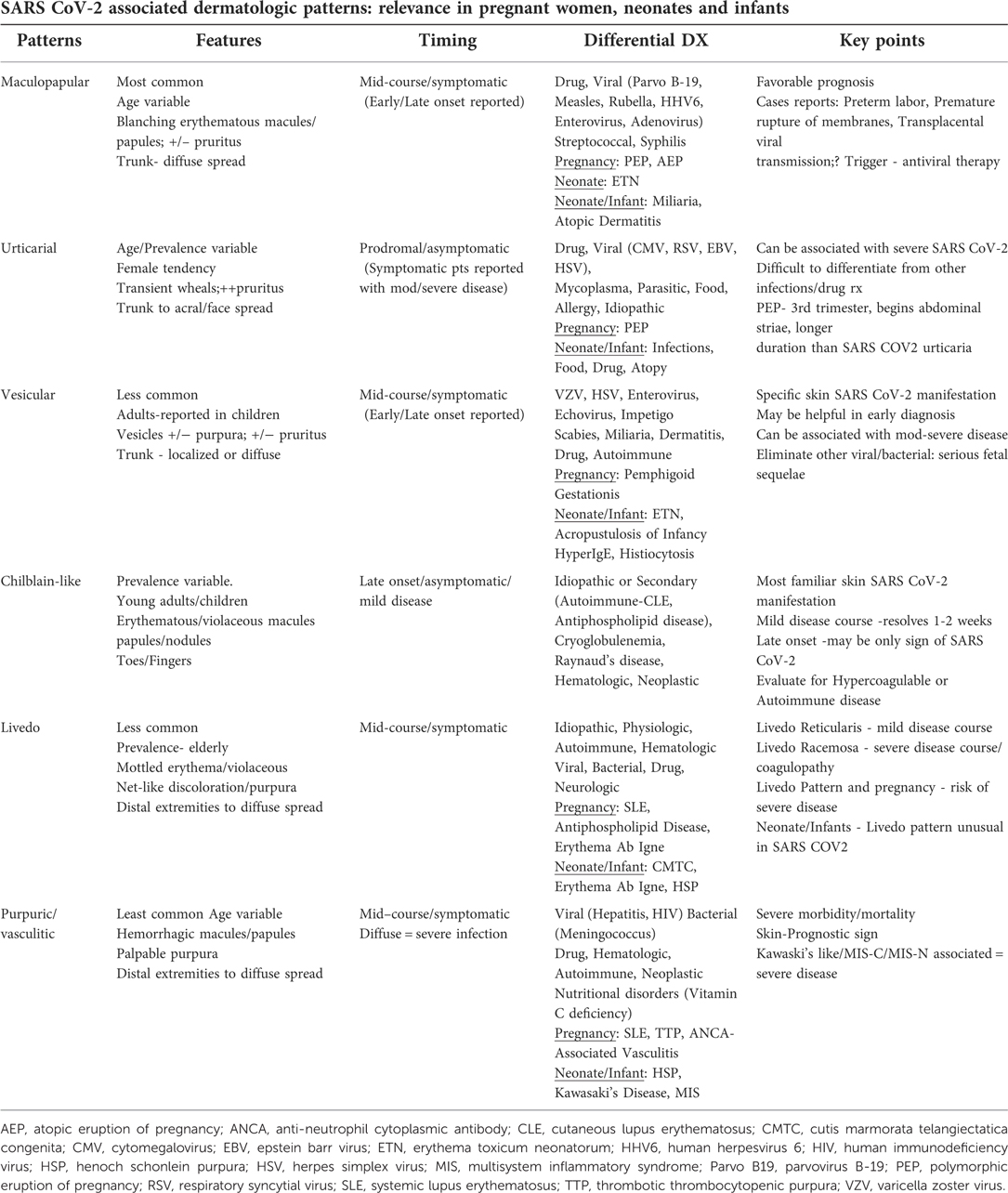
Table 1. Summarizes SARS CoV-2 associated dermatologic patterns and relevant clinical aspects in pregnant women, neonates, and infants.
Maculopapular
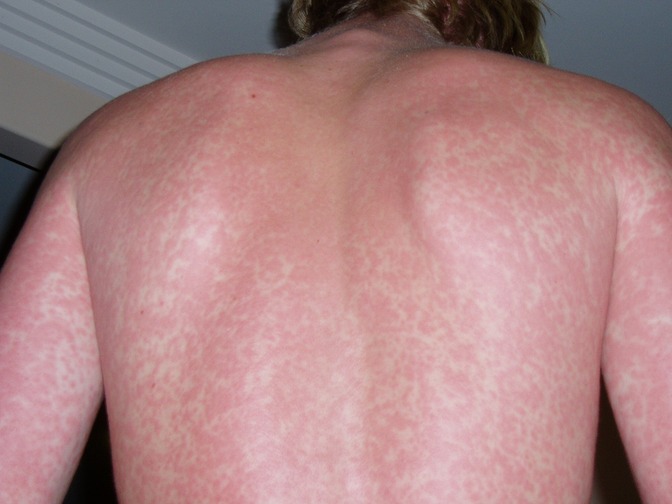
Maculopapular exanthems appear to be the most prevalent of all patterns. In a case series of 375 patients with SARS-CoV-2 infection, 47% presented with maculopapular lesions (17). In smaller case studies, the prevalence varied from 5%–70% (19). The exanthem can occur at any age consisting of small red raised and flat lesions which typically appear on the trunk and spread to the extremities but may appear on the face and neck (Figure 1).
The eruption may or may not be associated with pruritus. The lesions are most notably observed during the mid course of infection when the patient is most symptomatic. A few studies have reported a latent onset up to 27 days after diagnosis (21, 22). Variants of this exanthem have been reported as purpuric-like, erythema-multiforme-like, pityriasis-rosea- like, erythema elevatum diutinum-like and perifollicular patterns (23).
The majority of patients with maculopapular lesions tend to have an uneventful course, but there are reports of SARS-CoV-2 infected pregnant women presenting with only maculopapular eruptions devoid of constitutional symptoms with premature rupture of membranes (24). Rare cases of transplacental transmission of SARS CoV-2 have been reported in maternal infections with maculopapular lesions (25–28). Oropez et al. (25) reported a case of a 34 year old pregnant woman with a diamniotic dichorionic twin pregnancy presenting with mild SARS Co-V-2 infection and a maculopapular eruption in the 3rd trimester. Healthy twins delivered by cesarean section revealed one twin was positive for SARS Co-V-2 IgG antibodies while the other twin was serologically negative. Placental pathology was negative for evidence of SARS-Co-V-2. Maculopapular exanthems have been reported in infants with SARS-CoV-2 infection in association with mild symptomatic disease (17). The primary differential diagnosis includes other viral infections or adverse drug eruptions (Table 1). SARS-CoV-2 antiviral therapy can produce drug reactions appearing identical to viral eruptions making identification of the inciting agent challenging (14).
Polymorphic Eruption of Pregnancy (PEP) (Synonym-PUPP Pruritic Urticarial Papules and Plaques of Pregnancy) and Atopic Eruption of Pregnancy (AEP) may present in pregnant women as maculopapular lesions (29). Erythema toxicum neonatorum (ETN), miliaria, and atopic dermatitis could appear with maculopapular lesions in neonates and infants (30, 31). It is imperative to consider SARS CoV-2 infections in the differential of maculopapular eruptions in pregnant women, neonates and infants for proper intervention to prevent complications and transmission.
Urticarial
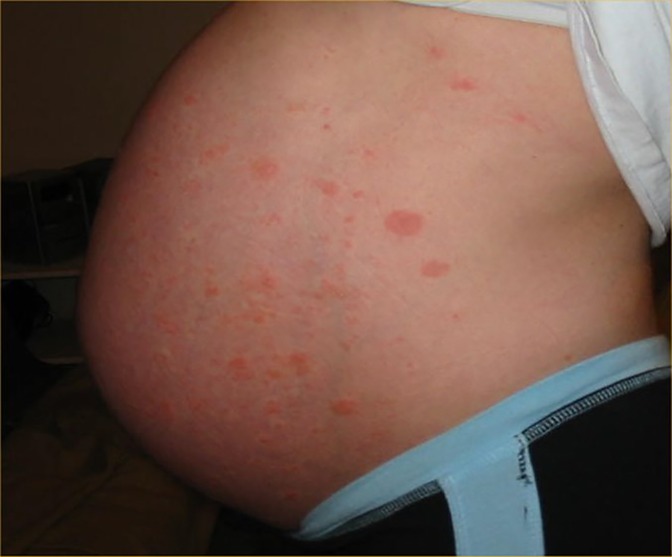
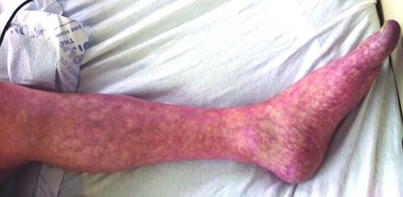
Urticarial lesions seen in SARS CoV-2 are generally encountered during the prodromal asymptomatic period of disease and may be the first sign of disease (Table 1). The incidence of urticaria in SARS-CoV-2 infection ranges from 16.7%–19% and has a higher prevalence in females (17, 32, 33). Although several studies have reported SARS CoV-2 associated urticaria primarily in adults, several cases have been documented in children (34). The lesions generally last a week and have been associated with moderate to severe complications in some patients (17). Hive-like, blanching thin plaques which are transient and changeable in shape with severe pruritus typically present on the trunk and spread to the extremities possibly affecting the face and acral areas (Figure 2). Angioedema and urticarial vasculitis may also occur (33).
Urticaria secondary to SARS CoV-2 may be difficult to differentiate from other causes such as medications, food, bacterial, parasitic, other viral infections, allergic reactions and idiopathic urticaria (33, 34). Pathophysiologically, SARS-CoV-2 stimulates mast cell degranulation through either direct viral contact or complement activation and cytokine release. It is theorized serious end organ damage in SARS-CoV-2 infection is due to mast cell activation (13, 35, 36).
With respect to pregnancy, the primary skin disease to differentiate other than drug or infection is PEP (29, 37). PEP tends to occur late in the 3rd trimester or in the post-partum period usually in primigradas and begins within the abdominal striae. Unlike PEP, SARS-CoV-2 associated urticaria tends to resolve around 7 days (17). Newer reports have shown that chronic urticaria can develop particularly in young women after SARS-CoV-2 infection or SARS-CoV-2 vaccines (38, 39). The most common etiologic factors in the differential of urticaria in neonates and infants include infection, food, medications and atopy (40).
SARS-CoV-2 should be considered in the differential diagnosis of urticaria in pregnant women, neonates, and infants without constitutional symptoms due to the possibility of moderate to severe disease complications.
Vesicular
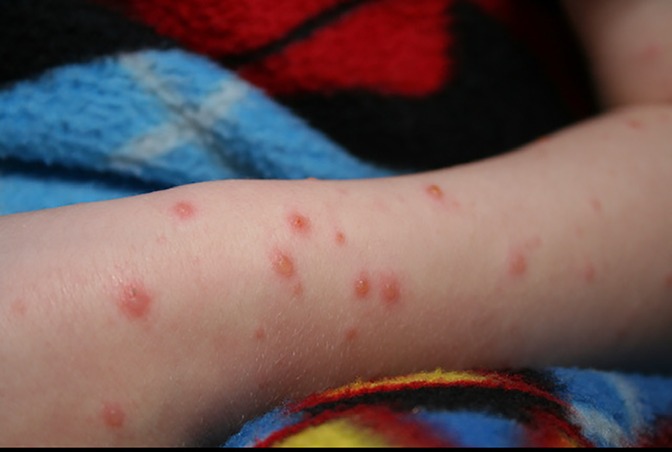
Vesicular eruptions in SARS-CoV-2 were first described as “varicella-like” in April 2020 by Marzano (20). Usually appearing on the trunk, scattered fluid filled blisters may appear localized or diffusely with or without purpura (Figure 3).
The prevalence ranges from 3.7%–15% occurring primarily in adults but has been reported in children (17, 19, 32, 41). SARS-CoV-2 vesicular exanthems are associated with moderate severity of illness and occur when patients are symptomatic in mid course of disease. Cases of early or late onset of vesicular lesions have been reported. The median duration of the eruption is approximately 8–10 days (17, 19, 20, 32). Vesicular eruptions associated with SARS CoV-2 are considered to be specific to the virus and may be useful diagnostically. In a systematic review, Jamshidi reports that vesicular lesions may be associated with neurologic symptoms including headache, dysgeusia, and confusion (42). The pathogenesis of vesicular lesions in SARS-CoV-2 is felt to be related to either a direct cytotoxic effect on dermal vessel endothelium or exaggerated release of cytokines (19, 41).
SARS-CoV-2 infection must be considered in the differential diagnosis of vesicular eruptions in pregnant women, neonates and infants. Causes to be eliminated include viral and bacterial infections, infestations, miliaria, irritant or contact dermatitis, and autoimmune diseases (19, 43, 44) (Table 1). Viruses such as herpes simplex virus (HSV), varicella zoster virus (VZV), measles, and rubella are associated with serious fetal sequelae and must be differentiated (45). HSV and VZV reactivation has been associated with SARS-CoV-2 infection with some patients developing more severe illness (46–48). Flaring of atopic dermatitis can present as vesicular lesions in pregnancy and infancy with secondary staphylococcal or HSV infection requiring immediate therapeutic intervention. Elevated IL-4 levels in pregnancy may be a factor in the exacerbation of atopic dermatitis (49). Common transient conditions in neonates may present with vesicular lesions (ETN, acropustulosis of infancy) or rare conditions such as Hyper IgE syndrome, or histiocytosis (50, 51). Pemphigoid Gestationis, a rare immunobullous disease in pregnancy, usually occurring in the 3rd trimester with periumbilical bullae should be included in the differential diagnosis of SARS-CoV-2 vesicular exanthems (29).
Chilblain-like
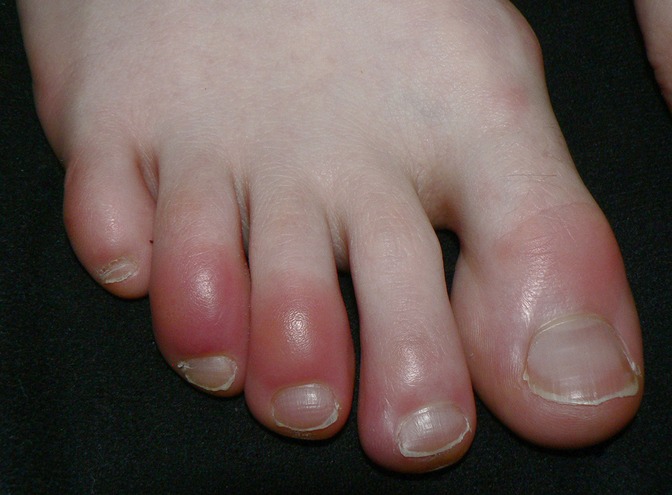
Chilblain-like or “pernio-like” vascular skin lesions of the hands and feet are probably the most recognized of all skin lesions associated with SARS-CoV-2. Multiple reports of non-blanching erythematous or violaceous lesions of the toes associated with SARS-CoV-2 infection help to coin the term “Covid toes” (19, 52) (Figure 4).
Usually seen in asymptomatic or mildly infected young adults and children, SARS-CoV-2 chilblain-like lesions tend to appear late in the course of infection. Numerous studies report the prevalence varies from 14.3%–72% (19). Chilblain and pernio diseases are vascular inflammatory reactive skin disorders to environmental stimuli such as cold exposure or damp humid environments. The hands and feet are primarily affected with a vasoconstrictive response and resultant erythematous, violaceous macules, papules or nodules of the fingers or toes. The most common symptoms are pain and pruritus. SARS-COV-2 associated chilblain-like lesions tend to last 1–2 weeks after the onset of symptoms and resolve without incident (32). Primary chilblain/pernio disease is idiopathic but secondary causes include autoimmune [systemic lupus erythematosus (SLE), antiphospholipid disease, Raynaud's disease] cryoglobulinemia, and hematologic diseases (52). There is controversy whether there is a direct association between SARS-CoV-2 infection and chilblain-like lesions. Colmenero (53) demonstrated the presence of SARS-CoV-2 in endothelial cells of pernio-like lesions by electron microscopy. On the contrary, many patients presenting with chilblain-like skin lesions tested negative with reverse transcription polymerase chain reaction test (RT-PCR), had negative serology, or were not tested at all (54, 55). This may be explained by robust protective levels of IFN-1 in younger patients or the significant variability of current testing. It has been proposed that chilblain-like lesions represent late manifestations of SARS-CoV-2 due to a delayed immunological reaction or an inappropriate type 1 interferon response (56). A literature review by Cappel et al. (56) suggested that the pathogenesis of SARS-CoV-2 chilblain-like lesions involves complex interactions between the virus, angiotension converting enzyme-2 (ACE-2), the renin-angiotension-aldosterone system, sex hormones, and interferon type 1 responses causing endothelial cell dysfunction (56–58).
Histopathology of skin lesions is similar to that found in idiopathic chilblains with epidermal necrotic keratinocytes, dermal edema, perivascular and perieccrine lymphocytic inflammation and microthrombi in the vasculature and endothelial cell inflammation (58).
Chilblain-like lesions may be the only sign of SARS CoV-2 in pregnant women, neonates and infants late in the disease course so it is important to properly diagnose this pattern and differentiate from other primary or secondary causes.
Livedo
Livedo patterns are less common manifestations of SARS CoV-2 ranging from 4%–6% (17, 19, 41). Infected patients presenting with livedo reticularis-like lesions tend to have milder disease and transiently clear over a period of 2 weeks with the average duration approximately 9–10 days (17, 19, 59, 60). The lesions appear as a mottled red-blue-purple net-like discoloration on the trunk, flexor forearm surface, dorsal hands and feet (Figure 5).
A pauci-inflammatory thrombogenic vasculopathy is noted on histopathology with serologic elevated D-Dimer levels (14, 61). It is theorized that the SARS CoV-2 virus directly infects endothelial or smooth muscle vessel cells causing low grade vascular inflammation and vasodilation. This process results in decreased blood flow with deoxygenated hemoglobin but no thromboembolism (60). Livedo reticularis must be differentiated from other causes including either physiologic, secondary, or idiopathic (Table 1).
Livedo racemosa is a more severe variant and is characterized by larger more widespread mottling of the skin that is generally secondary to a pathologic condition. Usually appearing in elderly patients with severe SARS-CoV-2 infection, the lesions can be transient or persistent and appear mid course during active symptoms. In contrast to livedo reticularis, patients presenting with livedo racemosa may develop severe coagulopathy and complications. Galvan Casas et al. (17) reported a mortality rate of 10% in patients presenting with livedo racemosa. Pathologically, vessels are partially occluded which leads to retiform purpura and complete vascular occlusion. Histologically, there is a micro thrombotic vasculopathy with possible dermal arterial thrombosis (62).The vasculopathy is thought to be due to direct viral effects or immune stimulation of the complement cascade with the release of proinflammatory cytokines (IL-6, IL-8, IFN γ, TNF-α) elevated D-Dimer levels, and fibrinogen degradation products which are associated with thrombosis and increased mortality (61, 62). Severity of SARS- CoV-2 illness in pregnant women may be correlated with elevated IFN γ, IL-6, and D Dimer levels (13).
In pregnant women, neonates and infants, livedo patterns can be seen associated with other hypercoagulable diseases including SLE and antiphospholipid antibody syndrome. Interestingly, antiphospholipid antibodies are found in SARS-CoV-2 patients with severe illness, livedo lesions, and severe thrombosis. In a study by Sangle et al. (63), widespread livedo reticularis is thought to be an independent factor of pregnancy complications in patients who have negative antiphospholipid antibodies with or without lupus. Rodriguez et al. (64) reported a case of an infant presenting with livedo racemosa and respiratory failure diagnosed as multisystem inflammatory syndrome (MIS-C). Given the high risk for severe complications of SARS-CoV-2, infected pregnant women, neonates and infants presenting with livedo patterns should closely be monitored and investigated for impending thrombotic events.
Purpuric/vasculitic
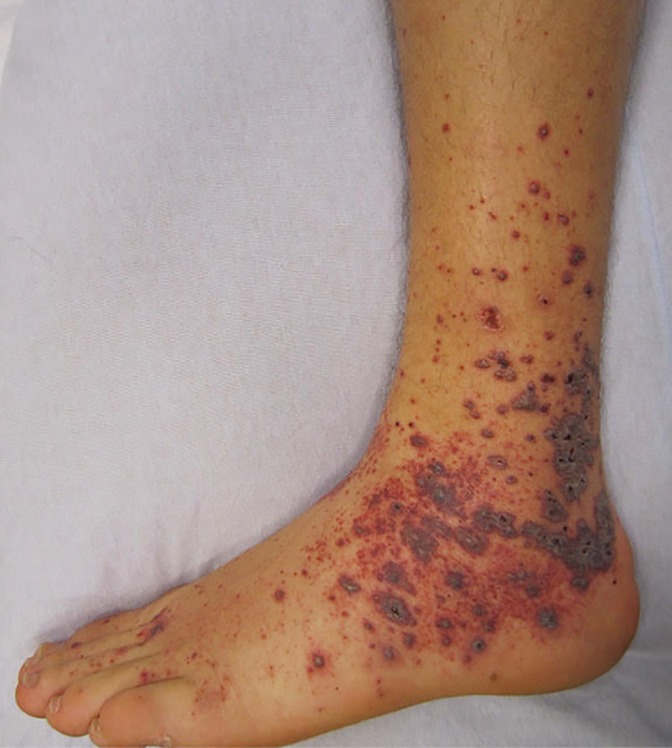
Vasculitic or purpuric lesions in SARS CoV-2 infected patients are associated with severe morbidity and mortality (17, 52). Less common than other patterns, various studies report a prevalence of 3%–8% and the lesions occurring during the symptomatic phase of infection(17, 19, 20, 32). The lesions appear as non-blanching hemorrhagic macules, patches, bullae, or palpable purpura on the extremities or acral areas (Figure 6).
Distal acral ischemia may occur leading to necrosis and gangrene of the digits (44). Diffuse spread of vasculitic lesions correlates with severe sequelae (65–67). Elevated D-Dimer and fibrinogen degradation products were found in patients with distal ischemia with some developing disseminated intravascular coagulation (68). Histologically, a true vasculitis is seen with a neutrophilic infiltrate within the small vessel walls, intense lymphocytic perivascular infiltrate, fibrin deposition, and endothelial swelling differentiating this pattern from livedo lesions (43).
Purpuric, petechial, or vasculitic lesions may be present in other viral infections in pregnant women, neonates and infants including hepatitis, human immunodeficiency virus, parvovirus B19 as well as bacterial infections, hematologic, autoimmune and nutritional disorders (45) (Table 1). Adverse drug reactions including antiviral agents are common causes of purpuric vasculitic eruptions in SARS Co-V-2 due to the multitude of therapeutics in severe illness (19). Systemic lupus erythematosus, thrombotic thrombocytopenic purpura, anti-neutrophil cytoplasmic antibody associated vasculitis, and parvo B-19 infections are included in the differential diagnosis of purpuric vasculitic lesions in pregnancy and can lead to serious perinatal and fetal complications (45, 69–71). Although rarely seen in this age group, Ig A vasculitis (Henoch Schonlein Purpura -HSP), Kawasaki's disease, and multisystem inflammatory syndrome should be considered in the differential diagnosis in neonates and infants with purpuric or vasculitic lesions and diagnosed promptly to prevent potential complications (72–74).
Pathogenesis of SARS-CoV2 petechiae/purpura/vasculitis is thought to be due to direct viral damage to endothelial cells causing endotheliitis and endothelial cell injury or a dysregulated inflammatory responses with immune complex deposition and massive cytokine release. Macrophage activation results and leads to the thrombotic lesions and events seen in SARS CoV02 coagulopathy (75).
Recognizing specific skin lesions may be prognostic in disease severity. Vasculitis, livedo racemosa, and distal ischemia are associated with more severe complications while chilblains-like lesions have the highest survival rates. The importance of identifying skin manifestations in SARS-CoV-2 infected pregnant women, neonates, and infants is imperative to allow early intervention and therapeutic management.
Multisystem inflammatory syndrome
Early reports of SARS-CoV-2 infection indicated that children and neonates tended to be spared of severe associated complications. In 2020, a hyperinflammatory syndrome with characteristics similar to Kawasaki's Disease (KD) was reported in children with concurrent or post SARS-CoV-2 infection (73, 74). The syndrome was labeled as Multisystem Inflammatory Syndrome in Children (MIS-C) or Paediatric Inflammatory Multisystem Syndrome temporarily associated with SARS-CoV-2 (PIMS-TS) and in neonates as Multisystem Inflammatory Syndrome in Neonates (MIS-N) (76–78). Although the incidence is rare, MIS-C is a potentially life threatening variant leading to severe complications including cardiac injury, multiorgan failure and death, The most commonly involved organ systems are gastrointestinal, cardiovascular, hematologic, mucocutaneous, and respiratory. Overall, pediatric mortality due to MIS-C is reported at 1.9% but in neonates and young infants it may be as high as 9% (79).
MIS-C usually occurs in children aged 9 years (ranging 1 month to 20 years) and in neonates (MIS-N) from within 7 days to 27 days post birth (77–79). The CDC criteria for MIS-C/MIS-N includes persistent fever(not MIS-N), 2 organ system involvement, laboratory evidence of inflammatory markers, laboratory evidence of current or recent SARS-CoV-2 infection or maternal infection, and no other plausible disease causing the syndrome (79, 80).
Cutaneous and mucocutaneous lesions are present in approximately 73% of children with MIS-C (81). Maculopapular exanthems and conjunctivitis are the most commonly reported skin signs. Facial erythema or periorbital edema (“Heliotrope rash”), hand and foot edema, perineal erythema, desquamation, and cracked lips are noted features. Retiform purpura, targetoid lesions, urticaria and erythroderma have been described (81). Godfred-Cato et al. (82) reported skin rash was the most common presenting sign of MIS-C in infants less than 12 months of age and 32.9% of these infants required ICU admission. The appearance of a maculopapular rash in MIS-C may have prognostic implications depending upon the presentation. In a small study by Rekhtman (83), some MIS-C patients specifically presenting with maculopapular lesions had lower levels of inflammatory markers, less ICU admission, less mechanical ventilator support, and less serious consequences. In contrast, isolated purpuric and necrotic lesions have been noted in neonates with MIS-N with cardiogenic shock, elevated inflammatory markers, and multiorgan failure (84).
MIS-C patients with a Kawasaki's disease-like (KD-like) presentation have been reported. Similar lesions include conjunctival injection, hyperemic cracked lips, strawberry tongue, and coronary artery disease with severe complications. While classic KD patients tend to be younger (less than 5 years), MIS-C with KD-like disease patients are usually older (5–13 years) and present with more gastrointestinal symptoms. Both MIS-C/KD-like disease and KD patients may present with severe cardiac involvement but KD patients tend to have severe persistent sequelae. There are reports of persistent cardiac dysfunction in some MIS-C patients (85, 86).
The pathogenesis of MIS-C and MIS-N is unknown but theorized to result from autoantibody mediated complexes to SARS-CoV-2 infection through the respiratory or gut mucosa (87). Neonates may develop immune complexes derived from exposure to maternal antibodies (79). Others postulate that SARS-CoV-2 virus acts as a superantigen causing an exaggerated release of inflammatory mediators leading to cytokine storm (88). Consiglio (87) reported that MIS-C patients had lower levels of TNF-α and normal IL-6 levels both of which are elevated in acute SARS-CoV-2 infection casting doubt on the cytokine storm theory. The efficacy of intravenous immunoglobulin therapy in MIS-C supports an autoantibody mediated pathogenesis (87).
Although there are no diagnostic skin manifestations, cutaneous and mucosal lesions may be the presenting signs of MIS-C or MIS-N, early recognition of dermatological manifestations can lead to timely diagnosis and intervention (85).
Less common skin manifestations of SARS-CoV-2
Unusual skin manifestations associated with SARS-CoV-2 have been reported with regard to maternal/fetal/infant health. Vertical transplacental transmission of SARS CoV-2 is rare but has been reported with possible associated skin manifestations. Generalized and local fetal skin edema diagnosed by ultrasound has been reported in pregnant women with SARS-CoV-2 infection (89). Associated elevated serological maternal levels of IL-6 and D-Dimer levels leading to cytokine stimulated inflammation or direct viral cytotoxicity is felt to alter the neonatal cutaneous microbiome resulting in fetal skin edema (89, 90). Necrotic lesions of the upper arm leading to amputation were noted in a neonate born to a SARS-CoV-2 infected mother and theorized that the virus may induce neonatal thrombotic events through exposure to maternal infection (91). Unusual orange discoloration of the skin was reported in a SARS-CoV-2 infected family in which yellow to brown macules were noted on the extremities of a newborn and yellow-brown discoloration of the palms and soles found on the other family members. The virus is thought to cause abnormalities in the conversion or transport of beta-carotene causing excess amounts to be deposited in the skin (92). Acute Hemorrhagic Edema of Infancy has been described in a SARS-CoV-2 infected infant which recurred 3 weeks after initial presentation and resolution (93). More evidence is needed to determine the relationship of neonatal infant skin eruptions and maternal SARS CoV-2 infections.
Conclusion
There are limited reports on the relationship of SARS-Co-V-2 infection and related dermatologic manifestations in pregnant women, neonates and infants but reports have demonstrated this patient population is at high risk for SARS-Co-V-2 complications. Skin lesions may be the first sign of infection and be prognostic for disease severity. Severe morbidity and mortality have been associated with the appearance of purpuric and vasculitic lesions and less commonly with chilblains-like lesions. By identifying skin manifestations in SARS-Co-V-2 infected pregnant women, neonates, and infants, asymptomatic infections may be properly diagnosed, disease transmission prevented, and severe disease complications averted. It is important to differentiate other skin diseases which can flare during pregnancy or in the neonatal/infancy period due to physiologic immunological shifts. The collaboration between dermatology, obstetrics and gynecology, neonatology, pediatrics, and infectious disease can optimize perinatal/maternal-fetal-infant health care in the diagnosis and treatment of SARS- CoV-2.
Author contributions
EY drafted the manuscript. The author was the sole contributor to the article and approved the submitted version.
Acknowledgments
I would like to gratefully thank Dr. Matthew Nudelman for his brilliant talent in graphics and expedient response to my inquiries. Thanks to Denise Smith, Administrative Assistant, at the Joan C. Edwards School of Medicine for her help in resources.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1071839/full#supplementary-material.
References
1. WHO COVID-19 Dashboard. Geneva: World Health Organization (2022). Available at: https://covid19.who.int/ (Last cited September 23, 2022).
2. Zambrano LD, Ellington S, Strid P, Galang RP, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy Status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1641–7. doi: 10.15585/mmwr.mm6944e3
3. Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, Oduyebo T, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy — sET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1635–40. doi: 10.15585/mmwr.mm6944e2
4. Marks KJ, Whitaker M, Agathis NT, Anglin O, Milucky J, Patel K, et al. Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19 — cOVID-NET, 14 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:429–36. doi: 10.15585/mmwr.mm7111e2
5. Devin J, Marano R, Mikhael M, Feaster W, Sanger T, Ehwerhemuepha L. Epidemiology of neonatal COVID-19 in the United States. Pediatrics. (2022) 150(4):e2022056297. doi: 10.1542/peds.2022-056297
6. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. (2020) 34(5):e212–3. doi: 10.1111/jdv.16387
7. Robertson SA. Immune regulation of conception and embryo implantation-all about quality control? J Reprod Immunol. (2010) 85(1):51–7. doi: 10.1016/j.jri.2010.01.008
8. Chow SSW, Craig ME, Jones CA, Hall B, Catteau J, Lloyd AR, et al. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine. (2008) 44(1):78–84. doi: 10.1016/j.cyto.2008.06.009
9. Piccinni MP, Raghupathy R, Saito S, Szekeres-Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front Immunol. (2021) 12:717808. doi: 10.3389/fimmu.2021.717808
10. Wilder RL. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci. (1998) 840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x
11. Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. (2007) 7(5):379–90. doi: 10.1038/nri2075
12. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. (2020) 383(23):2255–73. doi: 10.1056/NEJMra2026131
13. Tanacan A, Yazihan N, Erol SA, Anuk AT, Yetiskin FDY, Biriken D, et al. The impact of COVID-19 infection on the cytokine profile of pregnant women: a prospective case-control study. Cytokine. (2021) 140:155431. doi: 10.1016/j.cyto.2021.155431
14. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220:1–13. doi: 10.1016/j.trsl.2020.04.007
15. Lim RK, Kalagara S, Chen KK, Mylonakis E, Kroumpouzos G. Dermatology in a multidisciplinary approach with infectious disease and obstetric medicine against COVID-19. Int J Womens Dermatol. (2021) 7(5):640–6. doi: 10.1016/j.ijwd.2021.08.008
16. Kaya G, Kaya A, Saurat JH. Clinical and histopathological features and potential pathological mechanisms of skin lesions in COVID-19: review of the literature. Dermatopathology (Basel). (2020) 7(1):3–16. doi: 10.3390/dermatopathology7010002
17. Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. (2020) 183(1):71–7. doi: 10.1111/bjd.19163
18. Ortega-Quijano D, Jimenez-Cauhe J, Selda-Enriquez G, Fernandez-Guarino M, Fernandez-Nieto D. Algorithm for the classification of COVID-19 rashes. J Am Acad Dermatol. (2020) 83(2):e103–4. doi: 10.1016/j.jaad.2020.05.034
19. Singh H, Kaur H, Singh K, Sen CK. Cutaneous manifestations of COVID-19: a systematic review. Adv Wound Care (New Rochelle). (2021) 10(2):51–80. doi: 10.1089/wound.2020.1309
20. Marzano AV, Cassano N, Genovese G, Moltrasio C, Vena GA. Cutaneous manifestations in patients with COVID-19: a preliminary review of an emerging issue. Br J Dermatol. (2020) 183(3):431–42. doi: 10.1111/bjd.19264
21. Herrero-Moyano M, Capusan TM, Andreu-Barasoain M, Alcántara-González J, Salado MR-D, Uceda MES-L, et al. A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol. (2020) 34(9):e460–4. doi: 10.1111/jdv.16631
22. Reymundo A, Fernáldez-Bernáldez A, Reolid A, Butrón B, Fernández-Rico P, Muñoz-Hernández P, et al. Clinical and histological characterization of late appearance maculopapular eruptions in association with the coronavirus disease 2019. A case series of seven patients. J Eur Acad Dermatol Venereol. (2020) 34(12):e755–7. doi: 10.1111/jdv.16707
23. Català A, Galván-Casas C, Carretero-Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa A, et al. Maculopapular eruptions associated to COVID-19: a subanalysis of the COVID-piel study. Dermatol Ther. (2020) 33(6):e14170. doi: 10.1111/dth.14170
24. Sulistyowati S, Anggraini NWP. Skin manifestations of COVID-19 in a pregnant woman with premature rupture of membranes: a case report. Indones J Med Health. (2021) 12(1):98–104. doi: 10.20885/JKKI.Vol12.Iss1.art14
25. Chávez LO, Tinajero AS, Orozco JAM, Vargas EB, De la Merced AD, Santillán DPR, et al. A 34-year-old woman with a diamniotic dichorionic twin pregnancy presenting with an erythematous and papular skin rash associated with SARS-CoV-2 infection. Am J Case Rep. (2021) 22:e929489. doi: 10.12659/AJCR.929489
26. Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. (2020) 11(1):3572. doi: 10.1038/s41467-020-17436-6
27. Fenizia C, Biasin M, Cetin I, Vergani P, Mileto D, Spinillo A, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. (2020) 11(1):5128. doi: 10.1038/s41467-020-18933-4
28. Correia CR, Marçal M, Vieira F, Santos E, Novais C, Maria AT, et al. Congenital SARS-CoV-2 infection in a neonate with severe acute respiratory syndrome. Pediatr Infect Dis J. (2020) 39(12):e439–43. doi: 10.1097/INF.0000000000002941
29. Ambros-Rudolph CM. Dermatoses of pregnancy - clues to diagnosis, fetal risk and therapy. Ann Dermatol. (2011) 23(3):265–75. doi: 10.5021/ad.2011.23.3.265
30. Monteagudo B, Labandeira J, Cabanillas M, Acevedo A, Toribio J. Prospective study of erythema toxicum neonatorum: epidemiology and predisposing factors. Pediatr Dermatol. (2012) 29(2):166–8. doi: 10.1111/j.1525-1470.2011.01536.x
31. Dinulos JE, Dinulos JG. Cutaneous coronavirus disease 2019 in children: a clinical primer for diagnosis and treatment. Curr Opin Pediatr. (2021) 33(6):691–703. doi: 10.1097/MOP.0000000000001076
32. Freeman EE, McMahon DE, Lipoff JB, Rosenbach M, Kovarik C, Desai SR, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. (2020) 83(4):1118–29. doi: 10.1016/j.jaad.2020.06.10163
33. Algaadi SA. Urticaria and COVID-19: a review. Dermatol Ther. (2020) 33:e14290. doi: 10.1111/dth.14290
34. Andina D, Belloni-Fortina A, Bodemer C, Bonifazi E, Chiriac A, Colmenero I, et al. Skin manifestations of COVID-19 in children: part 2. Clin Exp Dermatol. (2021) 46(3):451–61. doi: 10.1111/ced.1448233
35. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. (2020) 19(6):102537. doi: 10.1016/j.autrev.2020.102537
36. Abuelgasim E, Dona ACM, Sondh RS, Harky A. Management of urticaria in COVID-19 patients: a systematic review. Dermatol Ther. (2021) 34(1):e14328. doi: 10.1111/dth.14328
37. Proietti I, Bernardini N, Tolino E, Mambrin A, Balduzzi V, Marchesiello A, et al. Polymorphic eruption of pregnancy as a possible COVID-19 manifestation. Dermatol Ther. (2020) 33(6):e14117. doi: 10.1111/dth.14117
38. Muntean IA, Pintea I, Bocsan IC, Dobrican CT, Deleanu D. COVID-19 Disease leading to chronic spontaneous Urticaria exacerbation: a Romanian retrospective study. Healthcare (Basel). (2021) 9(9):1144. doi: 10.3390/healthcare9091144
39. Strahan A, Ali R, Freeman EE. Chronic spontaneous urticaria after COVID-19 primary vaccine series and boosters. JAAD Case Rep. (2022) 25:63–6. doi: 10.1016/j.jdcr.2022.05.012
40. Shin M, Lee S. Prevalence and causes of childhood Urticaria. Allergy Asthma Immunol Res. (2017) 9(3):189–90. doi: 10.4168/aair.2017.9.3.189
41. Zhao Q, Fang X, Pang Z, Zhang B, Liu H, Zhang F. COVID-19 and cutaneous manifestations: a systematic review. J Eur Acad Dermatol Venereol. (2020) 34(11):2505–10. doi: 10.1111/jdv.16778
42. Jamshidi P, Hajikhani B, Mirsaeidi M, Vahidnezhad H, Dadashi M, Nasiri MJ. Skin manifestations in COVID-19 patients: are they indicators for disease severity? A systematic review. Front Med (Lausanne). (2021) 8:634208. doi: 10.3389/fmed.2021.634208
43. Genovese G, Moltrasio C, Berti E, Marzano AV. Skin manifestations associated with COVID-19: current knowledge and future perspectives. Dermatology. (2021) 237(1):1–12. doi: 10.1159/000512932
44. Gottlieb M, Long B. Dermatologic manifestations and complications of COVID-19. Am J Emerg Med. (2020) 38(9):1715–21. doi: 10.1016/j.ajem.2020.06.011
45. Auriti C, De Rose DU, Santisi A, Martini L, Piersigilli F, Bersani I, et al. Pregnancy and viral infections: mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim Biophys Acta Mol Basis Dis. (2021) 1867(10):166198. doi: 10.1016/j.bbadis.2021.166198
46. Polly S, Fernandez AP. Common skin signs of COVID-19 in adults: an update. Cleve Clin J Med. (2022) 89(3):161–7. doi: 10.3949/ccjm.89a.21126
47. Katz J, Yue S, Xue W. Herpes simplex and herpes zoster viruses in COVID-19 patients. Ir J Med Sci. (2022) 191(3):1093–7. doi: 10.1007/s11845-021-02714-z
48. Xu R, Zhou Y, Cai L, Wang L, Han J, Yang X, et al. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19 [published correction appears in Br J Dermatol. 2021 Sep;185(3):685]. Br J Dermatol. (2020) 183(6):1145–7. doi: 10.1111/bjd.19484
49. Eichenfield LF, Stripling S, Fung S, Cha A, O'Brien A, Schachner LA. Recent developments and advances in atopic dermatitis: a focus on epidemiology, pathophysiology, and treatment in the pediatric setting. Paediatr Drugs. (2022) 24(4):293–305. doi: 10.1007/s40272-022-00499-x1
50. Tarang G, Anupam V. Incidence of vesiculobullous and erosive disorders of neonates. J Dermatol Case Rep. (2011) 5(4):58–63. doi: 10.3315/jdcr.2011.1078
51. Howard RM, Frieden I. Vesiculopustular and erosive disorders in newborns and infants. In: Bologna JL, Schaffer JV, Cerroni L, editors. Dermatology. 4th ed. Philadelphia: Elsevier (2018). p. 562–79.
52. Zaladonis A, Huang S, Hsu S. COVID Toes or pernio? Clin Dermatol. (2020) 38(6):764–7. doi: 10.1016/j.clindermatol.2020.06.002
53. Colmenero I, Santonja C, Alonso-Riaño M, Noguera-Morel L, Hernández-Martín A, Andina D, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. (2020) 183(4):729–37. doi: 10.1111/bjd.193275
54. Caselli D, Chironna M, Loconsole D, Nigri L, Mazzotta F, Bonamonte D, et al. No evidence of SARS-CoV-2 infection by polymerase chain reaction or serology in children with pseudo-chilblain. Br J Dermatol. (2020) 183(4):784–5. doi: 10.1111/bjd.19349
55. Le Cleach L, Dousset L, Assier H, Fourati S, Barbarot S, Boulard C, et al. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. (2020) 183(5):866–74. doi: 10.1111/bjd.19377
56. Cappel MA, Cappel JA, Wetter DA. Pernio (chilblains), SARS-CoV-2, and COVID toes unified through cutaneous and systemic mechanisms. Mayo Clin Proc. (2021) 96(4):989–1005. doi: 10.1016/j.mayocp.2021.01.009
57. Kolivras A, Dehavay F, Delplace D, Feoli F, Meiers I, Milone L, et al. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. (2020) 6(6):489–92. doi: 10.1016/j.jdcr.2020.04.011
58. Hubiche T, Cardot-Leccia N, Duff FL, Seitz-Polski B, Giordana P, Chiaverini C, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. (2021) 157(2):202–6. doi: 10.1001/jamadermatol.2020.4324
59. Sajjan VV, Lunge S, Swamy MB, Pandit AM. Livedo reticularis: a review of the literature. Indian Dermatol Online J. (2015) 6(5):315–21. doi: 10.4103/2229-5178.164493
60. Verheyden M, Grosber M, Gutermuth J, Velkeniers B. Relapsing symmetric livedo reticularis in a patient with COVID-19 infection. J Eur Acad Dermatol Venereol. (2020) 34(11):e684–6. doi: 10.1111/jdv.16773
61. Droesch C, Do MH, DeSancho M, Lee EJ, Magro C, Harp J. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol. (2020) 156(9):1022–4. doi: 10.1001/jamadermatol.2020.2800
62. Sadeghzadeh-Bazargan A, Rezai M, Najar Nobari N, Mozafarpoor S, Goodarzi A. Skin manifestations as potential symptoms of diffuse vascular injury in critical COVID-19 patients. J Cutan Pathol. (2021) 48(10):1266–76. doi: 10.1111/cup.14059
63. Sangle S, D'Cruz DP, Hughes GR. Livedo reticularis and pregnancy morbidity in patients negative for antiphospholipid antibodies. Ann Rheum Dis. (2005) 64(1):147–8. doi: 10.1136/ard.2004.020743
64. Laza R, Musta VF, Nicolescu ND, Marinescu AR, Mocanu A, Vilceanu L, et al. Cutaneous manifestations in COVID-19: report on 31 cases from five countries. Biology (Basel). (2021) 10(1):54. doi: 10.3390/biology10010054
65. Wong K, Farooq Alam Shah MU, Khurshid M, Ullah I, Tahir MJ, Yousaf Z. COVID-19 associated vasculitis: a systematic review of case reports and case series. Ann Med Surg (Lond). (2022) 74:103249. doi: 10.1016/j.amsu.2022.103249
66. De Giorgi V, Recalcati S, Jia Z, Chong W, Ding R, Deng Y, et al. Cutaneous manifestations related to coronavirus disease 2019 (COVID-19): a prospective study from China and Italy. J Am Acad Dermatol. (2020) 83(2):674–5. doi: 10.1016/j.jaad.2020.05.073
67. Askin O, Altunkalem RN, Altinisik DD, Uzuncakmak TK, Tursen U, Kutlubay Z. Cutaneous manifestations in hospitalized patients diagnosed as COVID-19. Dermatol Ther. (2020) 33(6):e13896. doi: 10.1111/dth.13896
68. Baergen RN, Heller DS. Placental pathology in COVID-19 positive mothers: preliminary findings. Pediatr Dev Pathol. (2020) 23(3):177–80. doi: 10.1177/1093526620925569
69. Uva L, Miguel D, Pinheiro C, Freitas JP, Marques Gomes M, Filipe P. Cutaneous manifestations of systemic lupus erythematosus. Autoimmune Dis. (2012) 2012:834291. doi: 10.1155/2012/834291
70. Veltri NL, Hladunewich M, Bhasin A, Garland J, Thomson B. De novo antineutrophil cytoplasmic antibody-associated vasculitis in pregnancy: a systematic review on maternal, pregnancy and fetal outcomes. Clin Kidney J. (2018) 11(5):659–66. doi: 10.1093/ckj/sfy0117
71. Hashimoto H, Yuno T. Parvovirus B19-associated purpuric-petechial eruption. J Clin Virol. (2011) 52(3):269–71. doi: 10.1016/j.jcv.2011.08.004
72. Djakovic I, Butorac D, Vucicevic Z, Kosec V, Kuna AT, Lugović-Mihić L. Henoch-Schönlein purpura in the third trimester of pregnancy. Biochem Med (Zagreb). (2018) 28(1):010801. doi: 10.11613/BM.2018.010801
73. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395(10237):1607–8. doi: 10.1016/S0140-6736(20)31094-1
74. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 395(10239):1771–8. doi: 10.1016/S0140-6736(20)31103-X
75. González González F, Cortés Correa C, Peñaranda Contreras E. Cutaneous manifestations in patients with COVID-19: clinical characteristics and possible pathophysiologic mechanisms [manifestaciones cutáneas en pacientes con COVID-19: características clínicas y mecanismos fisiopatológicos postulados]. Actas Dermosifiliogr. (2021) 112(4):314–23. doi: 10.1016/j.adengl.2021.01.024
76. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324(3):259–69. doi: 10.1001/jama.2020.10369
77. Pawar R, Gavade V, Patil N, Mali V, Girwalkar A, Tarkasband V, et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children (Basel). (2021) 8(7):572. doi: 10.3390/children8070572
78. Shaiba LA, More K, Hadid A, Almaghrabi R, Al Marri M, Alnamnakani M, et al. Multisystemic inflammatory syndrome in neonates: a systematic review. Neonatology. (2022) 119(4):405–17. doi: 10.1159/000524202
79. De Rose DU, Pugnaloni F, Calì M, Ronci S, Caoci S, Maddaloni C, et al. Multisystem inflammatory syndrome in neonates born to mothers with SARS-CoV-2 infection (MIS-N) and in neonates and infants younger than 6 months with acquired COVID-19 (MIS-C): a systematic review. Viruses. (2022) 14(4):750. doi: 10.3390/v14040750
80. CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) and Adults (MIS-A): Centers for Disease Control and Prevention. (2022). Available at: https://www.cdc.gov/mis/about.html (Accessed September 29, 2022).
81. Esposito S, Principi N. Multisystem inflammatory syndrome in children related to SARS-CoV-2. Paediatr Drugs. (2021) 23(2):119–29. doi: 10.1007/s40272-020-00435-x
82. Godfred-Cato S, Tsang CA, Giovanni J, Abrams J, Oster ME, Lee EH, et al. Multisystem inflammatory syndrome in infants <12 months of age, United States, May 2020-January 2021 [published correction appears in Pediatr Infect Dis J. 2022 Mar 1;41(3):274]. Pediatr Infect Dis J. (2021) 40(7):601–5. doi: 10.1097/INF.0000000000003149
83. Rekhtman S, Tannenbaum R, Strunk A, Birabaharan M, Wright S, Garg A. Mucocutaneous disease and related clinical characteristics in hospitalized children and adolescents with COVID-19 and multisystem inflammatory syndrome in children. J Am Acad Dermatol. (2021 Feb) 84(2):408–14. doi: 10.1016/j.jaad.2020
84. Kappanayil M, Balan S, Alawani S, Mohanty S, Leeladharan SP, Gangadharan S, et al. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-CoV-2: a case report. Lancet Child Adolesc Health. (2021) 5(4):304–8. doi: 10.1016/S2352-4642(21)00055-9
85. Molloy EJ, Nakra N, Gale C, Dimitriades VR. Lakshminrusimha S. Multisystem inflammatory syndrome in children (MIS-C) and neonates (MIS-N) associated with COVID-19: optimizing definition and management [published online ahead of print, 2022 Sep 1]. Pediatr Res. (2022):1–10. doi: 10.1038/s41390-022-02263-w
86. Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. (2020) 76(17):1947–61. doi: 10.1016/j.jacc.2020.08.056
87. Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. (2020) 183(4):968–981.e7. doi: 10.1016/j.cell.2020.09.016
88. Sharma C, Ganigara M, Galeotti C, Burns J, Berganza FM, Hayes DA, et al. Multisystem inflammatory syndrome in children and kawasaki disease: a critical comparison. Nat Rev Rheumatol. (2021) 17:731–48. doi: 10.1038/s41584-021-00709-9
89. Garcia-Manau P, Garcia-Ruiz I, Rodo C, Sulleiro E, Maiz N, Catalan M, et al. Fetal transient skin edema in two pregnant women with coronavirus disease 2019 (COVID-19). Obstet Gynecol. (2020) 136(5):1016–20. doi: 10.1097/AOG.0000000000004059
90. Piccolo V, Mazzatenta C, Russo T, Morandi F, Bassi A, Argenziano G, et al. Late-onset pustular skin eruption in a healthy neonate born from COVID-positive mother: a coincidence or a new skin sign of the infection? J Eur Acad Dermatol Venereol. (2021) 35(12):e850–2. doi: 10.1111/jdv.17579
91. Perveen S, Millington K, Acharya S, Garg A, Boyar V. Neonate born with ischemic limb to a COVID-19 positive mother: management and review of literature. Case Rep Perinatal Med. (2021) 10(1):20200086. doi: 10.1515/crpm-2020-0086
92. Alario D, Bracaglia G, Franceschini G, Arcangeli F, Mecarini F. Orange discoloration of the skin in mother and newborn with SARS-CoV-2 infection: is hypercarotenosis a sign of COVID-19? J Pediatr Neonatal Individ Med. (2020) 10(1):e100101. doi: 10.7363/100101
Keywords: SARS-CoV-2, pregnant women, neonates, infants, dermatologic patterns, skin
Citation: Young EM (2022) Perinatal/maternal-fetal-infant dermatologic manifestations of SARS-CoV-2. An Overview and Implications for diagnosis, treatment, and prognosis. Front. Pediatr. 10:1071839. doi: 10.3389/fped.2022.1071839
Received: 17 October 2022; Accepted: 24 October 2022;
Published: 2 December 2022.
Edited by:
Balaji Govindaswami, Valley Medical Center Foundation, United StatesReviewed by:
Albert Alhatem, University of Aleppo, SyriaAnne Lucky, Cincinnati Children's Hospital Medical Center, United States
© 2022 Young. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elaine M. Young ZWxhaW5lLm0ueW91bmdtZEBnbWFpbC5jb20=
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Elaine M. Young
Elaine M. Young