- United States Drug Testing Laboratories, Des Plaines, IL, United States
The use, misuse, and abuse of substances are a continued public health concern in this country and around the world. Perinatal exposure to substances of abuse is associated with several long-term negative consequences for the neonate. Limited resources exist to assist perinatal health professionals on this very complex subject. The purpose of this document is to provide additional information about selecting monitoring protocols, the specifics of appropriate testing methodologies, and the interpretation of toxicological findings. Understanding these concepts better allows perinatal healthcare professionals to be a voice for the voiceless in order to protect and enrich lives during this unprecedented opioid epidemic.
1. Introduction
The use, misuse, and abuse of substances, including both prescription opioids and non-prescription opioids, are a continued public health concern (1). Prenatal exposure to these substances may lead to a number of negative health consequences, including neonatal opioid withdrawal syndrome (NOWS), a subcategory of neonatal abstinence syndrome (NAS); premature birth; stillbirth; and an array of other long term negative health consequences (2, 3). Additionally, children of parents suffering from substance use disorders are at a three-fold higher risk of experiencing child maltreatment (4, 5).
A long-standing objective of the HealthyPeople initiative has been to promote an increase of maternal abstinence from illicit substances. HealthyPeople 2030 (6) has targeted an increase from the baseline of 93% of pregnant women reporting abstinence in the National Survey on Drug Use and Health (NSDUH) to 95.3% reporting abstinence by 2030. The most recent findings published in the aggregated 2018–2019 NSDUH was 94.0% [95% CI: 92.9%, 95.6%] (CBHSQ, 2020). This improvement was not statistically significant but it is in the desired direction.
The 2020 NSDUH reported 8.3% (SE 2.05) of pregnant mothers claimed to have used an illicit substance in the past month, which was up from 5.8% (SE 1.04) in 2019 (7). A good portion of these mothers are at or near the poverty level and without private insurance. This highlights the fact that this population is very vulnerable with regard to inadequate access to prenatal care and treatment for substance use disorders and presents an opportunity for public health intervention efforts.
Fulfilling the objectives of HealthyPeople 2030 suggests that perinatal healthcare professionals must understand the scope and extent of prenatal substance exposure (8). Specifically needed are processes to provide effective prevention efforts, identify exposure in both an epidemiological and specific case perspective, recognize medical issues associated with perinatal exposure to substances, provide protection for the infant, and refer the exposed infants for appropriate follow-up when needed (8). To accomplish these objectives, the perinatology professional must obtain credible information. Questionnaires and the analysis of various biological specimen types are currently the two approaches for obtaining perinatal substance exposure information. Using questionnaires to obtain credible perinatal substance exposure information is very difficult due to the potential promotion of stigma and guilt which undermines a patient/healthcare professional trust relationship and the potential legal ramifications. Testing biological specimens have less than perfect sensitivity due primarily to detection window limitations.
Further complicating testing biological specimens is the complexity of the maternal-fetal dyad. The placenta, a temporary organ, resides between the mother and baby serving as an interface. Molecules, including substances of abuse and their metabolites, are transported through the placental barrier through simple diffusion (such as oxygen and carbon dioxide) and more complex transport mechanisms (9). The placenta is also a structure that is capable of metabolizing certain compounds that in some cases varies with gestational age (9). The structure of the human placenta is sufficiently different from other mammals which limits generalizability of the study of transport functions in animal models (9). Random controlled trials of prenatal exposure to substances of abuse are lacking due to the ethical considerations of providing pregnant persons a known toxic compound for research purposes.
Testing of biological specimens to monitor perinatal substance exposure is a very specialized field. Limited resources are available to perinatal health professionals to design perinatal substance exposure-monitoring strategies and assist with interpretation. The author consults routinely in cases where Child Protection Service action was taken based on specimens analyzed without chain of custody, presumptive positive results that have not been confirmed by a sufficiently specific method, and lacking review of the medical record to determine if the positive was due to hospital administered medicine. The aim of this manuscript is three-fold. We will review both commonly available options for perinatal substance monitoring and important concepts to consider when designing a monitoring policy, as well as discuss some frequently asked questions regarding the interpretation of newborn toxicology results.
2. Detection and monitoring of perinatal substance exposure
2.1. Questionnaire
The American College of Obstetricians and Gynecologists (ACOG) recommends screening all pregnant women for substance use with a validated questionnaire for the purpose of intervention and referral (10). There are several validated questionnaires available for use, such as the Drug Abuse Screening Test (DAST-10), the 4P's, Substance Use Risk Profile-Pregnancy, the CRAFFT screening tool, NIDA Quick Screen, and the Wayne Indirect Drug Use Screener (10). Strengths associated with the use of these tools are that they are inexpensive, quick to administer, and can monitor for substance use throughout the entire perinatal period (8, 10). However, limitations include recall bias and under-reporting due to stigma and fear of legal repercussions (8–12). Many unvalidated “local questionnaires” are in use, which may unwittingly negatively impact sensitivity and specificity (13).
2.2. Biological specimen types
2.2.1. Maternal urine
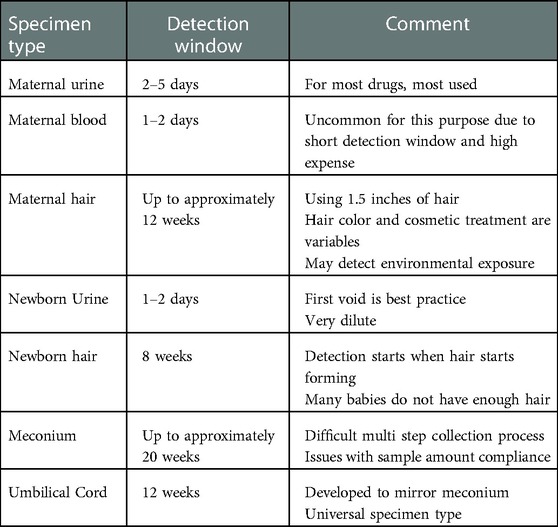
Maternal urine testing is the primary biological specimen type used for monitoring maternal substance use during the perinatal period, including at intake upon arrival at the birthing center (14, 15). Perinatal professionals have used urine testing for many decades. Urine testing has proven to be a reliable specimen type, many laboratories are proficient with the testing procedures, and costs are low compared to other specimen types. Additionally, many clinicians have sufficient experience with the interpretation of the results.
Many laboratories test specimens in a clinical environment as opposed to a forensic environment. Presumptive positive specimens are routinely unconfirmed using a definitive technique, processed without documented chain of custody, and destroyed in a few days regardless of the outcome of the test (which eliminates the possibility of a retest when there is a question about the accuracy of a result). Under these circumstances, these tests are satisfactory to utilize for research or as a screening tool to initiate brief intervention, further testing, or additional monitoring. These specimen results, if not performed using forensic protocols (maintaining a documented chain of custody and automatic confirmation of presumptive positive specimens), should not initiate negative action towards the mother and/or child.
2.2.2. Maternal blood
In this environment, maternal blood is typically not a specimen type of choice for drug testing. The collection protocol is invasive and presents an unnecessary biohazard risk to transportation and laboratory staff; the detection windows are very short (shorter than urine); and the analysis is very expensive. There are new tests that show promise in this environment, such as phosphatidylethanol (PEth) in whole blood or dried blood spots. PEth is a direct ethanol biomarker that detects prenatal ethanol exposure and has a detection window measured in weeks rather than days (16).
2.2.3. Maternal hair
Maternal hair is a specimen type that offers a very long detection window. Analytes incorporate into hair by three main routes. First is environmental exposure. In an environment where a drug is used, smoked, handled, manufactured, or prepared, the environment becomes contaminated, and the drug over time will transfer to the hair. Next is consumption. When a user consumes a drug, the sweat and sebum contain drug and drug metabolites, and as these fluids bathe the hair shaft these analytes deposit on the hair. Lastly, also following consumption, the blood, which contains drug and drug metabolites, deposits the analytes into the root. Once in or on the hair, the analytes bind to proteins and pigments in the hair and remain for an extended period.
Hair testing has several advantages. The collection procedure is simple and noninvasive. The collection may directly observe the donor without gender issues (15, 17). The collector may execute the collection outside of clinical settings. The detection window of drug in hair is months instead of days depending on the source of hair and the compound of interest.
There are several limitations of using hair as a specimen type for drug testing. Cosmetic treatment may interfere with analysis depending on the substance or treatment. These processes contain varying amounts of reducing and/or oxidizing agents, which may alter the structure of the compound of interest (18, 19). The analysis requires a complex specimen preparation, which makes the testing expensive (20). Lastly, there is a potential for observing a positive maternal hair test result due to external contamination or environmental exposure. While providing important information concerning the maternal environment, it does not provide specific evidence of prenatal exposure (15).
2.2.4. Newborn urine
For many years, newborn urine was the primary strategy to objectively identify prenatal drug exposure. The advantages of newborn urine testing are similar to the advantages listed for maternal urine testing, but there are several limitations to testing newborn urine.
Several limitations exist regarding newborn urine to monitor prenatal substance exposure (15). The ideal newborn urine specimen is the neonate's first urine void, and it is difficult to know if the specimen captured was indeed the first void. Missing the first urine void is commonplace. The newborn produces a limited volume of urine with the first void. This results in an excess of specimen rejections due to insufficient quantity for testing. Dilute newborn urine is typical, which shortens an already short detection window even further. Collection protocols are clumsy, and the adhesives are irritating to delicate newborn skin. These limitations led to the development of other testing strategies, such as newborn hair, meconium, and umbilical cord tissue segments as alternatives for monitoring prenatal substance exposure.
2.2.5. Newborn hair
Newborn hair testing offers many of the benefits mentioned in the maternal hair discussion above. Hair forms in the third trimester, and substances and their metabolites may become entrapped in the hair, thus offering a long window of detection (15). References to using newborn hair for prenatal drug exposure appears in the literature (21–24), but its use is not routine. While newborn hair provides a long window of detection and is a simple non-invasive collection process, newborn hair is routinely not present or in sufficient quantity to complete all testing. Approximately one-fourth of all children born do not have sufficient hair for testing. This obstacle limits the use of hair as a primary strategy for most routine prenatal substance monitoring programs (20).
2.2.6. Newborn meconium
The first alternative specimen to routinely replace newborn urine as the specimen of choice for newborn toxicology was meconium (14, 25–28). Meconium is the first fecal matter excreted by the newborn and is a complex and highly variable material composed primarily of mucopolysaccharides, water, bile, salts, bile acids, epithelial cells, and other lipids (29). Meconium begins to form near mid-term of the pregnancy with the majority forming after week 38. As the laboratory equipment and laboratory processes evolved to meet the demand and challenges of high throughput workplace drug testing resulting from the Federal Drug Free Workplace Act in the late 1980s, these processes and equipment were available to develop feasible and practical newborn toxicology testing strategies.
The primary advantage of meconium is the long detection window which includes the entire last trimester. Advantages include a non-invasive collection procedure. Additionally, enough specimen is available for testing in most cases and there are several laboratories available to perform the testing. These advantages have, over time, resulted in meconium becoming the gold standard of newborn toxicology (11).
As with any testing protocol, there are several limitations to using meconium. Limitations include the lack of detection of prenatal exposure in early pregnancy. The detection window is bound by the time of the formation of meconium and the fact that most of the meconium production occurs in the last few weeks, thus diluting earlier use (11). The distribution of analytes in meconium is heterogeneous. Therefore, the ideal collection procedure includes all passages of meconium. The transition from meconium to milk stool can be difficult to discern in some cases. The collection procedure is a multi-step process, which requires multiple collections by multiple collectors over multiple shifts and sometimes over multiple days. Meconium collection can be a very timely and expensive process. Lastly, all laboratories do not have the capability to adequately execute testing on this very difficult specimen type. While there are laboratories available for meconium testing, the number of competent laboratories remains limited.
2.2.7. Newborn umbilical cord
Concheiro and Huestis (11) noted that due to the number of limitations to using meconium in an organization's newborn toxicology program, umbilical cord tissue segment testing was developed as an alternative specimen type to meconium. Testing newborn umbilical cord for substances of abuse has been gaining traction in the newborn toxicology environment over the past 15 years. The development of umbilical cord testing was a direct response to an unacceptable number of meconium specimens rejected or canceled due to low sample volume (30, 31).
Umbilical cord as a specimen for newborn toxicology has several advantages (30–34). There is an abundance of specimen available for each birth, making umbilical cord collection truly universal. Table 1 provides a summary of examples in the literature demonstrating the extent of sample volume compliance when using meconium. The specimen collection and transfer to the laboratory occurs immediately following birth, which improves turnaround times. Analytes appear evenly distributed throughout the entire length of the cord. Analytes in meconium appear heterogeneously distributed, requires collection of the entire passage, and requires mechanical mixing (34, 39). Umbilical cord collection is a simple single-step procedure whereas meconium requires multiple collectors making multiple collections over multiple shifts and/or days.
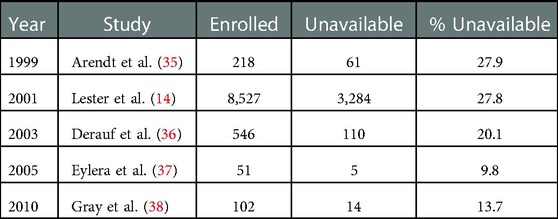
Table 1. Examples in the literature that demonstrate the extent of sample volume compliance using meconium for monitoring substances.
A disadvantage of using umbilical cord is that the concentrations of detectable substances and their metabolites are low, which requires more expensive laboratory equipment to achieve cutoffs that provide adequate sensitivity (30, 31, 40). This makes the analysis more expensive than meconium, but when controlling for the expense of multiple collections, missed opportunities, and turnaround time, the overall expense of using umbilical cord is comparable with meconium.
3. Concepts to consider when designing a perinatal substance monitoring policy
3.1. Questionnaire vs. biological specimen
An important question to ask when developing a newborn toxicology policy for your organization is whether to use a questionnaire and/or biological specimen. Several examples exist in the literature that compare the effectiveness of various self-report questionnaires and various validated biological specimen analyses (41–47). Following a review of the existing literature, the authors compared the rates of self-reported prenatal substance exposure and the presence of corresponding biomarkers using a variety of specimen types (13). In each instance in the literature reviewed, self-reported substance exposure was under-reported when compared to biological specimen analysis (13). Behnke et al. (8) noted that no single monitoring method was perfectly sensitive and specific and therefore recommended coupling questionnaire and biological analysis to improve the probability of identifying perinatal substance exposure.
3.2. Which biological specimen to choose
Take care when selecting the biological specimen type to use in a perinatal substance exposure program. Detection of a substance in a maternal specimen provides evidence of maternal exposure but does not necessarily provide conclusive evidence of substance exposure to the neonate (11). The detection of a substance or its metabolite in a specimen originating from the neonate provides conclusive evidence of prenatal exposure to a substance. Additionally, the Supreme Court of the United States (48) opined that using a maternal specimen that could result in legal repercussions requires the consent of the mother. This rationale does not extend to the specimens obtained from the neonate. The Keeping Children and Families Safe Act (Public Law 108-36) imposed a requirement to report the detection of prenatal illicit drug exposure to the State. Under these conditions, best practices necessitate that these tests, when ordered due to reasonable suspicion, should satisfy basic forensic tenants such as the maintenance of chain of custody and confirmation of presumptive positive results. Table 2 lists advantages and disadvantages for various specimen types used for monitoring prenatal substance exposure.
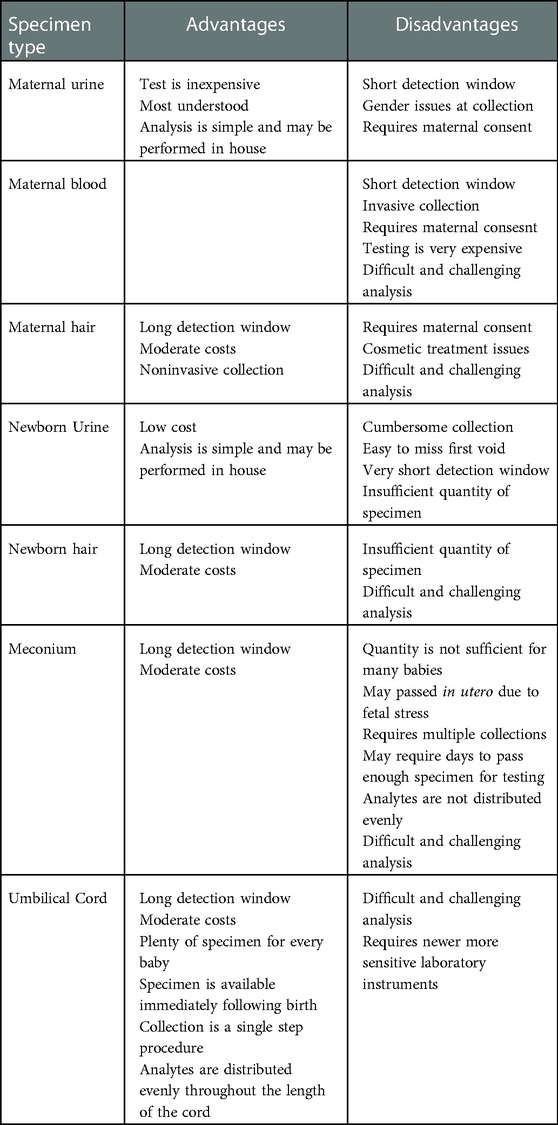
Table 2. Advantages and disadvantages of various specimen types commonly used for monitoring prenatal substance exposure.
3.3. Analysis of biological specimens
An effective substance exposure monitoring program requires a sensitive and specific testing strategy. While some research and clinical environments rely on a single immunoassay or single mass spectrometric protocol, which is adequate under research and/or clinical conditions. Newborn toxicology cases routinely transition from a clinical situation to a forensic situation (49). Policy makers who design workflows that rely on results generated without using commonly accepted forensic standard protocols (maintenance of chain of custody and confirmation of presumptive positive specimens) to reduce costs are acting in a scientifically irresponsible manner.
3.4. Screening or initial testing
Several techniques exist to monitor newborn specimens to preliminarily detect prenatal substance exposure. The most common initial tests utilize the sensitivity, speed, and cost effectiveness of a variety of immunoassay techniques, such as enzyme linked immunosorbent assay (ELISA), enzyme multiplied immunoassay test (EMIT®), cloned enzyme donor immunoassay (CEDIA), Diagnostics Reagents Inc immunoassay (DRI®), or homogenous enzyme immunoassay (HEIA™) (50–52). These methods provide a quick and economical way to identify negative specimens with adequate sensitivity, which in turn allows the laboratory to focus its attention on the presumptive positive specimens.
Several methods exist in the literature and commerce that utilize a mass spectrometric initial test protocol. Most common are liquid chromatography tandem mass spectrometry (LCMSMS) and liquid chromatography time of flight mass spectrometry (LCTOFMS) (11, 39, 53). These techniques allow for a higher degree of specificity over immunoassay at the expense of time and/or cost. However, the laboratory should confirm presumptive positive results obtained from these methods using a second protocol before reporting results to the State.
3.5. Confirmation testing
Once the laboratory obtains a presumptive positive test result by an adequate initial test, a confirmation test follows to confirm the presence of the specific analyte identified with the initial test. Currently, mass spectrometric techniques are the gold standard for this purpose due to the technique's high degree of sensitivity and specificity. Confirmation testing should use a second portion of the original specimen, regardless of the method used for initial testing. This is a best practice to rule out frame shift errors.
3.6. Importance of confirmation testing
Gray and Huestis (15) said, “Confirmation of positive screening results is essential.” Confirmation testing serves two primary purposes. First, the process of confirming an initial presumptive positive test by analyzing a second aliquot (a portion of a specimen used for analysis) mitigates the possibility of a frame shift error or sample switching in the initial testing process. Following a frame shift error during the initial testing process, the confirmation results will not agree with the initial test, thereby alerting the testing personnel of a potential error. Second, the use of two different analytical methodologies or procedures to arrive at the same result dramatically increases the analytical specificity of the entire process. This concept is even more important when considering that newborn biological tests represent a once in a lifetime opportunity to protect and enrich the life of the neonate. The use of a screen and confirm strategy while maintaining a documented chain of custody ensures the integrity of the identity of the specimen and ensures the accuracy of the result, thereby protecting the maternal-child dyad from erroneous results. These are the cornerstone principles of producing a forensically defensible result.
3.7. External oversight
External oversight of laboratory operations is an important best practice in our field, but all external oversight providers are not the same. There are multiple options of external oversight to choose from (such as CLIA, CAP, COLA), and the laboratory may select the oversight provider that best fits its geographic and/or regulatory needs. However, there are a select few options that provide oversight from the context of producing a forensically defensible result [such as CAP-FDT, NYDOH-Forensic Toxicology, ISO17025; (54)].
Clinical laboratories knowingly or unknowingly operating in a forensic environment without appropriate forensic oversight can expose all stakeholders involved to unexpected levels of risk (55). The Ontario Ministry of the Attorney General (55) reported how a well-respected laboratory staffed and managed by a highly competent team from a research and clinical perspective created a situation that required years of litigation and review of thousands of cases over multiple decades. Relevant external oversight would prevent this unfortunate outcome. It is important that newborn toxicology policymakers understand these differences and choose their testing laboratory accordingly.
4. Interpretation of biological specimen test results
Following the receipt of a biological specimen test result, you require an interpretation. Is the reported outcome the result expected? Is there a reasonable explanation for the result? Is a reasonable explanation lacking? These, among others, are very important questions that perinatal professionals address routinely.
4.1. Does a negative result infer abstinence?
A negative result is not conclusive evidence of abstinence. There are many reasons why a particular outcome is negative, especially considering the complex biology of a maternal-fetal system. The most common scenario in the experience of this authoris the test ordered does not include the specific substance in question. Standardization of newborn toxicology testing is currently lacking and each laboratory performing newborn toxicology testing have unique testing panels and cutoffs. The ordering and/or result interpretation professional should be knowledgeable of the substances included in the test ordered. Additionally, a negative result may be due to the use of the substance beyond the detection window of the specimen type or the donor consumed an insufficient amount of the substance to generate a positive result.
4.2. What are the detection windows?
The amount of time represented for a biological specimen result is the detection window or window of detection. Each specimen type has a commonly agreed upon detection window (17, 30, 31, 50). The windows of detection for each specimen type appear previously, and these detection windows appear in Table 3 for convenient comparison.
4.3. Is there a relationship between the reported concentration and the amount of substance consumed?
Several variables influence the observed concentration of any analyte in a reservoir specimen type, a specimen type where analytes may accumulate over time such as umbilical cord, meconium, hair, or urine. Currently, the scientific literature does not support using the reported concentrations to predict the amount of substance ingested, time of ingestion, or the frequency of ingestion (11, 56) even under tightly controlled research conditions (57).
4.4. Are medications provided to the mother or newborn detectable in newborn specimens?
Perinatal professionals should review the results to determine if the positive result aligns with the medical record. Detection of medications provided to the mother prior to birth may occur in newborn specimen types, including medications provided during labor and delivery (58). Medications given to the neonate postnatally may also appear in specimens collected following birth, such as meconium or newborn urine.
4.5. Was chain of custody documented, and was confirmation testing performed?
US physicians must notify state child protective services of prenatal exposure to illegal substances. Knowledge of the consequences of a positive test result creates a dilemma with the performance of reasonable suspicion testing. Under these circumstances testing procedures should include documented chain of custody and automatic confirmation testing of presumptive positives.
4.6. What if a donor refutes a positive test result?
Occasionally, a test result is unexpected, does not align with the case, and/or the mother refutes the result. It is a common practice of accredited forensic laboratories to retain positive specimens in an appropriate storage condition (depending on the type of specimen) for an extended period (typically one year) while maintaining chain of custody of the specimen. The purpose of this policy is to allow for the option of retesting the specimen, at the original laboratory or another designated laboratory, to verify the accuracy of the original reported results. This policy provides a safety net of protection for all stakeholders involved.
5. Conclusion
Maternal use, misuse, and abuse of substances is a very complicated problem that may initiate lifelong negative consequences for the neonate. Huestis and Choo (56) raised the concern years ago that we need to do more for infants exposed to opioids in utero regarding follow-up and appropriate interventions, if needed. It is our responsibility as perinatal healthcare professionals to be aware of the latest developments in the field of toxicology so that we can do the best for the maternal/infant dyad and enrich the lives of those living through the Opioid Epidemic.
Author contribution
JJ conceived and composed this manuscript.
Acknowledgments
I would like to thank Loretta Finnegan for the encouragement to prepare this manuscript. Additionally, I would like to thank Guida Brown for reviewing and editing the paper.
Conflict of interest
JJ is employed by United States Drug Testing Laboratories, a national commercial reference laboratory, that is in the business of selling some of the services mentioned in this manuscript. This relationship did not influence the design or composition of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ko JY, D’Angelo DV, Haight SC, Morrow B, Cox S, von Essen BS, et al. Vital signs: prescription opioid pain reliever use during pregnancy—34 US jurisdictions, 2019. Morb Mortal Wkly Rep. (2020) 69(28):897. doi: 10.15585/mmwr.mm6928a1
2. Sutter MB, Leeman L, Hsi A. Neonatal opioid withdrawal syndrome. Obstet Gynecol Clin. (2014) 41(2):317–34. doi: 10.1016/j.ogc.2014.02.010
3. Whiteman VE, Salemi JL, Mogos MF, Cain MA, Aliyu MH, Salihu HM. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J Pregnancy. (2014) 2014:1–8. doi: 10.1155/2014/906723
4. Chaffin M, Kelleher K, Hollenberg J. Onset of physical abuse and neglect: psychiatric, substance abuse, and social risk factors from prospective community data. Child Abuse Negl. (1996) 20(3):191–203. doi: 10.1016/S0145-2134(95)00144-1
5. Center on Addiction and Substance Abuse (CASA). No safe haven: Children of substance abusing parents. New York: Columbia University (1999).
6. U.S. Department of Health and Human Services (DHHS). Office of Disease Prevention and Health Promotion. HealthyPeople 2030: maternal, infant, and child health. MICH-11.1 Increase abstinence from illicit drugs among pregnant women. Available at: https://health.gov/healthypeople/objectives-and-data/browse-objectives/pregnancy-and-childbirth/increase-abstinence-illicit-drugs-among-pregnant-women-mich-11 (Retrieved October 08, 2022).
7. Center for Behavioral Health Statistics and Quality (CBHSQ). 2020 National survey on drug use and health: Detailed tables. MD, USA: Substance Abuse and Mental Health Services Administration (2017). Available at: https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables (Retrieved September 30, 2022)
8. Behnke M, Smith VC, Levy S, Ammerman S, Gonzalez P, Ryan S, et al. Prenatal substance abuse: short-and long-term effects on the exposed fetus. Pediatrics. (2013) 131(3):e1009–24. doi: 10.1542/peds.2012-3931
9. Blanco-Castañeda R, Galaviz-Hernández C, Souto PC, Lima VV, Giachini FR, Escudero C, et al. The role of xenobiotic-metabolizing enzymes in the placenta: a growing research field. Expert Rev Clin Pharmacol. (2020) 13(3):247–63. doi: 10.1080/17512433.2020.1733412
10. Ecker J, Abuhamad A, Hill W, Bailit J, Bateman BT, Berghella V, et al. Substance use disorders in pregnancy: clinical, ethical, and research imperatives of the opioid epidemic: a report of a joint workshop of the society for maternal-fetal medicine, American college of obstetricians and gynecologists, and American society of addiction medicine. Am J Obstet Gynecol. (2019) 221(1):B5–B28. doi: 10.1016/j.ajog.2019.03.022
11. Concheiro M, Huestis MA. Drug exposure during pregnancy: analytical methods and toxicological findings. Bioanalysis. (2018) 10(8):587–606. doi: 10.4155/bio-2017-0260
12. Chang G. Maternal substance use: consequences, identification, and interventions. Alcohol Res. (2020) 40(2):1–10. doi: 10.35946/arcr.v40.2.06
13. Chiandetti A, Hernandez G, Mercadal-Hally M, Alvarez A, Andreu-Fernandez V, Navarro-Tapia E, et al. Prevalence of prenatal exposure to substances of abuse: questionnaire versus biomarkers. Reprod Health. (2017) 14(1):1–2. doi: 10.1186/s12978-017-0385-3
14. Lester BM, ElSohly M, Wright LL, Smeriglio VL, Verter J, Bauer CR, et al. The maternal lifestyle study: drug use by meconium toxicology and maternal self-report. Pediatrics. (2001) 107(2):309–17. doi: 10.1542/peds.107.2.309
15. Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Anal Bioanal Chem. (2007) 388(7):1455–65. doi: 10.1007/s00216-007-1228-9
16. Finanger T, Spigset O, Gråwe RW, Andreassen TN, Løkken TN, Aamo TO, et al. Phosphatidylethanol as blood biomarker of alcohol consumption in early pregnancy: an observational study in 4,067 pregnant women. Alcohol Clin Exp Res. (2021) 45(4):886–92. doi: 10.1111/acer.14577
17. Concheiro M, Lendoiro E, de Castro A, Gónzalez-Colmenero E, Concheiro-Guisan A, Peñas-Silva P, et al. Bioanalysis for cocaine, opiates, methadone, and amphetamines exposure detection during pregnancy. Drug Test Anal. (2017) 9(6):898–904. doi: 10.1002/dta.2087
18. Takayama N, Tanaka S, Kizu R, Hayakawa K. High-performance liquid chromatography study on effects of permanent wave, dye and decolorant treatments on methamphetamine and amphetamine in hair. Biomed Chromatogr. (1999) 13(4):257–61. doi: 10.1002/(SICI)1099-0801(199906)13:4%3C257::AID-BMC830%3E3.0.CO;2-V
19. Stout P. Hair testing for drugs—challenges for interpretation. Forensic Sci Rev. (2007) 19(2):69–84. PMID: 26247284.26247284
20. Kwong TC, Ryan RM. Detection of intrauterine illicit drug exposure by newborn drug testing. Clin Chem. (1997) 43(1):235–42. doi: 10.1093/clinchem/43.1.235
21. Ursitti F, Klein J, Koren G. Clinical utilization of the neonatal hair test for cocaine: a four-year experience in Toronto. Neonatology. (1997) 72(6):345–51. doi: 10.1159/000244504
22. Bar-Oz B, Klein J, Karaskov T, Koren G. Comparison of meconium and neonatal hair analysis for detection of gestational exposure to drugs of abuse. Arch Dis Child Fetal Neonatal Ed. (2003) 88(2):F98–F100. doi: 10.1136/fn.88.2.F98
23. Vinner E, Vignau J, Thibault D, Codaccioni X, Brassart C, Humbert L, et al. Neonatal hair analysis contribution to establishing a gestational drug exposure profile and predicting a withdrawal syndrome. Ther Drug Monit. (2003) 25(4):421–32. doi: 10.1097/00007691-200308000-00002
24. Garcia-Bournissen F, Rokach B, Karaskov T, Koren G. Methamphetamine detection in maternal and neonatal hair: implications for fetal safety. Arch Dis Child Fetal Neonatal Ed. (2007) 92(5):351–5. doi: 10.1136/adc.2006.100156
25. Ostrea EM Jr, Parks PM, Brady MJ. Rapid isolation and detection of drugs in meconium of infants of drug-dependent mothers. Clin Chem. (1988) 34(11):2372–3. doi: 10.1093/clinchem/34.11.2372a
26. Ostrea EM Jr, Brady MJ, Parks PM, Asensio DC, Naluz A. Drug screening of meconium in infants of drug-dependent mothers: an alternative to urine testing. J Pediatr. (1989) 115(3):474–7. doi: 10.1016/S0022-3476(89)80860-1
27. Moore C, Negrusz A, Lewis D. Determination of drugs of abuse in meconium. J Chromatogr B Biomed Appl. (1998) 713(1):137–46. doi: 10.1016/S0378-4347(97)00479-9
28. Le NL, Reiter A, Tomlinson K, Jones J, Moore C. The detection of oxycodone in meconium specimens. J Anal Toxicol. (2005) 29(1):54–7. doi: 10.1093/jat/29.1.54
29. Concheiro-Guisan A, Concheiro M. Bioanalysis during pregnancy: recent advances and novel sampling strategies. Bioanalysis. (2014) 6(23):3133–53. doi: 10.4155/bio.14.278
30. Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. (2006) 26(1):11–4. doi: 10.1038/sj.jp.7211416
31. Montgomery DP, Plate CA, Jones M, Jones J, Rios R, Lambert DK, et al. Using umbilical cord tissue to detect fetal exposure to illicit drugs: a multicentered study in Utah and New Jersey. J Perinatol. (2008) 28(11):750–3. doi: 10.1038/jp.2008.97
32. Jones J, Rios R, Jones M, Lewis D, Plate C. Determination of amphetamine and methamphetamine in umbilical cord using liquid chromatography–tandem mass spectrometry. J Chromatogr B. (2009) 877(29):3701–6. doi: 10.1016/j.jchromb.2009.09.021
33. Jones J, Magri R, Rios R, Jones M, Plate C, Lewis D. The detection of caffeine and cotinine in umbilical cord tissue using liquid chromatography–tandem mass spectrometry. Anal Methods. (2011) 3(6):1310–5. doi: 10.1039/c0ay00625d
34. Jones JT, Jones M, Jones B, Sulaiman K, Plate C, Lewis D. Detection of codeine, morphine, 6-monoacetylmorphine, and meconin in human umbilical cord tissue: method validation and evidence of in utero heroin exposure. Ther Drug Monit. (2015) 37(1):45. doi: 10.1097/FTD.0000000000000104
35. Arendt RE, Singer LT, Minnes S, Salvator A. Accuracy in detecting prenatal drug exposure. J Drug Issues. (1999) 29:203–14. doi: 10.1177/002204269902900203
36. Derauf C, Katz AR, Easa D. Agreement between maternal self-reported ethanol intake and tobacco use during pregnancy and meconium assays for fatty acid ethyl esters and cotinine. Am J Epidemiol. (2003) 158(7):705–9. doi: 10.1093/aje/kwg215
37. Eyler FD, Behnke M, Wobie K, Garvan CW, Tebbett I. Relative ability of biologic specimens and interviews to detect prenatal cocaine use. Neurotoxicol Teratol. (2005) 27(4):677–87. doi: 10.1016/j.ntt.2005.04.001
38. Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Huestis MA. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine Tob Res. (2010) 12(6):658–64. doi: 10.1093/ntr/ntq068
39. Marin SJ, Christensen RD, Baer VL, Clark CJ, McMillin GA. Nicotine and metabolites in paired umbilical cord tissue and meconium specimens. Ther Drug Monit. (2011) 33(1):80–5. doi: 10.1097/FTD.0b013e3182055f14
40. Moore CM, Brown S, Negrusz A, Tebbett I, Meyer W, Jain L. Determination of cocaine and its major metabolite, benzoylecgonine, in amniotic fluid, umbilical cord blood, umbilical cord tissue, and neonatal urine: a case study. J Anal Toxicol. (1993) 17(1):62. doi: 10.1093/jat/17.1.62
41. Bessa MA, Mitsuhiro SS, Chalem E, Barros MM, Guinsburg R, Laranjeira R. Underreporting of use of cocaine and marijuana during the third trimester of gestation among pregnant adolescents. Addict Behav. (2010) 35(3):266–9. doi: 10.1016/j.addbeh.2009.10.007
42. García-Serra J, Ramis J, Simó S, Joya X, Pichini S, Vall O, et al. Alternative biological materials to detect prenatal exposure to drugs of abuse in the third trimester of pregnancy. An Pediatr (Barc). (2012) 77(5):323–8. doi: 10.1016/j.anpedi.2012.02.019
43. Lendoiro E, González-Colmenero E, Concheiro-Guisán A, de Castro A, Cruz A, López-Rivadulla M, et al. Maternal hair analysis for the detection of illicit drugs, medicines, and alcohol exposure during pregnancy. Ther Drug Monit. (2013) 35(3):296–304. doi: 10.1097/FTD.0b013e318288453f
44. Hutson JR, Magri R, Gareri JN, Koren G. The incidence of prenatal alcohol exposure in Montevideo Uruguay as determined by meconium analysis. Ther Drug Monit. (2010) 32(3):311–7. doi: 10.1097/FTD.0b013e3181dda52a
45. Friguls B, Joya X, Garcia-Serra J, Gómez-Culebras M, Pichini S, Martinez S, et al. Assessment of exposure to drugs of abuse during pregnancy by hair analysis in a Mediterranean island. Addiction. (2012) 107(8):1471–9. doi: 10.1111/j.1360-0443.2012.03828.x
46. García-Algar O, Scaravelli G, Pacifici R, Pichini S. Prenatal exposure to drugs of abuse using meconium analysis in a low socioeconomic population in Barcelona. An Pediatr (Barc). (2008) 70(2):151–8. doi: 10.1016/j.anpedi.2008.08.008
47. Joya X, Gomez-Culebras M, Callejón A, Friguls B, Puig C, Ortigosa S, et al. Cocaine use during pregnancy assessed by hair analysis in a canary islands cohort. BMC Pregnancy Childbirth. (2012) 12(1):1–8. doi: 10.1186/1471-2393-12-2
49. Gray T. Meconium drug testing. In: Levine B, Kerrigan S, editors. Principles of forensic toxicology. 5th ed. Cham, Switzerland: Springer (2020). p. 657–63.
50. Elsohly MA, Stanford D, Murphy T, Lester B, Wright L, Smeriglio V, et al. Immunoassay and GC-MS procedures for the analysis of drugs of abuse in meconium. J Anal Toxicol. (1999) 23:436–45. doi: 10.1093/jat/23.6.436
51. Novikov N, Melanson SE, Ransohoff JR, Petrides AK. Rates of fentanyl positivity in neonatal urine following maternal analgesia during labor and delivery. J Appl Lab Med. (2020) 5(4):686–94. doi: 10.1093/jalm/jfaa027
52. Jones J, Coy D, Mitacek R, Thompson S, Maxwell S. Neonatal cord tox panel and maternal fentanyl exposure: a retrospective chart review. Am J Anal Chem. (2021) 12:324–31. doi: 10.4236/ajac.2021.129020
53. Marin SJ, Metcalf A, Krasowski MD, Linert BS, Clark CJ, Strathmann FG, et al. Detection of neonatal drug exposure using umbilical cord tissue and liquid chromatography time-of-flight mass spectrometry. Ther Drug Monit. (2014) 36(1):119–24. doi: 10.1097/FTD.0b013e3182a0d18c
54. Bissell MG. Regulatory issues in accreditation of toxicology laboratories. Clin Lab Med. (2012) 32(3):525–42. doi: 10.1016/j.cll.2012.06.007
55. Ontario Ministry of the Attorney General. The Honorable Susan B. Lang: Report of the Motherisk Hair Analysis Independent Review (2015). Available at: http://m-hair.ca/docs/default-source/default-document-library/motherisk_enbfb30b45b7f266cc881aff0000960f99.pdf?sfvrsn=2 (Retrieved October 09, 2022).
56. Huestis MA, Choo RE. Drug abuse's Smallest victims: in utero drug exposure. Forensic Sci Int. (2002) 128(1–2):20–30. doi: 10.1016/S0379-0738(02)00160-3
57. Kacinko SL, Jones HE, Johnson RE, Choo RE, Huestis MA. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clin Pharmacol Ther. (2008) 84(5):604–12. doi: 10.1038/clpt.2008.156
Keywords: newborn toxicology, maternal substance use, substance abuse, prenatal drug exposure, umbilical cord testing, meconium testing, forensic testing
Citation: Jones J (2023) Toxicology as a diagnostic tool to identify the misuse of drugs in the perinatal period. Front. Pediatr. 10:1071564. doi: 10.3389/fped.2022.1071564
Received: 16 October 2022; Accepted: 23 December 2022;
Published: 8 February 2023.
Edited by:
Loretta Finnegan, Finnegan Consulting, LLC, United StatesReviewed by:
Pilar Saenz Gonzalez, La Fe Hospital, SpainLeslie Edinboro, Edinboro Consulting, LLC, United States
© 2023 Jones. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Jones Sm9lLmpvbmVzQHVzZHRsLmNvbQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Joseph Jones
Joseph Jones