- 1Shenzhen Children's Hospital, Shenzhen, China
- 2College of Medicine, Shantou University, Shantou, China
Objective: Enteral feeding after intestinal atresia has always been a concern for clinicians. But the present studies mainly focused on single factors. This research aimed to comprehensively analyze the multiple factors on complete enteral nutrition after primary anastomosis, and establish the convenient prediction model.
Methods: We retrospectively collected reliable information in neonates with intestinal atresia form January 2010 to June 2022. The cox regression analysis was performed to select independent risk factors and develop nomogram. Subsequently, ROC curve, calibration curve and decision curve were drawn to thoroughly evaluate the accuracy and applicability of the model.
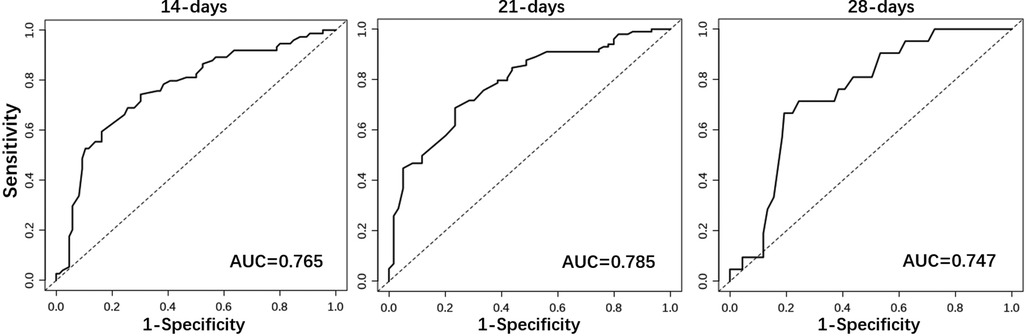
Results: The predictors finally included in the model were gestational age, meconium peritonitis, distance from the anastomosis to the ileocecal region, diameter ratio of proximal to distal bowels, and time of initial feeding. The nomogram of predicting the probability of week 2, week 3 and week 4 was drawn and their area under the curve were 0.765, 0.785 and 0.747, respectively. Similarly, calibration and decision curve indicated that the prediction model had a great prediction performance.
Conclusion: The clinical value of predictive models can be recognized. The hope is that the predictive model can help pediatricians reduce hospital costs and parental anxiety.
Introduction
Intestinal atresia is a congenital disease characterized by the interruption of intestinal continuity. The main clinical manifestations are persistent vomiting, meconium missing and progressive abdominal distension after birth (1, 2). Complete intestinal obstruction can be resolved by immediate surgery, but oral feeding after operation will consume tremendous amount of hospital stay and cost, because children require continuous intravenous nutrition until full enteral feeding (3, 4). However, current studies mainly discussed the influence of the single factor on the recovery of postoperative intestinal function in children, which made it impossible for clinicians to comprehensively consider the prognosis. Therefore, by collecting information of neonates with intestinal atresia, this study expected establishing the clinical prediction model to investigate the risk factors for complete enteral nutrition after primary anastomosis.
Patients and methods
Patients selection
Inclusion criteria: newborns were diagnosed with intestinal atresia (jejunum and ileum) according to the intraoperative performance and postoperative pathology.
Exclusion criteria: incomplete case records; patients had other severe malformations such as gastroschisis and omphalocele; the patient developed serious postoperative complications, such as anastomotic leakage or stenosis; children suffered from serious systemic diseases such as sepsis or hypoxic ischemic encephalopathy during feeding; the operation was performed for enterostomy.
Postoperative feeding strategy
The primary surgery of intestinal atresia is relatively onefold. The continuity of the bowel is reconstructed by removing the abnormal bowels and suturing the two broken ends. For the healing of the anastomosis and the recovery of intestinal function, regular and continuous intravenous nutrition should be used to meet the normal consumption and growth of newborns requiring prolonged fasting (2). According to the children's abdominal manifestations, exhaust and defecation, and abdominal x-ray findings, enteral nutrition will be implemented, and the intake should be gradually increased by observing the feeding tolerance. Significantly, nasal feeding can be temporarily applied for premature babies with poor swallowing function. When the children show feeding intolerance such as bloating and vomiting, pediatricians will avoid increasing the feeding amount, even abandon feeding again (3, 4). Finally, the terminus was identified when the patients were completely removed from parenteral nutrition. In addition, censored data was defined when full enteral nutrition had not been achieved before collection, and the children were withdrawn from feeding because of abandoning treatment or death.
Information collection
This study was approved by Ethics Committee of Shenzhen Children's Hospital (No.202104202). Neonates with enterostomy were not considered because the continuity of bowels were not reestablished. Eventually, a total of 163 cases that met the requirements were retrospectively collected from January 2010 to June 2022 in Shenzhen Children's Hospital. The contents with high credibility were selected, such as gestational age, multifetation, gender, weight, preoperative examination, operation time, location of atresia, mesenteric angiodysplasia, proportion and number of anastomoses, time of first defecation, time of starting feeding, time to complete enteral nutrition, length of hospital stay and complications, etc. The classification of intestinal atresia is based on intestinal septum (type I), fibrous band (type II), mesenteric defect (type IIIa), mesenteric angiodysplasia (type IIIb) and multiple atresia (type IV).
Statistical method
All data were statistically analyzed by SPSS20.0 and R software (version 3.34). Continuous variables satisfying normal distribution were expressed as mean (standard deviation), otherwise median (quartile) was applied. Moreover, categorical variables were represented as frequency (percentage). For further avoiding the potential bias caused by classification, stratified cox regression was applied in this study to eliminate the influence of confounding factors. Therefore, with complete enteral nutrition as the dependent variable, and pathological type as the stratification variable, all potential risk factors were initially screened by univariate COX regression analysis (P < 0.1). Then, the selected variables were subjected to multivariate COX stepwise regression. Meanwhile, visualized nomogram was drawn to facilitate the calculation of the predictive probability. Finally, the area under the curve (AUC) at different time point were calculated to evaluated the accuracy of the prediction model. The applicability of nomogram was assessed by the calibration and decision curve.
Results
General information
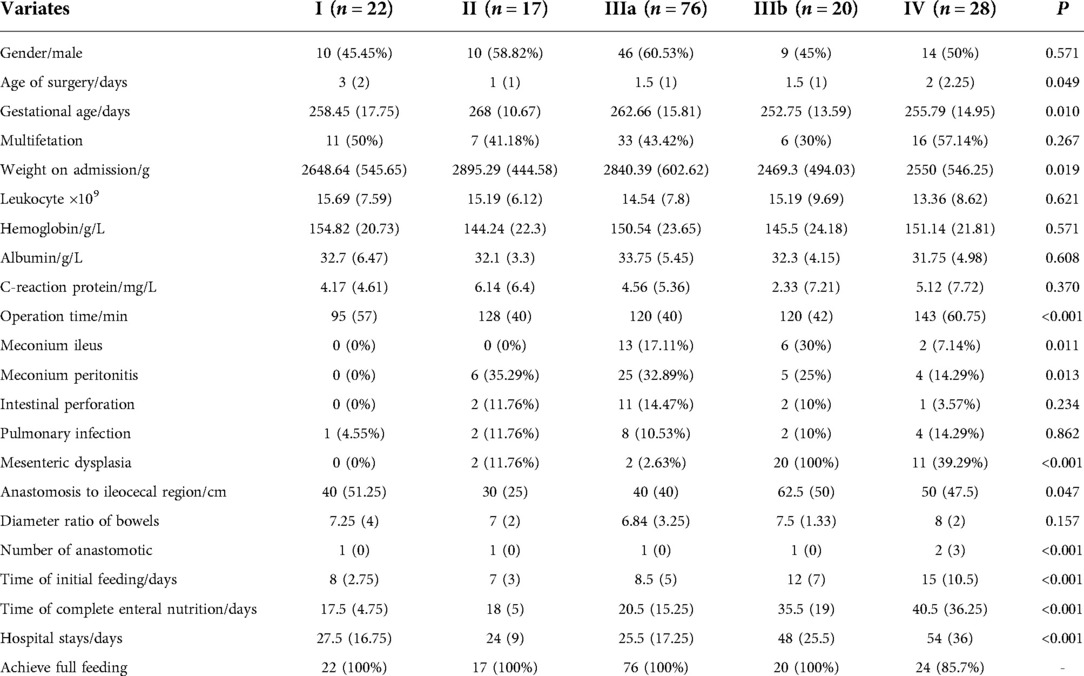
Based on inclusion and exclusion criteria, a total of 163 cases of intestinal atresia were included, of which 4 were interrupted because of treatment abandonment. All cases were divided into 5 groups according to pathological types, including 22 cases of type I, 17 cases of type II, 76 cases of type IIIa, 20 cases of type IIIb and 28 cases of type IV. The clinical characteristics of each group are shown in Table 1. There were no significant differences in gender, multifetation, preoperative blood examination, intestinal perforation, pulmonary infection, and diameter ratio of proximal to distal bowels (P > 0.05).
Univariate analysis
Univariate cox regression analysis was performed for all potential risk factors. The relationship between each variable and complete enteral nutrition was shown in Table 2. Age of surgery, multifetation, weight on admission, preoperative leukocytes, albumin and C-reaction protein, operation time, meconium ileus, intestinal perforation, pulmonary infection, mesenteric dysplasia, and number of anastomosis were initially excluded (P > 0.1).
Multivariate analysis
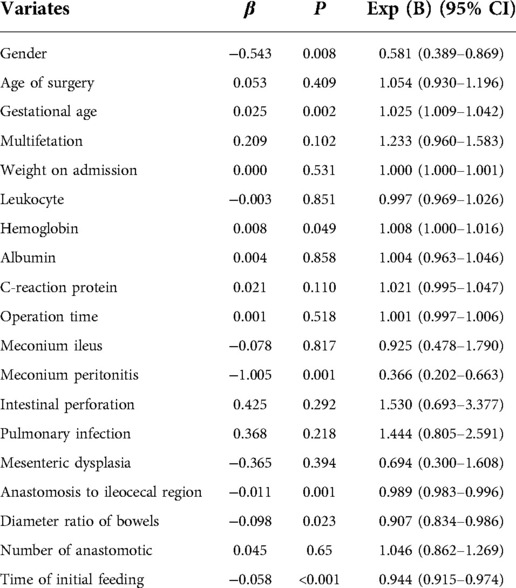
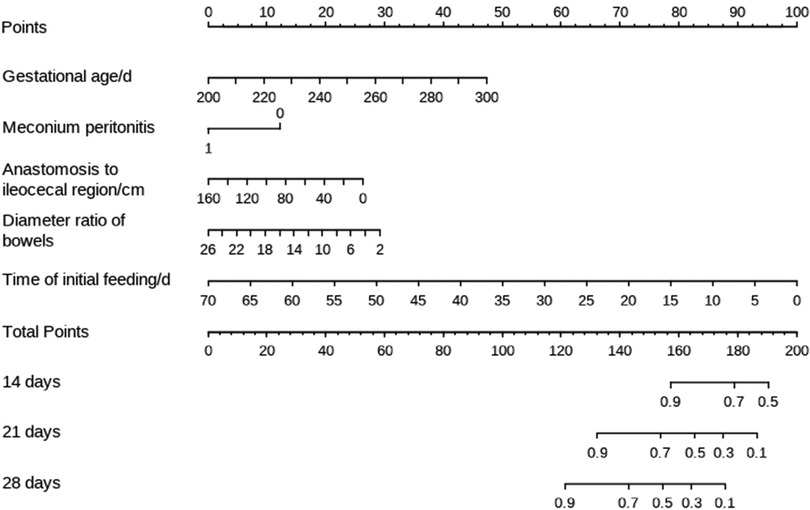
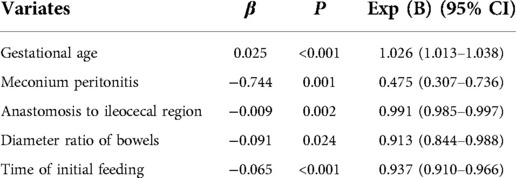
The filtered variables were analyzed again by multivariate stepwise COX regression (forward: LR). The final independent risk factors included in the prediction model were gestational age, meconium peritonitis, distance from the anastomosis to the ileocecal region, diameter ratio of proximal to distal bowels, and time of initial feeding, as shown in Table 3. Meanwhile, the nomogram predicting the weekly probability of achieving complete enteral nutrition was plotted, based on the β coefficient and hazard ratio (Figure 1).
Model evaluation
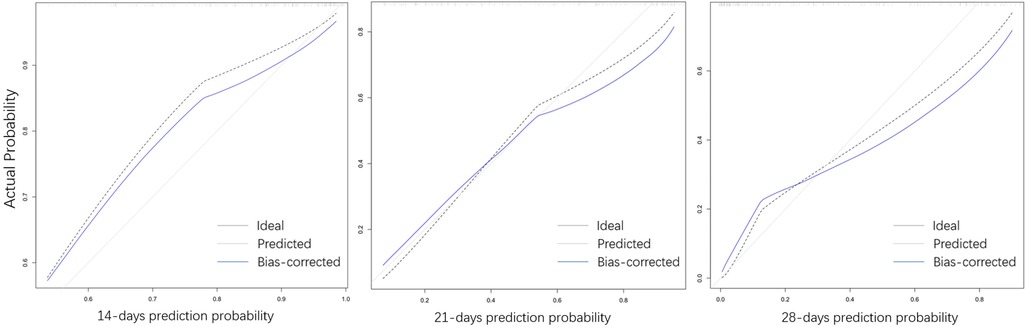
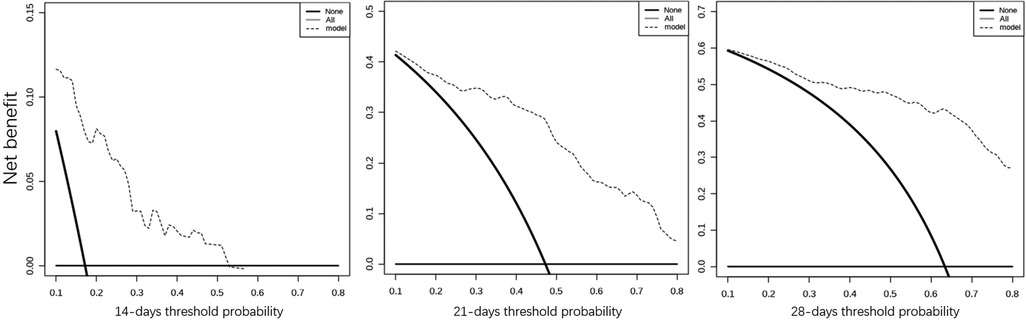
Considering the outcome metrics, the ROC curves were drawn at the time points of week 2, week 3 and week 4 (Figure 2). The areas under the curves of the three were 0.765, 0.785 and 0.747, respectively, which indicates that the accuracy of the model was satisfactory. Then, the calibration curve (Figure 3) drawn by multiple random overall sampling shows that the predicted curve fit well with the ideal curve, which proved that there was no overfitting performance of the prediction model. The decision curve (Figure 4) evaluating the predicted return suggested that the net benefit curve did not intersect the null line, revealing that patients can benefit from the predicted outcome.
Discussion
With the development of prenatal ultrasound, more children with intestinal atresia are identified and treated early, and intestinal anastomosis has been quite skilled for qualified hospitals (5). Besides, mature intravenous nutrition can meet the needs of newborns for a long time. Therefore, the main current obsession for children with intestinal atresia is how to restore enteral nutrition as soon as possible. After all, only after the children get rid of intravenous nutrition can they be considered for discharge (6, 7). Lacking sufficient cases and detailed medical records, the existing studies only focused on the impact of a single factor. In this study, we retrospectively collected the clinical information of 163 children with intestinal atresia after primary anastomosis, so that multivariate analysis of risk factors affecting complete enteral nutrition can be implemented. We hoped that the prediction model can be useful for pediatrician's judgment and intervention, and prognostic inference can also promote communication with family members.
Statistical analysis showed that gestational age, meconium peritonitis, distance from anastomosis to ileocecal region, diameter ratio of proximal to distal bowels, and time of initial feeding were independent risk factors for complete enteral nutrition in children with intestinal atresia after primary anastomosis. For the convenience of computing, the nomogram that directly calculated the weekly probability of achieving enteral nutrition was drawn. Moreover, both calibration and decision curves indicated that the model had a good prediction effect. These results indicate that the clinical prediction model can meet the conditions of clinical application.
The influence of gestational age on feeding has been studied in many studies. Because of the incomplete swallowing function and gastrointestinal development, many infants without gastrointestinal diseases still relies on parenteral nutrition (8, 9). Especially for preterm infants with low birth weight, the feeding strategy will be more conservative in order to prevent neonatal necrotizing enterocolitis (10). In addition, meconium peritonitis is usually caused by perforation of the dilated proximal bowel. The feces entering the abdominal cavity not only greatly increases the chance of infection, but also results in intestinal damage and paralysis, both of which are not conducive to the recovery of the intestinal function. Under the circumstances, many surgeons give priority to enterostomy in the case of possible serious infection (11, 12). Furthermore, the interruption of intestinal continuity makes the distal intestinal tract smaller because of long-term disuse atrophy, while the proximal intestinal tract expands passively due to obstruction. The diameter ratio of proximal and distal intestinal tubes reflects the size of distal intestinal tubes, and the distance from the anastomotic site to the ileocecal region represents the length of the distal slender bowels, all of which impair the efficiency of excretion (13, 14). Many studies have shown that early feeding is beneficial for the recovery of gastrointestinal function. The secretion of hormones and the stimulation of digesta can contribute to peristalsis of the bowel and growth of intestinal villi. At the same time, earlier initiation of feeding leads to faster attainment of complete enteral nutrition, because clinicians tend to gradually increase feeding amounts (15).
There is no significant difference in the operation timing of intestinal atresia because complete intestinal obstruction usually requires emergency surgery (16). Moreover, in order to improve the surgical tolerance of children, surgeons will artificially add albumin and hemoglobin or prophylactic antibiotics, which reduces the impact of abnormal preoperative indicators (17). With the development of anastomotic techniques and materials, anastomotic leakage and stenosis have become minimal. The reason why the number of anastomosis was excluded may be that the increased number did not increase the probability of complications (18, 19). The short mesenteries can generate small and twisted bowels, and it also weaken the blood supply to the intestines. Intriguingly, mesangial dysplasia was ruled out in the initial screening. The possible explanation is that the determination of mesangial dysplasia depends on the subjective judgment of the surgeon, and the corresponding bowels are removed directly in severe cases, or even the treatment is given up (20).
Limited by the low incidence and long follow-up time, the accuracy of the model was only verified by internal cross-validation. Future multicenter studies need to be attempted because external validation can better illustrate the applicability and extrapolation of the prediction model. Meanwhile, the issue about feeding after enterostomy is worth exploring in the future.
In conclusion, this study has obvious reference value on enteral feeding after primary anastomosis of intestinal atresia. Pediatricians can evaluate and intervene timely to achieve compete enteral nutrition quickly and safely, according to the risk factors in the prediction model.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shenzhen Children's Hospital (No.202104202). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
YC and L-dZ proposed the ideas together. L-dZ and LZ were responsible for data collection. Then, YC performed statistical analysis and manuscript writing. Z-yW, A-hG and DX contributed to data organization and discussion. FR and X-pM participated in the modification and submittingof the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1071056/full#supplementary-material.
References
1. Morris G, Kennedy A Jr. Small bowel congenital anomalies: a review and update. Surg Clin North Am. (2022) 102(5):821–35. doi: 10.1016/j.suc.2022.07.012
2. Stanescu AL, Liszewski MC, Lee EY, Phillips GS. Neonatal gastrointestinal emergencies: step-by-step approach. Radiol Clin North Am. (2017) 55(4):717–39. doi: 10.1016/j.rcl.2017.02.010
3. Choi G, Je BK, Kim YJ. Gastrointestinal emergency in neonates and infants: a pictorial essay. Korean J Radiol. (2022) 23(1):124–38. doi: 10.3348/kjr.2021.0111
4. Sujka JA, Weaver KL, Lim JD, Gonzalez KW, Biondo DJ, Juang D, et al. A safe and efficacious preventive strategy in the high-risk surgical neonate: cycled total parenteral nutrition. Pediatr Surg Int. (2018) 34(11):1177–81. doi: 10.1007/s00383-018-4351-0
5. Ju H, Feng S, Huang Y. Diagnostic value of the microcolon using ultrasonography in small bowel atresia. BMC Pediatr. (2022) 22(1):576. doi: 10.1186/s12887-022-03629-z
6. Darmaun D, Lapillonne A, Simeoni U, Picaud JC, Rozé JC, Saliba E, et al. Committee on nutrition of the French society of pediatrics (CNSFP), and French society of neonatology (SFN). parenteral nutrition for preterm infants: issues and strategy. Arch Pediatr. (2018) 25(4):286–94. doi: 10.1016/j.arcped.2018.02.005
7. De Nardo MC, Mario CD, Laccetta G, Boscarino G, Terrin G. Enteral and parenteral energy intake and neurodevelopment in preterm infants: a systematic review. Nutrition. (2022) 97:111572. doi: 10.1016/j.nut.2021.111572
8. McNelis K, Fu TT, Poindexter B. Nutrition for the extremely preterm infant. Clin Perinatol. (2017) 44(2):395–406. doi: 10.1016/j.clp.2017.01.012
9. Lau C. Development of suck and swallow mechanisms in infants. Ann Nutr Metab. (2015) 66(Suppl 5):7–14. doi: 10.1159/000381361
10. Kwok TC, Dorling J, Gale C. Early enteral feeding in preterm infants. Semin Perinatol. (2019) 43(7):151159. doi: 10.1053/j.semperi.2019.06.007
11. Wong CWY, Wong KKY. Meconium peritonitis: a 22-year review in a tertiary referral center. J Pediatr Surg. (2022) 57(8):1504–8. doi: 10.1016/j.jpedsurg.2021.10.006
12. Ferrara F, Angotti R, Burgio A, Di Maggio G, Molinaro F, Messina M. Ileostomy in extremely low birth weight and premature neonates. Minerva Pediatr. (2013) 65(4):411–5.24051974
13. Hosokawa T, Tanami Y, Sato Y, Ishimaru T, Kawashima H, Oguma E. Incidence of late severe intestinal complications after bowel atresia/stenosis. Pediatr Int. (2022) 64(1):e15208. doi: 10.1111/ped.15208
14. Han J, Hao Z, Wang L, Yao T, Fan W, Zhao Z, et al. The role of preserved bowel and mesentery fixation in apple-peel intestinal atresia. BMC Pediatr. (2022) 22(1):407. doi: 10.1186/s12887-022-03475-z
15. Aroonsaeng D, Losty PD, Thanachatchairattana P. Postoperative feeding in neonatal duodenal obstruction. BMC Pediatr. (2022) 22(1):467. doi: 10.1186/s12887-022-03524-7
16. Adams SD, Stanton MP. Malrotation and intestinal atresias. Early Hum Dev. (2014) 90(12):921–5. doi: 10.1016/j.earlhumdev.2014.09.017
17. Basel A, Bajic D. Preoperative evaluation of the pediatric patient. Anesthesiol Clin. (2018) 36(4):689–700. doi: 10.1016/j.anclin.2018.07.016
18. Zheng Z, Jin Z, Gao M, Tang C, Gong Y, Huang L, et al. Comparison of hand-sewn with stapled anastomosis in neonatal intestinal atresia surgery: a randomized controlled study. J Laparoendosc Adv Surg Tech A. (2022) 32(6):696–701. doi: 10.1089/lap.2021.0714
19. Govindarajan KK, Annamalai M. Multiple small bowel atresia: resection or conservation? J Coll Physicians Surg Pak. (2021) 30(6):740–2. doi: 10.29271/jcpsp.2021.06.740
Keywords: intestinal atresia, cox regression, enteral nutrition, primary anastomosis, nomogram
Citation: Chen Y, Zhu L, Zhou L, Guan A, Wang Z, Xiao D, Ma X and Ren F (2022) The multivariate cox regression model for complete enteral nutrition after primary anastomosis in neonates with intestinal atresia. Front. Pediatr. 10:1071056. doi: 10.3389/fped.2022.1071056
Received: 15 October 2022; Accepted: 21 November 2022;
Published: 12 December 2022.
Edited by:
Antonino Morabito, University of Florence, ItalyReviewed by:
Shun Onishi, Kagoshima University, JapanSimone Frediani, Bambino Gesù Children's Hospital (IRCCS), Italy
© 2022 Chen, Zhu, Zhou, Guan, Wang, Xiao, Ma and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-peng Ma MTM0NTAxMjkwN0BxcS5jb20= Feng Ren cmVuZmVuZzIwMDBAc29odS5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 Yang Chen
Yang Chen Le-dao Zhu
Le-dao Zhu Ling Zhou1
Ling Zhou1 Zhi-yong Wang
Zhi-yong Wang