- 1Department of General Surgery, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, Beijing, China
- 2Department of Radiology, Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, Beijing, China
Objective: To compare the differences in clinical features, postoperative complications, and long-term bowel function outcomes of ultrashort-segment Hirschsprung disease (USHD) and short-segment Hirschsprung disease (SHD).
Methods: A retrospective study was conducted to compare patients with USHD or SHD who underwent transanal endorectal pull-through (TEPT) at Beijing Children's Hospital between January 2014 and June 2021. Clinical details were collected from medical records. A long-term bowel function questionnaire (age > 4 years old) was completed by the patients' parents.
Results: A total of 84 patients (USHD = 15, SHD = 69) were included. Age at diagnosis and radical surgery in the USHD group were significantly older than the SHD group (46 [38, 66] vs. 34 [6, 55] months, p = 0.002; 51 [39, 68] vs. 37 [10, 68] months, p = 0.001, respectively). Compared with the SHD group, patients with USHD are more likely to suffer anastomosis leakage and postoperative enterocolitis after TEPT ([3/15, 33.3%] vs. [1/69, 1.4%], p = 0.017; [5/15, 33.3%] vs. [6/69, 8.7%], p = 0.023). In addition, patients in the USHD group are inclined to suffer lower bowel function scores (12.0 [7.5, 18.3] vs. 17 [15, 19], p = 0.018).Patients in the USHD group were more likely to suffer poorer ability to hold back defecation (p = 0.023), soiling (p = 0.011), fecal accidents (p = 0.004), and social problems (p = 0.004).
Conclusion: Compared with patients with SHD, patients with USHD are diagnosed and performed TEPT at an older age. and they are inclined to suffer postoperative enterocolitis, anastomosis leakage, and poorer long-term bowel function following TEPT.
Highlights
• Compared with short-segment Hirschsprung disease (SHD), ultrashort-segment Hirschsprung disease (USHD) was diagnosed and performed radical surgery at an older age.
• USHD are inclined to suffer anastomosis leakage and enterocolitis after TEPT.
• USHD are inclined to suffer anastomosis leakage and enterocolitis after TEPT.
Introduction
Hirschsprung disease (HD), known as aganglionosis, is one of the most common congenital malformations, with an incidence of 1 in 5,000 live births (1, 2). More than 80% of HD patients had aganglionosis restricted to the rectum and sigmoid colon. However, there is minimal knowledge concerning ultrashort-segment HD (USHD), a rare variant of HD (3). So far, there has been no universally accepted definition for USHD. Some scholars considered USHD and internal anal sphincter achalasia (IASA) as the same entity, which could be alleviated by sphincter myotomy or botulinum toxin (4, 5). A recent study proposed that they were utterly different diseases (6, 7). IASA was characterized by normal ganglion cells in the rectal mucosal biopsy but the absence of nitrergic innervation or defective innervation of the neuromuscular junction, resulting in motility dysfunction. Anal sphincter myotomy or botulinum toxin might be the primary treatment (4, 7, 8). However, USHD, presenting as a suddenly dilated bowel without an obvious transition zone on barium enema, is characterized by aganglionosis extending proximal to the distal rectum, and transanal endorectal pull-through (TEPT) might be required (3, 7, 9). In our study, we chose the latter to define USHD.
In recent years, postoperative complications and long-term bowel function recovery of HD has become gradually attracted attention (10, 11). Previous studies had revealed the postoperative complications and long-term bowel function operated by Soave, Duhamel, or Swenson (12, 13). However, few studies were concerned about the treatment and prognosis of different types of HD, especially for patients with USHD. This study was designed to compare the differences in clinical features, postoperative complications, and long-term bowel function outcomes between USHD and SHD following TEPT, aiming to provide guidance for the diagnosis and treatment of USHD.
Materials and methods
Patient selection
Approved by the Ethics Committee of Beijing Children's Hospital, we reviewed the medical records of 84 consecutive patients with rectosigmoid HD who underwent primary TEPT at Beijing Children's Hospital, National Center for Children's Health, between January 2014 and June 2021. The operation was performed by either totally transanal performed TEPT (TTEPT) or laparoscopic/laparotomy-assisted TEPT (LTEPT). All patients underwent Soave TEPT without a stoma by the same surgeon team. Patients who underwent radical surgery in other hospitals or patients without a completely preoperative radiography examination were excluded. Rectal biopsies and postoperative histopathological examination verified the diagnosis of HD. Contrast enemas was used to estimate the extension of the aganglionosis segment in all patients.
Study design
Considering the significant imaging differences between USHD and SHD, the USHD was defined as presenting a suddenly dilated bowel without an obvious transition zone on preoperative barium enema based on the absence of ganglion cells 3–4 cm above the pectinate line by rectal mucosal biopsy. To distinguish the presence of a transitional zone, all preoperative radiographs of HD were independently and randomly reviewed by an experienced pediatric radiologist and two practiced doctors. For the disputed imaging results, these radiography examinations were reviewed again to detect the presence of a transitional zone. Rectosigmoid HD was divided into two groups according to whether a transition zone presented on barium enema: the USHD group was defined as presenting a suddenly dilated bowel without an obvious transition zone (Figures 1A,B), and the short-segment (SHD) group as aganglionosis extending proximal to the rectum and sigmoid with a transition zone (Figures 1C,D). A comparative study was performed to analyze the difference in clinical manifestations, postoperative complications, and long-term bowel function outcomes between the two groups.
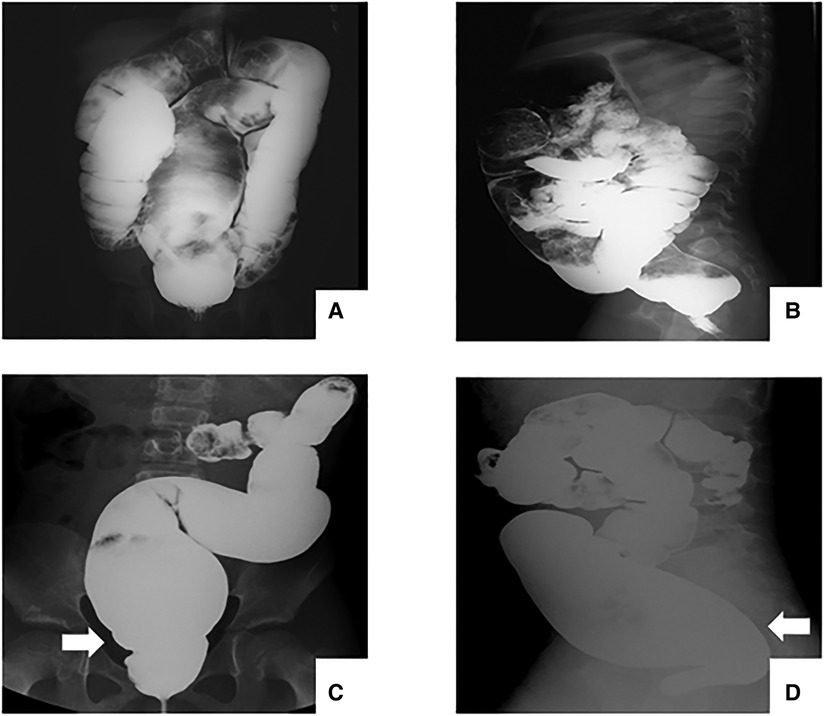
Figure 1. Radiography examination before TEPT. (A-B) patients with USHD shows a suddenly dilated bowel without an obvious transition zone. (C-D) patients with SHD shows a clear transition zone.
Acquisition of data
The patient's characteristics and clinical details were recorded retrospectively from medical records, including gender, birth weight, gestational age, congenital malformations, age at diagnosis, presenting symptoms, surgical details, and postoperative complications.
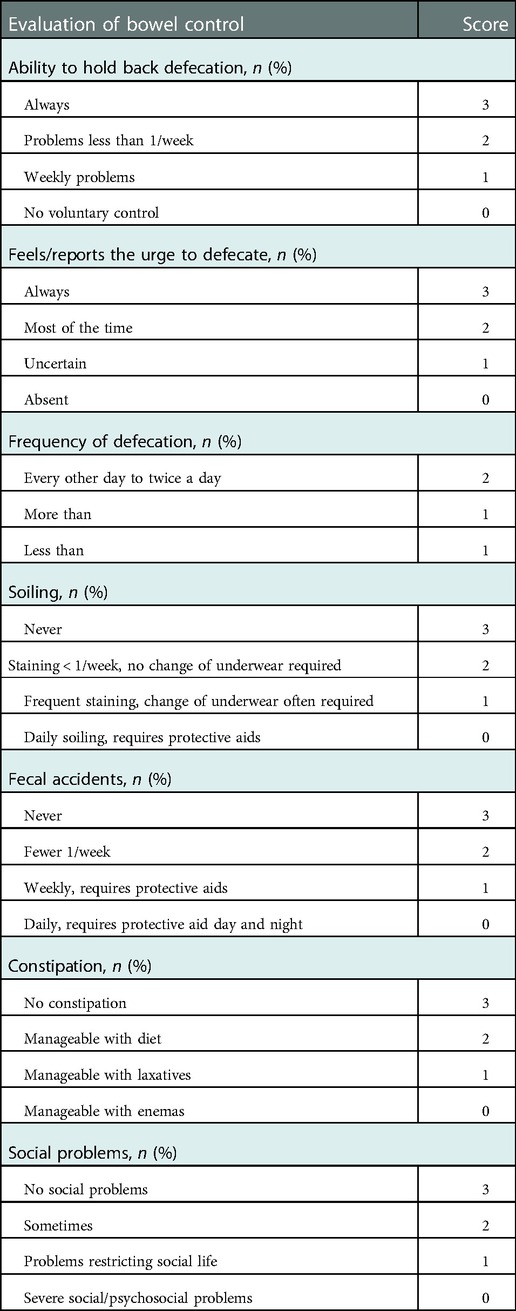
Bowel function outcome was evaluated by a bowel function score (BFS) questionnaire established by 7-item scoring systems with a maximum score of 20 (Table 1). Patients' parents filled out all the questionnaires. The BFS was only assessed when the children were older than 4 years old. Patients were also inquired about the history of enterocolitis and other postoperative complications following TEPT. Enterocolitis was diagnosed when patients presented with clinical signs of bowel inflammation, such as abdominal distension, diarrhea, fever, or lethargy.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics for Statistics ver. 26.0 Software. Data were presented as frequency (percentage) for qualitative variables and median and interquartile range (IQR) for continuous variables. All the statistical tests were two-sided, with a significant level of p < 0.05. Continuous Chi-squared tests or Fisher's exact tests (Fisher's exact if >25% of cell have expected counts less than 5) were applied for categorical variables. Independent sample t-test or Mann-Whitney U test (Mann-Whitney if the data did not meet normal distribution) for continuous variables.
Results
Patient characteristics
A total of 84 patients were enrolled in our study, including 15 patients with USHD and 69 with SHD. The clinical manifestations of all patients before admission are presented in Figure 2. No significant difference was observed between the two groups. Abdominal pain, constipation, and delayed meconium were the predominant clinical manifestations.
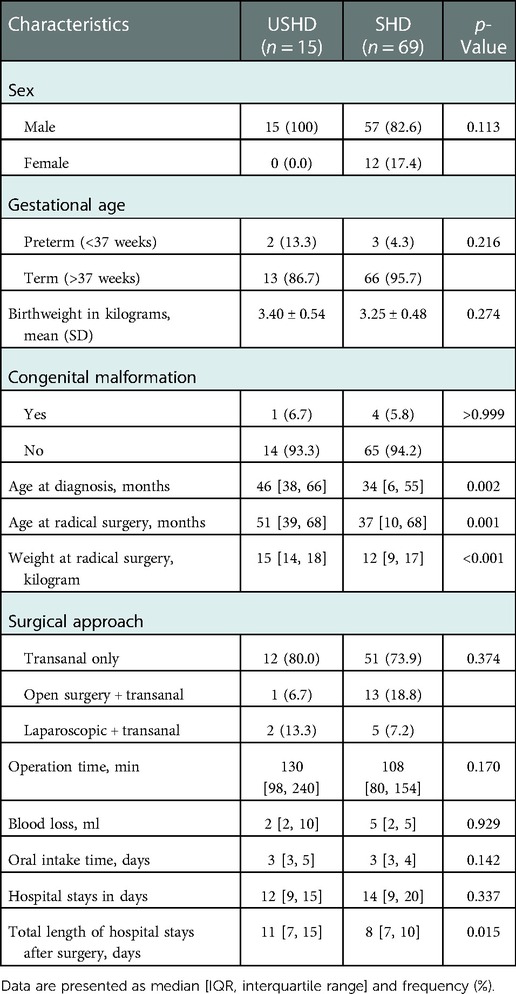
The baseline characteristics of all patients are presented in Table 2. The age at diagnosing HD, age at radical surgery, and weight at radical surgery in the USHD group were significantly higher than in the SHD group, respectively (age at diagnosis, 46 [38, 66] vs. 34 [6, 55] months, p = 0.002; age at surgery, 51 [39, 68] vs. 37 [10, 68] months, p = 0.001; weight at surgery, 15 [14, 18] vs. 12 [9, 17] Kg, p < 0.001). The frequency of TTEPT or LTEPT was similar in both groups. However, there were significantly prolonged hospital days following TEPT in the USHD group despite no statistically significant differences in operation time, blood loss, oral intake time, and hospital days.
Postoperative complications
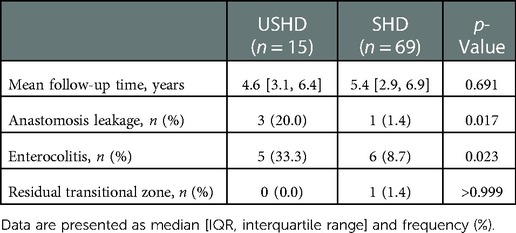
Table 3 shows postoperative complications between the two groups. All the patients were followed up for more than one year with a median follow-up time of 5.3 years (4.9 [3.1, 6.4] vs. 5.4 [2.9, 6.9], p = 0.691). Postoperative complications included anastomotic leakage or stricture, postoperative enterocolitis, and residual transitional zone. Compared with the SHD group, anastomosis leakage after TEPT was more likely to occur in the USHD group ([3/15, 33.3%] vs. [1/69, 1.4%], p = 0.017). These patients with anastomosis leakage often presented fever, abdominal pain, abdominal distention, blood stool, and severe infection, and ileostomies and anastomotic resuturing were performed. In addition, the frequency of postoperative enterocolitis in the USHD group was significantly higher than in the SHD group ([5/15, 33.3%] vs. [6/69, 8.7%], p = 0.023). No significant difference in both groups was observed in terms of residual transitional zone.
Long-term bowel function outcomes
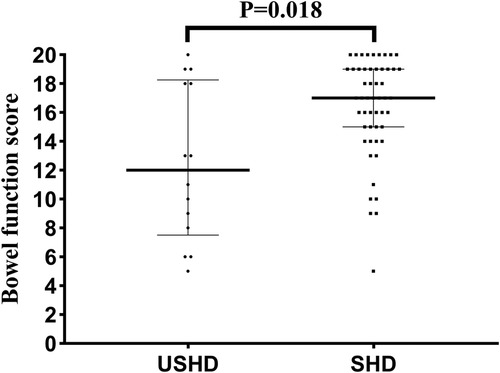
Table 4 presents the results of the BFS questionnaire completed by patients' parents. There was no statistically significant difference between the two groups in terms of follow-up age (8.7 [7.3, 11.9] vs. 7.8 [5.9, 9.4] years, p = 0.119). As shown in Figure 3, the median total bowel function score in the USHD group was significantly lower than the SHD group (12.0 [7.5, 18.3] vs. 17 [15, 19], p = 0.018). Besides, the percentage of poorer bowel function in the USHD group was significantly higher than SHD group (p = 0.01). Further, compared with the SHD group, we found that patients in the USHD group were more likely to suffer poorer ability to hold back defecation (p = 0.023), daily soiling (p = 0.011), fecal accidents (p = 0.004), and social problems (p = 0.004). There was no statistically significant difference between the two groups regarding feeling the urge to defecate, frequency of defecation, and constipation.
Discussion
This is the first single-center retrospective study to show that patients with USHD were diagnosed and performed TEPT at an older age, mainly related to non-specific symptoms. We have also shown that anastomosis leakage and postoperative enterocolitis are more likely to occur in the USHD group rather than SHD group after TEPT. In addition, our results suggest that compared with SHD, a higher proportion of patients with USHD may be predisposed to suffering long-term bowel functional defects.
Our study found that the age at diagnosis and radical surgery in both groups was significantly higher than in previous literature reports (14, 15). It might be related to the fact that most patients with HD in our study did not initially develop serious clinical symptoms, and most of them improved after conservative treatments (glycerine enema, polyethylene glycol, et al.) at local hospitals. Most of them would not be transferred to our center for radical surgery until presenting more severe symptoms. Moreover, the age at diagnosing HD and performing radical surgery in the USHD group was significantly older than in the SHD group. This condition was mainly due to the fact that patients with shorter-segment aganglionosis were more likely to relieve the obstructive symptoms by conservation treatment (3, 9, 16). However, the older age at performing radical surgery was an important factor for more severe dilated colon, leading to a higher frequency of early postoperative complications, which could also explain prolonged hospitalization following TEPT in the USHD group (17, 18).
Regarding postoperative complications, the most striking difference in terms of short-term postoperative complications between the USHD group and the SHD group was the incidence of anastomotic leakage. The frequency of anastomosis leakage in the USHD group (20%) was significantly higher than in the SHD group (1.4%). A plausible explanation for this phenomenon might be related to problems of a severely dilated distal rectum (17, 19). It could significantly contribute to the difficulty in dissection during the dissociation above the dentate line and hemodynamic disorder in the distal bowel, leading to a high incidence of anastomosis leakage (17, 20, 21). Some studies even recommended a preoperative stroma to decompress the distal colon to prevent anastomosis leakage (17, 18). Unlike previous studies, our study did not observe anastomotic stricture following TEPT, which might be related to regular anal dilatation two weeks after radical surgery (22, 23). Enterocolitis is the most common postoperative complication. The incidence of postoperative enterocolitis ranged from 10% to 44% based on heterogeneity in case definitions and geographical differences (24–26). However, the incidence of postoperative HAEC in the USHD group (33.3%) was significantly higher than in the SHD group (8.7%). A possible explanation was related to the higher preoperative enterocolitis frequency in the USHD group (26.7% vs. 13.0%) (15, 26). Preoperative enterocolitis episodes could change the gut microbial system, making patients susceptible to the development of further episodes of enterocolitis (27, 28).
Compared with the SHD group, the patients in the USHD group were inclined to suffer lower bowel function scores, and the percentage of poor bowel function in the USHD group (50%) was significantly higher than in the SHD group (11.5%). After careful analysis of the bowel function of these patients with low scores, we found that fecal incontinence was the main reason for poorer bowel function outcomes. Fecal incontinence, which refers to the poor ability to hold back defecation, daily soiling, and fecal accidents, is the most common problem following pull-through, predisposing children to impaired social functioning and emotional psychosocial well-being (11, 29, 30). The reason could explain the high frequency of fecal incontinence that more prolonged and extensive anal dilatation was required due to a severely dilated bowel below the peritoneal reflection, resulting in excessive stretching of the sphincter during TEPT (29, 31–33). To minimize the damage to the anal sphincter, some studies also advocated LTEPT as the primary treatment for HD rather than TTEPT (11, 29, 33). A previous study found internal anal sphincter defects occurred more often following TEPT by endosonography (32). However, recent studies did not find a significant difference in long-term bowel function outcomes between the two surgical approaches (34,35). In our study, three patients in the USHD group who underwent laparoscopic/laparotomic-assisted TEPT did not achieve good long-term bowel function. The reason was that the dissection of the severely dilated distal rectum was equally tricky whether choosing LTEPT or TTEPT.
It was the first study to identify that USHD was prone to suffering anastomosis leakage, postoperative enterocolitis, and poorer long-term bowel function outcomes than SHD. In addition, all the TEPT procedures were performed by the same surgeon, avoiding deviation due to the discrepancy in surgical details. However, this study has some limitations. First and foremost, there is no universally accepted definition for USHD. In our study, the USHD was defined as s presenting a suddenly dilated bowel without an obvious transition zone without transitional zone on preoperative barium enema based on the absence of ganglion cells of the distal rectum by rectal mucosal biopsy and postoperative histopathology examination. Second, a small number of cases of the USHD group might lead to a potential selection bias. Third, this study was retrospective in nature and included patients treated at a single center, leading to a particular deviation. Last but not least, despite a dropout of 16.7%, it remained unknown whether their bowel function result differed in those who completed and those who did not.
Conclusion
In conclusion, our study suggests that compared with SHD, USHD presenting as a suddenly dilated bowel without an obvious transition zone on barium enema has a greater delay in the age of diagnosis and radical surgery. After TEPT, USHD is more likely to suffer anastomosis leakage, enterocolitis, and poor long-term bowel function outcome, which was related to the problem of a severely distal dilated rectum. For USHD, it is a therapeutic challenge for pediatric surgeons to enhance earlier diagnosis rates and improve long-term bowel function following TEPT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by 2021-E-132-R. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
All authors read and confirmed the final manuscript. CPX and JYY participated the data collections, data analysis, and drafting the article; CPX and GJL contributed to the data collections; YKL contributed to revising the article. YJC was the major surgeon who conducted the surgery and contributed to reviewing and drafting the article. All authors contributed to the article and approved the submitted version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
CPX thanks his parents for always accompanying him to grow up.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ryan ET, Ecker JL, Christakis NA, Folkman J. Hirschsprung's disease: associated abnormalities and demography. J Pediatr Surg. (1992) 27:76–81. doi: 10.1016/0022-3468(92)90111-j
2. Löf Granström A, Svenningsson A, Hagel E, Oddsberg J, Nordenskjöld A, Wester T. Maternal risk factors and perinatal characteristics for hirschsprung disease. Pediatr. (2016) 138:e20154608. doi: 10.1542/peds.2015-4608
3. Butler Tjaden NE, Trainor PA. The developmental etiology and pathogenesis of hirschsprung disease. Trans Res. (2013) 162(1):1–15. doi: 10.1016/j.trsl.2013.03.001
4. Wildhaber BE, Pakarinen M, Rintala RJ, Coran AG, Teitelbaum DH. Posterior myotomy/myectomy for persistent stooling problems in Hirschsprung's Disease. J Pediatr Surg. (2004) 39:920–6. doi: 10.1016/j.jpedsurg.2004.02.016
5. Puri P, Rolle U. Variant Hirschsprung's Disease. Semin Pediatr Surg. (2004) 13:293–9. doi: 10.1053/j.sempedsurg.2004.10.017
6. Meier-Ruge WA, Bruder E, Holschneider AM, Lochbühler H, Piket G, Posselt HG, et al. Diagnosis and therapy of ultrashort Hirschsprung's Disease. Eur J Pediatr Surg. (2004) 14:392–7. doi: 10.1055/s-2004-830354
7. Obata S, Fukahori S, Yagi M, Suzuki M, Ueno S, Ushijima K, et al. Internal anal sphincter achalasia: data from a nationwide survey of allied disorders of Hirschsprung's Disease in Japan. Surg Today. (2017) 47:1429–33. doi: 10.1007/s00595-017-1532-8
8. Friedmacher F, Puri P. Comparison of posterior internal anal sphincter myectomy and intrasphincteric botulinum toxin injection for treatment of internal anal sphincter achalasia: a meta-analysis. Pediatr Surg Int. (2012) 28:765–71. doi: 10.1007/s00383-012-3123-5
9. Neilson IR, Yazbeck S. Ultrashort Hirschsprung's Disease: myth or reality. J Pediatr Surg. (1990) 25:1135–8. doi: 10.1016/0022-3468(90)90748-x
10. Davidson JR, Kyrklund K, Eaton S, Pakarinen MP, Thompson DS, Cross K, et al. Long-term surgical and patient-reported outcomes of hirschsprung disease. J Pediatr Surg. (2021) 56:1502–11. doi: 10.1016/j.jpedsurg.2021.01.043
11. Fosby MV, Stensrud KJ, Bjørnland K. Bowel function after transanal endorectal pull-through for hirschsprung disease - does outcome improve over time? J Pediatr Surg. (2020) 55:2375–8. doi: 10.1016/j.jpedsurg.2020.04.010
12. Onishi S, Nakame K, Kaji T, Kawano M, Moriguchi T, Sugita K, et al. The bowel function and quality of life of hirschsprung disease patients who have reached 18 years of age or older - the long-term outcomes after undergoing the transabdominal soave procedure. J Pediatr Surg. (2017) 52:2001–5. doi: 10.1016/j.jpedsurg.2017.08.036
13. Mao YZ, Tang ST, Li S. Duhamel operation vs. Transanal endorectal pull-through procedure for hirschsprung disease: a systematic review and meta-analysis. J Pediatr Surg. (2018) 53:1710–5. doi: 10.1016/j.jpedsurg.2017.10.047
14. Onishi S, Kaji T, Nakame K, Yamada K, Murakami M, Sugita K, et al. Optimal timing of definitive surgery for Hirschsprung's Disease to achieve better long-term bowel function. Surg Today. (2022) 52(1):92–7. doi: 10.1007/s00595-021-02356-9
15. Pruitt LCC, Skarda DE, Rollins MD, Bucher BT. Hirschsprung-associated enterocolitis in children treated at US children's Hospitals. J Pediatr Surg. (2020) 55:535–40. doi: 10.1016/j.jpedsurg.2019.10.060
16. Rodas A, Barillas S, Ardebol J. Ultrashort-segment hirschsprung disease in a 4-year-old female. J Surg Case Rep. (2020) 2020:320. doi: 10.1093/jscr/rjaa320
17. Stensrud KJ, Emblem R, Bjørnland K. Late diagnosis of hirschsprung disease-patient characteristics and results. J Pediatr Surg. (2012) 47:1874–9. doi: 10.1016/j.jpedsurg.2012.04.022
18. Ekenze SO, Ngaikedi C, Obasi AA. Problems and outcome of Hirschsprung's Disease presenting after 1 year of age in a developing country. World J Surg. (2011) 35(1):22–6. doi: 10.1007/s00268-010-0828-2
19. Podevin G, Lardy H, Azzis O, Branchereau S, Petit T, Sfeir R, et al. Technical problems and complications of a transanal pull-through for Hirschsprung's Disease. Eur J Pediatr Surg. (2006) 16:104–8. doi: 10.1055/s-2006-923995
20. Elhalaby EA, Hashish A, Elbarbary MM, Soliman HA, Wishahy MK, Elkholy A, et al. Transanal one-stage endorectal pull-through for Hirschsprung's Disease: a multicenter study. J Pediatr Surg. (2004) 39:345–51. doi: 10.1016/j.jpedsurg.2003.11.038
21. Lu C, Hou G, Liu C, Geng Q, Xu X, Zhang J, et al. Single-stage transanal endorectal pull-through procedure for correction of hirschsprung disease in neonates and nonneonates: a multicenter study. J Pediatr Surg. (2017) 52:1102–7. doi: 10.1016/j.jpedsurg.2017.01.061
22. Lin Z, Lin Y, Bai J, Wu D, Fang Y. Outcomes of preoperative anal dilatation for hirschsprung disease. J Pediatr Surg. (2021) 56(3):483–6. doi: 10.1016/j.jpedsurg.2020.05.008
23. Soh HJ, Nataraja RM, Pacilli M. Prevention and management of recurrent postoperative Hirschsprung's Disease obstructive symptoms and enterocolitis: systematic review and meta-analysis. J Pediatr Surg. (2018) 53(12):2423–9. doi: 10.1016/j.jpedsurg.2018.08.024
24. Pakarinen M. Perioperative complications of transanal pull-through surgery for Hirschsprung's Disease. Eur J Pediatr Surg. (2018) 28:152–5. doi: 10.1055/s-0038-1632393
25. Roorda D, Oosterlaan J, van Heurn E, Derikx JPM. Risk factors for enterocolitis in patients with hirschsprung disease: a retrospective observational study. J Pediatr Surg. (2021) 56:1791–8. doi: 10.1016/j.jpedsurg.2021.04.020
26. Le-Nguyen A, Righini-Grunder F, Piché N, Faure C, Aspirot A. Factors influencing the incidence of hirschsprung associated enterocolitis (HAEC). J Pediatr Surg. (2019) 54(5):959–63. doi: 10.1016/j.jpedsurg.2019.01.026
27. Demehri FR, Frykman PK, Cheng Z, Ruan C, Wester T, Nordenskjöld A, et al. Altered fecal short chain fatty acid composition in children with a history of hirschsprung-associated enterocolitis. J Pediatr Surg. (2016) 51:81–6. doi: 10.1016/j.jpedsurg.2015.10.012
28. Shen DH, Shi CR, Chen JJ, Yu SY, Wu Y, Yan WB. Detection of intestinal bifidobacteria and lactobacilli in patients with Hirschsprung's Disease associated enterocolitis. World J Pediatr. (2009) 5:201–5. doi: 10.1007/s12519-009-0038-x
29. Bjørnland K, Pakarinen MP, Stenstrøm P, Stensrud KJ, Neuvonen M, Granström AL, et al. A nordic multicenter survey of long-term bowel function after transanal endorectal pull-through in 200 patients with rectosigmoid hirschsprung disease. J Pediatr Surg. (2017) 52(9):1458–64. doi: 10.1016/j.jpedsurg.2017.01.001
30. Svetanoff WJ, Kapalu CL, Lopez JJ, Fraser JA, Briggs KB, Rentea RM. Psychosocial factors affecting quality of life in patients with anorectal malformation and hirschsprung disease-a qualitative systematic review. J Pediatr Surg. (2022) 57:387–93. doi: 10.1016/j.jpedsurg.2021.05.004
31. Neuvonen MI, Kyrklund K, Rintala RJ, Pakarinen MP. Bowel function and quality of life after transanal endorectal pull-through for hirschsprung disease: controlled outcomes up to adulthood. Ann Surg. (2017) 265:622–9. doi: 10.1097/SLA.0000000000001695
32. Stensrud KJ, Emblem R, Bjørnland K. Anal endosonography and bowel function in patients undergoing different types of endorectal pull-through procedures for hirschsprung disease. J Pediatr Surg. (2015) 50:1341–6. doi: 10.1016/j.jpedsurg.2014.12.024
33. Langer JC, Rollins MD, Levitt M, Gosain A, Torre L, Kapur RP, et al. American Pediatric surgical association hirschsprung disease interest group. Guidelines for the management of postoperative obstructive symptoms in children with hirschsprung disease. Pediatr Surg Int. (2017) 33:523–6. doi: 10.1007/s00383-017-4066-7
34. Thomson D, Allin B, Long AM, Bradnock T, Walker G, Knight M. Laparoscopic assistance for primary transanal pull-through in Hirschsprung's Disease: a systematic review and meta-analysis. BMJ Open. (2015) 5:e006063. doi: 10.1136/bmjopen-2014-006063
35. Karlsen RA, Hoel AT, Fosby MV, Ertresvåg K, Austrheim AI, Stensrud KJ, et al. Comparison of clinical outcomes after total transanal and laparoscopic assisted endorectal pull-through in patients with rectosigmoid hirschsprung disease. J Pediatr Surg. (2022) 57:69–74. doi: 10.1016/j.jpedsurg.2022.01.011
Keywords: ultrashort-segment hirschsprung disease, transanal endorectal pullthrough, complications, bowel function outcomes, clinical features
Citation: Xie C, Yan J, Guo J, Liu Y and Chen Y (2023) Comparison of clinical features and prognosis between ultrashort-segment and short-segment hirschsprung disease. Front. Pediatr. 10:1061064. doi: 10.3389/fped.2022.1061064
Received: 4 October 2022; Accepted: 9 December 2022;
Published: 6 January 2023.
Edited by:
Chang Xu, Sichuan University, ChinaReviewed by:
Tianqi Zhu, Huazhong University of Science and Technology, ChinaRui Dong, Fudan University, China
© 2023 Xie, Yan, Guo, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajun Chen Y2hlbnlhanVubWRAMTI2LmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 Chuanping Xie
Chuanping Xie Jiayu Yan1,†
Jiayu Yan1,† Yajun Chen
Yajun Chen