- 1Department of Paediatrics: Child and Youth Health, University of Auckland, Auckland, New Zealand
- 2Newborn Metabolic Screening Programme, LabPlus, Te Whatu Ora Te Toka Tumai Auckland, Auckland, New Zealand
- 3Liggins Institute, University of Auckland, Auckland, New Zealand
- 4School of Nursing, Midwifery and Health Practice, Faculty of Health, Victoria University of Wellington, Wellington, New Zealand
Severe and prolonged neonatal hypoglycemia can cause brain injury, while the long-term consequences of mild or transitional hypoglycemia are uncertain. As neonatal hypoglycemia is often asymptomatic it is routine practice to screen infants considered at risk, including infants of mothers with diabetes and those born preterm, small or large, with serial blood tests over the first 12–24 h after birth. However, to prevent brain injury, the gold standard would be to determine if an infant has neuroglycopenia, for which currently there is not a diagnostic test. Therefore, screening of infants at risk for neonatal hypoglycemia with blood glucose monitoring does not meet several screening test principles. Specifically, the long-term neurodevelopmental outcomes of transient neonatal hypoglycemia are not well understood and there is no direct evidence from randomized controlled trials that treatment of hypoglycemia improves long-term neurodevelopmental outcomes. There have been no studies that have compared the long-term neurodevelopmental outcomes of at-risk infants screened for neonatal hypoglycemia and those not screened. However, screening infants at risk of hypoglycemia and treating those with hypoglycaemic episodes to maintain the blood glucose concentrations ≥2.6 mmol/L appears to preserve cognitive function compared to those without episodes. This narrative review explores the evidence for screening for neonatal hypoglycemia, the effectiveness of blood glucose screening as a screening test and recommend future research areas to improve screening for neonatal hypoglycemia. Screening babies at-risk of neonatal hypoglycemia continues to be necessary, but as over a quarter of all infants may be screened for neonatal hypoglycemia, further research is urgently needed to determine the optimal method of screening and which infants would benefit from screening and treatment.
Introduction
Neonatal hypoglycemia is common with 50% of at-risk infants, 15% of all newborns, having one or more low blood glucose concentrations in the first 48 h after birth (1). Severe or prolonged hypoglycemia, while rare, can cause severe brain injury with lifelong disability (2). Transitional hypoglycemia, defined as low blood glucose concentrations in otherwise well late preterm and term neonates, in the absence of metabolic, endocrine or genetic disorders (3), is much more common, and not confined to infants with risk factors. Although the long-term effects are less well understood (4), transitional hypoglycemia is associated with adverse effects on neurodevelopment (5). Neonatal hypoglycemia is commonly asymptomatic, and it is standard practice to screen infants considered at increased risk, including infants of mothers with diabetes (IDM) and those born preterm, large and small for gestational age (LGA, SGA), with serial blood tests in the first 12–24 h after birth (6). However, what began 50 years ago as a pragmatic measure in a small proportion of IDM or growth restricted infants, has expanded through changes to the diagnostic thresholds, expansion of the risk criteria and a rapid increase in the incidence of diabetes in pregnancy to become a routine targeted screening test in more than a quarter of newborn infants, without having been evaluated or formally implemented as a screening programme.
Population screening
Medical screening is the systematic application of a test or inquiry, to identify individuals at sufficient risk of a specific disorder to benefit from further investigation or direct preventive action, among those who have not sought medical attention on account of symptoms of that disorder (7). Targeted or selective screening is the screening of high-risk groups in the population, which may still be at large scale and can be considered as a form of population screening.
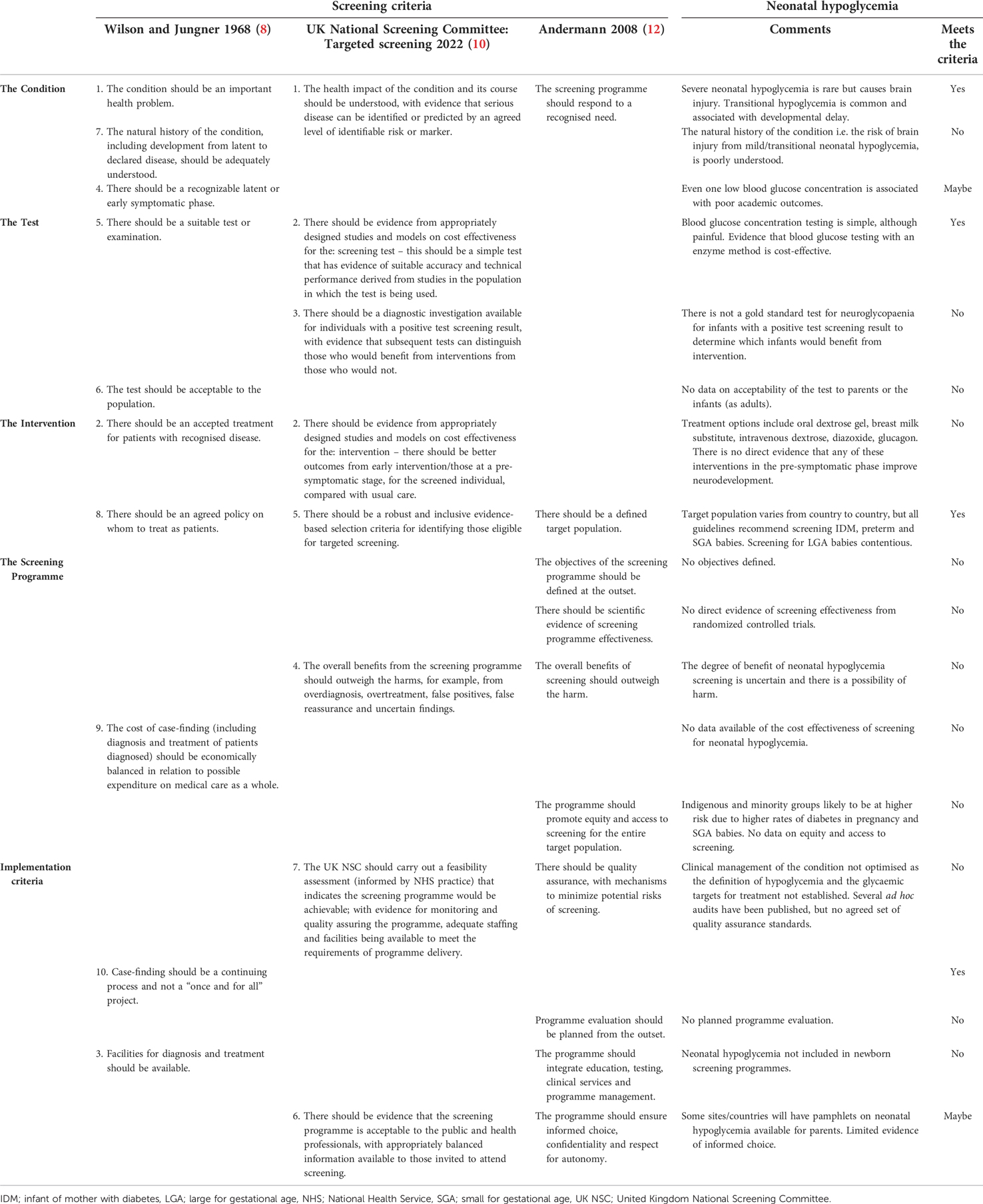
All screening is harmful to some degree, and costly, so before a screening programme is introduced it should meet the principles of a screening programme to ensure that the screening will be more beneficial than harmful (8–10). The original principles outlined by Wilson and Jungner in 1968 (8) have been updated and modified (10–12). The key principles of a screening programme include that the condition should be important, and its natural history well understood; there should be a simple safe and validated test, with an agreed, well defined, cut-off threshold which is acceptable to the target population; the intervention should be effective at improving outcomes when given in the pre-symptomatic phase; and the screening programme should have a clear objective, with data from randomized controlled trials demonstrating its effectiveness at improving outcomes, be equitable and well-resourced with good quality assurance measures.
In the current era there are rigorous steps to ensure a new screening programme meets these criteria before a screening programme is introduced. National or state committees rigorously review the evidence before recommending the introduction of a new screening programme, including screening tests for newborn infants (13). Newborn screening includes population screening not only for various metabolic, immune and endocrine conditions through a routine blood test after birth, but also screening for congenital cyanotic heart disease using pulse oximetry (14); hearing impairment using auditory stimulation; and screening for cataracts and developmental dysplasia of the hip by newborn examination. Some of the conditions screened for in the newborn screening programme, e.g., medium-chain acyl-CoA dehydrogenase deficiency, can cause neonatal hypoglycemia. However, targeted screening of newborn infants at high-risk for neonatal hypoglycemia, including IDM, preterm, SGA and LGA infants, who make up around 30% of all newborn infants (15), is not officially included in national newborn screening programmes.
Screening for neonatal hypoglycemia
History
Symptomatic neonatal hypoglycemia was first associated with poor neonatal outcomes in 1959 (16). In 1965, Cornblath and Reisner described that glucose has been measured in the blood of newborns since 1911, but “there is still disagreement over which levels of blood sugar are normal in the neonate and which are hypoglycaemic or hyperglycaemic” (17). Cornblath and Reisner also noted that low blood glucose concentrations could be observed in IDM and premature infants without obvious symptoms, but that “whether or not low levels of glucose without clinical manifestations produced brain damage remained to be elucidated” (17). While neuroglycopenia causes harm, it still remains to be fully elucidated if asymptomatic neonatal hypoglycemia in at-risk infants is a reliable marker of neuroglycopenia sufficient to cause brain damage. However, evidence with a low grade of certainty, in at-risk asymptomatic infants tested and treated for hypoglycemia, shows an association between hypoglycemia and impaired neurodevelopment (4, 5).
Concern that asymptomatic hypoglycemia may lead to neurodevelopmental sequalae led to the introduction of widespread screening of asymptomatic at-risk (IDM, SGA and asphyxiated) infants for neonatal hypoglycemia in the 1970s (18). Unlike the recommendations for the introduction of screening tests in the modern era (9), a standardised process involving the generation of data from randomized controlled trials on the efficacy and cost-effectiveness of blood glucose concentration screening to prevent brain damage was not performed. Instead, screening for neonatal hypoglycemia was facilitated by the availability of point of care testing which led to the screening of an ill-characterised clinical entity, with little evidence that the infants involved benefitted from screening (18).
Diagnostic thresholds and at-risk groups
In the 1950s, the initial thresholds below which low blood glucose concentrations would not be tolerated were less than 20 mg/100 ml (1.1 mmol/L) in growth restricted and 30 mg/100 ml (1.7 mmol/L) in well grown infants. There was wide variation in the definition of hypoglycemia in term infants among both textbooks and paediatricians in the 1980s, ranging from blood glucose concentrations of <1.0 to <4.0 mmol/L (19). In 1988, two studies defining neonatal hypoglycemia were published, one a secondary analysis of a randomized controlled trial of feeding in preterm infants (20), and the other a small observational study in 17 children, five of whom were neonates (21). Both studies found that a blood glucose concentration below 2.6 mmol/L was associated with worse neurodevelopmental outcomes. This led to a blood glucose concentration of 2.6 mmol/L being widely, although not uniformly, adopted as the blood glucose concentration threshold at which to define hypoglycemia (22). Subsequently, the Pediatric Endocrine Society published guidelines recommending that the threshold be 2.8 mmol/L in the first 48 h after birth, rising to 3.3 mmol/L thereafter (23).
The initial screening selected mainly IDM and SGA infants for blood glucose testing, with a frequency of low blood glucose concentrations of 4.4/1,000 live births in an era when diabetes in pregnancy was rare (24, 25). The criteria expanded over time to include LGA and preterm infants. In addition, some guidelines recommended testing infants of women with obesity and those exposed to maternal beta blocker or antenatal corticosteroid therapy. However, few guidelines recommend testing infants born to pregnant people with pre-eclampsia, despite the original paper from Cornblath in 1959 describing pre-eclampsia as a risk for hypoglycemia (16).
Data on the normal blood glucose concentrations of term newborns was originally published in the 1965, showing an initial dip after birth, with a lower mean blood glucose concentration for approximately the first 2 to 3 days (17). Subsequently, it was reported that 38% of uncomplicated term infants in Nepal had a blood glucose concentration of <2.6 mmol/L in first 50 h. In this paper, hypoglycemia was discussed as being a common problem in Nepal, rather than considering, that at 38% of normal births, this was potentially within the normal range (26). A recent study of uncomplicated term infants, appropriate weight for gestational age and born to non-obese mothers without diabetes confirmed that 39% of infants have at least one blood glucose concentration less than 2.6 mmol/L in the first 5 days after birth (27). This would mean that 40% of normal infants would be considered in need of treatment on the first day after birth according to recommendations by the Pediatric Endocrine Society (23).
Does neonatal hypoglycemia meet the principles for a screening programme?
Screening for neonatal hypoglycemia does not meet the majority of the original or updated principles for a screening test (Table 1).
The condition
Neonatal hypoglycemia is an important health problem (2, 4). Neonatal hypoglycaemia is not a disease in its own right, but a symptom of multiple diseases. Most babies perceived to be at-risk of neonatal hypoglycaemia e.g infants of diabetic mothers, have transitional hypoglycemia due to prolonged postnatal adaptation. In addition, infants with hypoglycemia are all treated to increase the blood glucose concentration, using the same treatments, with the same goal, to prevent brain damage. The natural history of transitional hypoglycemia is reasonably well understood, with resolution and a slightly delayed inflexion point in metabolic transition. However, it is unclear under what conditions there is net clinical benefit from interventions aimed at increasing the blood glucose concentration. At-risk infants with neonatal hypoglycemia who were tested and treated to maintain their blood glucose concentration >2.5 mmol/L had similar risks for neurodevelopmental impairment to at-risk infants who did not develop neonatal hypoglycemia (28–30). However, there are no data on the natural history of infants who had blinded screening for neonatal hypoglycemia and were not diagnosed or treated. Infants in the CHYLD study had blinded CGM measurements of their interstitial glucose concentrations but were also tested and treated for hypoglycemia with intermittent blood glucose concentration monitoring. There was an increased risk of abnormal neurodevelopment in infants with undiagnosed low interstitial glucose concentrations at 4.5 years after birth (29), but not at 2 years after birth or in mid childhood (28, 30).
It is an important principle of screening that there is a recognizable latent or early symptomatic phase. Even one or two low blood glucose concentrations has been associated with neurocognitive impairment (31) and worse academic performance (32), suggesting that there may not be a recognizable latent phase in which treatment of neonatal hypoglycemia can prevent neurodevelopmental impairment. However, screening may be worthwhile even if the initial latent phase is missed if further harm can be prevented.
The test
The test for neonatal hypoglycemia, a capillary blood glucose measurement, analysed by an enzymatic (glucose oxidase or hexokinase) method of analysis is relatively easy to obtain, although painful for the infant. However, there is no gold standard test for neuroglycopenia, a state of low glycolysis in neurons leading to excitotoxicity and reactive oxidative species and eventual neuronal death (33). Therefore, blood glucose concentrations cannot be validated as an effective screening test for neuroglycopenia. Moreover, while the distribution of test values in at-risk infants (IDM, preterm, SGA, LGA) is known (1) the threshold at which neonatal hypoglycemia is defined remains controversial. The accepted threshold ranges from <25 mg/100 ml (1.4 mmol/L) in the first 4 h (6) to <50 mg/100 ml (2.8 mmol/L) in the first 48 h (23). The uncertainty in international guidelines reflects the lack of evidence guiding the threshold at which neonatal hypoglycemia should be defined, as the frequently utilised threshold of 2.6 mmol/L is without scientific justification (6). However, there is reasonable indirect evidence that a blood glucose concentration threshold of 2.6 mmol/L is an adequate operational threshold provided it is used within a proactive framework of close blood glucose monitoring and protocol-based management (28).
The intervention
There are several commonly used interventions to treat neonatal hypoglycemia. However, only a few of the interventions have been shown to be effective at treating neonatal hypoglycemia in randomized controlled trials, including oral dextrose gel (34, 35) and diazoxide (36). The effectiveness of other interventions, including breastmilk substitute, intravenous dextrose, and glucagon (37) has not been determined in randomized controlled trials. However, in a randomized controlled trial of different glycemic treatment targets, infants randomized to maintain a blood glucose concentration of ≥2.6 mmol/L using supplementary oral feeding, tube feeding or intravenous glucose administration, had higher blood glucose concentrations than infants randomized to maintain a blood glucose concentration of ≥2.0 mmol/L (38), confirming that these interventions increase blood glucose concentrations in hypoglycemic newborns. However, while there is evidence that treatment with oral dextrose gel reduces short-term harm (34), currently there is no direct evidence that any intervention for neonatal hypoglycemia improves long term neurodevelopmental outcomes, although treatment with dextrose gel reduces short-term harm (neonatal intensive care unit admission, breastmilk substitute use) without worsening developmental outcomes (39, 40). Of concern, there is some evidence that rapidly increasing the blood glucose concentration after neonatal hypoglycemia is associated with a higher incidence of neurosensory impairment (30, 41).
In addition to uncertainty on the threshold at which neonatal hypoglycemia is defined, there is also uncertainty about the glycemic target that should be maintained with treatment. While most guidelines recommend maintaining the blood glucose concentrations at 2.6 mmol/L, a lower target of 2.0 mmol/L is non-inferior based on neurodevelopmental assessment at 18 months corrected age (38).
Currently, testing for neonatal hypoglycemia is a targeted intervention, with only infants considered at risk for hypoglycemia offered screening. International guidelines are consistent in recommending that IDM, SGA and preterm infants are at increased risk for neonatal hypoglycemia, although the thresholds to define SGA and preterm vary between guidelines (42, 43). IDM and SGA are at increased risk of hyperinsulinaemic hypoglycemia, which not only reduces the blood glucose concentration, but may also reduce alternative cerebral fuels, with ketones largely absent in infants with hypoglycemia in the first 48 h after birth (44).
Whether LGA infants are at increased risk for neonatal hypoglycemia is more contentious, with only half of international/state guidelines considering them at increased risk such as to recommend testing (15). There is no evidence that otherwise healthy LGA infants are at increased risk of neurodevelopmental impairment due to neonatal hypoglycemia (45, 46). A review of cases of neonatal hypoglycemia resulting in brain damage which resulted in litigation, found that all the infants were either IDM or SGA, with none of the infants LGA (47).
The screening programme
The principles for screening programmes assume that the programme is being applied for and is not yet in place and recommend that the objectives of a screening programme should be defined at the outset. However, screening for neonatal hypoglycemia “crept in through the back door”, and despite more than a quarter of all newborns being screened for neonatal hypoglycemia (15), it is not recognised as an official screening programme. Therefore, objectives are often vague or assumed to be the prevention of brain injury. There is also no evidence from randomized controlled trials that screening for neonatal hypoglycemia is effective at reducing mortality or morbidity and no evidence that the screening pathway, which includes multiple painful blood tests, is acceptable to health professionals or the public.
Raffle and Gray have been quoted as saying “All screening programmes do harm. Some do good as well and, of these, some do more good than harm at reasonable cost” (48). Therefore, a key principle of screening programmes is that the benefit gained from the screening programme should outweigh any harms. There is benefit from screening to identify at-risk infants for neonatal hypoglycemia. Severe or prolonged hypoglycemia causes brain injury (2, 47), and even mild or transitional hypoglycemia is associated with neurodevelopmental impairment although the evidence is of low certainty (4, 31). However, the degree of benefit is uncertain, and it is not known how many at-risk infants need to be screened to prevent one case of neurodevelopmental impairment.
As there have been no randomized trials in at-risk infants of testing compared to not testing for neonatal hypoglycemia, there are neither data on the benefits nor the harm of this approach, but there are likely to be harms in unnecessary testing of infants incorrectly identified as at increased risk for hypoglycemia. In addition to multiple painful blood tests, there are also concerns that introducing potentially unnecessary breastmilk substitute may reduce breastfeeding rates (49), contributing to infants at risk of hypoglycemia, such as IDM and preterm infants, being less likely to be exclusively breastfed on discharge from hospital (50, 51). In addition, infants may be potentially unnecessarily separated from their mothers.
Newborn screening programmes have previously been conflated by positive tests for a severe disease for those with a mild or benign variant which causes no long-term effects, with ill effects from the intervention as a result. For example, the first newborn screening programme was for phenylketonuria, where high concentrations of phenylalanine on a dried blood spot were predictive of the condition which could lead to severe intellectual disability if not recognised early and treated with a restricted diet. However, initially it was not recognised that people who are heterozygotes for the mutation have higher than normal concentrations of phenylalanine, which does not cause long term effects, but a restricted diet in these patients could be harmful (52). It is concerning that many infants that are screened for neonatal hypoglycemia, will have one or several low blood glucose concentrations, and as a result receive breastmilk substitute and/or intravenous dextrose, potentially be separated from their mother, resulting in failure to achieve full breastfeeding, with potentially long-term effects on health (53). However, they may have had transitional hypoglycemia that was self-resolving, with blood glucose concentrations less than 2.6 mmol/L common in healthy infants (27), with no effects on their long-term development, i.e., they have been harmed because they were tested for neonatal hypoglycemia.
The potential costs of neonatal hypoglycemia, including postnatal hospital costs and the costs of neurodevelopmental impairment are expensive (54). It is cheaper to use an accurate enzymatic point of care device than a less accurate non-enzymatic device which needs to have low results confirmed at the laboratory (55). Buccal dextrose gel as a treatment for neonatal hypoglycemia reduces the cost for management (56). However, the cost effectiveness of screening for neonatal hypoglycemia has not been established.
Screening is more likely to occur in higher socioeconomic groups with a lower risk of severe disease (9); therefore, it is important that screening programmes are designed to be equitable. There are few data available on whether neonatal hypoglycemia screening is equitable, although in one study there was no difference in adherence to neonatal hypoglycemia screening guidelines by ethnicity in a multi-ethnic population, although adherence was low overall (57). Nevertheless, indigenous and minority groups are likely to be at higher risk of neonatal hypoglycemia due to higher rates of risk factors, including diabetes in pregnancy (58) and preterm birth (59).
Implementation
As with any medical intervention, a screening programme will only be as successful as its implementation. A screening programme should be feasible, with a quality assurance programme, adequate staffing and facilities being available to meet the requirements of programme delivery and should integrate education, testing, clinical services and programme management.
There are currently no agreed set of quality assurance standards for a neonatal hypoglycemia screening programme, nor a plan for monitoring or evaluating the programme. Several audits have shown low adherence to neonatal hypoglycemia guideline recommendations (57, 60). However, it is also important to consider the accuracy of blood glucose analysers, timeliness of results and, appropriate follow-up algorithms including actions after a low blood glucose measurement. Without a gold standard test it is not possible to define true and false screen-positives to calculate standard screening metrics including sensitivity, specificity and positive predictive value.
It is unknown if parents are given the opportunity to make an informed choice regarding neonatal hypoglycemia screening for their baby. While some risk factors are known during pregnancy, such as maternal diabetes, other risk factors, such as preterm birth or SGA, may only be recognised at the time of birth, giving parents little time to make an informed decision about testing for hypoglycemia, which is commonly recommended to begin in the first 1 to 2 h after birth.
Future research areas to improve neonatal hypoglycemia screening
Screening at-risk infants for neonatal hypoglycemia is standard practice, and it would now be difficult, but not impossible, to conduct a randomized controlled trial of screening compared to no screening in infants at risk of neonatal hypoglycemia. Such a trial could be justified on the grounds of the high cost of screening, that screening may be causing harm, and that the current screening approach doesn't prevent severe hypoglycemia. There is a lack of consensus on whether LGA infants whose mothers do not have diabetes in pregnancy benefit from testing for neonatal hypoglycemia. Therefore, in contrast to other at-risk infants, it would be feasible to conduct a randomized controlled trial to assess whether screening and treating LGA infants for neonatal hypoglycemia improves their longer-term neurodevelopment.
It would also be difficult to conduct accurate retrospective studies on screening for neonatal hypoglycemia and neurodevelopmental outcome, as there would be significant confounders between babies who were tested for hypoglycaemia and those who were not tested. More research is also needed to determine the views of parents on screening their infants for neonatal hypoglycemia.
Conclusions
Screening for neonatal hypoglycemia does not meet the principles for a screening test, due to inadequate data on the natural history of transitional hypoglycemia; lack of an agreed, evidence-based definition of damaging hypoglycemia; lack of high quality data on interventions that improve long-term outcomes, and an equitable and quality assured screening programme. However, as at-risk babies are at risk of significant brain injury, testing of babies at increased risk of neonatal hypoglycemia continues to be necessary. Further research is needed to determine which infants benefit from screening for neonatal hypoglycemia.
Author contributions
JA performed the literature search, wrote the original draft and the final paper. NH, DH and CM contributed to the original draft and approved the final paper. JA takes overall responsibility for the work as guarantor. All authors contributed to the article and approved the submitted version.
Funding
Funds for open access publication were received from the University of Auckland.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. (2012) 161(5):787–91. doi: 10.1016/j.jpeds.2012.05.022
2. Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. (2008) 122(1):65–74. doi: 10.1542/peds.2007-2822
3. McKinlay CJD, Alsweiler JM, Bailey MJ, Cutfield WS, Rout A, Harding JE. A better taxonomy for neonatal hypoglycemia is needed. J Perinatol. (2021) 41(5):1205–6. doi: 10.1038/s41372-021-01058-x
4. Shah R, Harding J, Brown J, McKinlay C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology. (2019) 115(2):116–26. doi: 10.1159/000492859
5. Edwards T, Alsweiler JM, Crowther CA, Edlin R, Gamble GD, Hegarty JE, et al. Prophylactic oral dextrose gel and neurosensory impairment at 2-year follow-up of participants in the hPOD randomized trial. JAMA. (2022) 327(12):1149–57. doi: 10.1001/jama.2022.2363
6. Adamkin DH, Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. (2011) 127(3):575–9. doi: 10.1542/peds.2010-3851
8. Wilson JMG, Jungner G. Principles and practice of screening for disease. WHO Chron. (1968) 22(11):281–393.
9. WHO Regional Office for Europe. Screening programmes: a short guide. Increase effectiveness, maximize benefits and minimize harm. Copenhagen: World Health Organisation: Regional Office for Europe. (2020). Report No.: ISBN 978 92 890 5478 2. Available at: https://www.who.int/europe/publications/i/item/9789289054782
10. UK National Screening Committee. Criteria for a population screening programme. (2022). Available at: https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes/criteria-for-appraising-the-viability-effectiveness-and-appropriateness-of-a-screening-programme (Accessed August 4, 2022).
11. Standard Committee on Screening. Population Based Screening Framework. (August 2018). Report No.: ISBN: 978-1-76007-370-1.
12. Andermann A BI, Beauchamp S, Dery V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Clin Exp Hypertens. (2008) 86(4):317–9. doi: 10.2471/BLT.07.050112
13. Advisory Committee on Heritable Disorders in Newborns and Children. Recommended Uniform Screening Panel. (2018). Available at: https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp/index.html (Accessed August 4, 2022).
14. Plana MN, Zamora J, Suresh G, Fernandez-Pineda L, Thangaratinam S, Ewer AK. Pulse oximetry screening for critical congenital heart defects. Cochrane Database Syst Rev. (2018) 3(3):CD011912. doi: 10.1002/14651858.CD011912.pub2
15. O’Brien M, Gilchrist C, Hegarty J, Sadler L, Alsweiler J. Incidence of infants eligible for neonatal hypoglycaemia screening in a postnatal ward setting: systematic review and retrospective observational cohort study. Paediatric society of New Zealand 72nd annual scientific meeting; Virtual (2021).
16. Cornblath M, Odell GB, Levin EY. Symptomatic neonatal hypoglycemia associated with toxemia of pregnancy. J Pediatr. (1959) 55:545–62. doi: 10.1016/S0022-3476(59)80239-0
17. Cornblath M, Reisner SH. Blood glucose in the neonate and its clinical significance. N Engl J Med. (1965) 273(7):378–81. doi: 10.1056/NEJM196508122730707
18. Williams AF. Hypoglycaemia of the newborn: a review. Bull World Health Organ. (1997) 75(3):261–90.9277014
19. Koh TH, Eyre JA, Aynsley-Green A. Neonatal hypoglycaemia–the controversy regarding definition. Arch Dis Child. (1988) 63(11):1386–8. doi: 10.1136/adc.63.11.1386
20. Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. Br Med J. (1988) 297(6659):1304–8. doi: 10.1136/bmj.297.6659.1304
21. Koh THHG, Aynsley-Green A, Tarbit M, Eyre JA. Neural dysfunction during hypoglycemia. Arch Dis Child. (1988) 63:1353–8. doi: 10.1136/adc.63.11.1353
22. Harris DL, Weston PJ, Battin MR, Harding JE. A survey of the management of neonatal hypoglycaemia within the Australian and New Zealand neonatal network. J Paediatr Child Health. 2014 50(10):E55–62. doi: 10.1111/j.1440-1754.2009.01599.x
23. Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, et al. Recommendations from the pediatric endocrine society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr. (2015) 167(2):238–45. doi: 10.1016/j.jpeds.2015.03.057
24. Wilkerson HLC, Remein QR. Studies of abnormal carbohydrate metabolism in pregnancy: the significance of impaired glucose tolerance. Diabetes. (1957) 6(4):324–9. doi: 10.2337/diab.6.4.324
25. Gutberlet RL, Cornblath M. Neonatal hypoglycemia revisited, 1975. Pediatrics. (1976) 58(1):10–7. doi: 10.1542/peds.58.1.10
26. Anderson S, Shakya KN, Shrestha LN, Costello AM. Hypoglycaemia: a common problem among uncomplicated newborn infants in Nepal. J Trop Pediatr. (1993) 39(5):273–7. doi: 10.1093/tropej/39.5.273
27. Harris D, Weston PJ, Gamble G, Harding JE. Glucose profiles in healthy term infants in the first five days: the glucose in well babies (GLOW) study. J Pediatr. (2020) 223:34–41. doi: 10.1016/j.jpeds.2020.02.079
28. Shah R, Dai DWT, Alsweiler JM, Brown GTL, Chase JG, Gamble GD, et al. Association of neonatal hypoglycemia with academic performance in mid-childhood. JAMA. (2022) 327(12):1158–70. doi: 10.1001/jama.2022.0992
29. McKinlay CJD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Peds. (2017) 7:7. doi: 10.1001/jamapediatrics.2017.1579
30. McKinlay CJD, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, et al. Neonatal glycemia and neurodevelopmental outcomes at two years. N Engl J Med. (2015) 373:1507–18. doi: 10.1056/NEJMoa1504909
31. Edwards T, Alsweiler JM, Gamble G, Giffith R, Lin L, McKinlay CJD, et al. Neurocognitive outcomes at two years after neonatal hypoglycemia: cohort analysis of the hPOD randomized trial. JAMA Network Open. (2022) 5(10):e2235989. doi: 10.1001/jamanetworkopen.2022.35989
32. Kaiser JR, Bai S, Gibson N, Holland G, Lin TM, Swearingen CJ, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: a population-based study. JAMA Peds. (2015) 169(10):913–21. doi: 10.1001/jamapediatrics.2015.1631
33. McGowan JE, Chen L, Gao D, Trush M, Wei C. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett. (2006) 399(1–2):111–4. doi: 10.1016/j.neulet.2006.01.034
34. Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. Dextrose gel for neonatal hypoglycaemia (the sugar babies study): a randomised, double-blind, placebo-controlled trial. Lancet. (2013) 382:2077–83. doi: 10.1016/S0140-6736(13)61645-1
35. Edwards T, Liu G, Battin M, Harris DL, Hegarty JE, Weston PJ, et al. Oral dextrose gel for the treatment of hypoglycaemia in newborn infants. Cochrane Database Syst Rev. (2022) 3:CD011027. doi: 10.1002/14651858.CD011027.pub3
36. Laing D, Hanning SM, Harding JE, Mravicich LC, McKinlay CJ. Diazoxide for the treatment of transitional neonatal hypoglycemia: a systematic review. J Neonatol. (2021) 35(4):203–8. doi: 10.1177/09732179211059607
37. Walsh EPG, Alsweiler JM, Ardern J, Hanning SM, Harding JE, McKinlay CJD. Glucagon for neonatal hypoglycaemia: systematic review and meta-analysis. Neonatology. (2022) 119(3):285–94. doi: 10.1159/000522415
38. van Kempen AAMW, Eskes PF, Nuytemans DHGM, van der Lee JH, Dijksman LM, van Veenendaal NR, et al. Lower versus traditional treatment threshold for neonatal hypoglycemia. N Engl J Med. (2020) 382(6):534–44. doi: 10.1056/NEJMoa1905593
39. Harris DL, Alsweiler JM, Ansell JM, Gamble GD, Thompson B, Wouldes TA, et al. Outcome at 2 years after dextrose gel treatment for neonatal hypoglycemia: follow-up of a randomized trial. J Pediatr. (2016) 170:54–9.e2. doi: 10.1016/j.jpeds.2015.10.066
40. Harris D, Gamble G, Harding J. Outcome at 4.5 years after dextrose gel treatment of hypoglycaemia: follow-up of the sugar babies randomised trial. Arch Dis Child Fetal Neonatal Ed. (2022). doi: 10.1136/archdischild-2022-324148
41. Burakevych N, McKinlay CJD, Harris DL, Alsweiler JM, Harding JE. Factors influencing glycaemic stability after neonatal hypoglycaemia and relationship to neurodevelopmental outcome. Sci Rep. (2019) 9(1):8132. doi: 10.1038/s41598-019-44609-1
42. British Association of Perinatal Medicine. Identification and management of neonatal hypocycaemia in the full term infant: framework for practice. (2017). Available at: https://www.bapm.org/sites/default/files/files/Identification%20and%20Management%20of%20Neonatal%20Hypoglycaemia%20in%20the%20%20full%20term%20infant%20-%20A%20Framework%20for%20Practice%20revised%20Oct%202017.pdf (Accessed September 23, 2020).
43. Wight N, Marinelli KA, Academy of Breastfeeding M. ABM Clinical protocol #1: guidelines for blood glucose monitoring and treatment of hypoglycemia in term and late-preterm neonates, revised 2014. Breastfeed Med. (2014) 9(4):173–9. doi: 10.1089/bfm.2014.9986
44. Harris DL, Weston PJ, Harding JE. Lactate, rather than ketones, may provide alternative cerebral fuel in hypoglycaemic newborns. Arch Dis Child Fetal Neonatal Ed. (2015) 100(2):F161–4. doi: 10.1136/archdischild-2014-306435
45. Alsweiler JM, Harris DL, Harding JE, McKinlay CJD. Strategies to improve neurodevelopmental outcomes in babies at risk of neonatal hypoglycaemia. Lancet Child Adolesc Health. (2021) 5(7):513–23. doi: 10.1016/S2352-4642(20)30387-4
46. Brand PLP, Molenaar NLD, Kaaijk C, Wierenga WS. Neurodevelopmental outcome of hypoglycaemia in healthy, large for gestational age, term newborns. Arch Dis Child. (2005) 90(1):78–81. doi: 10.1136/adc.2003.039412
47. Hawdon JM, Beer J, Sharp D, Upton M. NHS Improvement patient safety programme ‘reducing term admissions to neonatal units’. Neonatal Hypoglycaemia. (2017) 102(2):F110–F5. doi: 10.1136/archdischild-2016-310936
48. Pollitt RJ. Evidence or enthusiasm? Why yields from UK newborn screening programmes for congenital hypothyroidism are increasing. Arch Dis Child. (2016) 101(2):120–3. doi: 10.1136/archdischild-2015-309546
49. Harris DL, Gamble GD, Weston PJ, Harding JE. What happens to blood glucose concentrations after oral treatment for neonatal hypoglycemia? J Pediatr. (2017) 190:136–41. doi: 10.1016/j.jpeds.2017.06.034
50. Longmore DK, Barr ELM, Wilson AN, Barzi F, Kirkwood M, Simmonds A, et al. Associations of gestational diabetes and type 2 diabetes during pregnancy with breastfeeding at hospital discharge and up to 6 months: the PANDORA study. Diabetologia. (2020) 63(12):2571–81. doi: 10.1007/s00125-020-05271-9
51. Jonsdottir RB, Jonsdottir H, Skuladottir A, Thorkelsson T, Flacking R. Breastfeeding progression in late preterm infants from birth to one month. Matern Child Nutr. (2020) 16(1):e12893. doi: 10.1111/mcn.12893
52. Wilcken B. The consequences of extended newborn screening programmes: do we know who needs treatment? J Inherit Metab Dis. (2008) 31(2):173–7. doi: 10.1007/s10545-008-0843-8
53. Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. (2016) 387(10017):475–90. doi: 10.1016/S0140-6736(15)01024-7
54. Glasgow MJ, Edlin R, Harding JE. Cost burden and net monetary benefit loss of neonatal hypoglycaemia. BMC Health Serv Res. (2021) 21(1):121. doi: 10.1186/s12913-021-06098-9
55. Glasgow MJ, Harding JE, Edlin R. Cost analysis of cot-side screening methods for neonatal hypoglycaemia. Neonatology. (2018) 114(2):155–62. doi: 10.1159/000489080
56. Glasgow MJ, Harding JE, Edlin R, CHYLD Study Team. Cost analysis of treating neonatal hypoglycemia with dextrose gel. J Pediatr. (2018) 198:151–5.e1. doi: 10.1016/j.jpeds.2018.02.036
57. Alsweiler JM, Gomes L, Nagy T, Gilchrist CA, Hegarty JE. Adherence to neonatal hypoglycaemia guidelines: a retrospective cohort study. J Paediatr Child Health. (2019) 22:22. doi: 10.1111/jpc.14544
58. Yuen L, Wong VW, Simmons D. Ethnic disparities in gestational diabetes. Curr Diab Rep. (2018) 18(9):68. doi: 10.1007/s11892-018-1040-2
59. Edmonds LK, Sibanda N, Geller S, Cram F, Robson B, Filoche S, et al. He tamariki kokoti tau: tackling preterm incidence and outcomes of preterm births by ethnicity in Aotearoa New Zealand 2010–2014. Int J Gynecol Obstet. (2021) 155:239–46. doi: 10.1002/ijgo.13855
Keywords: neonatal, hypoglycemia, screening, neurodevelopment, neuroglycopenia
Citation: Alsweiler JM, Heather N, Harris DL and McKinlay CJD (2022) Application of the screening test principles to screening for neonatal hypoglycemia. Front. Pediatr. 10:1048897. doi: 10.3389/fped.2022.1048897
Received: 20 September 2022; Accepted: 21 November 2022;
Published: 7 December 2022.
Edited by:
Amuchou S. Soraisham, University of Calgary, CanadaReviewed by:
MIchael Narvey, University of Manitoba, CanadaAzanna Ahmad Kamar, University of Malaya, Malaysia
Thomas Meissner, University Hospital of Düsseldorf, Germany
© 2022 Alsweiler, Heather, Harris and McKinlay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jane Alsweiler ai5hbHN3ZWlsZXJAYXVja2xhbmQuYWMubno=
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 J. M. Alsweiler
J. M. Alsweiler N. Heather
N. Heather D. L. Harris
D. L. Harris C. J. D. McKinlay
C. J. D. McKinlay