- Department of Cardiology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China
A 6-year-old girl was diagnosed with Kawasaki disease and bilateral giant coronary artery aneurysms at four months old and was subsequently referred to our hospital due to chest pain and T wave changes on electrocardiography. After admission, stress myocardial perfusion imaging showed reversible ischemia in multiple areas of the left ventricle. Coronary angiography revealed complete proximal segment occlusion of the left circumflex artery (LCX). The occluded LCX was recanalized by a Gaia 3rd micro-wire successfully passing through the occluded section to the distal end of the LCX, followed by sequential balloon dilation and drug-coated balloon angioplasty. Coronary angiography immediately after post-dilation and one-year follow-up angiography showed that the structure and blood flow of LCX was good. Although percutaneous coronary intervention (PCI) in pediatric patients with Kawasaki disease is limited in practice, PCI remains one of the treatment options for selected patients.
Introduction
Kawasaki disease (KD) is a self-limiting vasculitis predominantly occurring in children from 6 months to 5 years old. Coronary artery lesions (CALs) occur in 25% of untreated children and 5%–10% treated children (1, 2). It is reported that 30%–50% of coronary artery aneurysms (CAAs) regressed to normal within two years. However, giant aneurysms with a diameter of ≥8.0 mm rarely revert. Patients with giant aneurysms may develop coronary artery thrombosis even with antiplatelet and anticoagulant therapy. Chronic coronary artery stenosis and occlusion due to thrombus formation in the lumen and/or thickening of the intima results in myocardial ischemia, myocardial infarction, and even sudden death (3). Coronary artery bypass grafting (CABG) is the mainstay therapy for such patients due to the complexity and severity of coronary artery disease caused by KD. The experience of percutaneous coronary intervention (PCI) for children is quite limited, especially in children with chronic total occlusion (CTO) of the coronary artery. Here we report a 6-year-old girl who developed a CTO of her left circumflex artery (LCX) and was successfully recanalized by an antegrade technique followed by drug-coated balloon dilatation.
Case presentation
A 6-year-old girl was referred to our hospital for chest pain with fatigue (CCS class I). She was diagnosed with KD accompanied by coronary lesions at four months of age at a local hospital. Bilateral CAAs further aggravated to 10 mm in the LAD and 8.2 mm in the RCA at week six after onset and then remained stable. She was given oral aspirin and warfarin, with an international normalized ratio maintained at approximately 2.0. However, no further examination was done to evaluate CALs and myocardial ischemia except for regular echocardiography and electrocardiography until 30 months after onset, when coronary computed tomography angiography (CTA) demonstrated a locally dilated left coronary artery with a thickened wall and calcification. The diameters of the left main coronary artery (LMCA), LAD, LCX, and RCA were 3.8, 6.3, 2.6, and 3.7 mm, respectively. As such, warfarin was discontinued while aspirin was commenced at 62.5 mg once daily. She was then followed up irregularly at the local hospital. Six years after onset, a week before admission to our hospital, the patient had chest pain, dyspnea without syncope, and profuse sweating, which resolved after 20 min. An electrocardiogram at the local hospital demonstrated T wave changes. She was referred to our hospital for further evaluation and treatment.
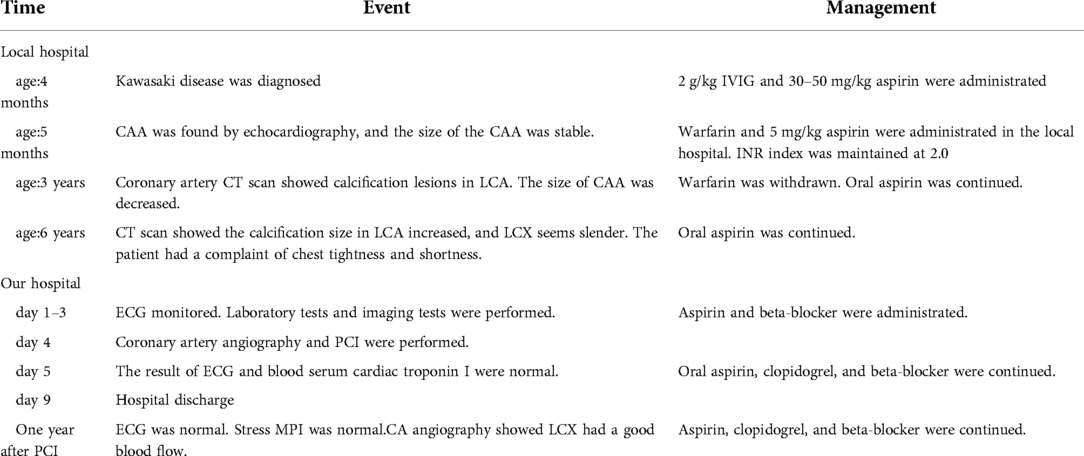
On the day of admission, the patient was in good condition, and her body weight was 19.5 kg. Blood troponin I and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were within normal limits. Electrocardiogram and treadmill test were normal while ATP stressed myocardial perfusion imaging (MPI) demonstrated reversible ischemia in the anterior wall near the apex and inferior, posterior, and lateral walls of the left ventricle (Figures 1A,B). Coronary angiography was performed and revealed a small coronary aneurysm (4.8 mm in diameter) in the LAD with severe calcification around the aneurysm wall, total occlusion of the proximal LCX without calcification, and collateral vessels from the LAD to the distal LCX (Figure 2A). The diameter of the RCA was near average with slight distortion in the main branch, suggesting a regression of previous aneurysms (Figure 2B). Following discussion amongst the cardiac multidisciplinary team (MDT), percutaneous revascularization for occlusive LCX was considered a suitable strategy for this patient. The procedure was performed under general anesthesia via the right femoral artery. She was heparinized (100 µ/kg), and the activated clotting time was monitored every hour, maintained at over 200 s. A 6-French JL3.5 guide catheter (Cordis) was advanced to the opening of the left coronary artery with great effort. A 0.014-in guidewire was introduced to the proximal segment of the LAD for support. A 2.6F (Corsair, Asahi) microcatheter was then introduced to the opening of the LCX (Figure 2A). Afterwards, attempts were made to pass Sion/Fielder XT-R/Fielder XT-A guide wires (Asahi) through the occlusion, but all attempts failed. Ultimately, a Gaia3rd guidewire (Asahi) was successfully passed across the occlusion site (Figure 2B) to the distal LCX (Figure 2C). We gently performed pre-dilations of the occluded lesion with a 2.0 mm × 12 mm Emerge™ PTCA balloon (Boston Scientific) (Figure 2D) and further dilations with a 2.5 mm × 30 mm paclitaxel-coated balloon (Bingo, Yinyi Biological) at 7 atm for 60 s (Figure 2E). The final coronary angiography showed that the occluded segment of the LCX was successfully recanalized, with the contrast agent evenly filling and no arterial dissection detected (Figure 2F). The procedure concluded free of complications. After the procedure, the child continued to be administered aspirin 100 mg and clopidogrel 20 mg per day. Postoperative troponin I remained normal, and color doppler ultrasound showed good blood flow in the LCX. She was discharged from the hospital five days after the procedure. At one year of follow-up, the patient had no symptoms with good exercise tolerance. Her electrocardiogram, treadmill test and ATP stressed MPI were normal. Coronary artery angiography demonstrated good blood flow in the LCX (Figures 3A,B) and the complete blood count as within normal limits. The timeline of therapy for this patient is listed in (Table 1).
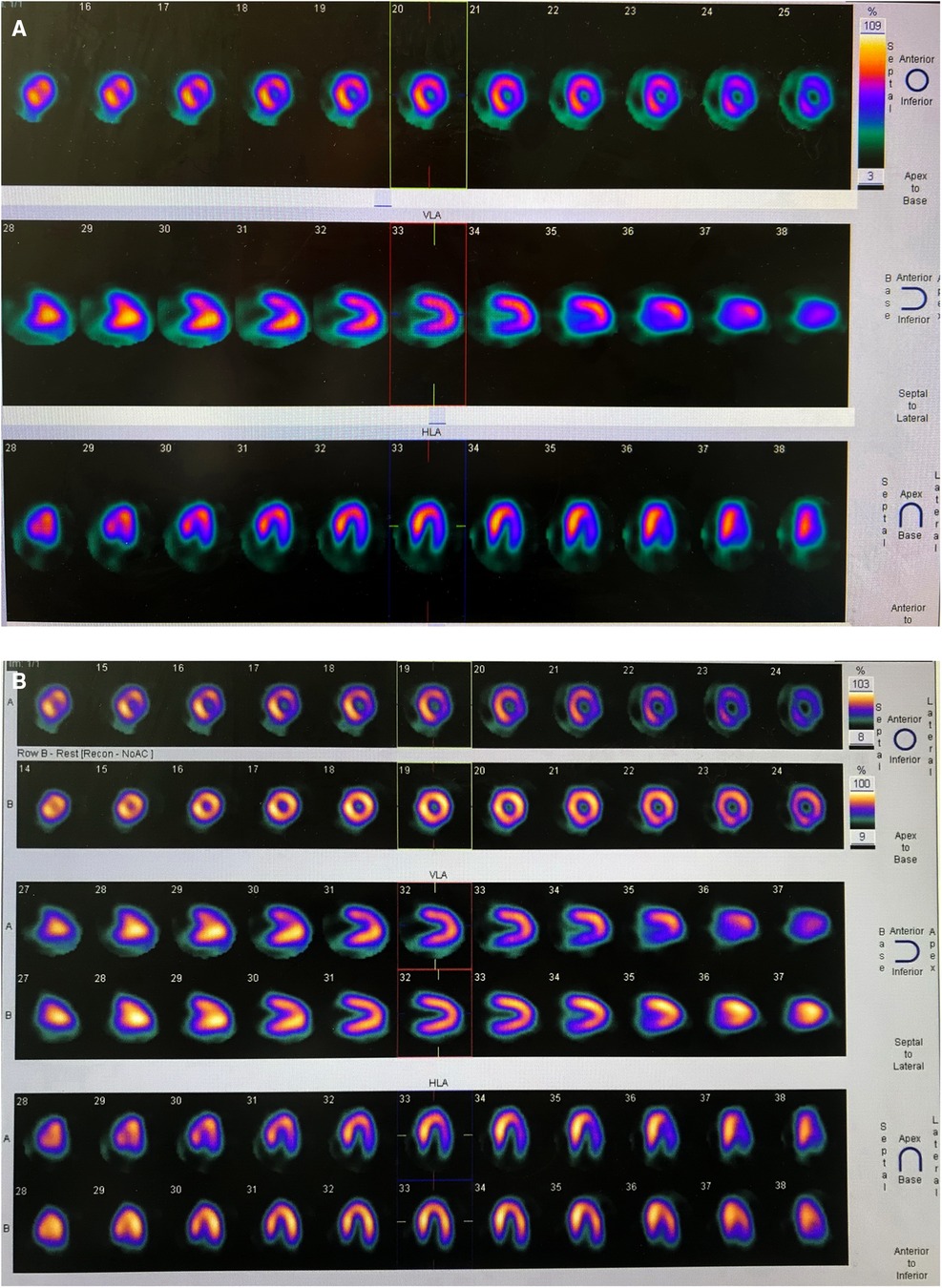
Figure 1. (A) ATP stressed myocardial perfusion imaging (MPI) demonstrated ischemia in the anterior wall near the apex, inferior wall, posterior wall, and lateral wall of the left ventricle. (B) Routine MPI showed uniform myocardial distribution.
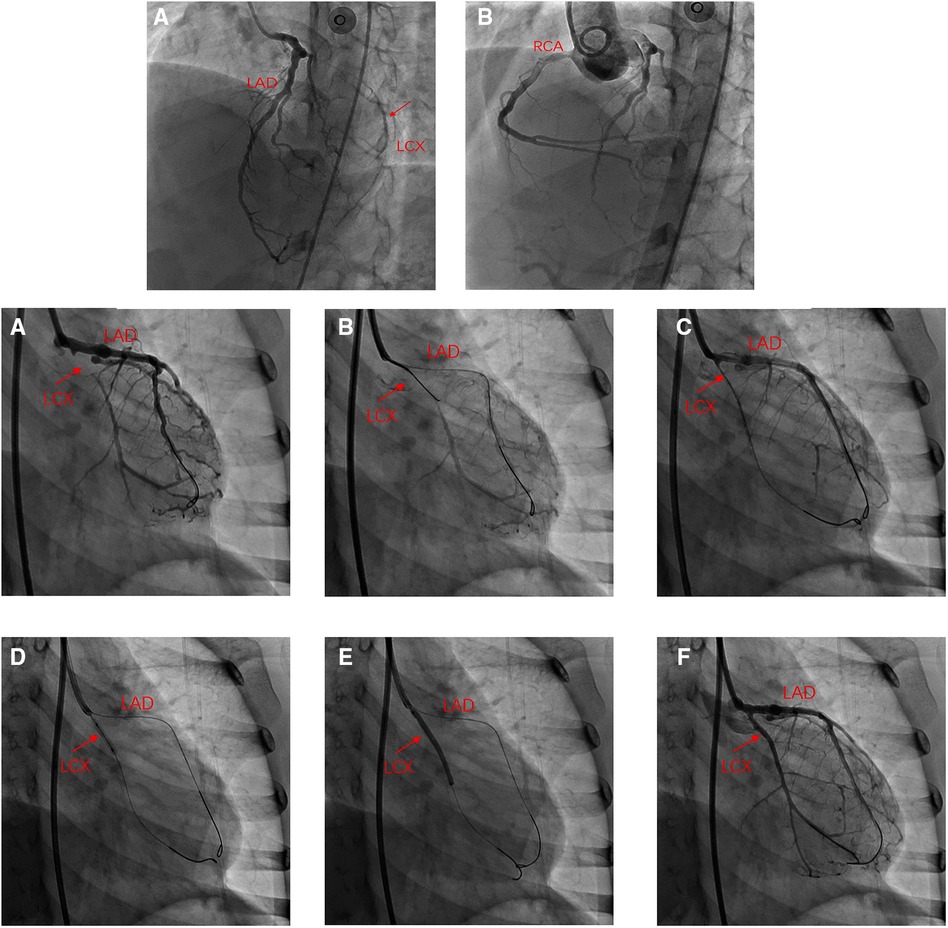
Figure 2. Angiography in left anterior oblique(LAO) 45° and cranial 25°: (A) total occlusion of the proximal LCX without calcification and collateral vessels from the LAD to distal LCX. (B) Internal diameter of the RCA was nearly average. (A) Angiography in right anterior oblique (RAO) 30°: LCX occluded at the proximal end, and distal blood supply has relied on collateral vessels; (B,C) Recanalization of occluded LCX using antegrade crossing technique; (D) Angiography after balloon angioplasty showed patent LCX; (E) DCB was attached to the LCX for 60 s; (F) Angiography of the LCX after DCB angioplasty.
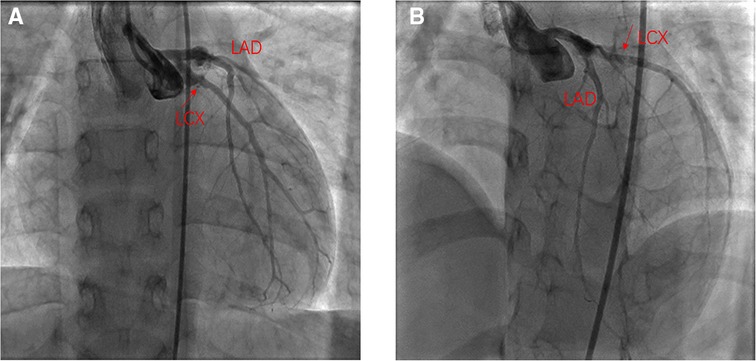
Figure 3. One year after the PCI angiography showed good flow in the LCX. (A) Angiography in right anterior oblique15° and Caudal 15°; (B) Angiography in left anterior oblique 45° and Cranial 25°.
Discussion
PCI is an important treatment method for acute coronary syndrome and chronic occlusive coronary artery disease in adults. However, the experience of PCI in KD children with CALs is minimal and reports are predominantly from Japan. The current PCI methods for KD specifically include balloon angioplasty, stent implantation, and intravascular rotational ablation (4). According to the JCS and AHA guidelines for KD, the indications for PCI in KD patients include the evidence of myocardial ischemia or inducible myocardial ischemia with coronary stenosis greater than 75%. CABG may be more beneficial for younger children or patients with the multiple-vessel disease (1, 2). In our case, ATP stressed MPI demonstrated reversible ischemia in multiple areas of the left ventricle, and coronary angiography showed complete occlusion of the proximal LCX. Therefore, indications for coronary intervention for this patient were absolute. Although the child had complex bifurcation lesions involving the LAD and LCX, the LAD was not indicated for coronary intervention. There was no significant reduction of blood flow in LAD, and the left ventricular function was as expected. Considering the focal lesion and the absence of calcification in the LCX, percutaneous revascularization for total occlusive LCX may be the first consideration. However, if the lesions in the LCX and LAD progress in the future, the procedure of CABG should be considered.
Reports on PCI in children with KD complicated with CTO of coronary arteries are rare. To our knowledge, there are only four reports in the literature. The recanalization method of occluded coronary arteries in children is the same as in adults, mainly relying on forward or reverse dissection reentry or forward true cavity to true cavity pathfinding technology. The youngest KD patient with total occlusion of coronary arteries undergoing PCI was reported to be six years old. After recanalization of the occluded coronary arteries was achieved, two patients underwent stent implantation, and the other two patients underwent balloon angioplasty. The longest follow-up period was 6 months. The short-term clinical effects were promising, but the long-term results still need follow-up (5–8). Our patient underwent paclitaxel-coated balloon angioplasty after recanalizing the LCX using the antegrade crossing technique. Her immediate and short-term postoperative outcome was also excellent. Drug-coated balloons (DCB) are a novel therapeutic strategy for coronary artery disease based on the rapid release of antiproliferative drugs into the local vessel wall during balloon inflation to inhibit intimal hyperplasia. In theory, DCB can effectively reduce the re-intervention rate of coronary lesions compared with angioplasty only. Recently, several large and adequately designed trials for the treatment of coronary small-vessel disease have confirmed the efficiency of the drug-coated balloon (9). However, the use of DCB in children is limited, especially in Kawasaki disease coronary cases. In 2021, Xu et al. reported the first successful percutaneous revascularization using a paclitaxel-coated balloon for coronary sequelae of KD in the pediatric population (8). In 2022, Zheng et al. reported another case about an 18-year-old girl who had severe stenosis in the proximal RCA sequelae with KD was treated with DCB and had an excellent result at one-year follow-up (10). Previously, limited case reports about paclitaxel-coated balloon treatment for renal artery, hepatic vein, and pulmonary artery stenosis in children had been published (11–13). To date, no severe adverse effects of the paclitaxel-coated balloon have been found in children. In our case, at one year after PCI, the clinical outcome of PCI and the blood tests were favorable and the patient had no obvious symptoms of immunosuppression. Nevertheless, longer follow-up on our patients and more studies on this topic is needed.
Stent implantation in patients with coronary arterial lesions secondary to KD should be considered carefully and be avoided or delayed as much as possible because coronary artery stenting in this population had some long-term adverse effects, including complete occlusion, restenosis, and stent migration. In 2020, Tsuda reviewed 33 patients who underwent stent implantations and reported that late-period adverse effects were found in 19 (68%) of 28 vessels with follow-up angiograms. The rate of being free of adverse effects at three years after the procedure was only 25% (14).
To date, few publications have compared the outcomes between CABG and PCI in KD children. Data is mainly derived from retrospective studies. Detailed comparisons between PCI and CABG were summarized in Table 2. Most studies affirm that KD patients undergoing PCI had a higher rate of re-intervention than those undergoing CABG (15, 16). However, PCI has the absolute advantages of being minimally invasive with a shorter recovery time for children. Therefore, PCI in selected KD patients with CALs can improve the prognosis and benefit these patients.
Conclusion
Current advances in coronary artery intervention provide a broader spectrum of nonsurgical therapeutic options for young children with severe KD-related cardiac sequelae. Percutaneous recanalization of the coronary CTO followed by drug-coated ballooning is effective for pediatric patients with KD. More clinical experience and long-term follow-up are needed to assess the safety and efficacy of drug-coated balloons in this patient population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
FL conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. LZ and L-pX conceptualized and designed the study, collected data, carried out the analyses, drafted the initial manuscript, and reviewed and revised the manuscript. CC, X-cL, LH carried out the initial analyses and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by a grant from the Innovation of Clinical Research Plan of SHDC (SHDC2020CR5011-002).
Acknowledgments
We are grateful to X-cL, CC, and Y-xL from the Department of Cardiovascular Center for their treatment of this patients. We express sincere thanks to J-yM from Zhongshan Hospital of Fudan University for his clinical intervention technique guidance. We also thank Conway Niu from Perth Children's Hospital, Australia for his contribution to language polishing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
2. Fukazawa R, Kobayashi J, Ayusawa M, Hamada H, Miura M, Mitani Y, et al. JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J. (2020) 84:1348–407. doi: 10.1253/circj.CJ-19-1094
3. Newburger JW, Fulton DR. Coronary revascularization in patients with Kawasaki disease. J Pediatr. (2010) 157:8–10. doi: 10.1016/j.jpeds.2010.04.008
4. Akagi T. Interventions in Kawasaki disease. Pediatr Cardiol. (2005) 26:206–12. doi: 10.1007/s00246-004-0964-2
5. Dahdah N, Ibrahim R, Cannon L. First recanalization of a coronary artery chronic total obstruction in an 11-year-old child with Kawasaki disease sequelae using the CROSSER catheter. Pediatr Cardiol. (2007) 28:389–93. doi: 10.1007/s00246-006-0083-3
6. Hsu YC, Liang KW, Lin MC, Fu YC, Jan SL. Stent implantation for a totally occluded right coronary artery in a six-year-old boy after Kawasaki disease: a case report. J Med Case Rep. (2012) 6:111. doi: 10.1186/1752-1947-6-111
7. Steinberg ZL, Jones TK, Lombardi WL. Novel percutaneous coronary intervention techniques for revascularizing chronically occluded giant coronary aneurysms in a patient with Kawasaki disease. Pediatr Cardiol. (2016) 37:1392–5. doi: 10.1007/s00246-016-1446-z
8. Xu X, Jin S, Liu T. Drug-coated balloon angioplasty for coronary stenotic lesions in a paediatric patient after Kawasaki disease. Cardiol Young. (2022) 32(2):340–2. doi: 10.1017/S104795112100295X
9. Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, et al. Drug-coated balloons for coronary artery disease: third report of the international DCB consensus group. JACC Cardiovasc Interv. (2020) 13:1391–402. doi: 10.1016/j.jcin.2020.02.043
10. Zheng B, Yi T, Wu Q, Bai F, Li J. Drug-coated balloon treatment for possible sequelae of Kawasaki disease evaluated by multi-modalities. Int Heart J. (2022) 63:773–6. doi: 10.1536/ihj.21-593
11. Hecht T, Esmaeili A, Behnke-Hall K. Balloon angioplasty of the bilateral renal arteries by Takayasu arteritis with a paclitaxel-eluting balloon. Cardiol Young. (2015) 25:1431–4. doi: 10.1017/S1047951114001930
12. Cohen JL, Glickstein JS, Crystal MA. Drug-coated balloon angioplasty: a novel treatment for pulmonary artery in-stent stenosis in a patient with Williams syndrome. Pediatr Cardiol. (2017) 38:1716–21. doi: 10.1007/s00246-017-1646-1
13. Parra DA, Brandao L. Use of drug-coated balloons in the management of a recalcitrant postsurgical hepatic vein stenosis in a pediatric patient. Radiol Case Rep. (2020) 15:1864–9. doi: 10.1016/j.radcr.2020.07.047
14. Tsuda E. Insights into stent implantation for coronary artery lesions caused by Kawasaki disease. Cardiol Young. (2020) 30:911–8. doi: 10.1017/S104795112000133X
15. Muta H, Ishii M. Percutaneous coronary intervention versus coronary artery bypass grafting for stenotic lesions after Kawasaki disease. J Pediatr. (2010) 157:120–6. doi: 10.1016/j.jpeds.2010.01.032
Keywords: kawasaki disease, CTO - percutaneous coronary intervention, CTO (chronic total occlusion), cornary artery, children
Citation: Zhao L, Xie L, He L, Liang X, Chu C and Liu F (2022) Case Report: Interventional therapy for coronary artery occlusion in a 6-year-old child with Kawasaki disease. Front. Pediatr. 10:1048178. doi: 10.3389/fped.2022.1048178
Received: 19 September 2022; Accepted: 17 November 2022;
Published: 5 December 2022.
Edited by:
Emanuele Monda, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Katsuhiro Hosoyama, Tohoku University, JapanMarco Antonio Yamazaki-Nakashimada, National Institute of Pediatrics, Mexico
© 2022 Zhao, Xie, He, Liang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu bGl1ZmFuZ0BmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Lu Zhao
Lu Zhao Li-ping Xie
Li-ping Xie Lan He
Lan He Xue-cun Liang
Xue-cun Liang Chen Chu Dr
Chen Chu Dr Fang Liu
Fang Liu