- 1Division of Pediatric Imaging, Department of Radiology, MassGeneral Hospital, Boston, MA, USA
- 2Harvard Medical School, Boston, MA, USA
- 3Division of Pediatric Gastroenterology, MassGeneral Hospital for Children, Boston, MA, USA
- 4Division of Gastroenterology, Hepatology, and Nutrition, Boston Children's Hospital, Boston, MA, USA
- 5Takeda Pharmaceuticals International Co, Cambridge, MA, USA
Purpose: The aim of this study was to explore potential correlation of the MR imaging features and clinical characteristics with formation of perianal abscess in children with Crohn's perianal fistulas (CPF).
Methods: From 2010 to 2020, pediatric patients with CPF diagnosis on their first pelvic MRI were identified retrospectively. All patients were divided into two groups based on the presence or absence of perianal abscess. Baseline clinical and MRI characteristics were recorded for each patient. All the statistical calculations were performed using R (version 3.6.3).
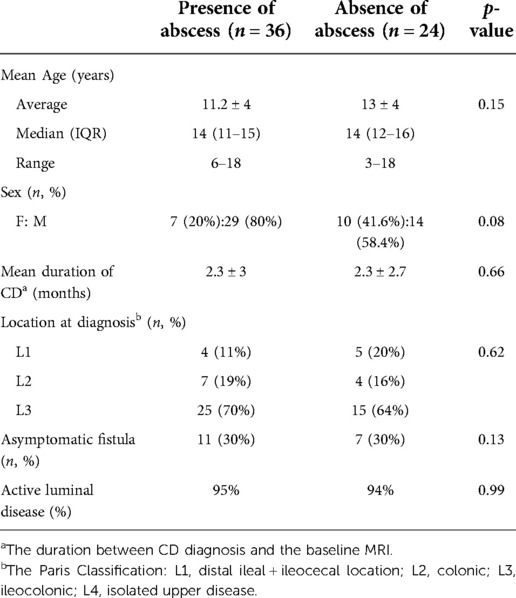
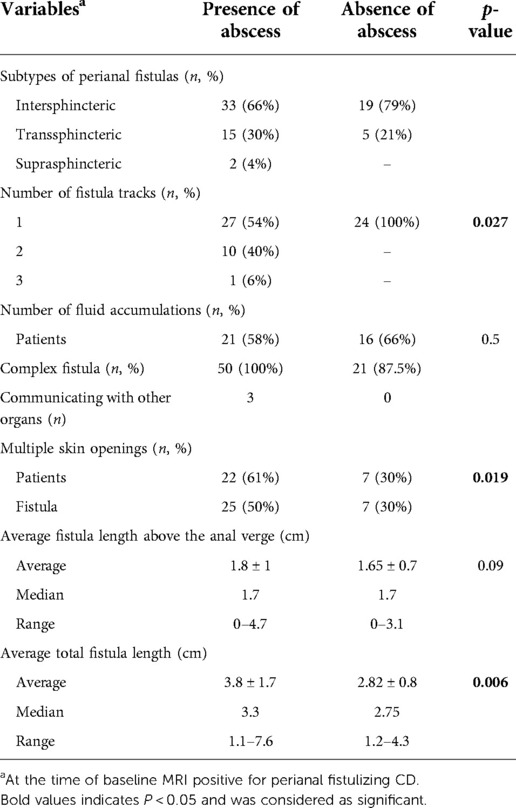
Results: A total of 60 patients [F:M 17:43, median age 14 years (IQR 10-15), ranging 3–18 years] were included in this study. Forty-four abscesses were identified in 36/60 children (mean volume 3 ± 8.6 ml, median 0.3 ml). In 24/60 patients with perianal disease, no abscess was detected on the MRI. Ten patients (28%) showed perianal abscess on pelvic MRI at the initial diagnosis. The rate of active disease on colonoscopy (visible ulcerations/aphthous ulcers) was similar in both groups (95% vs. 94%). With regards to disease location, the majority of patients (40/60, 66.6%) in both groups had ileocolonic CD. All patients without abscess had a single perianal fistula (n = 24; 3 simple and 21 complex fistulae), however, patients with perianal abscess tended to have >1 fistulous tracts (n = 50 fistulas; all complex, 27 single, 10 double and 1 triple). Intersphincteric fistula was the most common fistula type in both groups (79% and 66%, p = 0.1). The total length of fistula (3.8 ± 1.7 vs. 2.8 ± 0.8 cm, p = 0.006) and presence of multiple external openings (n = 25 vs. 7, p = 0.019) were significantly higher in patients with abscesses, and fistula length >3.3 cm showed 80% specificity and 83% PPV for the presence of perianal abscess. Fistulas were symptomatic (pain, bleeding or drainage) at similar rates in both groups (68% and 70%, p = 0.1).
Conclusion: Pediatric patients with CPF who develop perianal abscess have a distinct imaging phenotype defined by longer fistula length (>3.3 cm), multiple skin openings and multiple fistulous tracts (≥2) on MRI. Patients who have these features but does not have an abscess on imaging may merit more aggressive treatment (and close monitoring) to prevent the development of an abscess.
Introduction
Approximately 15% of children with Crohn's disease (CD) have perianal symptoms at the time of diagnosis, and 49%–62% develop perianal complications at some point during the course of the disease (1, 2). Despite optimized treatment, almost one-third of patients develop Crohn's perianal fistulae (CPF) and complications such as strictures and abscesses, necessitating surgery within 5 years of diagnosis (3, 4). The presence of penetrating perianal disease complications has been proven to predispose to a greater inflammatory burden. Therefore, significantly impact the quality of life in these children including higher risk of associated psychological distress and disability (5, 6).
Imaging evaluation of the perianal region and accurate depiction of CPF are important for planning treatment strategies. Perianal abscesses often require surgical incision and drainage, potentially followed by seton or catheter placement and antibiotics. The decision to perform surgery, especially for pediatric patients with complex CPF, is based on a number of psychosocial, developmental, and anatomic considerations (7–10). Pelvic MRI is able to identify inflammation and clinically silent abscesses with reported accuracy of 91%, and may enable identification of small abscesses that can respond to antibiotic treatment without surgical intervention (11, 12). In addition, abscess formation in the supralevator space may not be painful or palpable during physical examination (13, 14). For all these reasons, it is important to identify clinical and imaging phenotypes associated with abscess formation. We hypothesized that pediatric patients with perianal CD and fistulizing disease who develop perianal abscess may have distinct imaging-based and clinical features. In this study we aimed to identify MRI features and patient clinical characteristics associated with perianal abscess formation in children with CPF.
Materials and methods
Patients
This is a HIPAA compliant, institutional review board approved, retrospective, single institution study. From 2010 to 2020, all pediatric patients, who had perianal CD diagnosis and showed fistulizing perianal involvement on their first pelvic MRI performed for perianal assessment, were identified using an institutional radiology report database. Data were extracted from the electronic medical records, and MR images were then reviewed. Patients were excluded if (1) they did not have a diagnosis of CD based on the colonoscopy, or (2) perianal fistula was not detected on pelvic MRI.
MRI protocol
MRI examinations were performed using a 1.5 T magnet (HD Excite; GE Healthcare, USA) or on a 3 T scanner (Magnetom Trio; Siemens Healthineers, Erlangen, Germany) with a 16-channel phased-array coil. The standard MR protocol involves a tri-plane localizer followed by triplane fast spin-echo T2-weighted images using a full field of view (FOV) through the entire pelvis, small FOV short tau inversion recovery (STIR) images through the perianal region in the axial and coronal planes, T1-W fat suppressed 3-D gradient echo images before and after administration of intravenous contrast agent in the coronal and axial planes, as well as T1-W fat-suppressed high-resolution 2-D spoiled gradient echo axial delayed images post-contrast administration (15).
Definition of MRI based features and clinical variables
All pelvic MRI exams were reviewed by a radiologist, blinded to the image findings and disease diagnosis, with subspecialty training in pediatric and abdominal radiology with 12 years of experience reading pelvis MRI. The radiologic features such as: perianal fistula type (based on Parks classification), fistula length (defined as the length from anal canal to skin or the longest measurable distance if the tract does not reach the skin), fistula length above the anal verge (described as distance between anal canal site of fistula egress and anal verge) were recorded. Additional evaluation included the presence of fluid (determined as T2 hyperintense fluid that focally widens the fistula), skin openings and multiple fistulous tracts, perianal abscess (a circumscribed fluid collection with peripheral enhancement >3 mm in diameter in at least 2 dimensions). Abscess volume was calculated using formula: 0.52 × long axis diameter × (short axis diameter) (2). Fistulae were defined as complex if (1) high intersphincteric (IS, Parks' type 1) or transsphincteric (TS, Parks' type 2) fistulae or (2) any suprasphincteric (SS, Parks' type 3) nor extrasphincteric (ES, Parks' type 4) fistulae or (3) presence of abscesses and fistula terminating into the adjacent organs.
Two pediatric gastroenterologists (with 9 and 10 years of experience) blinded to the pathology, independently reviewed the demographic, clinical and endoscopic data from the time of presentation to our institution. Data collected including disease activity (visible ulcerations/aphthous ulcers), CD location and symptomatic fistula (if the patient reported drainage or pain), within 30 days from MRI. Luminal disease was classified according to the Paris classification (16). Diagnosis of perianal disease was established according to clinical symptoms and physical and radiological examinations.
Statistical analysis
Continuous variables were represented as mean ± standard deviation or median and range and categorical variables as the percentage of the total number. The Fisher's exact test was performed to compare the differences between the categorical variables. Continuous variables were compared using Student's t-test. For all of the statistical analyses, a p value of <0.05 was considered statistically significant. The optimum threshold for total fistula length discriminating presence or absence of abscess on MRI was calculated based on receiver operating curve analysis (12). All statistical analyses were carried out using R statistical computing software (version 3.6.3, The R Foundation for Statistical Computing).
Results
Presentation
A total of 60 patients were included in the study (F:M 17:43, median age 14 years (IQR 10-15). Patients were divided into 2 groups based on presence (n = 36) or absence of perianal abscess (n = 24). Patients’ demographics and clinical characteristics are summarized in Table 1.
A total of 74 fistulae and 44 abscesses were detected through MRI exams. Seventy percent of fistulae overall were intersphincteric (52/74,) and majority of the fistulae were complex (71/74). With regard to disease location, the majority of patients (40/60) had ileocolonic involvement at the time of CPF diagnosis in both groups.
In 36 patients with perianal abscess seen on MRI, 10 showed abscess at the time of initial CD diagnosis (CD-0). A total of fifty perianal fistula tracts (27 single, 10 double and 1 triple) corresponded to 44 abscesses (average volume 3 ± 8.6 ml) on pelvic MRI. Out of 50 fistulae; 33 were IS, 15 TS and 2 were SS. All fistulae in the patients with abscesses were complex. Twenty-one patients with 26 fistulae had fluid accumulation on T2 images. Three fistulae terminated into the other organs (n = 2 into the scrotum, n = 1 into the vagina).
In 24 patients, perianal abscess was not observed on MRI. A total of 24 single fistulous tracts (3 simple and 21 complex fistulae) were detected. Out of 24 fistulae; 19 were IS and 5 TS fistulae. Sixteen patients with 16 fistulae had fluid accumulation detected on T2 images. None of the fistulae were communicating with other organs or had external openings. Table 2 shows MRI features of the final cohort stratified by absence/presence of perianal abscess.
Overall outcome
Among the MRI features examined, total perianal fistula length (3.8 ± 1.7 vs. 2.8 ± 0.8 cm, p = 0.006) and number of external openings (n = 25 vs. 7, p = 0.019) were found to be significantly higher in patients with abscesses. Total fistula length <3.3 cm was shown to have a significant correlation with lower risk of abscess formation (sensitivity = 50%, specificity = 80%, positive predictive value (PPV) = 83%, negative predictive value (NPV) = 43%).
Discussion
The present study is the first study to combine clinical and MR imaging features associated with perianal abscess formation in pediatric patients with CPF. For the management of CPF it is essential to locate the fistula origin, establish the anatomical course of the fistula tract and exclude or identify the presence of associated perianal abscess (17–21). Patients with CPF who develop perianal abscess may subsequently require major operative intervention (1, 22). Studies to determine the appropriate timing of operative intervention for children with perianal abscess are lacking, leading to significant variability of treatment plans based upon surgeon preference and experience (23–25). Thus, early rule out of presence of perianal abscess is essential and should be discussed with the surgeon when drainage is detected.
In our analysis on perianal abscess in children with CPF, intersphincteric fistulae were the most common (66% on MRI) and transsphincteric fistulae were the second most common (30%) types. There were no extrasphincteric fistulae in the present study. In previous studies on adult population with CPF, transsphincteric fistulae were most frequently found (25, 26). In our study, one possible explanation for the finding that intersphincteric fistula was the most common type might be that for pediatric patients, the duration of disease was relatively short as compared with that in adults. Patients who develop perianal abscess were more likely to show perianal fistula with ≥2 fistulous tracts (n = 31, p = 0.027), and multiple skin openings (n = 22, p = 0.019). In addition, the fistula length was significantly shorter in patients who did not form perianal abscess (p = 0.006). A study by Choshen et al. highlighted that the best imaging findings indicating the severity of pediatric perianal CD were presence of collections >3 mm, location and length of the fistulas (27). Also, a previous study on MRI predictors of treatment response in children with CPF reported that maximum fistula length ≥2.5 cm was a predictor of disease progression (15). Our study extends that work by identifying features associated with abscess formation. A fistula length cut-off of <3.3 cm for perianal fistula showed 80% specificity and 83% PPV in patients with perianal abscess.
In this study, 16/60 (26.6%) of patients with perianal fistulae and 10/36 (28%) of patients with perianal abscess were CD-0 (presence of abscess at the time of initial CD diagnosis). Although not significant because of the small sample size, the patients who developed perianal abscess were more likely to be males (80% vs. 58.4%) and younger. This may be in part due to the known greater male > female predominance of pediatric Crohn's disease in the pre-pubescent age group. A study by Short et al. demonstrated that children who presented with perianal perforating CD at the time of diagnosis, were mostly young (mean 9.3 years old) and males (81%) (28).
The prevalence of perianal and anorectal abscesses, in general, are underestimated, since most patients do not seek medical attention, or are dismissed as having asymptomatic CPF. In situations where the child has perianal pain and discomfort and the abscess formation is suspected, prompt evaluation under anesthesia, including drainage is the procedure of choice to minimize damage to the sphincter. However, we found no correlation between the patients having symptomatic fistula and the presence of a perianal abscess (p = 0.1). Moreover, there was no significant association between the activity of luminal CD and development of perianal abscess in our patient population.
Strengths of our study include describing both MRI and clinical characteristics of patients with CPF associated with perianal abscess formation based on one of the largest pediatric datasets. The limitations were single-center retrospective nature of the study. In addition, a few patients were excluded because imaging was performed after specific surgical procedure/medical therapy to decrease the heterogeneity of the data. Additionally, our analysis focused on individual MRI features of CPF (e.g., fistula length, location, complexity) rather than scoring systems of perianal fistula severity that have previously been reported. Finally, a single radiologist (our institution's local expert on perianal fistula MRI interpretation) reviewed all the MRIs, and further studies on patients undergoing serial MR imaging exams reviewed by 2 independent radiologists would be helpful to assess the predictive value of these imaging features in terms of clinical management.
Conclusion
MRI quantitative parameters including fistula number and length have a high association with abscess development and should be reported when interpreting these imaging exams in pediatric patients with Crohn's disease. Given these findings, patients who have these features but does not have an abscess on imaging may merit more aggressive treatment (and close monitoring) in order to prevent the development of an abscess.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Radiology Department at Massgeneral Hospital-IRB # 2016P002784. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
All authors contributed to the article and approved the submitted version.
Conflict of interest
Author SH was employed by company Takeda Pharmaceuticals International Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Takeda Pharmaceuticals International Co. Takeda was involved in reviewing the final draft and the decision to submit this study for publication.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adegbola SO, Pisani A, Sahnan K, Tozer P, Ellul P, Warusavitarne J. Medical and surgical management of perianal Crohn's disease. Ann Gastroenterol. (2018) 31(2):129–39. doi: 10.20524/aog.2018.0236
2. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. (2015) 169(11):1053–60. doi: 10.1001/jamapediatrics.2015.1982
3. Gasparetto M, Angriman I, Guariso G. The multidisciplinary health care team in the management of stenosis in Crohn's disease. J Multidiscip Healthc. (2015) 8:167–79. doi: 10.2147/JMDH.S38729
4. Shen B. Interventional IBD: the role of endoscopist in the multidisciplinary team management of IBD. Inflamm Bowel Dis. (2018) 24(2):298–309. doi: 10.1093/ibd/izx058
5. Assa A, Amitai M, Greer ML, Castro DA, Kuint RC, Martínez-León M, et al. Perianal pediatric crohn disease is associated with a distinct phenotype and greater inflammatory burden. J Pediatr Gastroenterol Nutr. (2017) 65(3):293–8. doi: 10.1097/MPG.0000000000001484
6. Sýkora J, Pomahačová R, Kreslová M, Cvalinova D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. (2018) 24(25):2741–63. doi: 10.3748/wjg.v24.i25.2741
7. Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. Br Med J. (2017) 357:j2505. doi: 10.1136/bmj.j2505
8. Aardoom MA, Veereman G, De Ridder L. A review on the use of anti-TNF in children and adolescents with inflammatory bowel disease. Int J Mol Sci. (2019) 20(10):2529. doi: 10.3390/ijms20102529
9. Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. (2018) 11:215–26. doi: 10.2147/JIR.S165330
10. De Zoeten EF, Pasternak BA, Mattei P, Kramer RE, Kader HA. Diagnosis and treatment of perianal Crohn disease: NASPGHAN clinical report and consensus statement. J Pediatr Gastroenterol Nutr. (2013) 57(3):401–12. doi: 10.1097/MPG.0b013e3182a025ee
11. Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, et al. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s Perianal fistulas. Gastroenterology. (2001) 121(5):1064–72. doi: 10.1053/gast.2001.28676
12. Habibzadeh F, Habibzadeh P, Yadollahie M. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb). (2016) 26(3):297–307. doi: 10.11613/BM.2016.034
13. Seemann NM, Elkadri A, Walters TD, Langer JC. The role of surgery for children with perianal Crohn's disease. J Pediatr Surg. (2015) 50(1):140–3. doi: 10.1016/j.jpedsurg.2014.10.034
14. Jarchin L, Spencer EA, Khaitov S, Greenstein A, Jossen J, Lai J, et al. De novo Crohn's disease of the pouch in children undergoing ileal pouch-anal anastomosis for ulcerative colitis. J Pediatr Gastroenterol Nutr. (2019) 69(4):455–60. doi: 10.1097/MPG.0000000000002406
15. Shenoy-Bhangle A, Nimkin K, Goldner D, Bradley WF, Israel EJ, Gee MS. MRI predictors of treatment response for perianal fistulizing Crohn disease in children and young adults. Pediatr Radiol. (2014) 44(1):23–9. doi: 10.1007/s00247-013-2771-5
16. Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. (2011) 17(6):1314–21. doi: 10.1002/ibd.21493
17. Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A, et al. Management of complex perianal Crohn's disease. Ann Gastroenterol. (2017) 30(1):33–44. doi: 10.20524/aog.2016.0099
18. Gecse K, Khanna R, Stoker J, Jenkins JT, Gabe S, Hahnloser D, et al. Fistulizing Crohn's disease: diagnosis and management. United European Gastroenterol J. (2013) 1(3):206–13. doi: 10.1177/2050640613487194
19. Seemann NM, King SK, Elkadri A, Walters T, Fish J, Langer JC, et al. The operative management of children with complex perianal Crohn’s disease. J Pediatr Surg. (2016) 51(12):1993–7. doi: 10.1016/j.jpedsurg.2016.09.021
20. Kantor N, Wayne C, Nasr A. What is the optimal surgical strategy for complex perianal fistulous disease in pediatric Crohn’s disease? A systematic review. Pediatr Surg Int. (2017) 33(5):551–7. doi: 10.1007/s00383-017-4067-6
21. Sahnan K, Tozer PJ, Adegbola SO, Lee MJ, Heywood N, McNair AGK, et al. Developing a core outcome set for fistulising perianal Crohn’s disease. Gut. (2019) 68(2):226–38. doi: 10.1136/gutjnl-2017-315503
22. Kim S. Surgery in pediatric Crohn’s disease: indications, timing and post-operative management. Pediatr Gastroenterol Hepatol Nutr. (2017) 20(1):14–21. doi: 10.5223/pghn.2017.20.1.14
23. Amil-Dias J, Kolacek S, Turner D, Parregaard A, Rintala R, Afzal NA, et al. Surgical management of crohn disease in children: guidelines from the paediatric IBD porto group of ESPGHAN. J Pediatr Gastroenterol Nutr. (2017) 64(5):818–35. doi: 10.1097/MPG.0000000000001562
24. Garrick V, Stenhouse E, Haddock G, Russell RK. A multidisciplinary team model of caring for patients with perianal Crohn’s disease incorporating a literature review, topical therapy and personal practice. Frontline Gastroenterol. (2013) 4(2):152–60. doi: 10.1136/flgastro-2012-100160
25. Choi YS, Kim DS, Lee DH, Lee JB, Lee EJ, Lee SD, et al. Clinical characteristics and incidence of perianal diseases in patients with ulcerative colitis. Ann Coloproctol. (2018) 34(3):138–43. doi: 10.3393/ac.2017.06.08
26. Panes J, Reinisch W, Rupniewska E, Khan S, Forns J, Khalid JM, Bojic D, et al. Burden and outcomes for complex perianal fistulas in Crohn’s disease: systematic review. World J Gastroenterol. (2018) 24(42):4821–34. doi: 10.3748/wjg.v24.i42.4821
27. Choshen S, Turner D, Pratt LT, Precel R, Greer ML, Castro DA, et al. Development and validation of a pediatric MRI-based perianal Crohn disease (PEMPAC) Index-A report from the ImageKids study. Inflamm Bowel Dis. (2022) 28(5):700–9. doi: 10.1093/ibd/izab147
Keywords: pediatrcis, Crohn’s disease, fistula, absces, inflammatory bowel diseas
Citation: Tabari A, Kaplan JL, Huh SY, Moran CJ and Gee MS (2022) Clinical characteristics and MRI-based phenotypes of perianal abscess formation in children with fistulizing Crohn's Disease. Front. Pediatr. 10:1045583. doi: 10.3389/fped.2022.1045583
Received: 15 September 2022; Accepted: 18 October 2022;
Published: 18 November 2022.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Firas Rinawi, Technion Israel Institute of Technology, IsraelPaul Henderson, University of Edinburgh, United Kingdom
© 2022 Tabari, Kaplan, Huh, Moran and Gee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Azadeh Tabari YXRhYmFyaUBtZ2guaGFydmFyZC5lZHU=
Specialty Section: This article was submitted to Pediatric Gastroenterology, Hepatology and Nutrition, a section of the journal Frontiers in Pediatrics
 Azadeh Tabari
Azadeh Tabari Jess L. Kaplan2,3
Jess L. Kaplan2,3 Susanna Y. Huh
Susanna Y. Huh Christopher J. Moran
Christopher J. Moran Michael S. Gee
Michael S. Gee