- West China Hospital, Sichuan University, Chengdu, China
The patient was a male neonate, and a prenatal ultrasound had detected a right lung mass. He was born at term and after delivery had tachypnea and feeding difficulties. A chest x-ray and a computed tomography (CT) scan revealed a large mass in the right chest with compression on the right lung after birth. We initially considered congenital pulmonary airway malformation (CPAM). After conservative treatment, his respiratory symptoms worsened gradually, and he required continuous supplemental oxygen. The symptoms could not be relieved by puncturing due to a postnatal ultrasound having shown a mass with anechoic microcystic spaces. He therefore underwent an emergency thoracotomy and lobectomy at 14 days of age. The pathology was consistent with fetal lung interstitial tumor (FLIT). The patient remained healthy at the three-month follow-up. We reviewed the literature on FLIT and found that, to date, 23 cases have been reported worldwide.
Introduction
Fetal lung interstitial tumor (FLIT) is a newly-defined disease first reported by Dishop et al. (1, 2). Reading the literature on FLIT revealed that 23 cases have been reported worldwide. Of the 23 reported cases, 14 had an incorrect initial diagnosis, and 9 cases an ambiguous one. Shah et al. report that the initial diagnosis for one patient was congenital pulmonary airway malformation (CPAM) (3). Yoshida et al. report that one patient was initially considered to have CPAM or pulmonary sequestration (4). We can see that FLIT is easily misdiagnosed.
Misdiagnosis may increase clinical risk because the progression and treatment of FLIT differs from other conditions. Dishop et al. report that, in one case, FLIT was misdiagnosed as airway obstruction and did not receive surgery until doctors observed that the mass had increased (1). Prompt and accurate diagnosis of FLET can avoid both a delay in treatment and the administering of unsuitable treatment. However, the lack of a uniform standard at present leads to difficulties in diagnosing FLIT. We are therefore retrospectively analyzing a patient with FLIT who was transferred to our hospital, and reviewing the literature in order to summarize the characteristics of the disease with the aim that doctors might better diagnose FLIT.
Case report
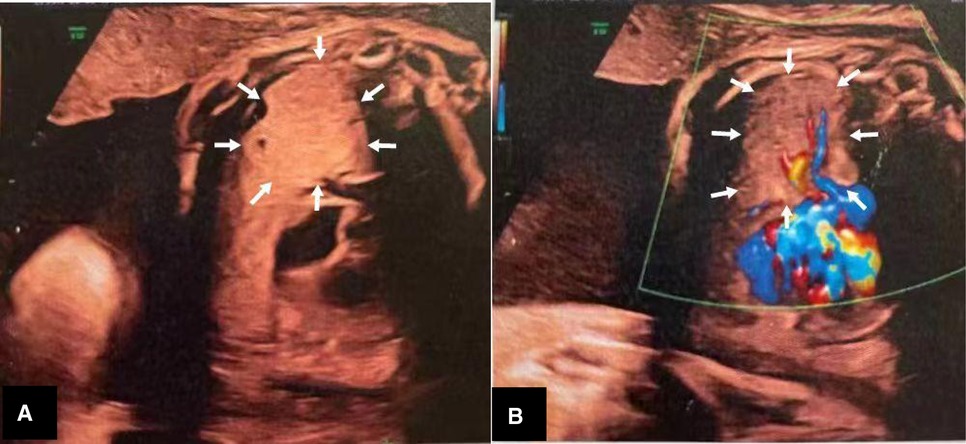
The patient was a male neonate (gestation, 37 weeks and 3 days; birth weight, 3,100 g). A prenatal ultrasound, at 33 weeks and 2 days of gestation, showed a solid, minimally hyperechoic, right-sided lung mass with cystic components, which suggested CPAM (Figure 1). The CPAM volume ratio (5) was 0.41 at 33 weeks, and 2.94 at 37 weeks of gestation. The pregnancy was otherwise uneventful. The patient was born at term by vaginal delivery at a local hospital. After delivery, he quickly developed tachypnea and was administered supplemental oxygen with a nasal catheter to stabilize his respiratory status. He also had feeding difficulties. At five hours of age he was transferred to our hospital for further evaluation and management.
On admission, the patient had tachypnea and decreased breath sounds. A chest x-ray and CT scan confirmed a large, solid mass with compression on the right lung (Figure 2). We initially considered CPAM. A postnatal ultrasound revealed a well-circumscribed, isoechoic mass (5.7 × 5.5 × 6.1 cm3) with anechoic cystic spaces (Figure 3). The patient underwent an emergency thoracotomy and lobectomy at 14 days of age.
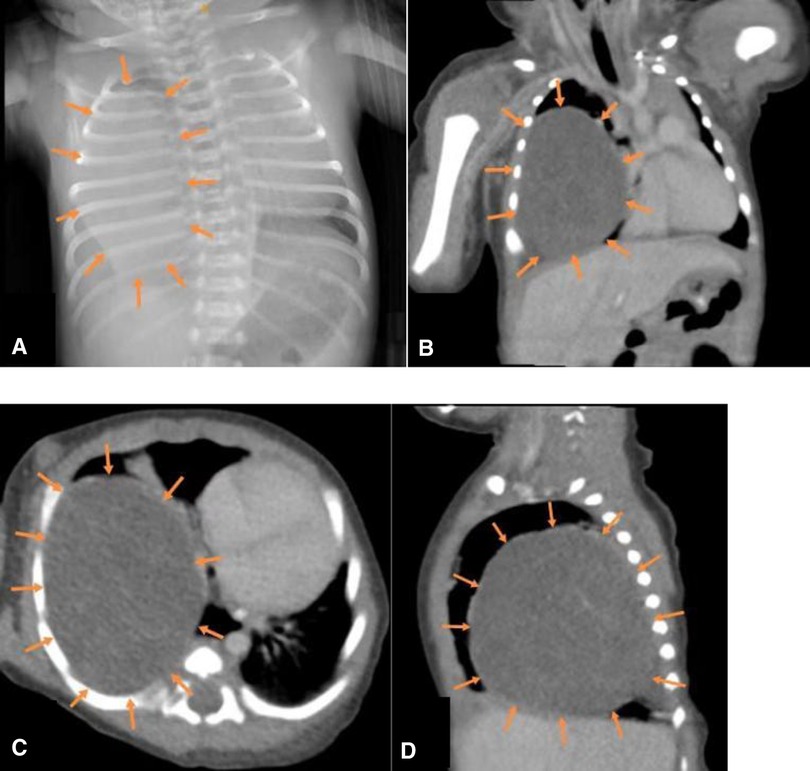
Figure 2. (A) x-ray and (B–D) CT (coronal, transverse and sagittal sections) showing a large mass in the right lower lobe.
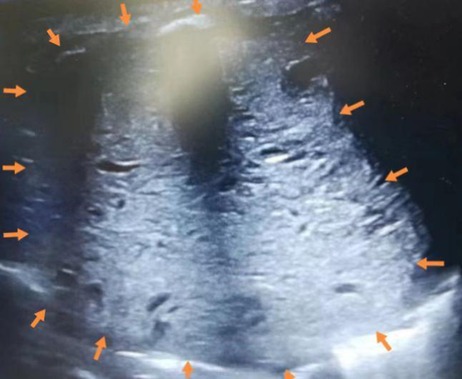
Figure 3. Postnatal ultrasound showing a well-circumscribed, isoechoic mass (arrows) (5.7 × 5.5 × 6.1 cm3) with anechoic cystic spaces.
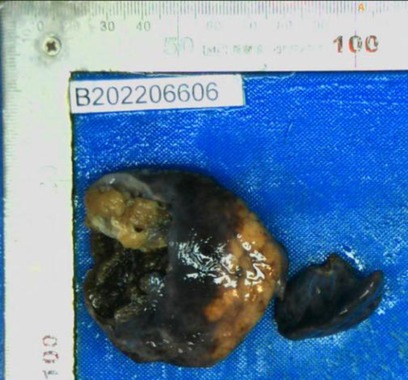
During surgery, we found a large tumor in the right upper lobe with a mediastinal shift and so we performed a right upper lobectomy. The solid mass pressed on the airway, leading to abnormal an airway position. As a consequence, the right main bronchus was accidentally injured and reconstructed. The patient remained healthy approximately three months post-surgery with no adjuvant radiotherapy or chemotherapy. The excised specimen showed a fuscous, spongy, and well-circumscribed mass with cystic spaces (Figure 4). The pathology met the diagnostic criteria for FLIT.
Discussion
Fetal lung interstitial tumor (FLIT) is easily misdiagnosed, especially as being CPAM (3, 4). In our case, the mass was misdiagnosed as CPAM and the doctors did not recognize the severe risk, resulting in the patient being born at a local hospital, rather than a specialist center. There are two main reasons for misdiagnosis. First, FLIT is rare, so doctors seldom consider this disease. Second, CPAM and FLIT have similar imaging manifestations. A prenatal ultrasound or magnetic resonance imaging (MRI) can detect FLIT. A prenatal ultrasound will show a solid, hyperechoic lung mass with or without cystic spaces (6, 7). After the prenatal ultrasound finds the lesion, an MRI can be performed for further evaluation. In our case the patient did not undergo an MRI because the price of an MRI is higher than that of an ultrasound, and there is no clear evidence to show that the additional information provided by an MRI influences the management of the patient. FLIT presents as a solid, well-circumscribed mass (1, 7), and the overall signal is slightly greater than that of the normal lung in a T2-weighted MRI (6). Some literature suggests that doctors should pay closer attention to the examination in the third trimester, in an effort to distinguish FLIT from CPAM. In CPAM, the maximum size appears at approximately 26–30 weeks of gestation (7–9), and the lesion may shrink or even disappear with the progression of pregnancy. However, in FLIT, the mass continues to grow and can lead to fetal edema in later pregnancy: Lazar et al. found a case of FLIT with fetal edema and heart failure at 37 weeks of gestation, which is later than CPAM would generally cause fetal edema (7).
Of the 23 reported cases, four were detected before birth, 18 were detected when symptoms developed after birth, and one was not described (Table 1). These figures demonstrate that doctors should remain vigilant for symptoms post-delivery. Most FLIT patients were born at term. All signs appeared within three months, and the most common symptom was respiratory difficulty (Table 1). Some patients also experienced feeding difficulties. Some patients with jaundice or a provisional diagnosis of dextrocardia are accidentally found to have a lung mass (3, 10). Our patient had also developed jaundice at nine days of age. Among these symptoms, feeding difficulties are relatively specific. A lung mass will generally lead to respiratory problems but not feeding problems (11). The reason that FLIT can lead to feeding difficulties may be that the lesion is large enough to compress the esophagus. The maximum diameter ranges from 2 cm to 9.5 cm, with the mean being 5.5 cm in FLIT (Table 1). A postnatal ultrasound and CT scan may also help in distinguishing between FLIT and CPAM. We find that the cystic content in FLIT is fluid, while the cystic content in CPAM is gas; so a postnatal ultrasound shows anechoic cystic spaces in FLIT (Figure 3), and a CT scan shows low-density cystic areas in CPAM (Figure 5).
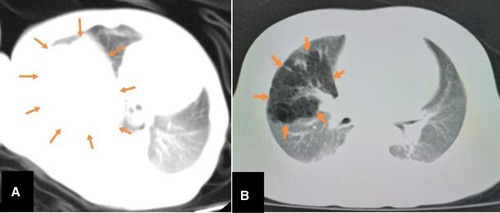
Figure 5. The comparison between CT features FLIT and CPAM. CT shows a mass (arrows) with uniform density in FLIT (A) and a mass (arrows) with low-density cystic spaces in CPAM (B).
A correct FLIT diagnosis can enable patients to receive timely and proper treatment. In the case of fetal edema, doctors can use hormones to try and relieve it. Phillips et al. report a case treated with betamethasone but that where polyhydramnios remained (12). Doctors might also perform an ex utero intrapartum treatment (EXIT) or early delivery. Lazar et al. report a fetus with fetal edema that received EXIT at 37 weeks gestation (7). Waelti et al. report that a patient with progressive fetal edema underwent an emergency cesarean section (13). When symptoms develop after birth, FLIT should be surgically resected as early as possible. The mean age at the time of surgery is 33 days in FLIT (Table 1). However, because the cystic content in CPAM is gas, in these cases percutaneous thoracic catheter drainage (PTCD) is an option (14). If symptoms ease, a patient with CPAM can receive watchful observation until their condition is improved and the likelihood of a positive outcome from thoracoscopic surgery increased.
Most FLIT cases received a lobectomy (Table 1). In our experience, doctors should pay close attention to the airway anatomy: we found that the solid mass put pressure on the airway, leading to an abnormal airway position. However, in CPAM there is less movement and deformity in anatomical structures. This may be because the cystic content and the size of the mass differ between FLIT and CPAM. Those patients with FLIT did not need radiotherapy or chemotherapy. The prognosis was good (15), and no recurrences were reported (Table 1). Waelti et al. report a case with a hilar remnant after surgery, this patient was followed up for three years and experienced no recurrence (13).
A pathological diagnosis cannot be confirmed by H&E staining alone, but with further specialist staining (16–18). To date, FLIT and pulmonary interstitial glycogenosis are the only diseases in which interstitial cells are rich in glycogen particles (4). In addition, the excised mass can be subjected to genetic testing to aid diagnosis (19). Zhao et al. report an initial pathological diagnosis of PPB in one case. However, no mutations were found in exons 24 and 25 of Dicer1, and so the final diagnosis was FLIT (12).
Conclusion
FLIT is easily misdiagnosed. Prenatal ultrasound can aid diagnosis and should be performed regularly. An increasing mass volume in later gestation can indicate FLIT. After birth, feeding difficulties is a relatively specific symptom. In FLIT, a postnatal ultrasound shows a mass with anechoic microcystic spaces. CT scans usually show a mass with uniform density in FLIT, but a mass with low-density cystic areas in CPAM. Correct diagnosis can enable patients to receive timely and proper treatment. FLIT should be excised as early as possible, and most surgeons perform lobectomy. During surgery, surgeons should carefully monitor airway anatomy in order to avoid airway damage.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Review Board of Sichuan University, West China Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
MY: collected data, ZW: wrote the manuscript. CX and TH: revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FLIT, fetal lung interstitial tumor; CPAM, congenital pulmonary airway malformation; CT, computed tomography; PPB, pleuropulmonary blastoma; PTCD, percutaneous thoracic catheter drainage; MRI, magnetic resonance imaging.
References
1. Dishop MK, McKay EM, Kreiger PA, Priest JR, Williams GM, Langston C, et al. Fetal lung interstitial tumor (FLIT): a proposed newly recognized lung tumor of infancy to be differentiated from cystic pleuropulmonary blastoma and other developmental pulmonary lesions. Am J Surg Pathol. (2010) 34(12):1762–72. doi: 10.1097/PAS.0b013e3181faf212
2. Mocayar Marón FJ, Oliva J, D'Angelo CR, Drago G, Sarabia E, Herón A, et al. Fetal lung interstitial tumor (FLIT): case report. Biocell. (2019) 43(Supplement 4).
3. Shah SN, Geetha N, Satheesan R, Parameswaran A. Fetal lung interstitial tumor: an uncommon pediatric pulmonary neoplasm. Lung India. (2021) 38(2):186–90. doi: 10.4103/lungindia.lungindia_646_20
4. Yoshida M, Tanaka M, Gomi K, Iwanaka T, Dehner LP, Tanaka Y. Fetal lung interstitial tumor: the first Japanese case report and a comparison with fetal lung tissue and congenital cystic adenomatoid malformation/congenital pulmonary airway malformation type 3. Pathol Int. (2013) 63(10):506–9. doi: 10.1111/pin.12098
5. Crombleholme TM, Coleman B, Hedrick H, Liechty K, Howell L, Flake AW, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg. (2002) 37(3):331–8. doi: 10.1053/jpsu.2002.30832
6. Phillips J, Blask A, DiPoto Brahmbhatt A, Lawrence A, Timofeev J, Badillo A, et al. Fetal lung interstitial tumor: prenatal presentation of a rare fetal malignancy. J Neonatal Perinatal Med. (2019) 12(4):473–7. doi: 10.3233/NPM-180059
7. Lazar DA, Cass DL, Dishop MK, Adam K, Olutoye OA, Ayres NA, et al. Fetal lung interstitial tumor: a cause of late gestation fetal hydrops. J Pediatr Surg. (2011) 46(6):1263–6. doi: 10.1016/j.jpedsurg.2011.02.056
8. Wang LM, Ma XY, Tu YP, Liu XJ, Shang N, Yu G, et al. Prenatal ultrasonographic diagnosis and prognosis of congenital cystic adenomatoid malformation. Zhonghua Yi Xue Chao Sheng Za Zhi. (2014) 11(02):155–9. (Chinese.)
9. Zhong SL, Deng YQ, Zhang DR. The analysis of ultrasonic features and follow-up outcome in 99 cases with fetal congenital cystic adenomatoid malformations. Zhongguo Fu Chan Ke Lin Chuang Za Zhi. (2018) 19(06):525–8. doi: 10.13390/j.issn.1672-1861.2018.06.013. (Chinese.)
10. Zhang LL, Liu L, Zhang JW, Zhang L, Wang LY, An HB. Newborn fetal lung interstitial tumor: report of a case. Zhonghua Bing Li Xue Za Zhi. (2020) 49(7):744–5. doi: 10.3760/cma.j.cn112151-20200214-00101. (Chinese.)32610391
11. Watanabe T, Ohno M, Tahara K, Tomonaga K, Fuchimoto Y, Fujino A, et al. An investigation on clinical differences between congenital pulmonary airway malformation and bronchial atresia. J Pediatr Surg. (2018) 53(12):2390–3. doi: 10.1016/j.jpedsurg.2018.08.031
12. Zhao JF, Dong S, Xi DW, Yu CZ, Zhu JM, Liu YF, et al. Neonatal fetal lung interstitial tumor: a case report and literature review. Zhonghua Xiao Er Wai Ke Za Zhi. (2020) 41(4):330–4. doi: 10.3760/cma.j.cn421158-20181212-00543. (Chinese.)
13. Waelti SL, Garel L, Soglio DD, Rypens F, Messerli M, Dubois J. Neonatal congenital lung tumors—the importance of mid-second-trimester ultrasound as a diagnostic clue. Pediatr Radiol. (2017) 47(13):1766–75. doi: 10.1007/s00247-017-3953-3
14. Kuroda Y, Fukuzawa H, Kawahara I, Morita K. Hemi-Clamshell approach for fetal lung interstitial tumor resection in a neonate: a case report. European J Pediatr Surg Rep. (2021) 9(1):e72–5. doi: 10.1055/s-0041-1735807
15. Oh SH, Kim CY, Lee BS, Kim DK, Kim EA, Kim KS. Transthoracic catheter drainage for large symptomatic congenital pulmonary airway malformation. Pediatr Pulmonol. (2017) 52(12):1572–7. doi: 10.1002/ppul.23835
16. Liu D, Wu XM, Lin YJ, Luo PF. Fetal lung interstitial tumor: a rare case report of newborn. Zhonghua Bing Li Xue Za Zhi. (2021) 50(3):268–70. doi: 10.3760/cma.j.cn112151-20200515-00389. (Chinese.)33677898
17. Goto A, Yoshioka T, Ito T, Yano M, Hebiguchi T, Yoshino H. A case of fetal lung interstitial tumor (FLIT) in a neonate. J Thorac Oncol. (2015) 10(9 SUPPL. 2 (S463)).25634009
18. Onoda T, Kanno M, Sato H, Takahashi N, Izumino H, Ohta H, et al. Identification of novel ALK rearrangement A2M-ALK in a neonate with fetal lung interstitial tumor. Genes Chromosomes Cancer. (2014) 53(10):865–74. doi: 10.1002/gcc.22199
Keywords: fetal lung interstitial tumor, pediatric lung tumor, fetal lung mass, diagnosis, surgery
Citation: Wang Z, Xu C, He T and Yuan M (2023) A case report of misdiagnosed fetal lung mass and review of the literature. Front. Pediatr. 10:1045037. doi: 10.3389/fped.2022.1045037
Received: 15 September 2022; Accepted: 30 December 2022;
Published: 10 February 2023.
Edited by:
Francesco Morini, Meyer Children's Hospital, ItalyReviewed by:
Hany Gabra, Newcastle Hospitals, United KingdomMichele ILARI, G. Salesi Children's Hospital, Italy
© 2023 Wang, Xu, He and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chang Xu aHVheGl4dWNoYW5nQDE2My5jb20=
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
 Zongyu Wang
Zongyu Wang Chang Xu
Chang Xu Taozhen He
Taozhen He