
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 17 January 2023
Sec. Children and Health
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1044954
 Xiaoxian Yang1,†
Xiaoxian Yang1,† Chuhui Zhou2,3,†
Chuhui Zhou2,3,† Chentao Guo4,†
Chentao Guo4,† Jie Wang2,3
Jie Wang2,3 Innie Chen5,6,7
Innie Chen5,6,7 Shi Wu Wen5,6,7
Shi Wu Wen5,6,7 Daniel Krewski6,8,9
Daniel Krewski6,8,9 Liqun Yue10*
Liqun Yue10* Ri-hua Xie2,11*
Ri-hua Xie2,11*
Purpose: Previous studies reported a higher risk of food allergy for cesarean-born children than vaginal-born children. This study aims to systematically compare the prevalence of food allergy among cesarean-born and vaginal-born children aged 0–3 years.
Methods: Three English and two Chinese databases were searched using terms related to food allergies and cesarean sections. Cohort studies that reported the prevalence of food allergy in cesarean-born and vaginal-born children aged 0–3 years were included. Two reviewers performed study selection, quality assessment, and data extraction. The pooled prevalence of food allergy in cesarean-born and vaginal-born children was compared by meta-analysis.
Results: Nine eligible studies, with 9,650 cesarean-born children and 20,418 vaginal-born children aged 0–3 years, were included. Of them, 645 cesarean-born children and 991 vaginal-born children were identified as having food allergies. The pooled prevalence of food allergy was higher in cesarean-born children (7.8%) than in vaginal-born children (5.9%). Cesarean section was associated with an increased risk of food allergy [odds ratio (OR): 1.45; 95% confidence interval (CI): 1.03–2.05] and cow's milk allergy (OR: 3.31; 95% CI: 1.98–5.53). Additionally, cesarean-born children with a parental history of allergy had an increased risk of food allergy (OR: 2.60; 95% CI: 1.28–5.27).
Conclusion: This study suggests that cesarean sections was associated with an increased risk of food and cow's milk allergies in children aged 0–3 years. Cesarean-born children with a parental history of allergy demonstrated a higher risk for food allergy than did vaginal-born children. These results indicate that caregivers should be aware of the risks of food allergies in cesarean-born children, reducing the risk of potentially fatal allergic events. Further research is needed to identify the specific factors affecting food allergies in young children.
Systematic Review Registration: http://www.crd.york.ac.uk/prospero, identifier: International Prospective Register of Systematic Reviews (NO. CRD42019140748).
Food allergy, defined as an adverse immune response to food proteins (1–3), is an important public health problem (4) that is becoming increasingly prevalent (2, 5). Specific foods that have been associated with allergic reactions include cow's milk, egg, wheat, soy, peanut, tree nuts, fish, and shellfish (6). At this time, the reasons for the apparent increase in food allergies in children are unclear (7).
The rate of cesarean section (CS) has been increasing in recent decades (2, 5). CS has been associated with an increased risk of developing asthma (8), allergic rhinitis (9), and other immune disorders in offspring (10). Although neonates acquire maternal vaginal and fecal microbiota during labor and delivery (11), CS interrupts this transfer process, thereby altering bacterial colonization of the gut (12). The altered gut flora of cesarean-born children has been shown to prolong immunological immaturity and thereby increase the risk of allergic diseases (13).
Previous studies that assessed the association between mode of delivery and food allergy have yielded inconsistent results (7, 14–16). One study revealed that CS might be a risk factor for food allergens up to the age of 2 years (effect estimates for allergic sensitization against food allergens [odds ratio (OR) = 1.64 (1.03–2.63)] (14), whereas other studies found no convincing evidence that CS increased the risk of developing allergic diseases in children (15, 16). A systematic review synthesized the available evidence on the association between mode of delivery and food allergy and revealed that cesarean-born children under 10 years of age might have a higher risk of food allergy than vaginal-born children (7). However, no systematic review has estimated the prevalence of food allergy from different sources of foods among cesarean-born and vaginal-born children. This study aims to quantify and compare the prevalence of food allergy for cesarean-born and vaginal-born children aged 0–3 years through a systematic review and meta-analysis.
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (17). The protocol was registered with the International Prospective Register of Systematic Reviews (NO. CRD42019140748) at http://www.crd.york.ac.uk/prospero (18).
With the support of two research librarians with expertise in systematic reviews in health services, a systematic search of the literature was conducted to identify relevant studies using EMBASE, MEDLINE, Web of Science, CNKI, and Wanfang. The literature search included all published articles from inception to May 31, 2022. A combination of key terms and/or subject headings was applied, including food allergy terms (food hypersensitivity or (food or egg or nut or nuts* or peanut* or cashew or pistachio or hazelnut* or almond* or fish or soy or legume* or kiwi or apple or fruit or peach or milk or dairy or shellfish or wheat) or (allerg* or hypersensitivit*)) and cesarean section-related terms (c section* or Cesarean* or Caesarean*) (Supplementary Table S1). The databases were then combined and searched using the Covidence (a web-based software platform).
Titles and abstracts of articles retrieved by searching the five electronic databases were screened independently by two reviewers (XY and CG) to determine if they satisfied the predetermined inclusion/exclusion criteria. Fulltext screening was then performed to identify studies included in the systematic review. Disagreements regarding eligibility were resolved by discussion with the third reviewer (R-hX).
Studies were included in this systematic review, if they: (1) targeted cesarean-born and vaginal-born children aged 0–3 years; (2) identified food allergy based on either self-report questionnaires (i.e., participants or their parents reported that they had any of the outcomes or not), or objective methods [i.e., skin prick test (SPT), specific immunoglobulin E (IgE), open food challenge (OFC)/double-blind placebo-controlled food challenge (DBPCFC), or convincing clinical history (i.e., outcomes confirmed by a physician)] (19); (3) had information about the sample size and prevalence of food allergy among cesarean-born and vaginal-born children aged 0–3 years; and (4) were published in English or Chinese.
Although food allergies typically appear around 1 year of age, there may be delays in identifying and reporting. By expanding the age range to include children up to 3 years old, we expected to achieve a more complete ascertainment of food allergies in young children. Due to the pre-specified age range in this study, with cesarean- and vaginal-born infants assessed in the same fashion; we did not expect bias to enter our review. Studies were excluded if they were abstracts, interviews, commentaries, or reviews. In addition, studies that did not report the prevalence of food allergy in children aged 0–3 were excluded.
Two reviewers (XY and CZ) independently extracted relevant data from eligible studies using a standard form, including: the last name of the first author, publication year, country of origin, age of children, the identified methods of food allergy, parental history of food allergy, the study period, the number of cesarean-born and vaginal-born children, and the number of food, cow's milk, and egg allergies occurring in cesarean-born, and vaginal-born children. Any disagreements between the two reviewers were resolved by discussion with the third reviewer (R-hX).
The risk of bias and quality of the study were assessed by two reviewers (CG and CZ) independently using the Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI) (Supplementary Table S2). Any disagreements with respect to validity were resolved by the third reviewer (LY).
Data analysis was conducted in the “meta” and “metaphor” modules using R software (version 3.6.2) (19). Heterogeneity across studies was assessed using the Higgins I2 statistic (20). In cases of significant heterogeneity (I2 > 0.5), the random effects model of Der Simonian and Laird (20) was used to obtain the prevalence of food allergy; otherwise, in cases of no inconsistency in the risk estimate (I2 < 0.5), a fixed effects model was used. When significant heterogeneity was observed, mixed-model meta-regression analysis was also conducted to explore the influence of potential moderators of heterogeneity using the restricted maximum-likelihood method. Subgroup analyses were conducted in terms of parental history of food allergy and identified methods of food allergy. Influence analysis was performed by serially removing each study one by one and excluding low-quality studies to examine their influence on the strength and stability of the pooled results. Potential publication biases were assessed graphically using funnel plots and statistically significance with P < 0.05 using Egger's tests (21).
A total of 937 studies were identified through the searching of the five electronic databases. After removing duplicates and title/abstract screening for eligibility, 88 studies were selected for full text review. Of which, 80 were excluded because of their inability to satisfy the inclusion and exclusion criteria; and one study identified through citation searching was included. Finally, nine eligible studies (14, 16, 22–28) were chosen for data analysis (Figure 1).
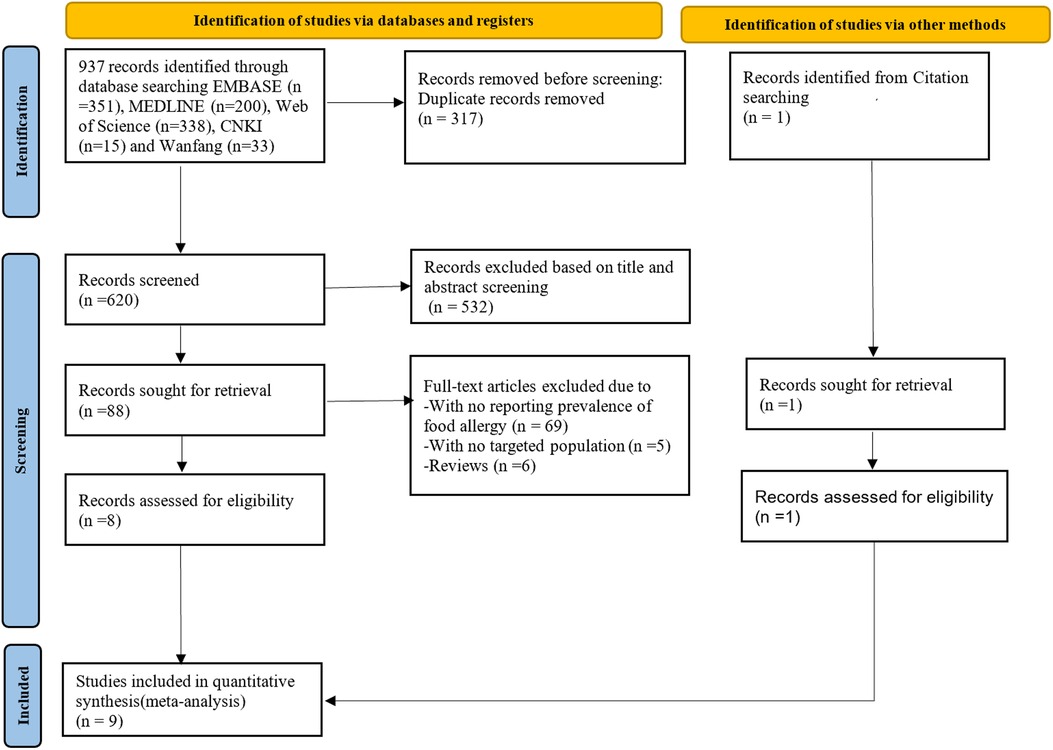
Figure 1. PRISMA flowchart showing selection of studies for review. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
The nine eligible studies (14, 16, 22–28) included in this review were published between 2003 and 2022 in seven countries: Norway, Germany, Australia, Sweden, China, and America. Their sample sizes ranged from 104 to 2,921, with a total of 9,650 cesarean-born children and 20,418 vaginal-born children aged 0–3 years. Of them, 645 cesarean-born children and 991 vaginal-born children had food allergies. Three studies (22, 23, 25) used exclusively perceived parental reactions to identify food allergy, and six (14, 16, 24, 26–28) used objective methods [SPT, IgE, OFC/DBPCFC, or clinical diagnosis (i.e., outcomes confirmed by a physician)]. Two studies (24, 25) recruited children whose parents had a history of allergy. Six studies (14, 16, 22–24, 26) reported the prevalence of any food allergy; three studies (23, 24, 28) reported cow's milk allergy; and two studies (23, 27) reported egg allergy in cesarean-born and vaginal-born children (Table 1).
Table 2 presents the results of the quality assessment of the nine studies (14, 16, 22–28) using the JBI-MAStARI quality scoring tool for cohort studies. The quality of these nine studies was generally high.
Figures 2, 3 summarize the pooled prevalence of food allergy of cesarean-born and vaginal-born children. The pooled prevalence of food allergy was 7.8% [95% confidence interval (CI): 4.7%–11.5%, I2 = 96.6%, 95% CI: 95.1%–97.7%] in cesarean-born children (Figure 2) and 5.9% (95% CI: 2.8%–10.1%; I2 = 99.3%, 95% CI: 99.1%–99.4%) in vaginal-born children (Figure 3). Overall, CS was associated with an increased risk of food allergy (OR: 1.45, 95% CI: 1.03–2.05; I2 = 86.0%, 95% CI: 75.5–92.1) (Figure 4).
Table 3 presents results from our subgroup analysis. The prevalence of food allergy was higher in cesarean-born children than in vaginal-born children aged 0–3 years according to identified methods of food allergy, type of food allergies, parental history of allergy, age group and country. In addition, cesarean-born children with cow's milk allergy (OR: 3.31; 95% CI: 1.98–5.53) and with a parental history of allergy (OR: 2.60; 95% CI: 1.28–5.27), had a higher risk of food allergy than did vaginal-born children.
Influence analysis based on one-by-one removal of the nine eligible studies (14, 16, 22–28) indicated that the pooled prevalence of food allergy in cesarean-born children varied from 7.1% (95% CI: 6.5%–7.7%) to 10.8% (95% CI:10.1%–11.5%), with the corresponding I2 statistic ranging from 91.8% to 96.1%, while the pooled prevalence of food allergy in vaginal-born children varied from 5.6% (95% CI: 5.3%–5.9%) to 8.2% (95% CI: 7.8%–8.7%), with the corresponding I2 statistic varying from 97.6% to 98.7% (Table 4). Although the number of the studies included in the final analysis was less than 10, the funnel plot was used to evaluate their potential publication bias (Figure 5), providing no evidence of potential publication bias, with P-value for the Egger's rank test being 0.6.
Meta-regression analysis revealed that the identified methods of food allergy and the infants' age were the significant sources of heterogeneity in estimates of the prevalence of food allergy (P < 0.05) (Table 5).

Table 5. Meta-regression analyses of the effects of potential modifying factors of the prevalence of food allergy in children.
Meta-analysis of the nine studies included in our systematic review found that the pooled prevalence of food allergy was 7.8% in cesarean-born children, which was higher than 5.9% in vaginal-born children, with an OR = 1.45 (95% CI: 1.03–2.05). Cesarean section was associated with an increased risk of both food allergy and cow's milk allergy in children. Cesarean-born children aged 0–3 years with a parental history of allergy also developed food allergies at a higher rate than vaginal-born children of the same age (OR: 2.60; 95% CI: 1.28–5.27).
To the best of our knowledge, this is the first systematic review/meta-analysis that compared the pooled prevalence of food allergy from various sources of foods for cesarean-born and vaginal-born children aged 0–3 years. Our consistent findings from the subgroup, influence analyses and meta-regression analysis indicated the robustness of the results. Also, we did not find any publication bias.
Several limitations of this study must be acknowledged. First, although the overall sample size was large, the small sample size for specific food allergies complicated the interpretation of these results. For example, only two studies (23, 27) included children who were allergic to eggs. Second, heterogeneities have been partly explained by our subgroup analyses or meta-regressions. Our influence analysis showed considerable variations in the results from one-by-one removal and the potential existence of influential cases/outliers. Thus, we could not adjust for potential confounders in the meta-analysis due to heterogeneity for specific foods, including important sources of food allergies such as peanuts, fish, and wheat, as this information was not available in the original studies. Third, the original studies lacked information regarding breastfeeding, formula feeding, eczema, and the timing to take complementary foods, precluding an analysis of the potential influence of these factors on our meta-analytic results.
Sensitization to food allergens during early childhood could be an important predictor for developing allergic airway diseases later in childhood (29). Our study found that more than 7.8% of cesarean-born children aged 0–3 years were allergic to any food sources, higher than the rate of 5.9% of vaginal-born children and higher than the 6% reported in children aged 0–3 years observed in another systematic review in the general population regardless of mode of delivery (30). These results support our hypothesis that CS may increase the risk of developing food allergies in children (31).
One possible explanation for the increased prevalence of food allergy in cesarean-born children aged 0–3 years is that their gut microbiota are different from those of vaginal-born children (15, 32). The intestinal microbiota and early-life microbial exposure play important roles in the development of the immune system (32), as well as in educating the immune system (33). CS could lead to delayed bacterial colonization of the gut because it limited newborn exposure to maternal vaginal and fecal microflora (34). The interruption of natural colonization has been speculated to derive from the altered immune development as well as allergic diseases among children (34). Previous studies demonstrated that mode of delivery is an imperative independent factor impacting natural colonization, especially in the first months of life (35–37). Thus, CS may be associated with an increased risk of immune and metabolic disorders, as compared with vaginal delivery (VD) (38). However, some studies showed conflicting results, namely, that CS was not associated with food allergy risk in infants (15, 16). These might be because there are diverse ways to distinguish whether an elective or emergency cesarean is with or without labor and to measure food allergy, leading to contradicting results (16).
Cow's milk protein allergy (CMPA) is considered the most common food allergy in infants (39). The subgroup analyses conducted in the present study suggest that cesarean-born children have a higher risk of cow's milk allergy than in vaginal-born children. The underlying mechanism is unknown but may be related to the gut microbiota composition in feces from children with food allergies (28, 40) which have higher proportions of the Clostridium coccoides group and Atopobium cluster as well as a higher proportion of the (sum of) different bacterial groups in comparison to healthy infant feces (40). It has also been shown that bacterial colonization of the gut in cesarean-born children aged 0–3 years differs from that in vaginal-born children (24).
The pooled prevalence of food allergy in cesarean-born children aged 0–3 years whose parents had a history of allergy was higher than that in vaginal-born infants, consistent with a previous study (7). Genetic predisposition seems to be the biggest risk factor for allergic diseases (41). Currently, it is widely accepted that children with a family history of “allergy” are at generally increased risk of food allergy (42). A family study has shown that food sensitization and allergy are more common in those with a first degree relative with food allergy (43). A study in Korea has demonstrated maternal allergy is associated with an increased risk of parent-reported food allergy in children's first year of life (44). However, genetics alone cannot explain the rising global prevalence of food allergy (41); CS and parental history of allergy may have cooperative effects on food allergy in children.
Cesarean section was associated with an increased risk of food allergy and cow's milk allergy in cesarean-born children aged 0–3. Cesarean-born children with a parental history of allergy developed a higher risk for food allergies than did vaginal-born children. These results indicate that caregivers should be aware of the risks of food allergy in cesarean-born children, reducing the risk of potentially fatal allergic events. Further research is needed to identify the specific factors affecting food allergies in young children.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
R-hX substantially contributed to the conception of the review. XY, CZ, and CG contributed to the acquisition. XY and CG contributed to the data analysis. XY, JW, and CZ contributed to the interpretation of these results. XY drafted the manuscript. R-hX, LY, IC, SWW, and DK critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by Jiangsu Provincial Department of Education (grant no. 20KJB330006), Canadian Institute of Health Research (grant nos. FDN-148438, PJT-178049, and P14-175351), and Clinical Research Startup Program of Southern Medical University by High-level University Construction Funding of Guangdong Provincial Department of Education (grant no. LC2019ZD019).
We thank all authors of the eligible articles and the two research librarians, Lindsey Sikora and Risa Shorr, from the University of Ottawa and Ottawa Hospital Research Institute, for assistance in designing the literature search.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1044954/full#supplementary-material.
CS, cesarean section; CI, confidence interval; OR, odds ratio; PRISMA, Preferred Reporting Item for Systematic Reviews and Meta-analyses; VD, vaginal delivery.
1. Sackeyfio A, Senthinathan A, Kandaswamy P, Barry PW, Shaw B, Baker M, et al. Diagnosis and assessment of food allergy in children and young people: summary of NICE guidance. Br Med J. (2011) 342:d747. doi: 10.1136/bmj.d747
2. Peters RL, Krawiec M, Koplin JJ, Santos AF. Update on food allergy. Pediatr Allergy Immunol. (2021) 32(4):647–57. doi: 10.1111/pai.13443
3. Cianferoni A, Spergel JM. Food allergy: review, classification and diagnosis. Allergol Int. (2009) 58(4):457–66. doi: 10.2332/allergolint.09-RAI-0138
4. Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. (2014) 69(1):62–75. doi: 10.1111/all.12305
5. Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. (2014) 69(8):992–1007. doi: 10.1111/all.12423
6. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. (2012) 32(1):35–50. doi: 10.1016/j.iac.2011.11.008
7. Koplin J, Allen K, Gurrin L, Osborne N, Tang ML, Dharmage S. Is caesarean delivery associated with sensitization to food allergens and IgE-mediated food allergy: a systematic review. Pediatr Allergy Immunol. (2008) 19(8):682–7. doi: 10.1111/j.1399-3038.2008.00731.x
8. Darabi B, Rahmati S, HafeziAhmadi MR, Badfar G, Azami M. The association between caesarean section and childhood asthma: an updated systematic review and meta-analysis. Allergy Asthma Clin Immunol. (2019) 15(1):1–13. doi: 10.1186/s13223-019-0367-9
9. Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. (2008) 122(2):274–9. doi: 10.1016/j.jaci.2008.05.007
10. Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol. (2016) 137(2):587–90. doi: 10.1016/j.jaci.2015.07.040
11. Hyman RW, Fukushima M, Diamond L, Kumm J, Giudice LC, Davis RW. Microbes on the human vaginal epithelium. Proc Natl Acad Sci U S A. (2005) 102(22):7952–7. doi: 10.1073/pnas.0503236102
12. Loo EXL, Sim JZT, Loy SL, Goh A, Chan YH, Tan KH, et al. Associations between caesarean delivery and allergic outcomes: results from the GUSTO study. Ann Allergy Asthma Immunol. (2017) 118(5):636–8. doi: 10.1016/j.anai.2017.02.021
13. Ly NP, Ruiz-Pérez B, Onderdonk AB, Tzianabos AO, Litonjua AA, Liang C, et al. Mode of delivery and cord blood cytokines: a birth cohort study. Clin Mol Allergy. (2006) 4(1):1–11. doi: 10.1186/1476-7961-4-13
14. Negele K, Heinrich J, Borte M, von Berg A, Schaaf B, Lehmann I, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. (2004) 15(1):48–54. doi: 10.1046/j.0905-6157.2003.00101.x
15. McKeever TM, Lewis SA, Smith C, Hubbard R. Mode of delivery and risk of developing allergic disease. J Allergy Clin Immunol. (2002) 109(5):800–2. doi: 10.1067/mai.2002.124046
16. Currell A, Koplin JJ, Lowe AJ, Perrett KP, Ponsonby AL, Tang MLK, et al. Mode of birth is not associated with food allergy risk in infants. J Allergy Clin Immunol Pract. (2022) 10(8):2135–43.e3. doi: 10.1016/j.jaip.2022.03.031
17. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRIS MA-P) 2015 statement. Syst Rev. (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
18. PROSPERO. Xiaoxian Yang YC, Rihua Xie SS. Wen: Prevalence of food allergy in cesarean-born infants: a systematic review (2019). Available at: https://wwwcrdyorkacuk/prospero/display_recordphp?ID=CRD42019140748. (Accessed September 18, 2019).
19. Laia-Dias I, Lozoya-Ibáñez C, Skypala I, Gama JMR, Nurmatov U, Lourenço O, et al. Prevalence and risk factors for food allergy in older people: protocol for a systematic review. BMJ Open. (2019) 9(8):e029633. doi: 10.1136/bmjopen-2019-029633
20. Higgins JPT, Green S, (editors). Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). The Cochrane Collaboration (2011). Available at: www.cochrane-handbook.org.
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
22. Adeyeye TE, Yeung EH, McLain AC, Lin S, Lawrence DA, Bell EM. Wheeze and food allergies in children born via cesarean delivery: the upstate kids study. Am J Epidemiol. (2019) 188(2):355–62. doi: 10.1093/aje/kwy257
23. Eggesbø M, Botten G, Stigum H, Nafstad P, Magnus P. Is delivery by cesarean section a risk factor for food allergy? J Allergy Clin Immunol. (2003) 112(2):420–6. doi: 10.1067/mai.2003.1610
24. Laubereau B, Filipiak-Pittroff B, von Berg A, Grübl A, Reinhardt D, Wichmann HE, et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch Dis Child. (2004) 89(11):993–7. doi: 10.1136/adc.2003.043265
25. Eggesbø M, Botten G, Stigum H, Samuelsen SO, Brunekreef B, Magnus P. Cesarean delivery and cow milk allergy/intolerance. Allergy. (2005) 60(9):1172–3. doi: 10.1111/j.1398-9995.2005.00857.x
26. Kvenshagen B, Halvorsen R, Jacobsen M. Is there an increased frequency of food allergy in children delivered by caesarean section compared to those delivered vaginally? Acta Paediatr. (2009) 98(2):324–7. doi: 10.1111/j.1651-2227.2008.01074.x
27. Koplin JJ, Dharmage SC, Ponsonby AL, Tang ML, Lowe AJ, Gurrin LC, et al. Environmental and demographic risk factors for egg allergy in a population-based study of infants. Allergy. (2012) 67(11):1415–22. doi: 10.1111/all.12015
28. Yang M, Tan M, Wu J, Chen Z, Long X, Zeng Y, et al. Prevalence, characteristics, and outcome of cow's milk protein allergy in Chinese infants: a population-based survey. JPEN J Parenter Enteral Nutr. (2019) 43(6):803–8. doi: 10.1002/jpen.1472
29. Kulig M, Bergmann R, Tacke U, Wahn U, Guggenmoos-Holzmann I. Long-lasting sensitization to food during the first two years precedes allergic airway disease. The mas study group, Germany. Pediatr Allergy Immunol. (2010) 9(2):61–7. doi: 10.1111/j.1399-3038.1998.tb00305.x
30. Panel NSE, Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. (2010) 126(6 Suppl):S1–58. doi: 10.1016/j.jaci.2010.10.007
31. Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. (2018) 392(10155):1349–57. doi: 10.1016/S01406736(18)31930-5
32. Zimmermann P, Messina N, Mohn WW, Finlay BB, Curtis N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: a systematic review. J Allergy Clin Immunol. (2019) 143(2):467–85. doi: 10.1016/j.jaci.2018.09.025
33. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. (2012) 336(6080):489–93. doi: 10.1126/science.1219328
34. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. (2016) 352(6285):539–44. doi: 10.1126/science.aad9378
35. Stokholm J, Thorsen J, Chawes BL, Schjørring S, Krogfelt KA, Bønnelykke K, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. (2016) 138(3):881–9.e2. doi: 10.1016/j.jaci.2016.01.028
36. Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. (2016) 8(343):343ra82. doi: 10.1126/scitranslmed.aad7121
37. Hoen AG, Coker MO, Madan JC, Pathmasiri W, McRitchie S, Dade EF, et al. Association of cesarean delivery and formula supplementation with the stool metabolome of 6-week-old infants. Metabolites. (2021) 11(10):702. doi: 10.3390/metabo11100702
38. Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. (2016) 22(3):250–3. doi: 10.1038/nm.4039
39. Vandenplas Y, Benninga M, Broekaert I, Falconer J, Gottrand F, Guarino A, et al. Functional gastro-intestinal disorder algorithms focus on early recognition, parental reassurance and nutritional strategies. Acta Paediatr. (2016) 105(3):244–52. doi: 10.1111/apa.13270
40. Thompson-Chagoyan OC, Fallani M, Maldonado J, Vieites JM, Khanna S, Edwards C, et al. Faecal microbiota and short-chain fatty acid levels in faeces from infants with cow's milk protein allergy. Int Arch Allergy Immunol. (2011) 156(3):325–32. doi: 10.1159/000323893
41. Yang HJ, Lee SY, Suh DI, Shin YH, Kim BJ, Seo JH, et al. The Cohort for Childhood Origin of Asthma and allergic diseases (COCOA) study: design, rationale and methods. BMC Pulm Med. (2014) 14:109. doi: 10.1186/1471-2466-14-109
42. Koplin JJ, Allen KJ, Gurrin LC, Peters RL, Lowe AJ, Tang ML, et al. The impact of family history of allergy on risk of food allergy: a population-based study of infants. Int J Environ Res Public Health. (2013) 10(11):5364–77. doi: 10.3390/ijerph10115364
43. Tsai HJ, Kumar R, Pongracic J, Liu X, Story R, Yu Y, et al. Familial aggregation of food allergy and sensitization to food allergens: a family-based study. Clin Exp Allergy. (2009) 39(1):101–9. doi: 10.1111/j.1365-2222.2008.03111
Keywords: food allergy, cesarean-born, children aged 0–3 years, meta-analysis, prevalence of food allergy
Citation: Yang X, Zhou C, Guo C, Wang J, Chen I, Wen SW, Krewski D, Yue L and Xie R (2023) The prevalence of food allergy in cesarean-born children aged 0–3 years: A systematic review and meta-analysis of cohort studies. Front. Pediatr. 10:1044954. doi: 10.3389/fped.2022.1044954
Received: 15 September 2022; Accepted: 28 December 2022;
Published: 17 January 2023.
Edited by:
Wenming Zhang, Stanford University, United StatesReviewed by:
Ayantika Sen, Stanford University, United States© 2023 Yang, Zhou, Guo, Wang, Chen, Wen, Krewski, Yue and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liqun Yue MjgwNzgxMjgzNkBxcS5jb20= Ri-hua Xie eGllcmlodWE5MjhAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.