- 1Neonatal Intensive Care Centre, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 2Women and Children’s Health, School of Life Course Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, United Kingdom
- 3Department of Radiology, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 4National Institute for Health Research (NIHR) Biomedical Research Centre Based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College, London, United Kingdom
Objective: To report values of the chest radiographic thoracic area (CRTA) in ventilated, term-born infants without respiratory disease and to evaluate whether CRTA is related to demographic data at birth.
Methods: Retrospective, observational cohort study in a tertiary neonatal unit at King's College Hospital NHS Foundation Trust, London, UK.
Newborn infants born after 36 completed weeks of gestation, ventilated for poor perinatal adaptation or hypoxic ischaemic encephalopathy without respiratory disease and admitted in a recent eight-year period (2014–2022).
The CRTA was assessed by free-hand tracing of the perimeter of the thoracic area as outlined by the diaphragm and the rib cage excluding the mediastinal structures and was calculated using the Sectra PACS software.
Results: One hundred and twenty-one infants (75 male) were included with a median (IQR) gestation of 40 (38–41) weeks and birth weight of 3.41 (3.04–3.75) kg. The median (IQR) CRTA was 2,589 (2,167–2,943) mm2 and was significantly related to birth weight (r = 0.316, p = 0.003), gestation at birth (r = 0.193, p = 0.032) and birth weight z-score (r = 0.187, p = 0.038).
Conclusions: We report values of the chest radiographic thoracic area in ventilated term-born infants which could be used as reference for determining respiratory disease severity.
Introduction
Quantitative assessment of the pulmonary parenchyma in neonatal intensive care has been undertaken by imaging and functional methods such as fetal magnetic resonance (1) or the measurement of the functional residual capacity (FRC) (2). An alternative and more accessible, surrogate method to estimate lung volumes is the chest radiographic thoracic area (CRTA), which is the measurement of the areas corresponding to the lungs on a two-dimensional chest radiograph. The CRTA has been shown to correlate well with the gold standard measurement of FRC by helium dilution (3). The measurement of the CRTA is important in diseases associated with decreased lung volumes. Low CRTA has been associated with failure to wean off invasive ventilation in preterm infants (4). The CRTA method has also been used in congenital diaphragmatic hernia (CDH) and predicted survival to discharge with an area under the receiver operator characteristic curve of 0.808–0.826 (5–7).
Although CRTA has been well-documented in pathological conditions, values in term infants without respiratory disease have not been reported. Such reference data would be important to establish the magnitude of the disease in respiratory disorders that are characterised by low lung volumes such as pulmonary hypoplasia due to prolonged preterm rupture of membranes (8) and diseases with hyperinflation such as bronchiolitis (9), severe asthma (10) or severe bronchopulmonary dysplasia (11). Our aim was to report values of CRTA in ventilated term-born infants without respiratory pathology and explore whether demographics at birth influence those values.
Methods
Subjects
Newborn infants treated for poor perinatal adaptation or hypoxic ischemic encephalopathy without concomitant respiratory pathology over 8 years (1/6/14–1/6/22) at King's College Hospital NHS Foundation Trust, London, UK were included in the study. The included infants were intubated and invasively ventilated for absence of respiratory drive at birth and had no supplemental oxygen requirement by 6 h of age. The starting point was selected as this was when the unit adopted volume-targeted ventilation as primary mode of mechanical ventilation. The infants were ventilated with a targeted tidal volume of 5 ml/kg and a positive end-expiratory pressure of 4 cm–5 cm H2O. The study was registered as a service evaluation with the Clinical Governance Department of KCH and, as it was not a research study, informed parental consent was not required. The following information was collected from the medical notes: sex, mode of delivery, gestation (completed weeks), birth weight (kg), birth weight z-score (12).
Chest radiographs
The chest radiographs in the first 24 h after birth were reviewed for each infant, and the one with the highest CRTA was included in the analysis. The chest radiographs were anterio–posterior, obtained in the supine position at end-inspiration and at a standard distance of one metre above the infant. Rotated radiographs and radiographs with evidence of a pneumothorax or concomitant respiratory disease were excluded from the analysis. Rotation was assessed by measuring the distance between the medial edges of the clavicles to the vertebral spinous processes. The radiographs were imported as digital image files by Sectra PACS software (Sectra AB, Linköping, Sweden). The software automatically adjusted for magnification errors. Free-hand tracing of the perimeter of the thoracic area as outlined by the diaphragm and the rib cage was undertaken and the CRTA was calculated by the software (Figure 1). The repeatability of the method has been previously described with an inter- and intra-observer coefficient of repeatability of 1.06 cm2 and 1.0 cm2 respectively (3).
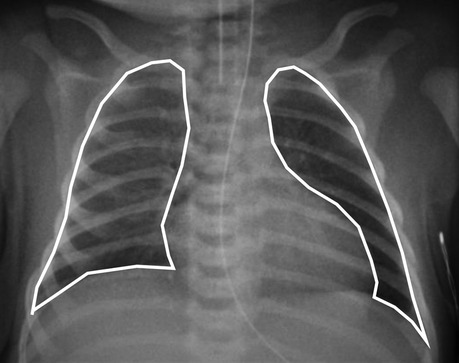
Figure 1. A chest radiograph of a term-born infant with poor perinatal adaptation on the first day of life. Method of free hand tracing of the perimeter of the CRTA excluding the mediastinal shadow.
Statistics
Data were tested for normality using the Kolmogorov–Smirnov test, found to be non-normally distributed and were presented as median (interquartile range). The relationships of CRTA with gestational age, birth weight and birth weight z-score were examined with the Spearman's ρ correlation coefficient. Differences in CRTA between male and female infants were assessed for statistical significance using the Mann-Whitney rank sum test. The relationship of the CRTA with birth weight was also examined with linear regression analysis to derive an equation to predict CRTA based on the birth weight. Statistical analysis was performed using SPSS software (SPSS Inc., Chicago IL).
Results
During the study period, 158 infants born after 36 completed weeks of gestation with poor perinatal adaptation or hypoxic ischemic encephalopathy were ventilated on the neonatal unit at KCH. Thirty-seven infants were excluded because of rotated radiographs, pneumothorax or concomitant respiratory disease. One hundred and twenty-one infants (75 male) were included with a median (IQR) gestational age of 40 (38–41) weeks, birth weight of 3.41 (3.04–3.75) kg and birth weight z-score of 0.03 (−0.59–0.75). The median (IQR) CRTA of the right lung was 1,486 (1,298–1,793) mm2 and of the left lung was 991 (852–1,202) mm2. The total CRTA was 2,589 (2,167–2,943) mm2 and was significantly related to birth weight (r = 0.316, p = 0.003 (figure 2)), gestational age (r = 0.193, p = 0.032) and birth weight z-score (r = 0.187, p = 0.038). The CRTA was not significantly different in male compared to female infants (p = 0.441). Following linear regression analysis, birth weight could predict the CRTA (R2 = 0.148, p < 0.001, 95% confidence intervals: 215–542). The regression equation was:
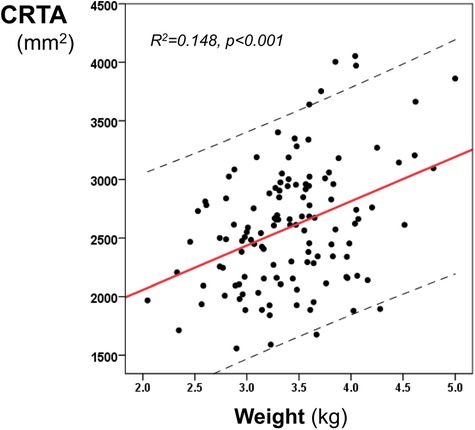
Figure 2. Linear regression analysis of the CRTA and the birth weight. The regression line and 95% confidence intervals (dashed lines) are depicted.
Discussion
We have reported CRTA values in ventilated term newborns without respiratory pathology. The CRTA was related to birth weight and gestation at birth. We report a median CRTA of 2,600 mm2 which is considerably higher than the 1,680 mm2 that we have previously reported in infants with CDH (7), reflecting that the latter infants had pulmonary hypoplasia. Amodeo and co-workers also studied 77 infants with CDH within 24 h after birth and reported that infants who died had lower CRTA values compared to the ones that survived, infants with hernia recurrence had lower CRTA and lower CRTA was associated with increased systolic pulmonary artery pressure (5). Weiss and co-workers measured the CRTA in 255 infants with CDH and demonstrated that the CRTA correlated significantly with survival and need for extracorporeal membrane oxygenation and that the CRTA displayed a higher ability to predict those outcomes compared with the widely used observed to expected lung/head ratio (6).
Other than in CDH infants, we have also reported that the median CRTA in 22 premature infants with bronchopulmonary dysplasia born at a median gestation of 26 weeks was 2,956 mm2, which is higher than our present study, but that result included the mediastinum, as in premature infants the mediastinal area sometimes cannot be clearly delineated from the lung fields (13). In our current study we reported that the ratio of the right to left lung CRTA is approximately 1.5. This might partially explain why unilateral right lung pathology is often clinically more severe than unilateral left, such as in right-sided CDH which has been associated with greater long-term morbidity (14), although clearly other factors might also play a role such as that the liver, which is herniating in right-sided CDH, is a non-compressible organ. In children and young adults, the volume of the right lung has been reported as 1.12 times larger than the volume of the left lung (15) and the weight as 1.14 times larger (16) but the projection of a three-dimensional structure to a two-dimensional radiograph might explain this discrepancy.
The correlation of the CRTA with the birth weight was significant, but arguably modest. This might be explained by the phase of breathing during which the radiograph was obtained. Although an effort is made to time the radiograph at end-inspiration, the high breathing rate of newborns might make this synchronisation challenging. There was, however, a clear separation of CRTA values in our study (interquartile range: 2,167–2,943 mm2), compared with infants with congenital lung pathology (interquartile range in CDH: 1,064–1,986 mm2) (7) which highlights that the possible impact of synchronisation on the CRTA would not affect the ability of CRTA to separate normal from pathological lungs. It is also possible that since the radiographs were performed on the first day of life in accordance with previous studies (5–7), some delayed clearance of lung fluid might be present in some infants (17). We should note that methodologically CRTA would be more appropriate to assess lung volumes in diseases that are characterised by lung hypoplasia, but would be of limited value for diseases with infiltrations such as pneumonia or neonatal acute respiratory distress syndrome. On the other hand CRTA would be expected to be increased in diseases that are characterised by hyperinflation or gas trapping such as emphysema or meconium aspiration syndrome respectively. In our population, the PEEP was kept relatively constant as per our internal protocol for term infants without respiratory disease. A higher PEEP, however, such as 6 or 7 cm H2O could affect the CRTA by producing artificially higher values.
Our study has strengths and some limitations. We included a large number of infants that were ventilated on volume-targeted ventilation. Infants ventilated for hypoxic ischemic encephalopathy are often receiving whole body hypothermia which might impact on respiratory function (18). Ventilation efficiency and tidal volumes have been reported to non-significantly increase during hypothermia (18), but in the era of volume targeted ventilation the effect of hypothermia on tidal volumes would be minimised.
In conclusion, we report values of the chest radiographic thoracic area in ventilated term newborn infants which could be used as a reference for determining respiratory disease severity in diseases that are characterised by lung hypoplasia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
TD: conceptualisation, analysis, writing of the original draft. JA: Data curation, methodology, software, validation, revising. AG: Analysis, methodology, critical revising and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was partially supported by King's College London 2018 Medical Research Council Confidence in Concept Award through the King's Health Partners' Research and Development Challenge Fund. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ward VL, Nishino M, Hatabu H, Estroff JA, Barnewolt CE, Felman HA, et al. Fetal lung volume measurements: determination with MR imaging–effect of various factors. Radiology. (2006) 240:187–93. doi: 10.1148/radiol.2393050583
2. Proietti E, Riedel T, Fuchs O, Pramana I, Singer F, Schmidt A, et al. Can infant lung function predict respiratory morbidity during the first year of life in preterm infants? Eur Respir J. (2014) 43:1642–51. doi: 10.1183/09031936.00149213
3. Dimitriou G, Greenough A, Kavvadia V, Shute M, Karani J. A radiographic method for assessing lung area in neonates. Br J Radiol. (1999) 72:335–8. doi: 10.1259/bjr.72.856.10474492
4. Dimitriou G, Greenough A. Computer assisted analysis of the chest radiograph lung area and prediction of failure of extubation from mechanical ventilation in preterm neonates. Br J Radiol. (2000) 73:156–9. doi: 10.1259/bjr.73.866.10884728
5. Amodeo I, Pesenti N, Raffaeli G, Macchini F, Condò V, Borzani I, et al. NeoAPACHE II. Relationship between radiographic pulmonary area and pulmonary hypertension, mortality, and hernia recurrence in newborns with CDH. Front Pediatr. (2021) 9:692210. doi: 10.3389/fped.2021.692210
6. Weis M, Burhany S, Perez Ortiz A, Nowak O, Hetjens S, Ket al Z. The chest radiographic thoracic area can serve as a prediction marker for morbidity and mortality in infants with congenital diaphragmatic hernia. Front Pediatr. (2021) 9:740941. doi: 10.3389/fped.2021.740941
7. Dassios T, Ali K, Makin E, Bhat R, Krokidis M, Greenough A. Prediction of mortality in newborn infants with severe congenital diaphragmatic hernia using the chest radiographic thoracic area. Pediatr Crit Care Med. (2019) 20:534–9. doi: 10.1097/PCC.0000000000001912
8. Kettle R, Subhedar NV. European I NOR. Nitric oxide in pulmonary hypoplasia: results from the European iNO registry. Neonatology. (2019) 116:341–6. doi: 10.1159/000501800
9. Seidenberg J, Masters IB, Hudson I, Olinsky A, Phelan PD. Disturbance in respiratory mechanics in infants with bronchiolitis. Thorax. (1989) 44:660–7. doi: 10.1136/thx.44.8.660
10. Tuxen DV, Williams TJ, Scheinkestel CD, Czarny D, Bowes G. Use of a measurement of pulmonary hyperinflation to control the level of mechanical ventilation in patients with acute severe asthma. Am Rev Respir Dis. (1992) 146:1136–42. doi: 10.1164/ajrccm/146.5_Pt_1.1136
11. May C, Kennedy C, Milner AD, Rafferty GF, Peacock JL, Greenough A. Lung function abnormalities in infants developing bronchopulmonary dysplasia. Arch Dis Child. (2011) 96:1014–9. doi: 10.1136/adc.2011.212332
12. Cole TJ, Williams AF, Wright CM, Group RGCE. Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts. Ann Hum Biol. (2011) 38:7–11. doi: 10.3109/03014460.2011.544139
13. Dassios T, Curley A, Krokidis M, Morley C, Ross-Russell R. Correlation of radiographic thoracic area and oxygenation impairment in bronchopulmonary dysplasia. Resp Physiol Neurobiol. (2016) 220:40–5. doi: 10.1016/j.resp.2015.09.009
14. Ali K, Dassios T, Khaliq SA, Williams EE, Tamura K, Davenport M, et al. Outcomes of infants with congenital diaphragmatic hernia by side of defect in the FETO era. Pediatr Surg Int. (2019) 35:743–7. doi: 10.1007/s00383-019-04484-3
15. Chun EM, Suh SW, Modi HN, Kang EY, Hong SJ, Song HR. The change in ratio of convex and concave lung volume in adolescent idiopathic scoliosis: a 3D CT scan based cross sectional study of effect of severity of curve on convex and concave lung volumes in 99 cases. Eur Spine J. (2008) 17:224–9. doi: 10.1007/s00383-019-04484-3
16. Matoba K, Hyodoh H, Murakami M, Saito A, Matoba T, Ishida L, et al. Estimating normal lung weight measurement using postmortem CT in forensic cases. Leg Med. (2017) 29:77–81. doi: 10.1016/j.legalmed.2017.10.014
17. Alhassen Z, Vali P, Guglani L, Lakshminrusimha S, Ryan RM. Recent advances in pathophysiology and management of transient tachypnea of newborn. J Perinatol. (2021) 41:6–16. doi: 10.1038/s41372-020-0757-3
Keywords: normal chest radiographic thoracic area newborn, infant, neonate, radiography, thoracic radiography
Citation: Dassios T, Adu J and Greenough A (2023) Brief research report: Chest radiographic thoracic area in term ventilated infants without respiratory disease. Front. Pediatr. 10:1042341. doi: 10.3389/fped.2022.1042341
Received: 12 September 2022; Accepted: 19 December 2022;
Published: 9 January 2023.
Edited by:
Francesco Morini, Meyer Children's Hospital, ItalyReviewed by:
Thomas Schaible, University Medical Centre Mannheim, University of Heidelberg, GermanyHanifi Soylu, Selcuk University, Turkey
Daniel Dirnberger, Alfred I. duPont Hospital for Children, United States
© 2023 Dassios, Adu and Greenough. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theodore Dassios dGhlb2RvcmUuZGFzc2lvc0BrY2wuYWMudWs=
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Theodore Dassios
Theodore Dassios John Adu3
John Adu3 Anne Greenough
Anne Greenough