- 1Department of Pediatrics, Neonatology, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Laboratory of Neonatal Disease, Institute of Pediatrics, Central South University, Changsha, China
Intrauterine growth restriction (IUGR) remains a great public health challenge as it affects neonatal survival and influences their normal biological development and metabolism. Several clinical researches have revealed the occurrence of metabolic syndrome, such as insulin resistance, obesity, type 2 diabetes mellitus, oxidative stress, dyslipidemia, as direct results of IUGR. Therefore, it is essential to understand its underlying mechanism, impact and develop effective therapies. The purpose of this work is to review the current knowledge on IUGR induced metabolic syndrome and relevant therapies. Here in, we elaborate on the characteristics and causes of IUGR by pointing out recent research findings. Furthermore, we discuss the impact of IUGR on different organs of the body, followed by preclinical studies on IUGR using suitable animal models. Additionally, various metabolic disorders with their genetic implications, such as insulin resistance, type 2 diabetes mellitus, dyslipidemia, obesity are detailed. Finally, the current therapeutic options used in the treatment of IUGR are summarized with some prospective therapies highlighted.
1. Introduction
Intrauterine growth restriction (IUGR), also called fetal growth restriction (FGR) is a condition in which the fetus is unable to develop to its full genetic and biological potential (1). This condition affects nearly 30%–50% of extremely preterm neonates (2). IUGR can be caused by naturally occurring or adverse environmental factors as revealed by studies conducted in livestock. To mimic human IUGR brought on by a variety of factors, several experimental techniques have been applied using animal models like sheep (3). Although hasty comparisons cannot be drawn between these animal models and humans, the impromptu occurrence of IUGR via placental insufficiency in pigs has been reported to be identical to that of humans (4, 5). IUGR causes great changes from abnormal lipid metabolism, liver inflammation to metabolic syndrome (MetS).
MetS is a constellation of morbidities such as insulin resistance, dyslipidemia, central obesity, hypertension, and glucose intolerance that includes impaired glucose tolerance or compromised fasting glycaemia and type 2 diabetes; all of which are well-documented cardiovascular disease risk factors. These metabolic abnormalities can simultaneously occur in an individual more often than expected (6). The “Developmental Origins of Health and Disease” theory evokes that a pernicious environment during early developmental periods (including fetal, infant and childhood) may lead to permanent alterations of both physiological and metabolic functions leading to adult metabolic syndrome (MetS) (7). In the principal etiology of these metabolic abnormalities, excessive fat storage in non-adipose tissues (e.g., liver) is a significant risk factor (8). The abnormal lipid metabolism in the liver has proven to be closely related to MetS (9). Except for diet and lifestyle, birth weight (BW) is considered the main relationship between abnormal hepatic accumulation of lipid and the increased incidence of MetS with its associated diseases (10).
Given the impact of IUGR on the development of MetS, there is a need to understand these conditions, review different parameters affected in IUGR models, and give an overview on current studied therapies.
2. Characteristics and causes of intrauterine growth restriction
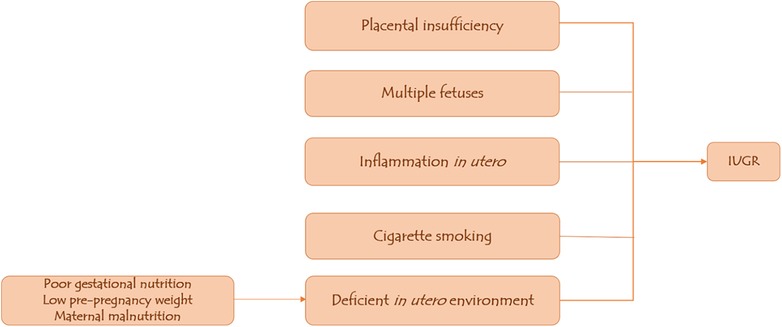
IUGR is characterized as symmetrical when the weight, length, and head circumference are low compared with the standard; and asymmetrical when the brain is spared, and the head circumference is within the normal limits (11). Chromosomal syndromes (trisomy 21, 13, and 18), congenital infections (toxoplasma, other viruses, rubella, cytomegalovirus, Herpes simplex infections), dwarfisms, maternal drug use (both prescription and illegal), and some inborn metabolic errors (fatty acid oxidation disorders) can all cause symmetrical IUGR. Asymmetrical causes of IUGR are typically associated with placental insufficiency, placental dysfunction, or nutrient deficiency (12, 13).
About 13% of children born with IUGR do not “catch up” with normal growth, i.e., do not reach body height greater than −2 z-score (below the third centile) during the first two to four years after birth (14). Many studies have demonstrated a clear correlation between deficient in utero environment leading to IUGR and the occurrence of cardiometabolic disease in adulthood (15, 16). Poor gestational nutrition and low pre-pregnancy weight are the strongest predictors of IUGR, according to data from developing countries. While in developed countries, cigarette smoking is the most important single factor implicated in IUGR, followed by poor gestational nutrition (17).
Multiple fetuses from twin pregnancies or assisted conception may result in nutrient competition between the two fetuses, predisposing them to IUGR; adolescent mothers are also at risk of having IUGR babies because their bodies compete for nutrients with the fetus (11, 17). Additional potential causes of intrauterine growth restriction (IUGR) include in utero inflammation, maternal malnutrition as low food intake and starvation result in a reduced nutrient stream from the mother to the fetus, thus restricting fetal growth; and placental insufficiency caused by fetal–placental perfusion dysfunction, which results in hypoxia and acidosis in the fetal circulation (18–20) (Figure 1).
3. Effects of IUGR on different organs
When exposed to in utero undernourishment, the available nutrients tends to be diverted to the growth and functional preservation of vital organs, e.g., brain, at the expense of organs such as the liver and pancreas (21). Even a slight reduction in nutrient supply limits fetal glycogen and fat formation, muscle and bone growth, with redistribution of cardiac output favoring delivery to the brain (2). The effects of environmental challenges are determined by their severity, duration, gestational age and probably the fetus's gender (22). The liver, the principal organ involved in the metabolism of dietary nutrients becomes a major target undergoing epigenetic, structural, and functional changes due to early exposure to unfavorable environment (23). The liver is essential in regulating gene expression for key transcription factors (24). Piglets with IUGR suffer from the hepatic disorder of lipid catabolism (25), with a significantly upregulated hepatic inflammatory factor under mRNA expression (26). It has been documented that IUGR piglets are presented with increased hepatic lipid concentrations, higher levels of serum pro-inflammatory cytokines, impaired growth, insulin resistance and inflammation (18).
Fatty hepatic infiltrates and cytoplasmic vacuolization has been reported in the livers of newborn IUGR piglets (27). An examination of the hepatic proteome revealed that the IUGR fetus had lower lipoprotein lipase activity (LPL) (28). IUGR piglets, compared to the normal birth weight (NBW) piglets, displayed low liver weight and compromised growth performance, decreased plasma free fatty acid (FFA) level, elevated hepatic oxidative stress (OS), abnormal hepatic lipid accumulation and compromised hepatic immune function. Some genes such as heme oxygenase 1, stearoyl-CoA desaturase 1, liver fatty acid- binding proteins 1, superoxide dismutase 1, toll-like receptor 4, sterol regulatory element-binding protein 1c, and tumor necrosis factor-alpha (TNF-α) have abnormal transcriptional expression in the liver of IUGR models (29). Therefore, it is a major requirement to improve the hepatic lipid metabolism, redox and immune status in the IUGR piglets’ model as it will be instrumental in developing new strategies for IUGR infants to prevent or/and slow the progression of non-alcoholic fatty liver disease (NAFLD) (29).
Through these findings, it is observed that among major defects occurring due to IUGR, lipid metabolism is greatly affected, followed by the immune function and its correlated genetic expressions. Thus, a hostile milieu can greatly impact an organism's normal biological function and development. The changes undergone by the organ for proper adaptation to the environment can be long-lasting and irreversible.
4. Preclinical IUGR investigations using different models
Diverse animal models of IUGR have been employed to investigate the mechanisms of intrauterine adaptation and programming, including maternal stress, uteroplacental insufficiency, hypoxia, nutrient (protein) restriction and glucocorticoid treatment (30). As the genetic background in animal models can be controlled, it is possible to explore the effects of the environment on long-term health during gestation or early postnatal life (22).
In multifetal mammals, IUGR has been reported to have lasting and harmful impacts on neonatal birth weight, postnatal growth, development, and liver function (31, 32). Pigs have been widely researched for human health regulation and animal nutrition. They have been extensively used as an animal model for IUGR studies due to their biological similarity to humans (33). Studies have shown that due to nutritional deficiencies during gestation, IUGR piglets displayed the most severe naturally occurring conditions; they have a higher risk of IUGR than other animals (25, 34).
The sheep model is attractive in studying the mechanistic basis of fetal adaptations that occur during fetal hypoxia, hypoglycemia, and IUGR caused by poor placentation (3). Other strengths of the sheep model include the availability of a singleton fetus, duration of pregnancy, similarities with humans in regard to organ and body development regulatory pathways and homeostasis. Most importantly, just like in humans, prenatal malnutrition in sheep has an extended impact on the endocrine, cardiovascular, and postnatal homeostasis systems (35).
Rodents offer a significant advantage in terms of relatively short gestation and lifespan (22). Malnutrition during critical early life periods has been shown to affect subsequent development (36) and perinatal adverse challenges have led to long-term consequences. For example, in both males and female rats, maternal caloric restriction results in low-birth-weight offspring with accelerated neonatal growth, early vaginal opening and sexual dimorphism (37, 38). However, some findings indicate that regardless of postnatal diet, male growth-restricted rats are resilient to impaired glucose homeostasis while female growth-restricted rats are more vulnerable to metabolic dysfunction (39). In another study the results demonstrated an intrauterine growth retardation and hypercholesterolemia in male adult offspring rats following prenatal nicotine exposure (PNE) (40). These findings highlight the role of gender in the development of IUGR associated Mets.
In summary the availability of various animal models makes it possible to study IUGR associated Mets. However, these models have their own advantages and disadvantages, therefore they are selected based on the research interest, need and available resources. Understanding the characteristics of each model is highly useful for the development of preventive, diagnostic and therapeutic strategies.
5. Impacts of intrauterine growth restriction
5.1. Restricted growth and development
IUGR is one of the leading causes of perinatal morbidity, affecting approximately 7%–15% of pregnancies worldwide (41, 42). Low birth weight (LBW) can be passed down through two generations; a low-birth-weight mother is 2.8 times more likely to have a low-birth-weight baby (43). LBW and IUGR are linked to preconception anemia (44). A large number of epidemiological and animal studies have reported that LBW caused by IUGR is mainly associated with an elevated risk for the development of NAFLD in both children and adults (45–48). IUGR can present with a SGA accompanied by LBW, enhancing lipid accumulation and other metabolic abnormalities. Stunted growth and development affects approximately 7%–9% of newborns, and may be responsible for up to 50% of unexplained stillbirths (19). About 86% of children born with small gestational age (SGA) catch-up with normal developmental pace and reach normal adult height with the others end up as short adults (49). Metabolic aberrations have been noticed in adult IUGR rats exhibiting catch-up growth (50). Children with IUGR, especially if they achieve catch-up growth in childhood, as well as SGA subjects, are at a higher risk for long-term developmental consequences or developing diseases later in life such as short stature, metabolic syndrome, Type 2 diabetes, hypertension, dyslipidemia, insulin resistance, and cardiovascular disease (7, 51). An enormous economic loss has been recorded in pig production due to the decreased survival rate of low-birth-weight piglets induced by IUGR (52).
5.2. Altered hepatic redox status
Oxidative stress is implicated in the IUGR-induced liver injury (53). Oxidative stress, particularly in the pancreas may be a common mechanism by which an adverse intrauterine milieu influences the development of the fetus and subsequent development of type 2 diabetes (54). An increase in the concentration of acidic metabolites (g-glutamyl leucine and 2 hydroxybutyric acids) may be associated with oxidative stress which might result in insulin resistance in the child (55). A recent study by Cheng et al. revealed increased plasma concentrations of malondialdehyde (MDA) and protein carbonyl (PC), and decreased total superoxide dismutase (T-SOD) in IUGR pigs (longissimus lumborum) compared with NBW pigs (52).
5.3. Genetic implications
The epigenetic adaptation of the fetus during the gestational period has lasting impacts on its growth and development. Maternal genes play a very important role in offspring development. For instance, pleckstrin homology-like domain-family A member (PHLDA2) regulates placental growth, growth receptor binding protein (GRB10) is associated with insulin receptor signal transmission, the IGF1 receptor inhibits the insulin receptor signal and placental acid-labile subunit (ALS) (56, 57). It has been reported that pigs suffering from IUGR displayed marked upregulation of mRNA expression for sterol regulatory element-binding protein-1 (SREBP-1), liver-x receptor α (LXRα), and peroxisome proliferator-activated receptor α (PPARα) (25). In mice, increased expression of CoA desaturase 1 (SCD1) gene, and decreased expression of carnitine palmitoyl transferase 1 alpha (CPT1α), acyl-CoA oxidase 1 (ACOX1), IGF-1 and IGF-2 genes have been reported (58).
Insulin and fatty acid regulation by lipogenic gene expression are largely mediated by transcription factors (such as SREBPs) and to a lesser extent, by nuclear receptors (such as LXRs) (59, 60). In mice, the insulin-like growth factor 2(IGF-2) gene is regulated by the maternal H19 gene, encodes IGF-2 synthesis and contributes to fetal growth. In contrast, the IGF2R gene, which encodes the IGF2R receptor, may cause intrauterine growth restriction (61).
5.4. Metabolic syndrome induced by IUGR
5.4.1. Insulin resistance and its genetic correlation
The concentration of insulin in the serum is increased significantly due to IUGR, leading to low serum glucose concentration and a significantly decreased glycogen concentration in the liver (25). Children exposed to IUGR have increased insulin resistance, and low birth weight has also been linked to altered insulin sensitivity (62, 63). Insulin sensitivity is commonly defined as insulin's ability to stimulate glucose uptake in peripheral target tissues (64). Insulin resistance is regarded as the damage to insulin signal transduction occurring when normal hormone concentrations in the blood are insufficient to regulate metabolic pathways (25). In white adipose tissue, insulin increases fatty acid flux to the liver, resulting in ectopic fat deposition in hepatocytes (65). The increased visceral fat deposition eventually lead to insulin resistance in patients with growth hormone deficiency (GHD) (66). Insulin resistance can develop as early as one year of age in SGA children (67). Young men born with IUGR have 30% lower insulin secretion in comparison to their insulin sensitivity, indicating a lower insulin deposition index (68). Additionally, when postnatal nutrient availability exceeds prenatal predictions, there is increased postnatal growth and fat deposition resulting in insulin resistance (69). The “survival” hypothesis claims that peripheral insulin resistance occurs in order to redistribute glucose to vital organs (e.g., the brain) of a malnourished fetus (70).
Insulin receptor dysfunction caused by abnormal phosphorylation of the β-receptor subunit's tyrosine kinase also leads to insulin resistance (71). IGF-1 levels are related to beta cell function and growth in the first year, and at age three, IGF-1 is related to BMI and insulin resistance (72). Studies have shown that insulin resistance in a child with IUGR is linked to higher IGF-1 and BMI levels during postnatal catch-up growth (14). Insulin resistance is also common in people with severe IGF-1 deficiency (73).
5.4.2. Type 2 diabetes mellitus and its genetic correlation
Infants born with severe IUGR and weighing less than 1.5 kg have lower beta cell mass resulting in Type 2 diabetes (74). Animals with impaired beta cell activity and low islet mass develop gestational diabetes, primarily caused by insulin deficiency (75). Fowden hypothesized that adult diseases, including diabetes, are caused, at least in part, by changes in the development of key endocrine axes, specifically the hypothalamic–pituitary–adrenal (HPA) axis, during suboptimal intrauterine conditions associated with impaired growth (76).
It has been demonstrated that intrauterine growth patterns can be linked to specific adult diseases; for example, a thin infant with a low ponderal index is more likely than a symmetrically small baby to develop type 2 diabetes as an adult (77). Low birth weight is associated with reduced expression of insulin signaling proteins in muscle and adipose tissue preceding the development of diabetes (22). In IUGR rats, altered hepatic glucose metabolism may contribute to the onset of fasting hyperglycemia prior to the development of obesity and diabetes (78). Fetal hyperglycemia is caused by severe maternal diabetes, puppies born to severely diabetic mothers with stunted intrauterine growth remain small until they reach adulthood (22).
Unfavorable intrauterine environment and the accumulation of DNA methylation errors over time may result in premature “epigenetic aging”, contributing to an increased susceptibility to diabetes in adulthood (79). Diabetes develops in IUGR rats with a phenotype similar to type 2 diabetes in humans, namely progressive dysfunction in insulin secretion and action (22). In adults with IUGR, Cyclin-dependent kinase inhibitor 1C (CDKN1C), a cell proliferation regulator that affects pancreatic β cells, can have a mutation at chromosome 11p15, resulting in decreased fetal growth, growth deficiency, and the onset of diabetes at a young age (80).
5.4.3. Dyslipidemia
The key steps in intra-fetal lipid synthesis and lipid catabolism are regulated by total cholesterol (TC) and total triglycerides (TG) (81). Hepatic lipid accumulation is caused by an imbalance between lipid availability and lipid disposal, eventually leading to lipoperoxidative stress and hepatic injury (82). The disproportion of lipid metabolism plays a critical role in hepatic defect and injury in IUGR individuals (29). Lipoprotein lipase (LPL) catalyzes the triacylglycerol hydrolysis in circulating chylomicrons and very low-density lipoproteins (83). Hepatic lipase (HL) is essential for the hydrolysis of circulating triglycerides and phospholipids (28). Lipogenesis is increased in IUGR piglets (84). Some studies on lipid parameters showed that the concentration of free fatty acids (FFA), total cholesterol (TC) and total triglycerides (TG) was increased while a decrease was found in the activity of lipoprotein lipase (LPL), hepatic lipase (HL) and total lipase (TL) in IUGR piglets' liver (25, 29, 52).
Furthermore, adipocytokines have been linked to adult diseases associated with IUGR. Leptin plays a permissive role in pubertal development and reproductive function maintenance. Adiponectin has been shown to play a role in linking energy homeostasis and hypothalamo-pituitary-gonadal axis control. Ghrelin, an orexigenic compound, stimulates growth hormone secretion as well. PYY 3e36 is a gastrointestinal hormone that regulates food intake and energy balance; it has also been shown in animal studies to modulate GnRH and gonadotropin release (51).
Also, hyperinsulinemia is known to increase hepatic very-low-density lipoprotein synthesis, which may contribute to higher plasma triglyceride and LDL-c levels, and resistance to insulin action on lipoprotein lipase in peripheral tissues may also contribute to higher triglyceride and LDL-c levels [56]. Brown adipose tissue, which protects against metabolic disorders, was found abnormal in SGA children (85).
5.4.4. Obesity
Obesity and overweight are linked to a variety of comorbid disorders, including cardiometabolic diseases (hypertension, diabetes, and insulin resistance), as well as some malignancies (86). The negative effects of “catch-up” growth in humans have been connected to the development of obesity (87). Insulin resistance in particular, is usually associated with obesity (88). Fetal malnutrition, pancreatic ß cell dysfunction, altered insulin metabolism, and, as a result, obesity are all associated to epigenetic regulation (89).
Obesity causes lower HDL cholesterol levels, higher systolic and diastolic blood pressures, higher triglyceride levels, and higher hemoglobin A1c (HbA1c) (90). Triglycerides are normally stored in adipocytes, but when energy intake exceeds the body's capacity, they can be stored in the liver, muscle cells, and visceral adipocytes (adipocytes surrounding vital organs), resulting in central obesity (91). Dams subjected to a much more severe food restriction of 30% of ad-lib intake results in IUGR offspring who develop hyperphagia and obesity as adults (92).
A cohort study showed that weight gain in the first three months of life was associated with more fat, central adiposity, lower insulin sensitivity, and higher insulin resistance in early adulthood, including in SGA children with catch-up growth (93). Also, low leptin levels, low or normal adiponectin levels, and higher ghrelin and visfatin levels in the IUGR sate may put an individual at risk for obesity development (94).
6. Recent and prospective IUGR therapies
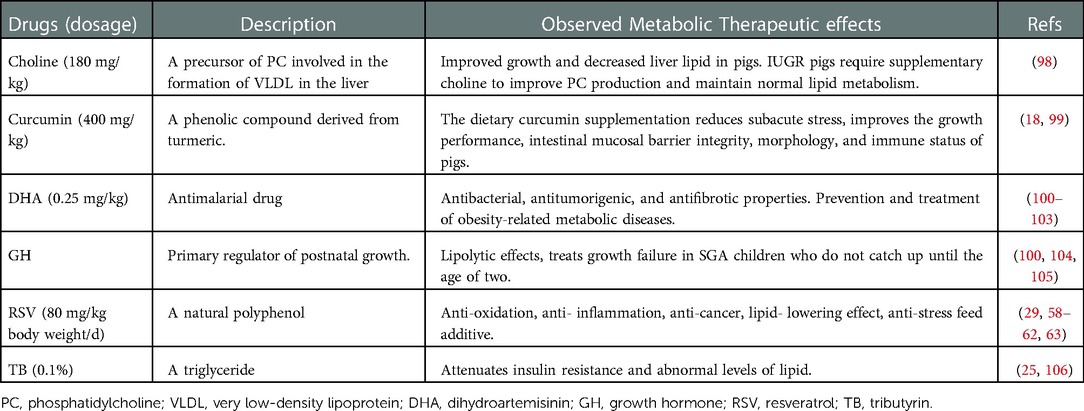
Presently, IUGR has no known effective therapies (95). However, numerous treatments are being investigated to support the improvement of growth performance, immune status, lipid and glucose metabolism, prevention as well as treatment of obesity-related metabolic diseases and so on. In humans, therapies such as antenatal corticoids, aspirin, low molecular weight heparin, phosphodiesterase type 5 inhibitors, nitric oxide donors, N-acetylcysteine, proton pump inhibitors, and Maternal vascular endothelial growth factor (VEGF) gene therapy have been used to improve poor placentation and uterine blood flow (95, 96). Other drugs such as statins and melatonin were reported to have anti-inflammatory and antioxidant properties (96). Magnesium sulfate on the other hand has been widely used as prophylaxis for neuroprotection (95, 97). Similarly, in preclinical animal experiments several compounds are being investigated and could pave the way to future effective therapy. Table 1 summarizes some of these drugs.
7. Concluding remark
Although the data used are not exhaustive and conflicting, an in-depth understanding of the occurrence and development of metabolic disorders in IUGR models is key to promoting therapies that will ultimately tackle and mediate the consequences caused by these metabolic aberrations. Even though a clear knowledge of the underlined mechanism is still a challenge to overcome, the present studies are helpful in grasping the clinical impact of IUGR.
Author contributions
Conceptualization, AKM, XH, TW. Writing—original draft preparation, AKM. Writing—review and editing, XH. Supervision and approval, TW. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by The National Natural Science Foundation of Hunan Province of the People's Republic of China (grant no. 2020JJ4785, 2022JJ30848), Ausnutria Food and Nutrition Science Research Fund (AU-YJY-B-LX-20-022), and The Changsha Municipal Natural Science Foundation of Hunan Province of the People's Republic of China (grant no. kq2007077).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shrivastava D, Master A. Fetal growth restriction. J Obstet Gynaecol India. (2020) 70(2):103–10. doi: 10.1007/s13224-019-01278-4
2. Rosenberg A. The IUGR newborn. Semin Perinatol. (2008) 32(3):219–24. doi: 10.1053/j.semperi.2007.11.003
3. Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. (2008) 35(7):730–43. doi: 10.1111/j.1440-1681.2008.04975.x
4. Ferenc K, Pietrzak P, Godlewski MM, Piwowarski J, Kiliańczyk R, Guilloteau P, et al. Intrauterine growth retarded piglet as a model for humans–studies on the perinatal development of the gut structure and function. Reprod Biol. (2014) 14(1):51–60. doi: 10.1016/j.repbio.2014.01.005
5. Burke C, Sinclair K, Cowin G, Rose S, Pat B, Gobe G, et al. Intrauterine growth restriction due to uteroplacental vascular insufficiency leads to increased hypoxia-induced cerebral apoptosis in newborn piglets. Brain Res. (2006) 1098(1):19–25. doi: 10.1016/j.brainres.2006.04.129
6. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 365(9468):1415–28. doi: 10.1016/s0140-6736(05)66378-7
7. Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. (2004) 58(2):114–5. doi: 10.1136/jech.58.2.114
8. Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. (2012) 142(4):711–25.e6. doi: 10.1053/j.gastro.2012.02.003
9. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. (2003) 37(4):917–23. doi: 10.1053/jhep.2003.50161
10. Pruis MG, van Ewijk PA, Schrauwen-Hinderling VB, Plösch T. Lipotoxicity and the role of maternal nutrition. Acta Physiol. (2014) 210(2):296–306. doi: 10.1111/apha.12171
11. Salam RA, Das JK, Bhutta ZA. Impact of intrauterine growth restriction on long-term health. Curr Opin Clin Nutr Metab Care. (2014) 17(3):249–54. doi: 10.1097/MCO.0000000000000051
12. Cox P, Marton T. Pathological assessment of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. (2009) 23(6):751–64. doi: 10.1016/j.bpobgyn.2009.06.006
13. Fu Q, McKnight RA, Callaway CW, Yu X, Lane RH, Majnik AV. Intrauterine growth restriction disrupts developmental epigenetics around distal growth hormone response elements on the rat hepatic IGF-1 gene. FASEB J. (2015) 29(4):1176–84. doi: 10.1096/fj.14-258442
14. Korpysz A, Szalecki M. What’s new in IUGR from the endocrinological point of view? Pediatr Endocrinol Diabetes Metab. (2019) 25(4):188–93. doi: 10.5114/pedm.2019.91547
15. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. (1986) 1(8489):1077–81. doi: 10.1016/s0140-6736(86)91340-1
16. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. (1989) 2(8663):577–80. doi: 10.1016/s0140-6736(89)90710-1
17. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. (2013) 382(9890):427–51. doi: 10.1016/S0140-6736(13)60937-X
18. Niu Y, He J, Zhao Y, Shen M, Zhang L, Zhong X, et al. Effect of curcumin on growth performance, inflammation, insulin level, and lipid metabolism in weaned piglets with IUGR. Animals. (2019) 9(12):1098. doi: 10.3390/ani9121098
19. Miller J, Turan S, Baschat AA. Fetal growth restriction. Semin Perinatol. (2008) 32(4):274–80. doi: 10.1053/j.semperi.2008.04.010
20. Roberts DJ, Post MD. The placenta in pre-eclampsia and intrauterine growth restriction. J Clin Pathol. (2008) 61(12):1254–60. doi: 10.1136/jcp.2008.055236
21. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. (2001) 60:5–20. doi: 10.1093/bmb/60.1.5
22. Martin-Gronert MS, Ozanne SE. Experimental IUGR and later diabetes. J Intern Med. (2007) 261(5):437–52. doi: 10.1111/j.1365-2796.2007.01800.x
23. Deodati A, Inzaghi E, Cianfarani S. Epigenetics and in utero acquired predisposition to metabolic disease. Front Genet. (2019) 10:1270. doi: 10.3389/fgene.2019.01270
24. Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. (2005) 135(11):2503–6. doi: 10.1093/jn/135.11.2503
25. He J, Dong L, Xu W, Bai K, Lu C, Wu Y, et al. Dietary tributyrin supplementation attenuates insulin resistance and abnormal lipid metabolism in suckling piglets with intrauterine growth retardation. PLoS One. (2015) 10(8):e0136848. doi: 10.1371/journal.pone.0136848
26. Liu J, He J, Yang Y, Yu J, Mao X, Yu B, et al. Effects of intrauterine growth retardation and postnatal high-fat diet on hepatic inflammatory response in pigs. Arch Anim Nutr. (2014) 68(2):111–25. doi: 10.1080/1745039x.2014.897532
27. Li W, Zhong X, Zhang L, Wang Y, Wang T. Heat shock protein 70 expression is increased in the liver of neonatal intrauterine growth retardation piglets. Asian-Australas J Anim Sci. (2012) 25(8):1096–101. doi: 10.5713/ajas.2012.12058
28. Liu C, Lin G, Wang X, Wang T, Wu G, Li D, et al. Intrauterine growth restriction alters the hepatic proteome in fetal pigs. J Nutr Biochem. (2013) 24(6):954–9. doi: 10.1016/j.jnutbio.2012.06.016
29. Cheng K, Ji S, Jia P, Zhang H, Wang T, Song Z, et al. Resveratrol improves hepatic redox status and lipid balance of neonates with intrauterine growth retardation in a piglet model. Biomed Res Int. (2020) 2020:7402645. doi: 10.1155/2020/7402645
30. Schröder HJ. Models of fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. (2003) 110(Suppl 1):S29–39. doi: 10.1016/s0301-2115(03)00170-2
31. Widdowson EM. Intra-uterine growth retardation in the pig. I. Organ size and cellular development at birth and after growth to maturity. Biol Neonate. (1971) 19(4):329–40. doi: 10.1159/000240427
32. Wang J, Chen L, Li D, Yin Y, Wang X, Li P, et al. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr. (2008) 138(1):60–6. doi: 10.1093/jn/138.1.60
33. Merrifield CA, Lewis M, Claus SP, Beckonert OP, Dumas ME, Duncker S, et al. A metabolic system-wide characterisation of the pig: a model for human physiology. Mol Biosyst. (2011) 7(9):2577–88. doi: 10.1039/c1mb05023k
34. Anderson LL, Parker RO. Distribution and development of embryos in the pig. J Reprod Fertil. (1976) 46(2):363–8. doi: 10.1530/jrf.0.0460363
35. Gonzalez-Bulnes A, Astiz S, Parraguez VH, Garcia-Contreras C, Vazquez-Gomez M. Empowering translational research in fetal growth restriction: sheep and swine animal models. Curr Pharm Biotechnol. (2016) 17(10):848–55. doi: 10.2174/1389201017666160519111529
36. Zambrano E, Guzman C, Rodriguez-Gonzalez GL, Durand-Carbajal M, Nathanielsz PW. Fetal programming of sexual development and reproductive function. Mol Cell Endocrinol. (2014) 382(1):538–49. doi: 10.1016/j.mce.2013.09.008
37. Sloboda DM, Howie GJ, Pleasants A, Gluckman PD, Vickers MH. Pre- and postnatal nutritional histories influence reproductive maturation and ovarian function in the rat. PLoS One. (2009) 4(8):e6744. doi: 10.1371/journal.pone.0006744
38. Sanchez-Garrido MA, Castellano JM, Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, Leon S, et al. Metabolic programming of puberty: sexually dimorphic responses to early nutritional challenges. Endocrinology. (2013) 154(9):3387–400. doi: 10.1210/en.2012-2157
39. Intapad S, Dasinger JH, Johnson JM, Brown AD, Ojeda NB, Alexander BT. Male and female intrauterine growth-restricted offspring differ in blood pressure, renal function, and glucose homeostasis responses to a postnatal diet high in fat and sugar. Hypertension. (2019) 73(3):620–9. doi: 10.1161/hypertensionaha.118.12134
40. Zhou J, Zhu C, Luo H, Shen L, Gong J, Wu Y, et al. Two intrauterine programming mechanisms of adult hypercholesterolemia induced by prenatal nicotine exposure in male offspring rats. FASEB J. (2019) 33(1):1110–23. doi: 10.1096/fj.201800172R
41. Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health. (2003) 16(2):230. doi: 10.1080/1357628031000116808
42. Maternal anthropometry and pregnancy outcomes. A WHO collaborative study. Bull World Health Organ. (1995) 73(Suppl):1–98.
43. Agnihotri B, Antonisamy B, Priya G, Fall CH, Raghupathy P. Trends in human birth weight across two successive generations. Indian J Pediatr. (2008) 75(2):111–7. doi: 10.1007/s12098-008-0066-x
44. Sukrat B, Wilasrusmee C, Siribumrungwong B, McEvoy M, Okascharoen C, Attia J, et al. Hemoglobin concentration and pregnancy outcomes: a systematic review and meta-analysis. Biomed Res Int. (2013) 2013:769057. doi: 10.1155/2013/769057
45. Nobili V, Marcellini M, Marchesini G, Vanni E, Manco M, Villani A, et al. Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes Care. (2007) 30(10):2638–40. doi: 10.2337/dc07-0281
46. Fraser A, Ebrahim S, Smith GD, Lawlor DA. The associations between birthweight and adult markers of liver damage and function. Paediatr Perinat Epidemiol. (2008) 22(1):12–21. doi: 10.1111/j.1365-3016.2007.00876.x
47. Newton KP, Feldman HS, Chambers CD, Wilson L, Behling C, Clark JM, et al. Low and high birth weights are risk factors for nonalcoholic fatty liver disease in children. J Pediatr. (2017) 187:141–6.e1. doi: 10.1016/j.jpeds.2017.03.007
48. Hyatt MA, Gardner DS, Sebert S, Wilson V, Davidson N, Nigmatullina Y, et al. Suboptimal maternal nutrition, during early fetal liver development, promotes lipid accumulation in the liver of obese offspring. Reproduction. (2011) 141(1):119–26. doi: 10.1530/rep-10-0325
49. Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. (1995) 38(5):733–9. doi: 10.1203/00006450-199511000-00017
50. Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab. (2005) 288(5):E935–47. doi: 10.1152/ajpendo.00342.2004
51. Darendeliler F. IUGR: genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract Res Clin Endocrinol Metab. (2019) 33(3):101260. doi: 10.1016/j.beem.2019.01.001
52. Cheng K, Yu C, Li Z, Li S, Yan E, Song Z, et al. Resveratrol improves meat quality, muscular antioxidant capacity, lipid metabolism and fiber type composition of intrauterine growth retarded pigs. Meat Sci. (2020) 170:108237. doi: 10.1016/j.meatsci.2020.108237
53. Lakshmy R. Metabolic syndrome: role of maternal undernutrition and fetal programming. Rev Endocr Metab Disord. (2013) 14(3):229–40. doi: 10.1007/s11154-013-9266-4
54. Simmons RA. Developmental origins of diabetes: the role of oxidative stress. Free Radic Biol Med. (2006) 40(6):917–22. doi: 10.1016/j.freeradbiomed.2005.12.018
55. Metrustry SJ, Karhunen V, Edwards MH, Menni C, Geisendorfer T, Huber A, et al. Metabolomic signatures of low birthweight: pathways to insulin resistance and oxidative stress. PLoS One. (2018) 13(3):e0194316. doi: 10.1371/journal.pone.0194316
56. Moore GE, Ishida M, Demetriou C, Al-Olabi L, Leon LJ, Thomas AC, et al. The role and interaction of imprinted genes in human fetal growth. Philos Trans R Soc Lond B Biol Sci. (2015) 370(1663):20140074. doi: 10.1098/rstb.2014.0074
57. Iniguez G, Argandona F, Medina P, Gonzalez C, San Martin S, Kakarieka E, et al. Acid-labile subunit (ALS) gene expression and protein content in human placentas: differences according to birth weight. J Clin Endocrinol Metab. (2011) 96(1):187–91. doi: 10.1210/jc.2010-0244
58. Verhaeghe J, Van Bree R, Van Herck E, Laureys J, Bouillon R, Van Assche FA. C-peptide, insulin-like growth factors I and II, and insulin-like growth factor binding protein-1 in umbilical cord serum: correlations with birth weight. Am J Obstet Gynecol. (1993) 169(1):89–97. doi: 10.1016/0002-9378(93)90137-8
59. Yamamoto T, Shimano H, Inoue N, Nakagawa Y, Matsuzaka T, Takahashi A, et al. Protein kinase A suppresses sterol regulatory element-binding protein-1C expression via phosphorylation of liver X receptor in the liver. J Biol Chem. (2007) 282(16):11687–95. doi: 10.1074/jbc.M611911200
60. Kim HJ, Miyazaki M, Man WC, Ntambi JM. Sterol regulatory element-binding proteins (SREBPs) as regulators of lipid metabolism: polyunsaturated fatty acids oppose cholesterol-mediated induction of SREBP-1 maturation. Ann N Y Acad Sci. (2002) 967:34–42. doi: 10.1111/j.1749-6632.2002.tb04261.x
61. Dunger DB, Petry CJ, Ong KK. Genetic variations and normal fetal growth. Horm Res. (2006) 65(Suppl 3):34–40. doi: 10.1159/000091504
62. Crume TL, Scherzinger A, Stamm E, McDuffie R, Bischoff KJ, Hamman RF, et al. The long-term impact of intrauterine growth restriction in a diverse U.S. Cohort of children: the EPOCH study. Obesity. (2014) 22(2):608–15. doi: 10.1002/oby.20565
63. Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. (2004) 15(4):183–7. doi: 10.1016/j.tem.2004.03.002
64. Wang X, Cui Y, Tong X, Ye H, Li S. Glucose and lipid metabolism in small-for-gestational-age infants at 72 h of age. J Clin Endocrinol Metab. (2007) 92(2):681–4. doi: 10.1210/jc.2006-1281
65. Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. (2004) 114(2):147–52. doi: 10.1172/JCI22422
66. Beshyah SA, Henderson A, Niththyananthan R, Skinner E, Anyaoku V, Richmond W, et al. The effects of short and long-term growth hormone replacement therapy in hypopituitary adults on lipid metabolism and carbohydrate tolerance. J Clin Endocrinol Metab. (1995) 80(2):356–63. doi: 10.1210/jcem.80.2.7852490
67. Soto N, Bazaes RA, Pena V, Salazar T, Avila A, Iniguez G, et al. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab. (2003) 88(8):3645–50. doi: 10.1210/jc.2002-030031
68. Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA. Early differential defects of insulin secretion and action in 19-year-old caucasian men who had low birth weight. Diabetes. (2002) 51(4):1271–80. doi: 10.2337/diabetes.51.4.1271
69. Gatford KL, Simmons RA. Prenatal programming of insulin secretion in intrauterine growth restriction. Clin Obstet Gynecol. (2013) 56(3):520–8. doi: 10.1097/GRF.0b013e31829e5b29
70. Jaquet D, Leger J, Levy-Marchal C, Czernichow P. Low birth weight: effect on insulin sensitivity and lipid metabolism. Horm Res. (2003) 59(1):1–6. doi: 10.1159/000067940
71. Jaquet D, Vidal H, Hankard R, Czernichow P, Levy-Marchal C. Impaired regulation of glucose transporter 4 gene expression in insulin resistance associated with in utero undernutrition. J Clin Endocrinol Metab. (2001) 86(7):3266–71. doi: 10.1210/jcem.86.7.7677
72. Iniguez G, Ong K, Bazaes R, Avila A, Salazar T, Dunger D, et al. Longitudinal changes in insulin-like growth factor-I, insulin sensitivity, and secretion from birth to age three years in small-for-gestational-age children. J Clin Endocrinol Metab. (2006) 91(11):4645–9. doi: 10.1210/jc.2006-0844
73. Guler HP, Zapf J, Froesch ER. Short-term metabolic effects of recombinant human insulin-like growth factor I in healthy adults. N Engl J Med. (1987) 317(3):137–40. doi: 10.1056/NEJM198707163170303
74. Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. (1977) 84(10):751–3. doi: 10.1111/j.1471-0528.1977.tb12486.x
75. Blondeau B, Avril I, Duchene B, Breant B. Endocrine pancreas development is altered in foetuses from rats previously showing intra-uterine growth retardation in response to malnutrition. Diabetologia. (2002) 45(3):394–401. doi: 10.1007/s00125-001-0767-4
76. Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. (2005) 81(9):723–34. doi: 10.1016/j.earlhumdev.2005.06.007
77. Phillips DI, Barker DJ, Hales CN, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. (1994) 37(2):150–4. doi: 10.1007/s001250050086
78. Vuguin P, Raab E, Liu B, Barzilai N, Simmons R. Hepatic insulin resistance precedes the development of diabetes in a model of intrauterine growth retardation. Diabetes. (2004) 53(10):2617–22. doi: 10.2337/diabetes.53.10.2617
79. Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet. (2006) 15(5):705–16. doi: 10.1093/hmg/ddi484
80. Kerns SL, Guevara-Aguirre J, Andrew S, Geng J, Guevara C, Guevara-Aguirre M, et al. A novel variant in CDKN1C is associated with intrauterine growth restriction, short stature, and early-adulthood-onset diabetes. J Clin Endocrinol Metab. (2014) 99(10):E2117–22. doi: 10.1210/jc.2014-1949
81. Girard JR, Cuendet GS, Marliss EB, Kervran A, Rieutort M, Assan R. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J Clin Invest. (1973) 52(12):3190–200. doi: 10.1172/JCI107519
82. Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. (2009) 48(1):1–26. doi: 10.1016/j.plipres.2008.08.001
83. Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J Mol Med. (2002) 80(12):753–69. doi: 10.1007/s00109-002-0384-9
84. He Q, Ren P, Kong X, Xu W, Tang H, Yin Y, et al. Intrauterine growth restriction alters the metabonome of the serum and jejunum in piglets. Mol Biosyst. (2011) 7(7):2147–55. doi: 10.1039/c1mb05024a
85. Malpique R, Gallego-Escuredo JM, Sebastiani G, Villarroya J, Lopez-Bermejo A, de Zegher F, et al. Brown adipose tissue in prepubertal children: associations with sex, birthweight, and metabolic profile. Int J Obes. (2019) 43(2):384–91. doi: 10.1038/s41366-018-0198-7
86. Saget S, Cong R, Decourtye L, Endale ML, Martinerie L, Girardet C, et al. Changes in circulating miRNA19a-3p precede insulin resistance programmed by intra-uterine growth retardation in mice. Mol Metab. (2020) 42:101083. doi: 10.1016/j.molmet.2020.101083
87. Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. (2004) 427(6973):411–2. doi: 10.1038/427411b
88. Suliburska J, Bogdanski P, Szulinska M, Pupek-Musialik D, Jablecka A. Changes in mineral status are associated with improvements in insulin sensitivity in obese patients following L-arginine supplementation. Eur J Nutr. (2014) 53(2):387–93. doi: 10.1007/s00394-013-0533-7
89. Vaiserman A, Lushchak O. Prenatal malnutrition-induced epigenetic dysregulation as a risk factor for type 2 diabetes. Int J Genomics. (2019) 2019:3821409. doi: 10.1155/2019/3821409
90. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. (2015) 373(14):1307–17. doi: 10.1056/NEJMoa1502821
91. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. (2000) 49(5):677–83. doi: 10.2337/diabetes.49.5.677
92. Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. (2000) 279(1):E83–7. doi: 10.1152/ajpendo.2000.279.1.E83
93. Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. (2009) 301(21):2234–42. doi: 10.1001/jama.2009.761
94. Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrinol. (2009) 160(3):337–47. doi: 10.1530/EJE-08-0621
95. Melamed N, Baschat A, Yinon Y, Athanasiadis A, Mecacci F, Figueras F, et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: best practice advice for screening, diagnosis, and management of fetal growth restriction. Int J Gynaecol Obstet. (2021) 152(Suppl 1):3–57. doi: 10.1002/ijgo.13522
96. Groom KM, David AL. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am J Obstet Gynecol. (2018) 218(2s):S829–40. doi: 10.1016/j.ajog.2017.11.565
97. Lees CC, Stampalija T, Baschat A, da Silva Costa F, Ferrazzi E, Figueras F, et al. ISUOG practice guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. (2020) 56(2):298–312. doi: 10.1002/uog.22134
98. Li W, Li B, Lv J, Dong L, Zhang L, Wang T. Choline supplementation improves the lipid metabolism of intrauterine-growth-restricted pigs. Asian-Australas J Anim Sci. (2018) 31(5):686–95. doi: 10.5713/ajas.15.0810
99. Wei S, Xu H, Xia D, Zhao R. Curcumin attenuates the effects of transport stress on serum cortisol concentration, hippocampal NO production, and BDNF expression in the pig. Domest Anim Endocrinol. (2010) 39(4):231–9. doi: 10.1016/j.domaniend.2010.06.004
100. Chen K, Hua H, Zhu Z, Wu T, Jia Z, Liu Q. Artemisinin and dihydroartemisinin promote β-cell apoptosis induced by palmitate via enhancing ER stress. Apoptosis. (2020) 25(3–4):192–204. doi: 10.1007/s10495-019-01587-z
101. Akpaloo W, Purssell E. Does the use of dihydroartemisinin-piperaquine in treating patients with uncomplicated falciparum malaria reduce the risk for recurrent new falciparum infection more than artemether-lumefantrine? Malar Res Treat. (2014) 2014:263674. doi: 10.1155/2014/263674
102. Lu P, Zhang FC, Qian SW, Li X, Cui ZM, Dang YJ, et al. Artemisinin derivatives prevent obesity by inducing browning of WAT and enhancing BAT function. Cell Res. (2016) 26(10):1169–72. doi: 10.1038/cr.2016.108
103. Zhang Z, Guo M, Zhao S, Shao J, Zheng S. ROS-JNK1/2-dependent activation of autophagy is required for the induction of anti-inflammatory effect of dihydroartemisinin in liver fibrosis. Free Radic Biol Med. (2016) 101:272–83. doi: 10.1016/j.freeradbiomed.2016.10.498
104. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. (1963) 1(7285):785–9. doi: 10.1016/s0140-6736(63)91500-9
105. Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. (2007) 92(3):804–10. doi: 10.1210/jc.2006-2017
Keywords: small gestational age (SGA), lipid metabolism, insulin resistance, type 2 diabetes, intrauterine growth restriction
Citation: Mutamba AK, He X and Wang T (2023) Therapeutic advances in overcoming intrauterine growth restriction induced metabolic syndrome. Front. Pediatr. 10:1040742. doi: 10.3389/fped.2022.1040742
Received: 9 September 2022; Accepted: 21 December 2022;
Published: 11 January 2023.
Edited by:
Andrea Hartner, University Hospital Erlangen, GermanyReviewed by:
Hiromichi Shoji, Juntendo University, JapanEkkehard Schleussner, University Hospital Jena, Germany
© 2023 Mutamba, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaori He aGV4aWFvcmlAY3N1LmVkdS5jbg== Tao Wang d2FuZ3RhbzAwMUBjc3UuZWR1LmNu
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Alpha Kalonda Mutamba
Alpha Kalonda Mutamba Xiaori He1*
Xiaori He1*