
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 21 November 2022
Sec. Pediatric Nephrology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1040304
This article is part of the Research TopicCase Reports in Pediatric Nephrology 2022View all 5 articles
Background: Congenital mesoblastic nephroma (CMN) is a rare renal tumour in children, the most common kidney tumour in the neonatal period. It can be divided into three types, classical, cellular and mixed.
Case presentation: A 31-year-old Chinese woman had no apparent foetal abnormality in regular prenatal care during the first and second trimesters. At 33 weeks of gestation, a solid mass in the right kidney was noted with echoes similar to liver and hypervascularity. It grew larger during late pregnancy. The infant was transferred to have a radical nephrectomy on the 9th day after birth. The postoperative histopathological result indicated classical CMN.
Conclusion: CMN could be detected prenatally, mainly during late pregnancy. The postnatal outcome is good.
Congenital mesoblastic nephroma (CMN) is known as Boland's tumour or foetal renal hamartoma. It is a rare renal tumour that accounts for approximately 3% of renal tumours in children (1) and is the most common kidney tumour in the neonatal period (2). Histopathology of CMN can be divided into three types: classical, cellular, and mixed. Classical CMN is benign, similar to infantile leiomyoma, without a capsule. Classical CMN could infiltrate towards the renal parenchyma and have no apparent haemorrhage or necrosis. The prognosis of classical CMN is good. Cellular CMN is similar to infantile leiomyosarcoma, with high mitotic activity and potential invasive behaviour. It is a low-grade malignant tumour with apparent haemorrhage, degeneration and necrosis. Mixed CMN is a mixture of classical and cellular CMN, with the morphological characteristics of the two (3, 4).
Although CMN is the most common kidney tumour in the first month of life (54%–66%) (2, 5), only 11%–14% of cases can be detected by prenatal ultrasound (5, 6). Therefore, this article reported a case of CMN noticed in the third trimester and confirmed by postnatal histopathology. It is necessary to summarise the ultrasound features during the prenatal scan to provide better counselling to the future parent.
A 31-year-old healthy Chinese woman with Gravida 3, parity 1, and abortion 1 received regular prenatal care at Peking University First Hospital. The first-trimester scan shows a crown-rump length (CRL) of 61.8 mm and a nuchal translucency (NT) of 1.77 mm. Maternal screening for Down's screening showed a low risk of trisomy 21 syndrome and trisomy 18 syndromes. An anomaly scan displayed no foetal structural abnormalities. The oral glucose tolerance test (OGTT) results were unremarkable. The pregnancy was uneventful until the growth scan at 29 weeks of gestation, considering susceptive large for gestation age (LGA) foetus. Hence, another OGTT was prescribed, and the result returned normal. During a referral ultrasound due to suspected LGA at 33 weeks of gestation, a medially echoic solid mass (27 mm × 28 mm × 22 mm) in the right kidney (Figures 1A,B) was detected. The echo of the mass was equivalent to that of the liver. Amniocentesis was recommended, but the patient refused. The following foetal magnetic resonance imaging (MRI) suggested a solid mass (28 mm × 25 mm × 21 mm) in the middle and upper right kidney with isointense on T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI). The lesion had a well-defined boundary, and there were no apparent swollen lymph nodes in the surrounding area (Figure 2). However, the MRI did not cite a clear conclusion. After counselling with the future parents, they decided to continue the pregnancy and denied any family history of renal disease. The follow-up growth scan showed a growing solid mass with a measurement of 36 mm × 34 mm × 30 mm at 35 gestational weeks and a size of 41 mm × 33 mm × 31 mm at 36 gestational weeks (Figure 1C). Rich blood flow signals were around and inside the mass (“ring” sign, Figure 1D). The RI of the blood flow was 0.60 and 0.71, respectively. At 37 weeks of gestation, the pregnant woman underwent a caesarean section due to gestational hypertension and previous history of caesarean section (CS). The Apgar score at 1 min and 5 min were both 10. During the neonatal routine physical examination, the mass was palpable in the right hypochondriac region, with a diameter of approximately 3 cm.
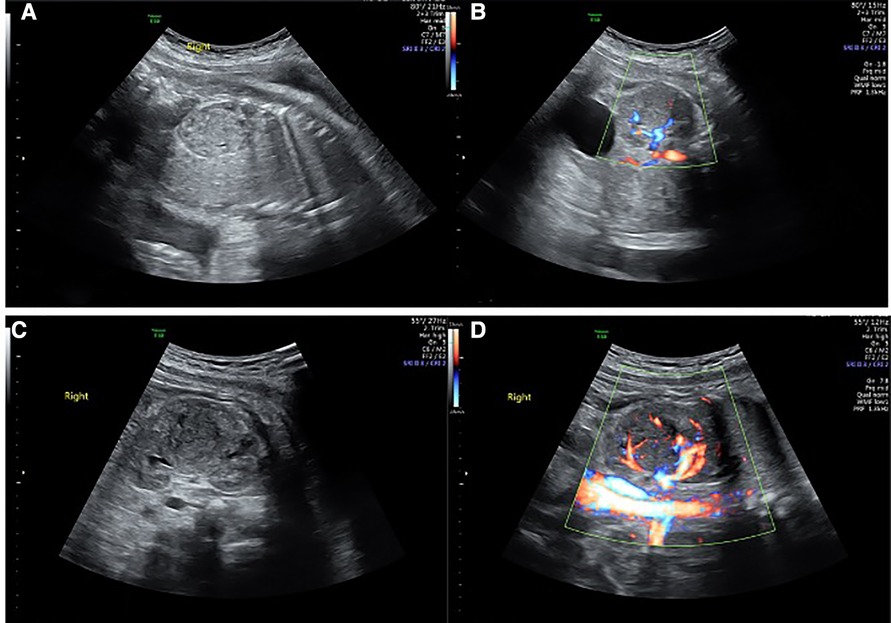
Figure 1. Renal solid mass at different gestational weeks. (A,B) showed the mass at 33 weeks and gestation by 2D and Colour Doppler. (C,D) showed the mass at 36 weeks of gestation by 2D and Colour Doppler.
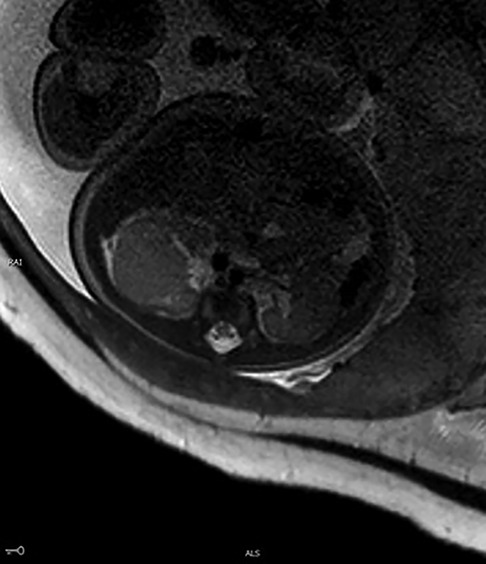
Figure 2. MRI of the foetus. A large, irregular mass (28 mm × 25 mm × 21 mm) was noted with isointense on T2WI.
The neonate was transferred to the paediatric ward directly. An enhanced computerized tomography (CT) was performed on the 2nd day after birth, which suggested nephroblastoma with a measurement of 26 mm × 26 mm × 25 mm (Figure 3). The neonate underwent radical nephrectomy on the 9th day after birth with the whole affected kidney, the fatty tissues surrounding the kidney, and a portion of the tube connecting the kidney to the bladder (ureter) removed (Figure 4). The postoperative pathological report indicated an intrarenal nodular mass (31 mm × 27 mm × 20 mm), similar to prenatal imaging and postnatal CT measurements. The pathologist reported that the mass was formed by clusters of spindle cells (mild-moderate pleomorphism). It invaded the surrounding nephrons. The microscope findings demonstrated visible mitotic figures (20 cells/10 HPF), but no necrosis, nerve invasion or vascular tumour thrombi were noted (Figure 5). Molecular pathology suggested the absence of ETV6-NTRK3 fusion signals. Above all, the neonate was diagnosed with classical CMN and discharged on the 9th day after surgery.
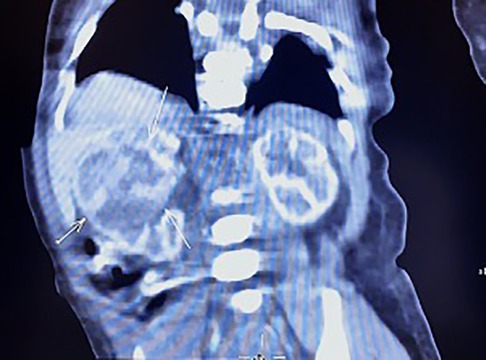
Figure 3. Enhance CT image of the newborn. A large, heterogeneous mass was noted in the middle of the right kidney with a size of 26 mm × 26 mm × 25 mm.
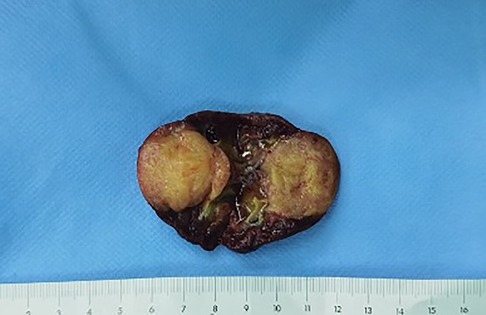
Figure 4. Right kidney was resected radically with a solid mass in the middle, yellow and white inside.
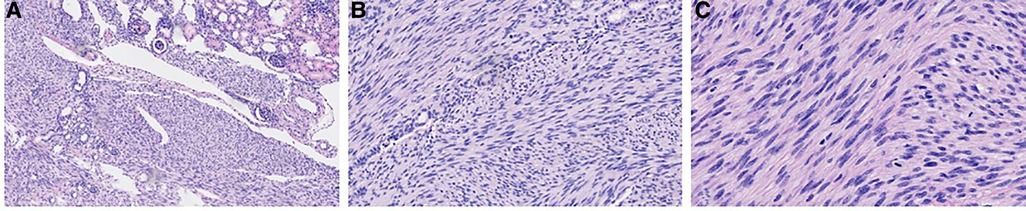
Figure 5. Microscopic images with haematoxylin and eosin (H&E) stain at different magnifications. (A) 100× magnification; (B) 200× magnification; (C) 400× magnification.
The use of all data was approved by the Institutional Review Board of the Peking University First Hospital with the project number of 2013 [572]. Written informed consent was obtained from the patient for publication of this case report.
Bolande et al. (7) first reported CMN in 1967 and described its pathological tissue morphology in 1973 (8). However, CMN is the most common benign tumour in the first six months of life (2). However, most studies on foetal CMN are case reports (9–12). Lin et al. reported the first case of live birth diagnosed with foetal CMN in Taiwan. Patil et al. demonstrated a fetal CMN complicated with oligohydramnios and small for gestational age (SGA) foetus. In our case, the foetus manifested polyhydramnios and LGA. However, an intrauterine death (IUD) occurred in the case of CMN due to renal failure (11). In a study by Chen et al., the perinatal characteristics of 11 CMN patients were retrospectively studied. The patients with CMN detected by prenatal ultrasound had an average gestational age of 35 weeks (25–39 weeks) (13). In our case, the renal mass was first seen at 33 weeks of gestational age and grew larger gradually. The literature reported that CMN was more common in male infants (male: female ratio 1.5–2:1) (14, 15); however, the neonate was female in this report.
The prenatal ultrasound characteristic of CMN is mainly a lateral, single round or oval mass, with a solid or cystic-solid nature and a well-defined boundary with the surrounding organs. On colour doppler imaging, rich blood flow signals are observed inside and around the mass, described as a “ring” sign (16).
Polyhydramnios occurs in 15%–36.4% of CMN cases, which increases the risk of premature birth. The cause of polyhydramnios may be high blood perfusion in the kidney and compression of the surrounding intestine (13, 17). A foetus with CMN and oligohydramnios died one week after discovering the renal mass. Therefore, decreasing amniotic fluid volume could indicate renal failure, a predictor of poor prognosis for foetuses with CMN (11). Moreover, CMN in some foetuses could be complicated with other structural abnormalities, such as digestive system abnormalities, polydactyly, hydrocephalus, and Beckwith-Wiedemann syndrome (17). No other structural abnormalities were observed in this case, and the amniotic fluid did not increase throughout the gestational period.
In a Chinese report, three pregnant women terminated pregnancies due to an erroneous prenatal ultrasound diagnosis of foetal renal mass, while the autopsy turned out to be CMN (18). Therefore, improving the ability to diagnose and differential diagnosis is essential. Apart from CMN, the other foetal renal masses included Wilm's tumour (nephroblastoma) and neuroblastoma. Although Wilm's tumour is more common among children aged 3–4, the foetal type was reported occasionally. Rampersad et al. (19) and Vadeyar et al. (20) wrote that Wilms tumour in foetuses clinically manifests as polyhydramnios, foetal oedema, and foetal distress. The prenatal ultrasound of Wilm's tumour demonstrated a solid and cystic mass (21). However, Wilms tumour was difficult to distinguish from cellular CMN on prenatal ultrasound. Neuroblastomas originate from the adrenal gland. Therefore, it is necessary to determine whether the ipsilateral adrenal gland is present and assess the relationship between the mass and the adrenal gland. Neuroblastomas' boundaries are poorly defined and often contain haemorrhage, cystic degeneration and calcification. It is challenging to distinguish renal neuroblastomas from CMN when neuroblastoma is located in the kidney (22).
The correlation between foetal imaging findings and the histopathological types of CMN has been reported in the literature. Therefore, the ultrasound features of foetal CMN could correspond to a specific histopathological type. For example, a solid renal mass suggested classical CMN, while a renal mass with cystic degeneration, necrosis, and haemorrhage indicated cellular CMN (23). The ultrasound finding, in this case, was a well-defined solid mass, which was consistent with the final pathological result of classical CMN.
In a review by Gooskens et al., the classical, cellular, mixed, and classical/mixed CMN accounted for 39%, 42%, 10%, and 9%, respectively. Among the 251 cases of CMN, 208 (83%) were stage I/II, and only 42 (17%) were stage III (17). In CMN, the most common chromosomal gene mutations are trisomy 11 and t (12;15) (P13; q25) (24). The postoperative pathology for this case suggested classical CMN, and molecular pathology did not reveal ETV6-NTRK3 fusion signals.
The prognosis of infants with CMN is good, and the factors affecting the outcome include the pathological type and stage (25). Surgical treatment is required for CMN after birth. The primary surgical method is radical nephrectomy. Adjuvant chemotherapy suits patients with relapsed, high-stage (stage III or higher), or late-onset cellular CMN (14). The 5-year survival rate is 95%. In a study focused on renal tumours in infants within seven months of birth, the 5-year event-free survival (EFS) was 94%, and the 5-year overall survival was 96% (2). In another study reported by the German Society of Paediatric Oncology and Haematology (GPOH), the EFS was 100% in children with CMN diagnosed within three months of birth, and 83% in children with CMN diagnosed beyond three months after birth (6). Since CMN recurrence occurs in 4% of patients within one year, postoperative follow-ups should be performed at least one year after surgery (17).
Although CMN has been reported and discussed in previous studies, our case detailed all relevant imaging and postnatal management. Meanwhile, our case especially highlighted the contribution of prenatal ultrasound in the prenatal diagnosis of renal mass. More experience should be accumulated in MRI and CT of CMN.
CMN could be detected prenatally, mainly during late pregnancy. Therefore, it is necessary to perform a detailed foetal ultrasound in the third trimester to detect structural anomalies, such as renal mass. Early diagnosis of foetal renal mass could favor the postnatal management and improve the neonatal prognosis.
The datasets obtained and/or analyzed during the current study are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by The Institutional Review Board of Peking University First Hospital. The patients/participants provided their written informed consent to participate in this study.
XZ and HZ: designed the research and drafted the manuscript. SW: performed a detailed chart review and literature search. YG: performed the surgery for the neonate and provided reviews on postnatal management. LL: provided detailed pathological results and explanation. HY: provided substantial edits and additions to the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Howell CG, Othersen HB, Kiviat NE, Norkool P, Beckwith JB, D'Angio GJ. Therapy and outcome in 51 children with mesoblastic nephroma: a report of the national Wilms’ tumor study. J Pediatr Surg. (1982) 17(6):826–31. doi: 10.1016/S0022-3468(82)80451-X
2. van den Heuvel-Eibrink MM, Grundy P, Graf N, Pritchard-Jones K, Bergeron C, Patte C, et al. Characteristics and survival of 750 children diagnosed with a renal tumor in the first seven months of life: a collaborative study by the SIOP/GPOH/SFOP, NWTSG, and UKCCSG Wilms tumor study groups. Pediatr Blood Cancer. (2008) 50(6):1130–4. doi: 10.1002/pbc.21389
3. Sandstedt B, Delemarre JF, Krul EJ, Tournade MF. Mesoblastic nephromas: a study of 29 tumours from the SIOP nephroblastoma file. Histopathology. (1985) 9(7):741–50. doi: 10.1111/j.1365-2559.1985.tb02860.x
4. Marsden HB, Newton WA Jr. New look at mesoblastic nephroma. J Clin Pathol. (1986) 39(5):508–13. doi: 10.1136/jcp.39.5.508
5. Isaacs H. Fetal and neonatal renal tumors. J Pediatr Surg. (2008) 43(9):1587–95. doi: 10.1016/j.jpedsurg.2008.03.052
6. Furtwaengler R, Reinhard H, Leuschner I, Schenk JP, Goebel U, Claviez A, et al. Mesoblastic nephroma—a report from the Gesellschaft fur Pädiatrische Onkologie und Hämatologie (GPOH). Cancer. (2006) 106(10):2275–83. doi: 10.1002/cncr.21836
7. Bolande RP, Brough AJ, Izant RJ Jr. Congenital mesoblastic nephroma of infancy. A report of eight cases and the relationship to Wilms’ tumor. Pediatrics (Evanston). (1967) 40(2):272–8. doi: 10.1542/peds.40.2.272
8. Bolande RP. Congenital mesoblastic nephroma of infancy. Perspect Pediatr Pathol. (1973) 1:227–50. PMID: 4363286
9. Lin L-C, Cheng Y-C, Chang C-H. Prenatal diagnosis of fetal congenital mesoblastic nephroma and neonatal follow-up. J Med Ultrasound. (2021) 29(2):126–8. doi: 10.4103/JMU.JMU_79_20
10. Patil PA, Vemarapu G. Fetal congenital mesoblastic nephroma: case report. J Fetal Med. (2021) 8(3):243–8. doi: 10.1007/s40556-021-00317-3
11. Guimaraes A, Antonelli J, Silva C, Tavares R, Sousa L, Menicucci F, et al. VP11.12: prenatal diagnosis of congenital mesoblastic nephroma associated with oligohydramnios. Ultrasound Obstet Gynecol. (2020) 56:97. doi: 10.1002/uog.22493
12. Che M, Yang F, Huang H, Zhang H, Han C, Sun N. Prenatal diagnosis of fetal congenital mesoblastic nephroma by ultrasonography combined with MR imaging: a case report and literature review. Medicine (Baltimore). (2021) 100(3):e24034. doi: 10.1097/MD.0000000000024034
13. Chen YX, Huang C, He QM, Wang Z, Huang L, Wang HY, et al. Prenatal diagnosis and postnatal management of congenital mesoblastic nephroma: experience at a single center in China. Prenat Diagn. (2021) 41(6):766–71. doi: 10.1002/pd.5942
14. Powis M. Neonatal renal tumours. Early Hum Dev. (2010) 86(10):607–12. doi: 10.1016/j.earlhumdev.2010.08.018
15. Pachl M, Arul GS, Jester I, Bowen C, Hobin D, Morland B. Congenital mesoblastic nephroma: a single-centre series. Ann R Coll Surg Engl. (2020) 102(1):67–70. doi: 10.1308/rcsann.2019.0111
16. Chung EM, Graeber AR, Conran RM. Renal tumors of childhood: radiologic-pathologic correlation part 1. The 1st decade: from the radiologic pathology archives. Radiographics. (2016) 36(2):499–522. doi: 10.1148/rg.2016150230
17. Gooskens SL, Houwing ME, Vujanic GM, Dome JS, Diertens T, Coulomb-l'Herminé A, et al. Congenital mesoblastic nephroma 50 years after its recognition: a narrative review. Pediatr Blood Cancer. (2017) 64(7):e26437. doi: 10.1002/pbc.26437
18. Zhao ML, Chang DE, Tang HF. The clinicopathological features and research progress of congenital mesoblastic nephroma. Zhonghua Bing li xue za zhi. (2021) 50(5):553–6. doi: 10.3760/cma.j.cn112151-20201224-00954
19. Rampersad F, Diljohn J, Goetz C. The clinical presentation, imaging features and differential diagnoses of congenital Wilms tumour. BMJ Case Rep. (2019) 12(5):e228651. doi: 10.1136/bcr-2018-228651
20. Vadeyar S, Ramsay M, James D, O'Neill D. Prenatal diagnosis of congenital Wilms’ tumor (nephroblastoma) presenting as fetal hydrops: prenatal diagnosis of congenital Wilms’ tumor. Ultrasound Obstet Gynecol. (2000) 16(1):80–3. doi: 10.1046/j.1469-0705.2000.00169.x
21. Linam LE, Yu X, Calvo-Garcia MA, Rubio EI, Crombleholme TM, Bove K, et al. Contribution of magnetic resonance imaging to prenatal differential diagnosis of renal tumors: report of two cases and review of the literature. Fetal Diagn Ther. (2010) 28(2):100–8. doi: 10.1159/000313655
22. Garnier S, Maillet O, Haouy S, Saguintaah M, Serre I, Galifer RB, et al. Prenatal intrarenal neuroblastoma mimicking a mesoblastic nephroma: a case report. J Pediatr Surg. (2012) 47(8):e21–3. doi: 10.1016/j.jpedsurg.2012.03.090
23. Bayindir P, Guillerman RP, Hicks MJ, Chintagumpala MM. Cellular mesoblastic nephroma (infantile renal fibrosarcoma): institutional review of the clinical, diagnostic imaging, and pathologic features of a distinctive neoplasm of infancy. Pediatr Radiol. (2009) 39(10):1066–74. doi: 10.1007/s00247-009-1348-9
24. Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6-NTRK3 Gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. (1998) 58(22):5046–8. PMID: 9823307
Keywords: congenital mesoblastic nephroma (CMN), prenatal ultrasound, solid renal mass, nephrorectomy, CLASSIC
Citation: Zhang X, Zhang H, Wang S, Gao Y, Liang L and Yang H (2022) Prenatal diagnosis and postnatal management of congenital mesoblastic nephroma: A case report and literature review. Front. Pediatr. 10:1040304. doi: 10.3389/fped.2022.1040304
Received: 9 September 2022; Accepted: 14 October 2022;
Published: 21 November 2022.
Edited by:
Claudio La Scola, Sant'Orsola-Malpighi Polyclinic, ItalyReviewed by:
Brendan Crawford, University of Arkansas for Medical Sciences, United States© Zhang, Zhang, Wang, Gao, Liang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huixia Yang eWFuZ2h1aXhpYUBiam11LmVkdS5jbg==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Nephrology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.