- 1Department of Endocrinology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
- 2Department of Endocrinology and Metabolic Disease, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 3Institute of Endocrine and Metabolic Diseases, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
Background: To investigate the effect of school life by comparing the glycemic control between holidays and schooldays in children and adolescents with type 1 diabetes (T1D).
Methods: This observational study enrolled school-aged students with T1D (aged 6–19) from September 2019 to July 2021. Continuous glucose monitoring (CGM) records were processed and divided into holidays and schooldays. Other information was collected via questionnaires. We compared the results using paired T-test, Wilcoxon paired test and logistic regression analysis.
Results: 78 paticipants were included (40 boys, mean age 9.95 years). A total of 142,945 h of CGM data were analyzed. Overall, TIR (3.9–7.8 mmol/L) during holidays was better than schooldays [56.97 (SD 15.03) vs. 55.87 (15.06), %, p = 0.039]. On nocturnal (0–6 am) glycemic fluctuation, TIR was longer in children aged 6–10 [60.54 (17.40) vs. 56.98 (SD 16.32), %, p = 0.012] during holiday and TAR (7.8 mmol/L) was shorter [31.54 (17.54) vs. 35.54 (16.95), %, p = 0.013], compared with schooldays. In adolescents aged 10–19 years, TAR was also significantly shorter during holidays. Stratified analysis showed that girls, patients with longer duration, and insulin pump users had more pronounced worsening of nighttime glycemia on schooldays. Logistic regression analysis showed that girls had higher risk of worse nocturnal glycemic control [3.26, 95% CI: (1.17, 9.72), p = 0.027] and nocturnal hyperglycemia [OR = 2.95, 95% CI: (1.08, 8.56), p = 0.039], compared to boys.
Conclusions: Children and adolescents with T1D were found to have worse glycemic control in nighttime during schooldays.
Introduction
Type 1 diabetes (T1D) is defined as an autoimmune disease characterized by absolute insulin deficiency (1). The burden of T1D is vast and is expected to increase rapidly. In 2021, there were about 8.4 million individuals worldwide with T1D, including an estimated 1.5 million children under the age of 20 years living with T1D worldwide (2). According to the International Diabetes Registry, the hemoglobin A1c (HbA1c) of most children and adolescents with T1D does not meet the ISPAD target (3). The data from International Pediatric Registry SWEET demonstrated that only 37% of patients attained the ISPAD former HbA1c target of less than 7.5%, and 21% achieved the current goal of less than 7% (3–5).
In children and adolescents, the management of diabetes is difficult due to various factors, including physiological factors such as changes in insulin resistance related to physical growth and puberty (6). In addition, there is an increasing need for self-care knowledge and behaviors, including an understanding of carbohydrate counting, insulin calculation, self-glucose monitoring, and the effects of exercise and stress (7). A distinctive feature is that the responsibilities of children and parents in diabetes management are dynamic (7). Over time, parents have less responsibility for diabetes management. In early childhood (0–5 years), care recommendations for this age group focus on parental management (8). When children turn school age (generally 6 years of age or older), they spend most of their day at school. Children begin gaining more independence in their lifestyle, and thus diabetes management starts transitioning from a family-centered model to a patient-centered one (9). Such a shift in diabetes management responsibility is a challenge for children with T1D and affects their glycemic control (10). Together, children in lower grades in elementary school may have poor glycemic control due to poor adherence to treatment on schooldays. In contrast, children spend more time with their parents on holidays. Many studies (11, 12) show that more parental engagement is associated with better glycemic outcomes. However, glucose management can also be challenging due to the increased caloric intake on holidays (13).
However, current studies have revealed contradicting findings regarding differences in glycemic control between schooldays and holidays in children and adolescents with T1D. Several investigations of T1D indicate that children and adolescents with T1D had higher HbA1c levels (14) or lower self-monitoring blood glucose (SMBG) (15) during holidays than during school semesters, possibly because children with T1D had reduced treatment compliance (16) and a change in lifestyle. In contrast, another study demonstrated (17) no significant difference in HbA1c levels between summer vacation and school semesters. However, HbA1c levels represent a longer term of glycemic control and may be insensitive to the short-term changes between schooldays and vacation. While SMBG can make up for some HbA1c limitations, such as short-term glycemic variability, it cannot fully capture actual glycemic fluctuation (18). Continuous glucose monitoring (CGM) overcomes the problems associated with HbA1c and SMBG and offers opportunities to better reflect short-term glycemic changes and other details of glycemic variations, which assists to achieve better glycemic control.
To date, few studies have been conducted to assess glycemic control in children and adolescents with T1D, especially in regard to schooldays and holidays, using data derived from CGM. Therefore, this study aimed to investigate the impact of school life by comparing the glycemic control of CGM between holidays and schooldays among children and adolescents with T1D. This will provide important information on glycemic control in children and adolescents with T1D on schooldays.
Methods
Study design and participants
This was an observational study. We recruited eligible participants from the Chinese Registry of T1D, which was launched in 2014 (ChiCTR2000034642) (19). This program is conducted with the assistance of the smartphone-based application Tangtangquan (TTQ). TTQ (20) is a Chinese mobile application designed to provide diabetes self-management education and support for patients with T1D and is available for download from major application markets and the registration is free of charge. We issued advertisements in the application to recruit potential participants. Eligibility criteria were as follows: (1) patients with T1D diagnosed by an endocrinologist; (2) children and adolescents aged 6–19 years; and (3) patients who were willing to donate their CGM data for analysis in this study. The exclusion criteria included (1) refusing to participate in the study; (2) patients who wore a personal CGM device (FGM or rtCGM) for less than three days during holidays and schooldays; (3) having uncontrolled psychiatric comorbidity; and (4) currently participating in other clinical studies. The participants were divided into children (aged 6–9.9 years) and adolescents (aged 10 years or older) according to the United Nations definition of adolescence (21).
The observation period of this study was from September 2019 to July 2021. We compared the CGM-based glycemic control of the eligible participants between schooldays and holidays to investigate the impact of school life. The observation period was divided into holidays (holidays during the semester including weekends and short holidays, excluding summer and winter vacations) and schooldays. Because long vacations tended to be associated with greater lifestyle changes, such as more outdoor activities and long trips, and thus summer vacations and winter vacations were excluded. Due to the COVID-19 outbreak, Chinese students were studying from home from February 2020 to August 2020, so this period was also excluded.
Data collection
In TTQ, a cloud platform that relies on the Nightscout system (22, 23) was established in September 2019. With this platform, TTQ users can upload their raw CGM data to the server in real time. We retrieved the CGM data of the participants during the designated study period from this platform.
We collected the following data from the participants at baseline from the T1D China Registry dataset: (1) demographic data: age, sex, education level, hometown, and household income per year; and (2) medical history: duration of T1D, age at T1D onset, diabetes complications, current HbA1c values, insulin treatment [multiple daily insulin injections (MDI), continuous subcutaneous insulin infusion (CSII), or others]. The MDI regimen consists of at least four insulin injections a day, including three premeal short-acting insulin shots and one bedtime long-acting insulin shot, and any add-on shots of short-acting insulin if necessary. In addition, information about lifestyle was obtained through a questionnaire that can be completed online or via telephone. Our questionnaire consists of six parts (20): (1) growth and development; (2) diet; (3) physical exercise; (4) sleep habits; (5) diabetes management; and (6) medical visits.
Outcome measurements: CGM metrics
We use the definition of CGM metrics according to the consensus statements the ATTD Congress issued in February 2017 on 14 CGM core metrics that may be most useful in clinical practice (24). Raw CGM data obtained from the cloud platform were processed using Glyculator 2.0 software (25).
In this study, we observed the following CGM core metrics: (1) primary outcome: the proportion of time spent in the target glucose range between 3.9 and 7.8 mmol/L (TIR 3.9–7.8) and the proportion of time spent with glucose levels above 7.8 mmol/L (TAR 7.8); and (2) secondary outcome: mean glucose levels, glucose management indicator (GMI), coefficient of variation (CV), and the proportion of time spent with glucose levels below 3.9 mmol/L (TBR 3.9). The formula of GMI (%) (26) was 3.31 + 0.02392 × mean glucose in mg/dl. CV, the main metric for the evaluation of glycaemic variability, was not significantly associated with HbA1c (27). Primary and secondary outcomes were calculated utilizing data from all observed subjects from at least three days. The household income group was split based on the annual per capita income data of China (28). Duration of T1D was categorized into groups based on the average value. Additionally, we assessed dietary management compliance with midnight snacks. Daytime referred to 6 am–12 pm, and nighttime referred to 0 am–6 am.
Statistical analysis
Data are presented as the means (standard deviations) or frequencies (proportions). Comparisons between two groups were conducted using the paired T test or Wilcoxon paired test, depending on whether the data followed a normal distribution. The χ2 test was used for categorical variables. Logistic regression analysis was used to analyze the association between the change in glycemic control and clinical and lifestyle-related variables. R (version 4.1.1) was used for statistical analyses. Statistical significance was defined as a two-sided p < 0.05.
Results
Participants and baseline characteristics
During the study period, 78 (40 boys, 38 girls) participants were enrolled. The participant characteristics are summarized in Table 1. A total of 87.2% of the participants (n = 68) used flash glucose monitoring (FreeStyle Libre; Abbott, North Chicago, IL, United States), and 12.8% of the patients (n = 10) used CGM (7 participants used Dexcom G5, 3 participants used Dexcom G6). Overall, the included patients were using CGM for 60.89% of the time during holidays, and 60.12% during school days, respectively.
The participants were divided into children and adolescents, according to an age cutoff of 10 years. The average age of the patients was 9.95 (1.79) years. The average duration of T1D was 2.63 (1.66) years. The average baseline HbA1c value was 7.13 (1.63) %. More of the participants were from households with a high annual income (n = 29, 60.24%). Among all participants, 43 (55.13%) were in the child group (6 ≤ age < 10 years), and 35 (44.87%) were in the adolescent group (10 ≤ age ≤ 19 years). Except age, onset age of T1D and BMI, there were no statistically significant differences observed in other basic characteristics between the two groups (Table 1).
Comparison of glycemic metrics: holidays vs. schooldays
Of the participants, a total of 142,945 h of CGM data were analyzed: 48,145 h during holidays and 94,800 h during schooldays. The characteristics of the glycemic metrics among the 78 participants are shown in Supplementary Table S1. For the whole 24-h period, TIR 3.9–7.8 during holidays was better than during schooldays [56.97 (15.03) vs. 55.87 (15.06), %, p = 0.039]. During the nighttime, TIR 3.9–7.8 during holidays was longer than that during schooldays [60.42 (17.06) vs. 56.92 (18.13), %, p = 0.001], and the TAR 7.8 during holidays was shorter than that during schooldays [31.81 (17.46) vs. 35.64 (18.55), %, p = 0.001]. No significant differences were found in daytime TIR 3.9–7.8 or daytime TAR 7.8 between holidays and schooldays.
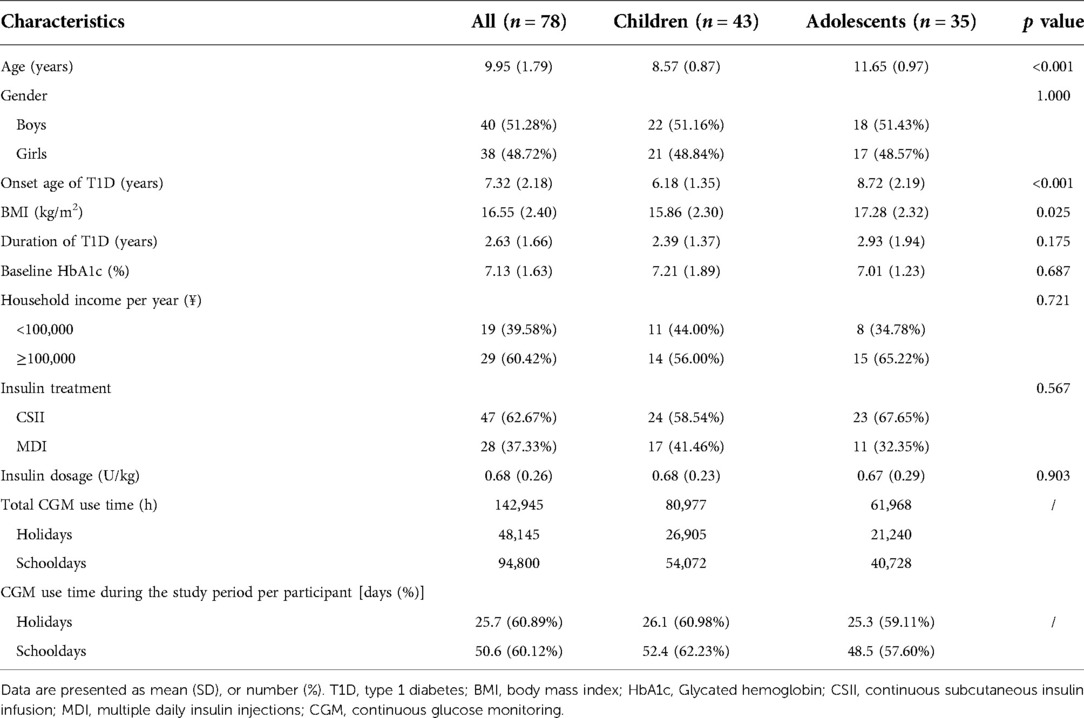
Then, we explored the impact of age on the difference in glycemic control between schooldays and holidays. We compared the CGM metrics between schooldays and holidays among children and adolescents. The results are presented in Figures 1,2 and Supplementary Table S2. For the child group, in the whole 24-h period, TIR 3.9–7.8 was higher during holidays than during schooldays [56.82 (14.91) vs. 55.14 (14.75), %, p = 0.011]. Moreover, the TAR 7.8 was lower during holidays than during schooldays [35.50 (15.20) vs. 37.46 (15.14), %, p = 0.017]. In the adolescent group, TIR 3.9–7.8 and TAR 7.8 also appeared to be worse on schooldays, but the differences were not significant (p > 0.05) for the whole day period. During the nighttime, TIR 3.9–7.8 was longer during the holidays than during schooldays in the child group [60.54 (17.40) vs. 56.98 (16.32), %, p = 0.012] and seemingly longer in the adolescent group [60.27 (16.88) vs. 56.83 (20.38), %, p = 0.051]. Additionally, the nighttime TAR 7.8 was lower during holidays than during schooldays, both in the child [31.54 (17.54) vs. 35.54 (16.95), %, p = 0.013] and adolescent [32.15 (17.61) vs. 35.77 (20.60), %, p = 0.028] groups. Notably, in the adolescent group, the mean glucose level was lower during holidays than during schooldays [7.07 (1.19) vs. 7.36 (1.47), mmol/L, p = 0.042], as was the GMI [6.36 (0.51) vs. 6.48 (0.63), %, p = 0.042]. During the daytime, there were no significant differences between holidays and schooldays for any CGM metrics in either the children or the adolescents.
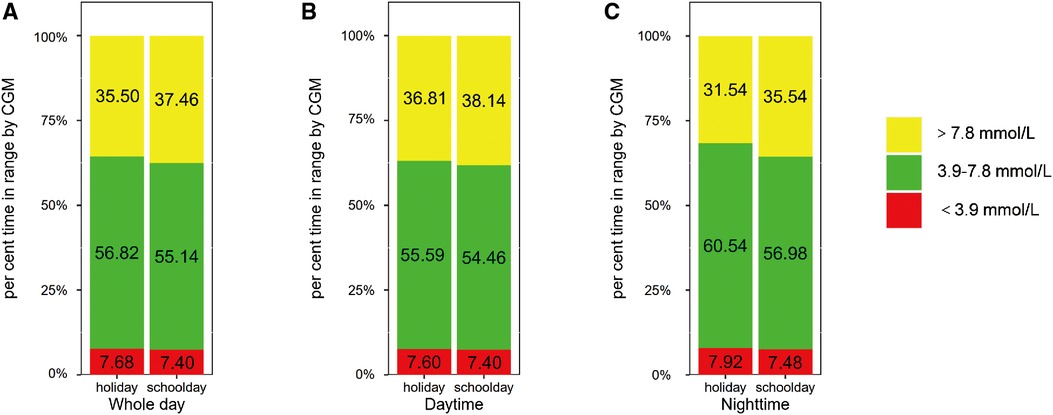
Figure 1. Comparison of glycemic metrics in children group: holidays vs. schooldays (A) Comparison of 24-hour CGM metrics between holidays and schooldays; (B) Comparison of daytime CGM metrics between holidays and schooldays; (C) Comparison of nighttime CGM metrics between holidays and schooldays; CGM, Continuous glucose monitoring; TIR 3.9–7.8, The time spent in target glucose range between 3.9–7.8 mmol/L; TAR 7.8, the proportion of time spent with glucose levels above 7.8 mmol/L; TBR 3.9, The proportion of time spent with glucose levels below 3.9 mmol/L.
Figure 2. Comparison of glycemic metrics in adolescents group: holidays vs. schooldays (A) Comparison of 24-hour CGM metrics between holidays and schooldays; (B) Comparison of daytime CGM metrics between holidays and schooldays; (C) Comparison of nighttime CGM metrics between holidays and schooldays; CGM, Continuous glucose monitoring; TIR 3.9–7.8, The time spent in target glucose range between 3.9–7.8 mmol/L; TAR 7.8, the proportion of time spent with glucose levels above 7.8 mmol/L; TBR 3.9, The proportion of time spent with glucose levels below 3.9 mmol/L.
Factors associated with nocturnal glycemic stability
We further analyzed factors associated with nocturnal glycemic deterioration on schooldays, and thus, we performed subgroup analysis for nocturnal TIR 3.9–7.8, stratified by gender, duration of T1D, insulin treatment, household income, and midnight snack consumption. The results are summarized in Table 2. In the pediatric group, a difference in nocturnal TIR 3.9–7.8 between schooldays and holidays was observed among girls [children: 58.85 (16.39) vs. 64.91 (17.61), %, p = 0.003], with a mean difference of 6.06% in favor of holidays. However, such differences were not observed among boys or adolescents. Nocturnal TIR 3.9–7.8 was lower during schooldays than during holidays in the adolescents with a duration of T1D longer than three years [40.99 (11.06) vs. 48.46 (11.90), %, p = 0.033] but not among those with a duration under three years or in the child group. Children treated with insulin pumps had better nocturnal glycemic control on holidays than on schooldays [nocturnal TIR 3.9–7.8, 64.70 (18.45) vs. 61.29 (16.89), %, p = 0.038]. Similar results were observed in adolescents treated with an insulin pump [57.52 (14.01) vs. 52.55 (18.45), %, p = 0.045]. In children in the low household income group, TIR 3.9–7.8 was higher during holidays than during schooldays [65.38 (19.99) vs. 58.61 (20.90), %, p = 0.026]. In contrast, in adolescents in the high household income group, TIR 3.9–7.8 was lower during schooldays than during holidays [53.97 (21.73) vs. 61.62 (16.06), %, p = 0.017]. In the children in the no midnight snacks group, TIR 3.9–7.8 was better during holidays than during schooldays [64.26 (18.29) vs. 58.34 (19.90), %, p = 0.023]. In adolescents in the midnight snack group, TIR 3.9–7.8 was higher on holidays [54.98 (13.43) vs. 48.43 (15.81), %, p = 0.013]. The results of the subgroup analysis for nocturnal TAR 7.8 are shown in Supplementary Table S3. Similar trends were found in these findings.
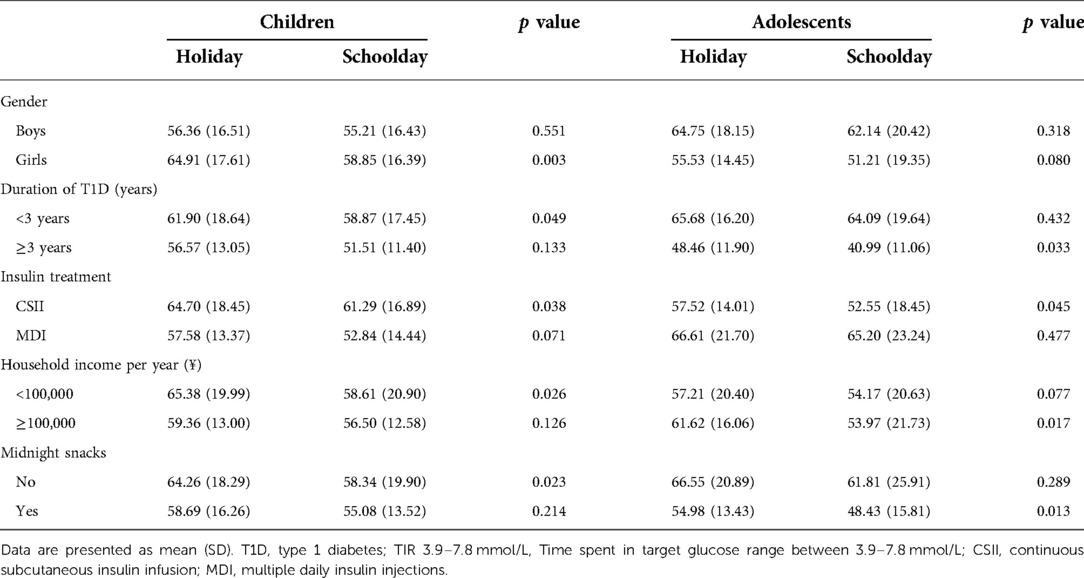
Table 2. Associated factors with nocturnal glycemic fluctuation (TIR 3.9–7.8 mmol/L) in participants with T1D.
To evaluate the associations between the aforementioned factors and the poorer nocturnal glycemic control identified during schooldays, logistic regression analysis was performed. We compared the patient's schoolday nocturnal glucose metrics with their holiday nocturnal glucose metrics. We coded the binary variable of deterioration of TIR as 1 when TIR 3.9–7.8 was lower on schooldays than on holidays and 0 otherwise. Likewise, we coded a binary variable of deterioration of TAR as 1 when TAR 7.8 was higher on schooldays than on holidays and 0 otherwise. (Details of the binary variable definition are presented in Supplementary Table S4) In our multivariate model, we adjusted for gender, age, duration of T1D and insulin treatment. The results are shown in Table 3. Girls were approximately three times more likely to have poorer nighttime glycemic control during schooldays than boys (OR = 3.26, 95% CI: 1.17–9.72, p = 0.027); simultaneously, girls were almost three times more likely to have a higher risk of nocturnal hyperglycemia during schooldays than boys (OR = 2.95, 95% CI: 1.08–8.56, p = 0.039).

Table 3. The relationship between the participants’ characteristics and the worse nocturnal glycemic control in schooldays by using binary logistic regression analysis.
Discussion
In this observational study, we found that school-aged children and adolescents with T1D had worse glycemic control at night on schooldays. Deterioration of nocturnal glycemic control on schooldays might be affected by patients' annual household income, the type of insulin therapy, and the duration of T1D. Notably, compared to boys, girls might have a higher risk of worse nocturnal glycemic control among both children and adolescents. This finding is potentially of clinical importance, suggesting that children and adolescents should pay more attention to glycemic control during schooldays. Our results echo previous research on SMBGs (15) and show better glycemic control during holidays. Moreover, we offered more details on glycemic variations and found a lower risk of nocturnal hyperglycemia during holidays.
We observed that the children and adolescents with T1D treated with CSII had a lower TIR 3.9–7.8 during schooldays than during holidays. One possible explanation is that children and adolescents spend most of their time away from their parents, making it difficult for parents to know the details of their children's school life; hence, it is difficult to adjust the pump settings properly. For example, parents may not be able to acquire a complete list of foods consumed by their children, resulting in inaccurate carbohydrate and insulin calculations that can affect daily insulin therapy. Although recent studies (29, 30) have shown that compared with MDI, the CSII group had a lower risk of severe hypoglycemia and better glycemic control and earlier CSII use was associated with better glycemic control in younger T1D patients, our findings raise a concern about the effectiveness of CSII treatment among school-aged children during schooldays. In a previous school survey, more than one-third of adolescents hid the fact that they had diabetes at school, which could lead to reduced glucose testing and insulin omission, especially during schooldays (31). One study of children and adolescents on CSII therapy reported that 10% of participants missed mealtime boluses (32). The omission of four injections of rapid-acting insulin per week can result in a 1% rise in HbA1c. Further studies should be performed to offer more guidance for children and adolescents regarding insulin pump use at school. Similar difficulties were faced by children and adolescence in China. A report shows that the patients with type 1 diabetes in China are facing stigma, fear, and guilt may discourage insulin pump use and multiple daily injections, especially for those who require pre-meal insulin injections at school (33).
Our data showed that nocturnal glycemic control in young T1D patients during holidays and schooldays was affected by the consumption of midnight snacks. The TIR values of the children and adolescents in the no-midnight-snacks group were higher than those of the children in the midnight-snacks group, regardless of whether the snacks were consumed on a holiday or a schoolday. Midnight snacks had a more pronounced effect on adolescents' blood glucose on schooldays, and the TIR on schooldays was significantly lower than that on holidays. We also showed differences between holidays and schooldays in nocturnal glycemic control among adolescents with a longer duration of T1D, and TIR was lower on schooldays. This finding might be explained by increased independence and more time away from parents. Responsibility for diabetes management gradually shifts from parents to children and their school. The recent ISPAD clinical practice consensus guidelines state that all students with T1D, regardless of age and ability, should receive the support, encouragement and supervision of school personnel (34). In the DAWN study (35) of an international network survey with more than 6,000 people with T1D from eight countries, the results showed that respondents rated the level of support provided by schools as significantly lower than that received elsewhere. Alarmingly, a recent study found that only 2% of physical education teachers had adequate knowledge of diabetes (36). The findings of this project indicate the importance of national attention and programs for the management of diabetes in schools.
The findings showed that girls had an almost three times higher risk of worse nocturnal glucose levels and nocturnal hyperglycemia than boys. One possible explanation is that puberty typically begins at the age of 8 years in girls, which is earlier than that in boys (37). Adolescence is the transition period between childhood and adulthood, which leads to dramatic changes both physically and mentally, and girls face these challenges earlier than boys. This period is characterized by rapid sexual maturation, which usually leads to insulin resistance, exacerbating diabetic hyperglycemia (7). In addition, another possible explanation is that girls are more likely to experience reduced insulin doses during schooldays than boys (38). One study showed a gender disparity regarding the location of insulin administration at school, with girls being significantly less likely to inject insulin in the classroom and more likely to inject in the bathroom (39). This difference in injection site increased the chances of girls missing insulin doses due to classes. Therefore, we need to consider the impact of adolescence and gender when formulating treatment plans.
Previous studies (40, 41) found a link between lower annual household income and poorer diabetes knowledge. However, our study found no significant difference in nocturnal glycemic control in young T1D patients between the high and low family annual income groups. It is possible that there is a complex relationship between the family's annual income level and the patient's glucose control, and lower income is often not equivalent to worse family support.
The strength of this study is that we captured an extended time frame of CGM data reflecting glycemic control in children and adolescents with T1D during holidays and schooldays in a real-world setting. We acknowledge that there are several limitations. First, the sample size was relatively small, but the post hoc analysis showed that each key endpoint had more than 80% power to detect standardized effect sizes equal to 0.30. Second, on the questionnaire, some information was missing, such as growth and sexual development status. Therefore, we could not assess the associations of each of these items with nocturnal glycemic stability in the present study. Finally, as in other observational studies, even though we adjusted for several covariates related to glycemic control, residual confounding by unidentified confounders is still possible.
The results of this observational study may provide references for schools to support the self-care of diabetic patients, such as training school staff on diabetes management and strengthening psychological support for children with diabetes. They can also provide advice for clinical treatment, such as diabetes education in school-age children and individuals in early adolescence.
Conclusions
Children and adolescents with T1D were found to have poor nighttime glycemic control during schooldays. These individuals may need more attention and guidance to improve their glucose control during schooldays.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The institutional review board approved the study protocol at the first affiliated hospital of University of Science and Technology of China (IRB no: 2019 KY-27). Electronic informed consent was obtained from each participant and their parent or legal guardian, as applicable, before enrollment. It is consistent with the provisions of the Declaration of Helsinki.
Author contributions
SL and XZ: conceptualized and designed research. YD and WZ: conducted the data collection, analyzed data, and wrote the manuscript. YD and WZ: contributed equally to this work. XW, TW, XW: provided insightful comments on the interpretation of the data, and the structure and revision of the manuscript. SL and XZ: also contributed to data interpretation and manuscript discussion. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the National Natural Science Foundation of China (Grant No. 82100822). This study was also supported by the Anhui Provincial Natural Science Foundation (Grant No. 2008085MH248 and 2008085MH278). The funders had no role in the design and conduct of the study, the collection, analysis, and interpretation of the data, or the preparation, review, or approval of the manuscript.
Acknowledgments
We thank all the participants for donating the CGM data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1037261/full#supplementary-material.
References
1. Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. (2017) 3:17016. doi: 10.1038/nrdp.2017.16
2. Gregory GA, Robinson TIG, Linklater SE, Wang F, Colagiuri S, de Beaufort C, et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. (2022) 10(10):741–60. doi: 10.1016/S2213-8587(22)00218-2
3. Cardona-Hernandez R, Schwandt A, Alkandari H, Bratke H, Chobot A, Coles N, et al. Glycemic outcome associated with insulin pump and glucose sensor use in children and adolescents with type 1 diabetes. Data from the international pediatric registry SWEET. Diabetes Care. (2021) 44(5):1176–84. doi: 10.2337/dc20-1674
4. Rewers MJ, Pillay K, de Beaufort C, Craig ME, Hanas R, Acerini CL, et al. ISPAD Clinical practice consensus guidelines 2014. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. (2014) 15(Suppl 20):102–14. doi: 10.1111/pedi.12190
5. DiMeglio LA, Acerini CL, Codner E, Craig ME, Hofer SE, Pillay K, et al. ISPAD Clinical practice consensus guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. (2018) 19(Suppl 27):105–14. doi: 10.1111/pedi.12737
6. Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. (1986) 315(4):215–9. doi: 10.1056/nejm198607243150402
7. Markowitz JT, Garvey KC, Laffel LM. Developmental changes in the roles of patients and families in type 1 diabetes management. Curr Diabetes Rev. (2015) 11(4):231–8. doi: 10.2174/1573399811666150421114146
8. Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 diabetes through the life span: a position statement of the American diabetes association. Diabetes Care. (2014) 37(7):2034–54. doi: 10.2337/dc14-1140
9. Lyons SK, Libman IM, Sperling MA. Clinical review: diabetes in the adolescent: transitional issues. J Clin Endocrinol Metab. (2013) 98(12):4639–45. doi: 10.1210/jc.2013-2890
10. Anderson BJ. Behavioral research in pediatric diabetes: putting the evidence to work for advocacy and education. Pediatr Diabetes. (2012) 13(1):77–80. doi: 10.1111/j.1399-5448.2011.00778.x
11. Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes self-care: links to health outcomes. J Pediatr Psychol. (2008) 33(5):497–508. doi: 10.1093/jpepsy/jsm081
12. Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. J Pediatr. (1997) 130(2):257–65. doi: 10.1016/s0022-3476(97)70352-4
13. Schlundt DG, Pichert JW, Rea MR, Puryear W, Penha ML, Kline SS. Situational obstacles to adherence for adolescents with diabetes. Diabetes Educ. (1994) 20(3):207–11. doi: 10.1177/014572179402000305
14. Boland EA, Grey M, Mezger J, Tamborlane WV. A summer vacation from diabetes: evidence from a clinical trial. Diabetes Educ. (1999) 25(1):31–40. doi: 10.1177/014572179902500105
15. Landau Z, Lebenthal Y, Boaz M, Pinhas-Hamiel O. Observational study of diabetes management in type 1 diabetic school-age children during holiday versus school days. J Pediatr Endocrinol Metab. (2013) 26(11-12):1083–6. doi: 10.1515/jpem-2013-0045
16. Leggett C, Giles L, Anderson JJA, Doogue M, Couper J, Pena AS. Adherence to metformin is reduced during school holidays and weekends in children with type 1 diabetes participating in a randomised controlled trial. Arch Dis Child. (2019) 104(9):890–4. doi: 10.1136/archdischild-2018-316303
17. Sherr JL, Boyle CT, Miller KM, Beck RW, Tamborlane WV. No summer vacation from diabetes: glycemic control in pediatric participants in the T1D exchange registry based on time of year. Diabetes Care. (2016) 39(12):e214–5. doi: 10.2337/dc16-1522
18. Yoo JH, Kim JH. Time in range from continuous glucose monitoring: a novel metric for glycemic control. Diabetes Metab J. (2020) 44(6):828–39. doi: 10.4093/dmj.2020.0257
19. Weng J, Zhou Z, Guo L, Zhu D, Ji L, Luo X, et al. Incidence of type 1 diabetes in China, 2010–13: population based study. Br Med J. (2018) 360:j5295. doi: 10.1136/bmj.j5295
20. Wu X, Luo S, Zheng X, Ding Y, Wang S, Ling P, et al. Glycemic control in children and teenagers with type 1 diabetes around lockdown for COVID-19: a continuous glucose monitoring-based observational study. J Diabetes Investig. (2021) 12(9):1708–17. doi: 10.1111/jdi.13519
21. Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. (2018) 2(3):223–8. doi: 10.1016/s2352-4642(18)30022-1
22. Kublin O, Stępień M. The nightscout system - description of the system and its evaluation in scientific publications. Pediatr Endocrinol Diabetes Metab. (2020) 26(3):140–3. doi: 10.5114/pedm.2020.95621
23. Nightscout. The nightscout project–we are not waiting. Available at: http://www.nightscout.info/(Accessed October 9, 2019).
24. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. (2019) 42(8):1593–603. doi: 10.2337/dci19-0028
25. Pagacz K, Stawiski K, Szadkowska A, Mlynarski W, Fendler W. Glyculator2: an update on a web application for calculation of glycemic variability indices. Acta Diabetol. (2018) 55(8):877–80. doi: 10.1007/s00592-018-1140-0
26. Leelarathna L, Thabit H, Hovorka R, Evans M. Estimated HbA(eA1c) and glucose management indicator (GMI): are they the same? Diabet Med. (2021) 38(2):e14423. doi: 10.1111/dme.14423
27. Piona C, Marigliano M, Mozzillo E, Rosanio F, Zanfardino A, Iafusco D, et al. Relationships between HbA1c and continuous glucose monitoring metrics of glycaemic control and glucose variability in a large cohort of children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. (2021) 177:108933. doi: 10.1016/j.diabres.2021.108933
28. Tang K, Zhang Y, Wang H, Tan SH, Bai L, Liu Y. Regional economic development, household income, gender and hypertension: evidence from half a million Chinese. BMC Public Health. (2020) 20(1):901. doi: 10.1186/s12889-020-09002-y
29. Kamrath C, Tittel SR, Kapellen TM, von dem Berge T, Heidtmann B, Nagl K, et al. Early versus delayed insulin pump therapy in children with newly diagnosed type 1 diabetes: results from the multicentre, prospective diabetes follow-up DPV registry. Lancet Child Adolesc Health. (2021) 5(1):17–25. doi: 10.1016/s2352-4642(20)30339-4
30. Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. Jama. (2017) 318(14):1358–66. doi: 10.1001/jama.2017.13994
31. Amillategui B, Calle JR, Alvarez MA, Cardiel MA, Barrio R. Identifying the special needs of children with type 1 diabetes in the school setting. An overview of parents’ perceptions. Diabet Med. (2007) 24(10):1073–9. doi: 10.1111/j.1464-5491.2007.02250.x
32. Pańkowska E, Skórka A, Szypowska A, Lipka M. Memory of insulin pumps and their record as a source of information about insulin therapy in children and adolescents with type 1 diabetes. Diabetes Technol Ther. (2005) 7(2):308–14. doi: 10.1089/dia.2005.7.308
33. Jaacks LM, Liu W, Ji L, Mayer-Davis EJ. Type 1 diabetes stigma in China: a call to end the devaluation of individuals living with a manageable chronic disease. Diabetes Res Clin Pract. (2015) 107(2):306–7. doi: 10.1016/j.diabres.2014.12.002
34. Bratina N, Forsander G, Annan F, Wysocki T, Pierce J, Calliari LE, et al. ISPAD Clinical practice consensus guidelines 2018: management and support of children and adolescents with type 1 diabetes in school. Pediatr Diabetes. (2018) 19(Suppl 27):287–301. doi: 10.1111/pedi.12743
35. Peyrot M. International DAWN youth survey group. The DAWN youth WebTalk study: methods, findings, and implications. Pediatr Diabetes. (2009) 10(Suppl 13):37–45. doi: 10.1111/j.1399-5448.2009.00612.x
36. Halpern LA, Agwu JC. Physical education teachers’ knowledge of type 1 diabetes. Arch Dis Child. (2009) 94(6):483–4. doi: 10.1136/adc.2008.150375
37. Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Bräuner EV, et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatr. (2020) 174(4):e195881. doi: 10.1001/jamapediatrics.2019.5881
38. Ottosson A B, Åkesson K, Ilvered R, Forsander G, Särnblad S. Self-care management of type 1 diabetes has improved in Swedish schools according to children and adolescents. Acta Paediatr. (2017) 106(12):1987–93. doi: 10.1111/apa.13949
39. McCollum DC, O'Grady MJ. Diminished school-based support for the management of type 1 diabetes in adolescents compared to younger children. Diabet Med. (2020) 37(5):779–84. doi: 10.1111/dme.14160
40. Hatherly K, Smith L, Overland J, Johnston C, Brown-Singh L, Waller D, et al. Glycemic control and type 1 diabetes: the differential impact of model of care and income. Pediatr Diabetes. (2011) 12(2):115–9. doi: 10.1111/j.1399-5448.2010.00670.x
Keywords: type 1 diabetes, children and adolescents, continuous glucose monitoring, glycemic control, schooldays
Citation: Ding Y, Zhang W, Wu X, Wei T, Wang X, Zheng X and Luo S (2022) Deterioration in glycemic control on schooldays among children and adolescents with type 1 diabetes: A continuous glucose monitoring-based study. Front. Pediatr. 10:1037261. doi: 10.3389/fped.2022.1037261
Received: 5 September 2022; Accepted: 22 November 2022;
Published: 7 December 2022.
Edited by:
Maurizio Delvecchio, Giovanni XXIII Children's Hospital, ItalyReviewed by:
Roque Cardona-Hernandez, Sant Joan de Déu Hospital, SpainClaudia Piona, University City Hospital of Verona, Italy
© 2022 Ding, Zhang, Wu, Wei, Wang, Zheng and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sihui Luo bHVvc2lodWlAdXN0Yy5lZHUuY24= Xueying Zheng bHh5emhlbmdAdXN0Yy5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Pediatric Endocrinology, a section of the journal Frontiers in Pediatrics
 Yu Ding1,†
Yu Ding1,† Xiumei Wu
Xiumei Wu Xueying Zheng
Xueying Zheng Sihui Luo
Sihui Luo