- Department of Neonatology, Taizhou Enze Medical Center (Group), Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Enze Hospital, Taizhou, China
Objective: To evaluate the effects of antenatal corticosteroids (ACS) on blood glucose fluctuations in late-preterm neonates.
Methods: A retrospective study was performed on 236 neonates with gestational age of 34+0 to 36+6 weeks who were admitted to the neonatology department of a tertiary general hospital in China's Zhejiang Province between April 2020 and February 2022. The neonates were divided into three groups: complete course, partial course, and control. Primary outcome was the neonatal blood glucose levels within the first 48 h of life.
Results: 134 (56.8%) newborns were exposed to a complete course of ACS, 56 (23.7%) had a to a partial course of ACS, and 46 (19.5%) had no exposure to ACS. The patients in the complete course group had the highest proportion of neonatal hypoglycemia (16.4% vs. 3.6% and 6.5%).The patients exposed to a complete course of dexamethasone had significantly lower blood glucose levels within 12 h of birth than the control group, although no significant differences were observed after 24 h. Differences in blood glucose levels were more significant among male infants, although blood glucose curves of the male and female infants remained close to the overall trend.
Conclusions: Blood glucose levels in late-preterm neonates may decrease after ACS administration, especially after exposure to a complete course. The effects are more pronounced in the first 12 h of life, with males being more severely affected; however, the effects on blood glucose levels were not significant 24 h after birth. This can provide a reference for future clinical studies.
Introduction
Maternal and neonatal health has improved over the past decades; nevertheless, with advances in reproductive assistance, the incidence of preterm birth has not declined (1). The global premature birth rate is estimated at approximately 10% (2). China has the second highest number of premature births worldwide, with >1 million babies born prematurely (1). The rate of premature births in China has reportedly increased by 1.3% annually from 2012 to 2018 (1, 3). Late-preterm infants are defined as those with a gestational age of 34 + 0 to 36 + 6 weeks, and they account for approximately 75% of preterm infants (1). They are at a relatively high risk of common preterm complications, such as respiratory distress syndrome, hypoglycemia, and hyperbilirubinemia (4).
Administration of antenatal corticosteroids (ACS) is an important antenatal intervention to promote fetal lung maturation and improve the prognosis of premature infants. A study by gyamfi Bannerman et al. targeting ACS in late preterm infants (ALPS) showed that antenatal betamethasone treatment significantly improved respiratory outcomes in late preterm infants. However, they reported significantly higher rates of hypoglycemia in these neonates who had experienced ACS (5). Pettit et al. reported the relationship between ACS use and neonatal hypoglycemia, speculating that ACS use may cause maternal hyperglycemia, which in turn leads to fetal pancreatic β-cell proliferation/hyperinsulinemia and then neonatal hypoglycemia (6). Pregnant women take large amounts of corticosteroids during pregnancy, and the drugs can pass through the placenta, resulting in inhibitory effects on the adrenal function of the fetus. Neonatal adrenal insufficiency can lead to hypoglycemic symptoms in newborns that also include symptoms such as hypotension and hyponatremia (7). In our study, the mother used dexamethasone prenatally. As far as we know, there is limited research data on changes in blood glucose levels in neonates exposed to dexamethasone. This study focused on neonatal blood glucose fluctuation in these late preterm infants exposed to ACS administration, and we attempted to explore the timing and severity of the effects of ACS on blood glucose in this population.
Methods
This retrospective study was conducted at a tertiary general hospital in Zhejiang Province, China. Patients were enrolled according to the following criteria: neonatal birth at this hospital, regular glucose level monitoring 48 h after birth, complete maternal ACS dosing data, and accurate maternal and neonatal birth date records. Weeks of gestation for mothers were imputed by last menstrual period. Neonates with congenital inherited metabolic disorders, congenital defects, chromosomal disorders, hospital stays of <2 days, and those who died were also excluded from the study.
The research group screened all neonates with a gestational age of 34+0 to 36+6 weeks who were admitted to the Department of Neonatology between April 2020 and February 2022 (Figure 1). Altogether, 347 premature infants were screened in the study, including 274 premature infants with a gestational age of 34 + 0 to 36 + 6 weeks. 73 preterm neonates with a gestational age <34 weeks were excluded. Because our study aimed to discuss the effects of ACS application on blood glucose level fluctuation within 48 h of birth, the neonates whose first blood glucose level was measured later than 60 min after birth were further excluded because they lack regular blood glucose monitoring.The parents of these premature infants, due to various factors, did not consent to their immediate hospitalization in the neonatal department. There were 38 premature infants excluded due to this criterion. Ultimately, 236 preterm infants were included for statistics. The study protocol was approved by Ethics Committee of Enze Hospital, Taizhou Enze Medical Center (Group)(K20220502).
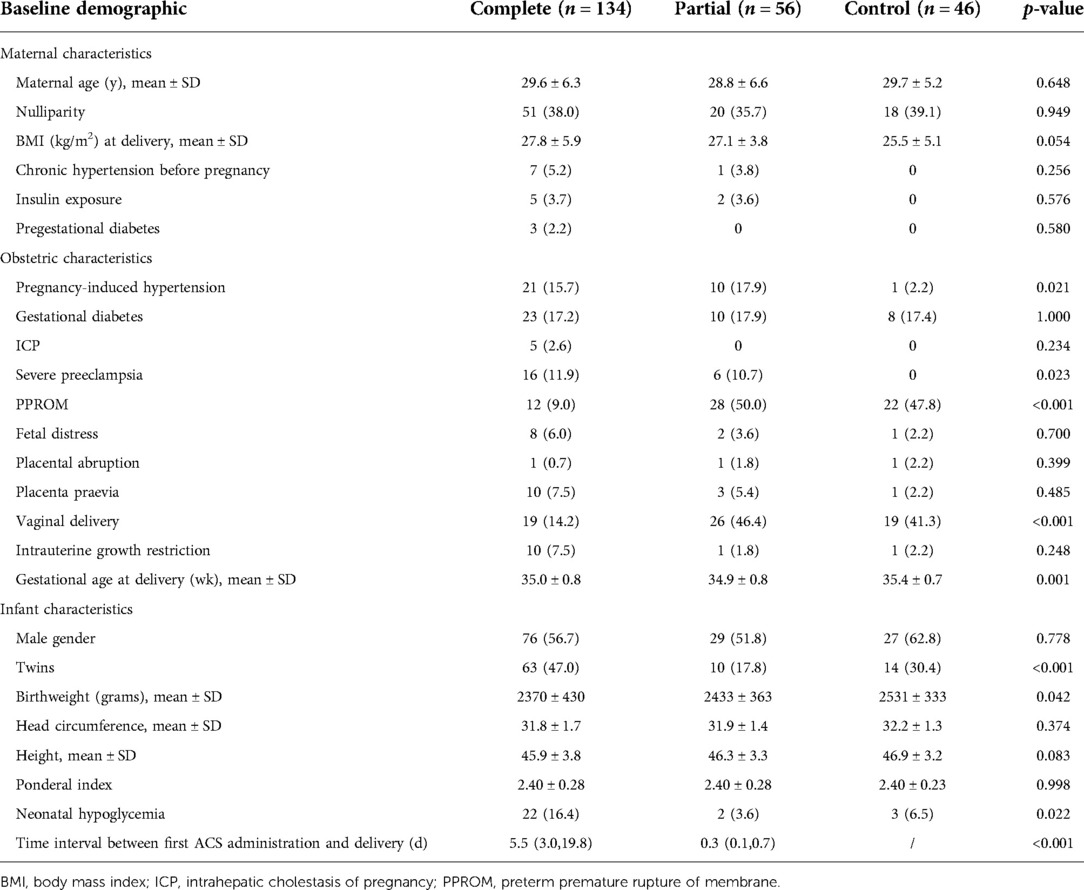
Demographic data such as the mother's age, mode of delivery, pregnancy history, delivery history, body mass index at delivery, pre-pregnancy history, current pregnancy history, cause of premature birth, neonatal gender, birth weight, head circumference, height, number of fetuses, gestational age at birth, neonatal blood glucose levels and glucose and calorie intake were obtained from the hospital's electronic medical record system.
The application method of ACS in obstetrics of our hospital is as follows: in the study,women with <34 gestational weeks and a risk of preterm birth within 1 week received one course of ACS treatment. Pregnant women with a tendency toward delivery via cesarean section and those with premature rupture of membranes or other conditions that might increase fetal risk were also treated with ACS even if the gestational age was >34 weeks (8–12).
The neonates were divided into three groups as follows: the complete course, partial course, and control groups. The complete course of ACS included four 6-mg doses of intramuscular dexamethasone every 12 h, and the partial course included one to three 6-mg doses. The mothers of the neonates in the control group were not exposed to dexamethasone before delivery,because some mothers did not meet the above indications for ACS medication, while some mothers did not have enough time to take medication during emergency delivery. Primary outcome was the neonatal blood glucose levels within the first 48 h of life. Baseline data and blood glucose levels were compared between the three groups.
Neonatal hypoglycemia was defined as a total blood glucose level <2.2 mmol/L (13). Blood glucose levels were measured using a FreeStyle Optium Neo H blood glucose monitor and test paper (Abbott Pharmaceutical Co., Ltd., USA). Each neonate was regularly monitored following admission. Blood glucose levels were screened within 1 h of birth and 2, 4, 12, 24, 36, and 48 h after birth.
Statistical analyses
A one-way analysis of variance or Mann-Whitney U-test was used to analyze the continuous variable data in the baseline data, and the chi-square test or Fisher's exact test was used to analyze the classified variable data (14). The differences in blood glucose levels between the complete course, partial course, and control groups were analyzed using a one-way analysis of variance. Post-hoc tests were performed between groups. All statistical data were analyzed using the IBM SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). A P value < 0.05 on both sides indicated a significant difference.
Results
Of the remaining 236 eligible preterm infants, 134 (56.8%) were exposed to a complete course of ACS, 56 (23.7%) had a partial course of ACS, and 46 (19.5%) had no exposure to ACS (Table 1).
Baseline characteristics
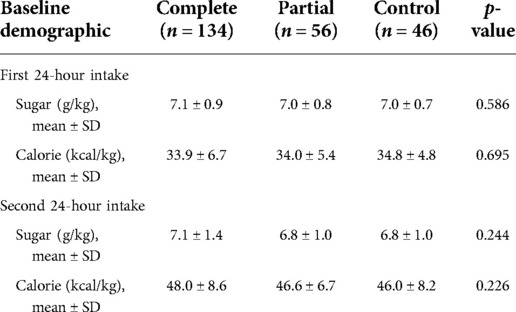
A comparison of the baseline characteristics of the three groups of preterm infants is presented in Table 1 Maternal characteristics differed minimally between the groups, with similar maternal age, body mass index at delivery, and medical history prior to pregnancy, followed by similar incidences of gestational diabetes mellitus, fetal distress, placental abruption, placenta previa, and intrauterine growth restriction.Compared with the control group, newborns exposed to ACS had lower birth weight (p = 0.042). Women who were treated with dexamethasone were more likely to have pregnancy-induced hypertension (15.7.0% and 17.9% vs. 2.2%, p = 0.021), severe preeclampsia (p = 0.023), and earlier gestational duration at delivery (35.0 ± 0.8 weeks and 34.9 ± 0.8 weeks vs. 35.4 ± 0.7 weeks). The complete course group was less likely to have preterm premature rupture of membrane (9% vs. 50.0% and 47.8%, p < 0.001) and vaginal delivery (14.2% vs. 46.4% and 41.3%, p < 0.001). In the complete course group, the incidence of neonatal hypoglycemia was the highest (16.4% vs. 3.6% and 6.5%). Hypoglycemia occurred within 2 h of birth in this study. Table 2 shows that there were no significant differences among the three groups in the intake of sugar and calories during the first 48 h after birth.
Comparison of blood glucose levels among all groups
The mean and standard deviation of the glucose levels are presented in Figure 2. Here, the patients exposed to the complete course of ACS had significantly lower blood glucose levels 12 h after birth than the control group (p < 0.001). All the groups had low blood glucose levels at birth, which then continuously increased and peaked at 4 h after birth. After 4 h, the blood glucose levels started to decline. The blood glucose curve of the complete course group rebounded earlier than those of the other two groups at 12 h after birth and then stabilized. The blood glucose levels of the partial course and control groups continued to decrease 12 h after birth; while the blood glucose curve of the control group became stable 24 h after birth, that of the partial course group entered an increasing phase.
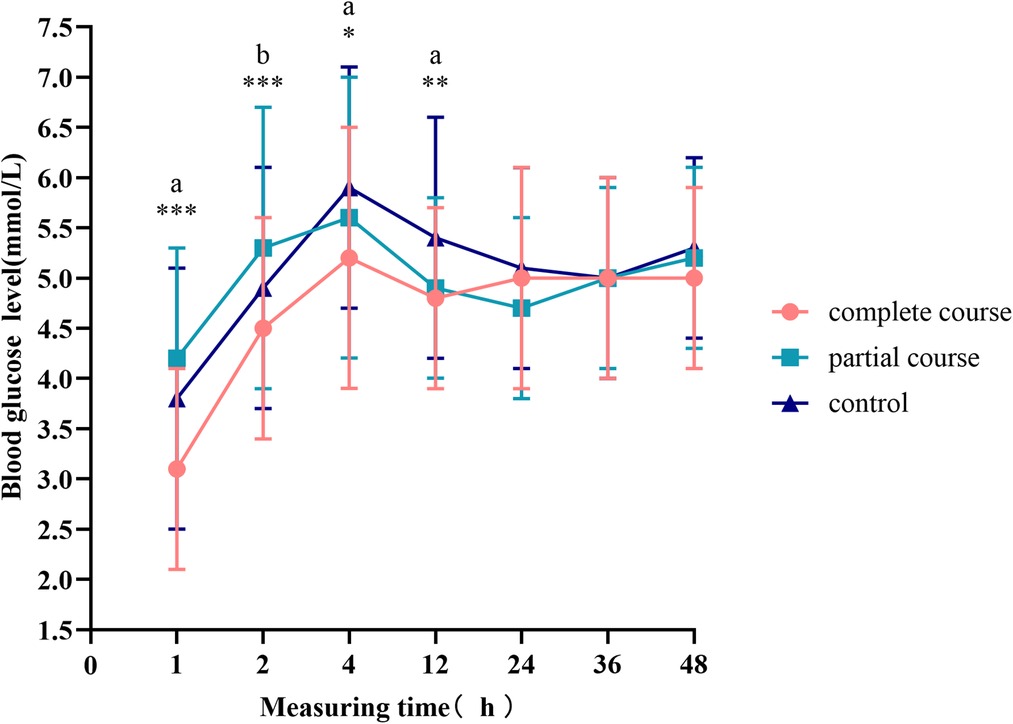
Figure 2. Blood glucose levels at different time points. The curves represent the mean and standard deviation of blood glucose at a specific time point. Comparison of blood glucose levels among all groups, *p < 0.05, **p < 0.01, ***p < 0.001; ap < 0.0167 for the comparison of the complete course or partial course group with the control group. bp < 0.0167 for the comparison of the complete course group with the partial group.
The complete course group had the highest proportion of twins in the baseline data. Compared with the control group, the proportion of pregnancy-induced hypertension syndrome in the complete course and partial course groups was higher. Binary logistic regression analysis showed that Pregnancyinduced hypertension, twins and gender had influence on neonatal hypoglycemia (Table 3). However, significant differences were noted as we excluded twins and neonates of mothers with pregnancy-induced hypertension from the general population. The blood glucose fluctuation was similar to that in Figure 2. Within 4 ∼ 24 h, the blood sugar in the control group was the highest. After 24 h, the blood glucose levels of all the groups tended to stabilize.

Table 3. The effect of pregnancy-induced hypertension, twins, sex and ACS administration on the risk of hypoglycemia.
The t-test demonstrated a difference in blood glucose levels between male and female infants; therefore, we divided the total population into male and female groups, performed statistics separately, and plotted the blood glucose fluctuation curves in Figures 3, 4. The blood glucose levels were more significantly different among male infants, with the fluctuation curve consistent with combined male and female population. The difference in the blood glucose levels among the three groups of female infants was reduced, and only the blood glucose levels of the complete course group and partial course group demonstrated statistical significance, within 1 and 2 h of birth, respectively. Meanwhile, the blood glucose levels of the complete course group were higher than those of the partial course group, and those of the three groups were close at 4 h after birth. Since then, the differences in the blood glucose levels among the three groups were not significant; however, the blood glucose curves of the female infants remained close to the overall trend.
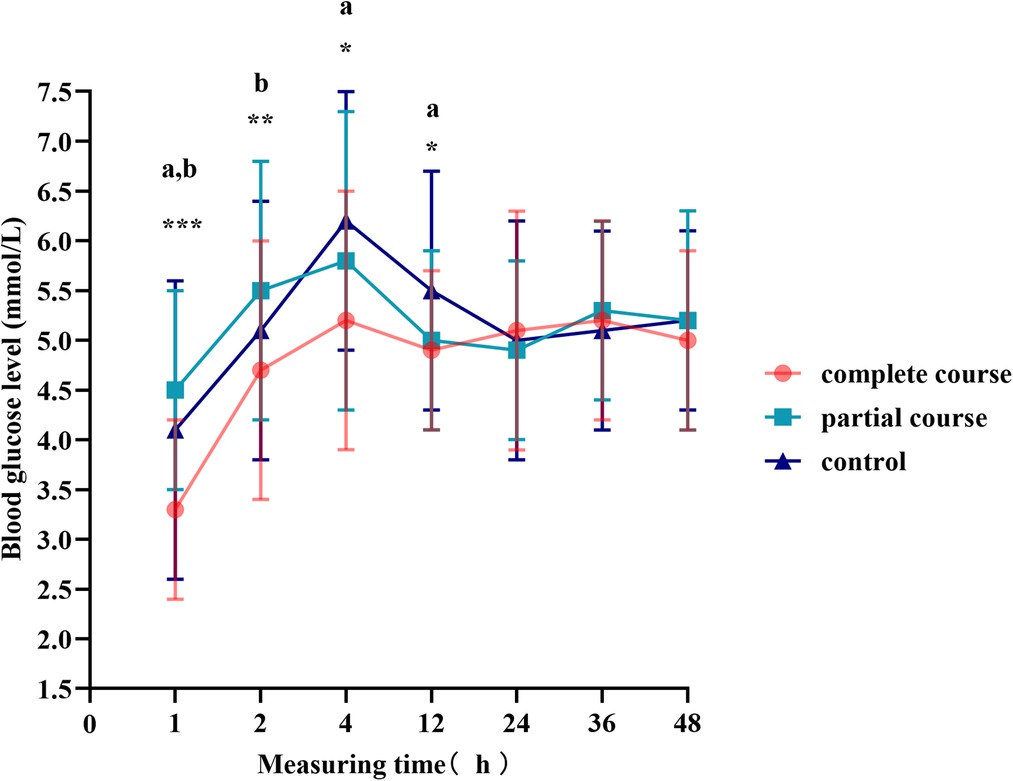
Figure 3. Blood glucose levels of male infants at different time points. Comparison of blood glucose levels of male infants among all groups, *p < 0.05, **p < 0.01, ***p < 0.001; ap < 0.0167 for the comparison of the complete or partial course group with the control group; bp < 0.0167 for the comparison of the complete course group with partial group.
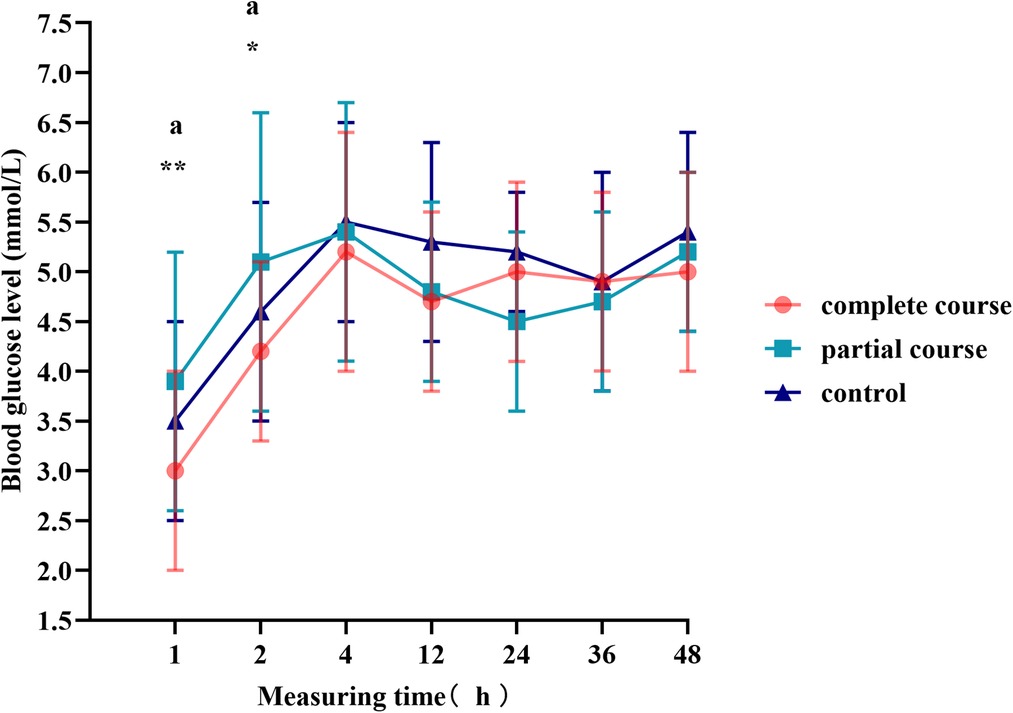
Figure 4. Blood glucose levels of female infants at different time points. Comparison of blood glucose levels of female infants among all groups, *p < 0.05, **p < 0.01, ***p < 0.001; ap < 0.0167 for the comparison of the partial course group with the control group.
Discussion
Main findings
The blood glucose levels in late-preterm neonates may decrease, and the probability of neonatal hypoglycemia may increase after ACS administration in mothers, especially after exposure to a complete course of treatment. In the neonates, the effects are more pronounced in the first 12 h of life and vary according to sex. Male infants tend to be more severely affected, although the effects on blood glucose levels are not significant 24 h after birth.
Clinical significance
At present, several studies on the benefits of ACS application have been reported in the literature. A prospective cohort study of 298 participants in Eastern India in 2021 had revealed that a complete course of ACS reduced the incidence of neonatal hypoglycemia (15). They reported the incidence of hypoglycemia at 12 and 72 h after birth was significantly lower in the complete course group. The mothers of the control group were not given antenatal dexamethasone treatment. This may be because glucose homeostasis is directly affected by dexamethasone, especially in infants born shortly after ACS treatment. We have different results from theirs. However, another retrospective cohort study by Uquillas et al. revealed a greater risk of hypoglycemia in newborns delivered by mothers receiving ACS (16). Mothers of newborns hospitalized with hypoglycemia in the NICU had a higher incidence of ACS (odds ratio adj 4.71, p adj = 0.01). Our study results are consistent with those of Uquillas et al. Here, the blood glucose levels of neonates with exposure to a complete course of ACS remained the lowest within 12 h, while those of neonates without ACS exposure remained peaked during the period of 4–12 h.
Interpretation
In our study, the incidence of hypoglycemia was higher in neonates exposed to ACS than in neonates without exposure to ACS and was relatively lower compared with those in some other antenatal betamethasone studies (4, 17). The pathophysiological mechanism of ACS-related neonatal hypoglycemia has not yet been fully elucidated. In the first 2 h, the blood glucose levels of the partial course groups were the highest, and the group with the highest glucose levels after 2 h was the control group. According to some experiments, muscle injection of dexamethasone reached peak plasma concentration at 6 h, and the median time to return to baseline blood glucose levels after dexamethasone treatment was 33.1 h (18). Hyperglycemia begins at approximately 3 h with the long-acting glucocorticoid dexamethasone, peaks at approximately 9–10 h, and resolves 48 h after discontinuation (19). We speculated that the fluctuation in blood glucose levels was related to the time required for maternal blood glucose and insulin levels to peak after antenatal dexamethasone application, time to delivery after dexamethasone application, and effect of maternal hyperglycemia on neonatal insulin levels (4, 13, 18, 20). After umbilical cord ligation, the glucose and dexamethasone transmitted by the umbilical cord were immediately terminated, and the neonates left the dexamethasone and maternal hyperglycemic environment and entered self-regulation. The stress state of delivery and postnatal energy through oral and intravenous administration may cause an increase in postnatal blood glucose.
Research implications
It was generally believed that long-term and severe hypoglycemia might affect the neonatal nervous system. Neonatal hypoglycemia may lead to seizures and may progress to coma, death, or severe neurodevelopmental abnormalities. These findings can be observed in patients with severe and persistent hypoglycemia (21). A systematic review has revealed that neonatal hypoglycemia is associated with a 2- to 3-fold increased risk of specific cognitive deficits in early childhood, including executive dysfunction and visual–motor dysfunction (22). A randomized clinical trial by Taygen Edwards et al. reported that compared with patients without hypoglycemia at birth, those children who experienced hypoglycemia in the early life had a significantly higher incidence rate of neurosensory impairment at the age of 2 years (23% vs. 18%) (23). In recent years, studies have found that transient asymptomatic hypoglycemia can also cause neurological sequelae. McKinlay et al. showed that children with a history of hypoglycemia within 12 h of birth were at a greater risk for combined neurosensory impairment (24). Kaiser Jr et al. examined the association of hypoglycemia within 3 h of birth with poor academic performance, proposing that early transient hypoglycemia may correlate with poor academic performance at 10 years (25). In our study, although hypoglycemia caused by antenatal dexamethasone treatment was mostly transient, it might carry the potential risks mentioned above. Therefore, early detection and management of neonatal hypoglycemia are important. In addition, transient neonatal hypoglycemia has been associated with post-natal hyperinsulinemia (26). By studying the fluctuation of blood glucose, we can indirectly reveal changes in insulin to some extent. The blood glucose levels of all three groups entered the steady state at 24 h, and no statistically significant difference was observed, indicating that blood glucose regulation was completed within 24 h as well as that of insulin. In subsequent studies, neonatal insulin levels may be analyzed to verify the effects of prenatal corticosteroids on neonatal insulin levels.
Strengths and limitations
To the best of our knowledge, this is the first study to examine the effects of ACS on blood glucose fluctuation in preterm infants in China as some studies only focused on abnormal outcomes and did not consider the effects of the blood glucose fluctuation curve. In this study, the blood glucose levels of new infants were regularly monitored, and the curve of blood glucose fluctuation was observed, including the differences between male and female infants. This can provide a reference for future clinical studies.
Nevertheless, this study had some limitations. First, it was limited to 48-h blood glucose monitoring without long-term monitoring and was not representative of long-term follow-up results. Second, only changes in blood glucose levels were observed, and no changes in blood glucose-regulating hormones, such as insulin, were observed. Third, the measurement specimen used was peripheral blood from the capillaries as collecting venous blood is difficult. Considering ethical issues, the blood glucose levels were measured with peripheral blood, and many studies have reported that the difference between peripheral blood glucose and venous blood glucose is insignificant and does not affect the blood glucose curve (27–30).
Conclusion
Antenatal dexamethasone treatment for mothers may have a temporary negative effect on neonatal blood glucose levels in late-preterm infants within 24 h of birth. Therefore, regular glucose monitoring is recommended for late-preterm infants with a history of ACS exposure, especially for the first 24 h after birth.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Enze Hospital, Taizhou Enze. Medical Center (Group) (K20220502). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
CZ designed the research. CZ and WZ drafted the first draft and reviewed and modified it. CZ, WZ and LW collected the data. MZ and TT provided guidance for data statistics and design ideas. LW and LW conducted strict review and modification on the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research received funding support from Basic public welfare research program of Zhejiang Province (LGF21H040006) and Science and Technology Plan Project of Taizhou (1802ky22).
Acknowledgments
The authors would like to acknowledge Basic public welfare research program of Zhejiang Province (LGF21H040006) and Science and Technology Plan Project of Taizhou (1802ky22), and also the staff working at the maternal and neonatal units in Enze Hospital, Taizhou Enze Medical Center (Group) including Meiling Ye, Lu Li, Dan Lin and Shuyao Lei for all their assistance in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACS, antenatal corticosteroids.
References
1. Deng K, Liang J, Mu Y, Liu Z, Wang Y, Li M, et al. Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Glob Health. (2021) 9(9):e1226–41. doi: 10.1016/S2214-109X(21)00298-9
2. Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7(1):e37–46. doi: 10.1016/S2214-109X(18)30451-0
3. Zhang J, Sun K, Zhang Y. The rising preterm birth rate in China: a cause for concern. Lancet Glob Health. (2021) 9(9):e1179–80. doi: 10.1016/S2214-109X(21)00337-5
4. Gulersen M, Gyamfi-Bannerman C, Greenman M, Lenchner E, Rochelson B, Bornstein E. Time interval from late preterm antenatal corticosteroid administration to delivery and the impact on neonatal outcomes. Am J Obstet Gynecol MFM. (2021) 3(5):100426. doi: 10.1016/j.ajogmf.2021.100426
5. Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. (2016) 374:1311–20. doi: 10.1056/NEJMoa1516783
6. Aydin M, Deveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. (2015) 28:892. doi: 10.3109/14767058.2014.936002
7. Kurtoğlu S, Sarıcı D, Akın MA, Daar G, Korkmaz L, Memur Ş. Fetal adrenal suppression due to maternal corticosteroid use: case report. J Clin Res Pediatr Endocrinol. (2011) 3:160–2. doi: 10.4274/jcrpe.v3i3.31
8. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. (2016) 215(2):B13–5. doi: 10.1016/j.ajog.2016.03.013
9. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. Br Med J. (2016) 355:i5044. doi: 10.1136/bmj.i5044
10. Yasser Y, Ann EB, Gyamfi-Bannerman C. Committee opinion No. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. (2017) 130(2):e102–9. doi: 10.1097/AOG.0000000000002237
11. Haviv HR, Said J, Mol BW. The place of antenatal corticosteroids in late preterm and early term births. Semin Fetal Neonatal Med. (2019) 24(1):37–42. doi: 10.1016/j.siny.2018.10.001
12. Hamm RF, Combs CA, Aghajanian P, Friedman AM. Society for maternal-fetal medicine special statement: quality metrics for optimal timing of antenatal corticosteroid administration. Am J Obstet Gynecol. (2022) 226:B2–2B10. doi: 10.1016/j.ajog.2022.02.021
13. Zigron R, Rotem R, Erlichman I, Rottenstreich M, Rosenbloom JI, Porat S, et al. Factors associated with the development of neonatal hypoglycemia after antenatal corticosteroid administration: it's all about timing. Int J Gynaecol Obstet. (2022) 158(2):385–9. doi: 10.1002/ijgo.1397
14. Kuper SG, Baalbaki SH, Parrish MM, Jauk VC, Tita AT, Harper LM. Association between antenatal corticosteroids and neonatal hypoglycemia in indicated early preterm births(). J Matern Fetal Neonatal Med. (2018) 31(23):3095–101. doi: 10.1080/14767058.2017.1364724
15. Pal S, Sardar S, Sarkar N, Ghosh M, Chatterjee S. Effect of antenatal dexamethasone in late preterm period on neonatal hypoglycemia: a prospective cohort study from a developing country. J Trop Pediatr. (2022) 68(2):fmac021. doi: 10.1093/tropej/fmac021
16. Uquillas KR, Lee RH, Sardesai S, Chen E, Ihenacho U, Cortessis VK, et al. Neonatal hypoglycemia after initiation of late preterm antenatal corticosteroids. J Perinatol. (2020) 40(9):1339–48. doi: 10.1038/s41372-020-0589-1
17. Gyamfi-Bannerman C, Jablonski KA, Blackwell SC, Tita A, Reddy UM, Jain L, et al. Evaluation of hypoglycemia in neonates of women at risk for late preterm delivery: an antenatal late preterm steroids trial cohort study. Am J Perinatol. (2021) :10.1055/s-0041-1729561. doi: 10.1055/s-0041-1729561
18. Jobe AH, Milad MA, Peppard T, Jusko WJ. Pharmacokinetics and pharmacodynamics of intramuscular and oral betamethasone and dexamethasone in reproductive age women in India. Clin Transl Sci. (2020) 13(2):391–9. doi: 10.1111/cts.12724
19. Zhang F, Karam JG. Glycemic profile of intravenous dexamethasone-induced hyperglycemia using continuous glucose monitoring. Am J Case Rep. (2021) 22:e930733. doi: 10.12659/AJCR.930733
20. Vaughan OR, Fisher HM, Dionelis KN, Jeffreys EC, Higgins JS, Musial B, et al. Corticosterone alters materno-fetal glucose partitioning and insulin signalling in pregnant mice. J Physiol. (2015) 593(5):1307–21. doi: 10.1113/jphysiol.2014.287177
21. Thompson-Branch A, Havranek T. Neonatal hypoglycemia. Pediatr Rev. (2017) 38(4):147–57. doi: 10.1542/pir.2016-0063
22. Shah R, Harding J, Brown J, McKinlay C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology. (2019) 115(2):116–26. doi: 10.1159/000492859
23. Edwards T, Alsweiler JM, Gamble GD, Griffith R, Lin L, McKinlay C, et al. Neurocognitive outcomes at age 2 years after neonatal hypoglycemia in a cohort of participants from the hPOD randomized trial. JAMA Netw Open. (2022) 5:e2235989. doi: 10.1001/jamanetworkopen.2022.35989
24. McKinlay C, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. (2017) 171:972–83. doi: 10.1001/jamapediatrics.2017.1579
25. Kaiser JR, Bai S, Gibson N, Holland G, Lin TM, Swearingen CJ, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: a population-based study. JAMA Pediatr. (2015) 169:913–21. doi: 10.1001/jamapediatrics.2015.1631
26. Stanescu DL, Stanley CA. Advances in understanding the mechanism of transitional neonatal hypoglycemia and implications for management. Clin Perinatol. (2022) 49(1):55–72. doi: 10.1016/j.clp.2021.11.007
27. Lee I, Lunt H, Chan H, Heenan H, Berkeley J, Frampton CM. Postprandial capillary-venous glucose gradient in type 1 diabetes: magnitude and clinical associations in a real world setting. Diabet Med. (2016) 33(7):998–1003. doi: 10.1111/dme.13025
28. Coetzee A, van de Vyver M, Hoffmann M, Hall DR, Mason D, Conradie M. A comparison between point-of-care testing and venous glucose determination for the diagnosis of diabetes mellitus 6-12 weeks after gestational diabetes. Diabet Med. (2019) 36(5):591–9. doi: 10.1111/dme.13903
29. Nevander S, Landberg E, Blomberg M, Ekman B, Lilliecreutz C. Comparison of venous and capillary sampling in oral glucose testing for the diagnosis of gestational diabetes mellitus: a diagnostic accuracy cross-sectional study using accu-chek inform II. Diagnostics (Basel). (2020) 10(12):1011. doi: 10.3390/diagnostics10121011
Keywords: late preterm, dexamethasone, gestational age, neonate, hypoglycemia
Citation: Zhou C, Zheng W, Zhang M, Tung T, Wang L and Wang L (2022) Effects of antenatal corticosteroids on neonatal blood glucose fluctuation in late-preterm infants. Front. Pediatr. 10:1036565. doi: 10.3389/fped.2022.1036565
Received: 4 September 2022; Accepted: 25 October 2022;
Published: 11 November 2022.
Edited by:
Daniele Trevisanuto, University Hospital of Padua, ItalyReviewed by:
Yair Kasirer, Shaare Zedek Medical Center, IsraelVarsha Bhatt-Mehta, Food & Drug Administration of Iran, Iran
© 2022 Zhou, Zheng, Zhang, Tung, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhen Wang d2FuZ2x6QGVuemVtZWQuY29t Linghua Wang d2FuZ2xoNjAxOUBlbnplbWVkLmNvbQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Cailing Zhou
Cailing Zhou Wanli Zheng
Wanli Zheng