
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 24 October 2022
Sec. Pediatric Hematology and Hematological Malignancies
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1034373
This article is part of the Research Topic Immunotherapy for Hematological Malignancies in Children View all 5 articles
A correction has been applied to this article in:
Corrigendum: Blinatumomab for treating pediatric B-lineage acute lymphoblastic leukemia: A retrospective real-world study
 Ying Wu1,†
Ying Wu1,† Yanming Li2,†
Yanming Li2,† Jia Fan1
Jia Fan1 Peijing Qi1
Peijing Qi1 Wei Lin1
Wei Lin1 Jie Yang1
Jie Yang1 Huiqing Liu1
Huiqing Liu1 Xiaoling Wang2
Xiaoling Wang2 Huyong Zheng1
Huyong Zheng1 Tianyou Wang1*
Tianyou Wang1* Ruidong Zhang1*
Ruidong Zhang1*
Objectives: Blinatumomab was shown to be safe and effective for consolidation therapy in B-cell acute lymphoblastic leukemia (B-ALL). This study aimed to investigate the effectiveness and safety of blinatumomab in pediatric B-ALL patients in a real-world setting.
Methods: This was a retrospective, observational study that included patients who initiated blinatumomab treatment between October 1, 2020 and June 20, 2022. Patients with B-ALL diagnosis, age below 18 years, and at least one blinatumomab treatment cycle were included. Treatment-related toxicities were assessed.
Result: Totally 23 pediatric patients were included in this study, with a median age of 6 years (range, 2 to 11 years). Blinatumomab therapy was applied for MRD-positive (disease ≥0.01%, n = 3) or chemotherapy-ineligible (n = 20) B-ALL cases. The median follow-up time was 9 months, and all evaluable patients achieved complete molecular remission with undetectable MRD. Four relapsed B-ALL cases proceeded to hematopoietic stem cell transplantation (HSCT) without further bridging therapy, while the others underwent maintenance chemotherapy after blinatumomab treatment. Grade ≥3 febrile neutropenia, white blood cell decrease and seizure were observed in 57%, 48% and 4.3% of patients, respectively. One case discontinued therapy due to neurologic toxicities. Elevated cytokine levels were observed in 4 patients. In all 23 patients, increased T-cell and low B-cell counts (<10/μl) were detected during blinatumomab therapy.
Conclusion: These encouraging results suggest blinatumomab in pediatric B-ALL patients with MRD+ or chemotherapy-related toxicities is effective and safe in the short run, although long-term follow-up is still needed.
B-lineage acute lymphoblastic leukemia (B-ALL) is a subtype of acute lymphoblastic leukemia, which is the most prevalent cancer among children and adolescents, with a 5-year overall survival (OS) rate currently approaching 90% (1–3). Despite significant improvements in the efficacy of multi-agent chemotherapy regimens, there are still about 15% of subsequent relapse cases and 10% of disease-related deaths, indicating poor survival and low quality of life (4, 5). Moreover, chemotherapy-related side effects occur, causing a major burden on patients and families (6). Thus, better treatment strategies with higher efficacy and lower toxicity are still needed.
In recent years, the emergence of monoclonal antibodies to surface antigens has represented an important advancement in ALL therapy (7, 8). Blinatumomab is a bispecific T-cell engager (BiTE) targeted to CD3 and CD19, with promising clinical efficacy as a monotherapy for eradicating persistent MRD in B-ALL (9). In a phase I/II, open-label trial, 27 (39%; 95% CI, 27% to 51%) patients achieved complete remission (CR) within the first two cycles, 14 (52%) of whom achieved complete minimal residual disease response (10). In a prospective, multicenter phase III trial, 108 patients with R/R B-ALL (<18 years) were randomly assigned to the blinatumomab or standard chemotherapy group (11). After a median follow-up of 31 months, overall survival (OS) was higher in the blinatumomab group compared with the chemotherapy group (83% vs. 60%, 95% CI: 0.15–0.72, p = 0.003) (11, 12). In another study of 208 B-ALL patients with high- and intermediate-risk first relapse receiving blinatumomab as post-reinduction consolidation prior to HSCT, Brown et al. found two-year OS rates of 71.3% in the blinatumomab group and 58.4% in the chemotherapy group (95% CI, 0.39–0.98; 1-sided p = 0.02) (13). The main adverse effects of blinatumomab are cytokine release syndrome and neurotoxicity (11, 12). Based primarily on these results, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved blinatumomab for the treatment of relapsed/refractory (R/R) B-ALL and CR with MRD ≥ 0.01% B-ALL (14). In a real-world study, Beneduce et al. retrospectively assessed the safety and efficacy of blinatumomab in 39 R/R B-ALL cases, of whom most achieved MRD negativity after blinatumomab treatment (15).
Despite these phase III trials, few reports have evaluated the real-world efficacy and safety of blinatumomab in treating relapsed or primary pediatric B-ALL. This report is a retrospective analysis of our single-center experience administering blinatumomab for the treatment of pediatric B-ALL patients.
This was a retrospective, observational study that included patients who initiated blinatumomab treatment between October 1, 2020 and June 20, 2022 in routine clinical practice at the Hematology Oncology Center of Beijing Children's Hospital, Capital Medical University, which is a unique, high-volume center integrating clinical care, scientific research, teaching, and training (16). Patients with B-ALL diagnosis, age below 18 years, and treatment with blinatumomab were included. This study was approved by the ethics committee of Beijing Children's Hospital ([2022]-E-090-R). The requirement for informed consent was waived by the committee because of the retrospective nature of the current study, which was performed in accordance with the Declaration of Helsinki.
Clinical records were reviewed to extract data, including baseline demographics, ALL-relevant clinical history, frontline therapy, lymphocyte subsets, peripheral cytokine levels and treatment patterns for blinatumomab.
This retrospective observational study evaluated complete MRD response, and the incidence and severity of adverse events (AEs). Hematologic and MRD assessments were performed of bone marrow aspirates and core biopsies obtained at screening, at the end of infusion on day 28 of each cycle. MRD was measured based on next-generation sequencing (NGS, threshold 10−6/0.0001%). Complete MRD response was defined as no detectable signal for leukemic cells by NGS. Adverse drug reactions were recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 proposed by the National Cancer Institute. Peripheral lymphocyte subsets were evaluated by FACS analyses. Peripheral blood levels of cytokines, including IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12p70, IL-17, tumor necrosis factor α (TNF-α) and interferon gamma γ (IFN-γ), were assessed by FACS-based analyses with a limit of quantitation of 2.44 pg/ml. Response, relapse, and genetic risk categories were defined according to the National Comprehensive Cancer Network (NCCN) guidelines: Pediatric Acute Lymphoblastic Leukemia (17). All data were collected from electronic medical records by two reviewers to decrease inconsistency.
Demographic and disease characteristics were assessed by descriptive statistics. Categorical variables were expressed as number and percentage (%). Continuous variables were expressed as mean/median and standard deviation (SD). SPSS statistics version 25.0 and GraphPad Prism version 9.0 were used for all statistical analyses.
From October 1, 2020 to June 20, 2022, a total of 23 patients were included in this study. Median age at the initiation of blinatumomab treatment was 6 years (range, 2 to 11 years). Most patients were male (70%). The baseline characteristics of the 23 patients are shown in Table 1. The enrolled patients were assigned to the MRD+ or chemotherapy-ineligible group. Of all patients, 20 (87%) with chemotherapy-associated toxicity precluding standard chemotherapy constituted the chemotherapy-ineligible group, while the remaining with MRD >0.01% constituted the MRD+ group. Patients in the chemotherapy-ineligible group had consolidation-associated toxicities, including pancreatitis (12/20), anal mucositis (3/20), cerebral venous thrombosis (1/20), septic shock (1/20), infection (1/20), liver damage (1/20), and hyperlipidemia (1/20). Most of these adverse reactions were caused by peg-asparaginase and high-dose methotrexate during consolidation therapy as reported in CCLG previously. Blinatumomab was administered to all chemotherapy-ineligible patients at the time of recovery from overwhelming toxicity.
Eight subjects had high-risk genetic abnormalities, including 1 MLL arrangement, 4 IKZF1 deletion, and 3 Philadelphia chromosome-like cases. Totally 19 (83%) and 4 (17%) patients were treated in the China Children's Leukemia Group (CCLG)-2018-preB-ALL (ChiCTR1800018935) and CCLG-2008 trials, respectively.
A cycle of blinatumomab treatment was 28 days (4 weeks) of continuous intravenous infusion followed by a 14-day (2 weeks) period without medication. Four of all patients received 2 blinatumomab cycles, and 19 underwent one cycle with one stopping blinatumomab therapy on the second day because of serious seizure. Patient 1 received a stepwise dose escalation from 5 to 15 µg/m2 per day after reinduction, while other patients received the full dosage (15 µg/m2) from day 1. For the prevention of severe cytokine release syndrome (CRS) and neurologic events, patients with MRD+ were treated with oral dexamethasone (5 mg/m2) for three days during the pretreatment period of blinatumomab.
Totally 22/23 patients were evaluated for efficacy outcome after blinatumomab therapy. Patient 20 did not complete one course of treatment due to neurotoxicity (Table 2). Hematologic CR had been achieved in all 23 patients prior to the first dose of blinatumomab. NGS-based MRD was detected at 2.33%, 1.04% and 0.28% in patients 2, 12, and 16, respectively, before blinatumomab therapy. The complete MRD response rate in MRD+ group was 100%, with 2 (66.7%) patients achieving undetectable MRD during the first cycle and 1 (33.3%) achieving after two cycles. Persistently undetectable MRD after blinatumomab treatment was found in chemotherapy-ineligible patients. Standard maintenance therapy was performed in these individuals following blinatumomab therapy. The mean follow-up time was 9 months, and 34.8% of all patients were followed up for more than 10 months (Figure 1). Four relapsed B-ALL cases proceeded to HSCT after blinatumomab therapy without lymphodepletion. The patients in MRD + group proceeded to maintenance therapy without HSCT due to the fear of their parents and other factors. Totally 20 patients with chemotherapy-associated toxicity recovered and bridged to maintenance therapy in the CCLG-2018-preB-ALL protocol (Figure 2).
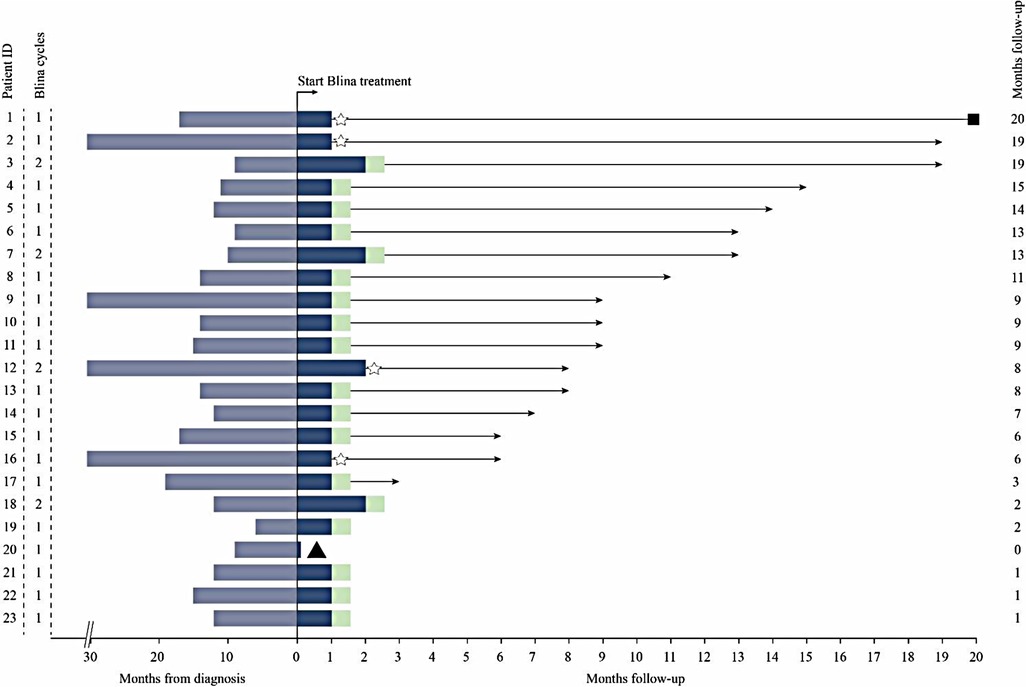
Figure 1. Clinical course of patients treated with blinatumomab (blina). Blue Shading, Time From diagnosis; Light Green Shading, bridge to maintenance therapy; Star, bridge to HSCT; Triangle, discontinuation of blinatumomab due to toxicity; Square, death.
Totally 22 out of 23 pediatric patients experienced at least one AE during blinatumomab therapy, regardless of cause. A total of 107 AEs were reported, including 58.9% of grade 1–2 and 42.0% of grade 3–4 (Table 3). The rates of grade 3–4 adverse events included febrile neutropenia (29%), white blood cell decrease (30%), seizure (2%), hepatotoxicity (2%) and fever (2%). None of the patients showed unexpected serious adverse events (SAEs). Sinus tachycardia requiring no medication was developed in eight patients. Five patients developed rash maculo-papular mainly on upper trunk within two weeks of blinatumomab initiation. Two patients showed increased liver enzyme levels (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) after infusion. Case 22 in the chemotherapy-ineligible group had liver damage, with elevated ALT and AST levels before blinatumomab treatment. Following blinatumomab treatment, glutathione was given as supportive therapy. No subsequent increase in aminotransferase occurred; ALT returned to normal level, and AST returned 1.5-fold to baseline level. Vomiting, diarrhea, abdominal pain, hypercalcemia, PICC-related venous thrombosis, and anal mucositis were observed once, respectively.
Among the 23 patients, CRS and immune effector cell-associated neurotoxicity syndrome (ICANS) occurred within the first week after administration. CRS was observed in 3 patients (10.5%), with fever and substantial elevations in laboratory parameters such as IL5, IL6, IL10 and IFN-γ. The degree of CRS was mild, and the disorder was spontaneously resolved without medication. Two of the patients experienced ICANS, including tremor (grade 1) and seizure (grade 3). Patient 20 discontinued blinatumomab after days in the first cycle due to ICANS.
Totally 20 assessable patients in the chemotherapy-ineligible group with low B-cell count (<10/μl) at baseline and after blinatumomab therapy. A slight increase in T-cells was observed in peripheral blood samples from 8 patients (Figure 3A). The median count of CD3+ T cells was 0.725 × 109 /L at baseline vs. 1.32 × 109 /L at the end of blinatumomab treatment (Supplementary Table S1). In all 8 assessable patients, the absolute CD4+ and CD8+ T cell counts increased during blinatumomab treatment (Figures 3B,C). Regulatory T cells (Tregs) were analyzed in 7 out of the 23 B-ALL patients. The absolute counts of Tregs slightly increased from a median of 7.59 at baseline to 10.06 on Day 27 (p = 0.128). The concentrations of cytokines were evaluated in all 20 patients. High levels of cytokines were observed in 4 patients (Figures 3, 4). The maximum cytokine levels were detected for IL-6 (1256.92 pg/ml) on day 7, followed by IL-5 (805.24 pg/ml) on day 1, IFN-γ (546.29 pg/ml) on day 11 and IL-10 (283.89 pg/ml) on day 1.
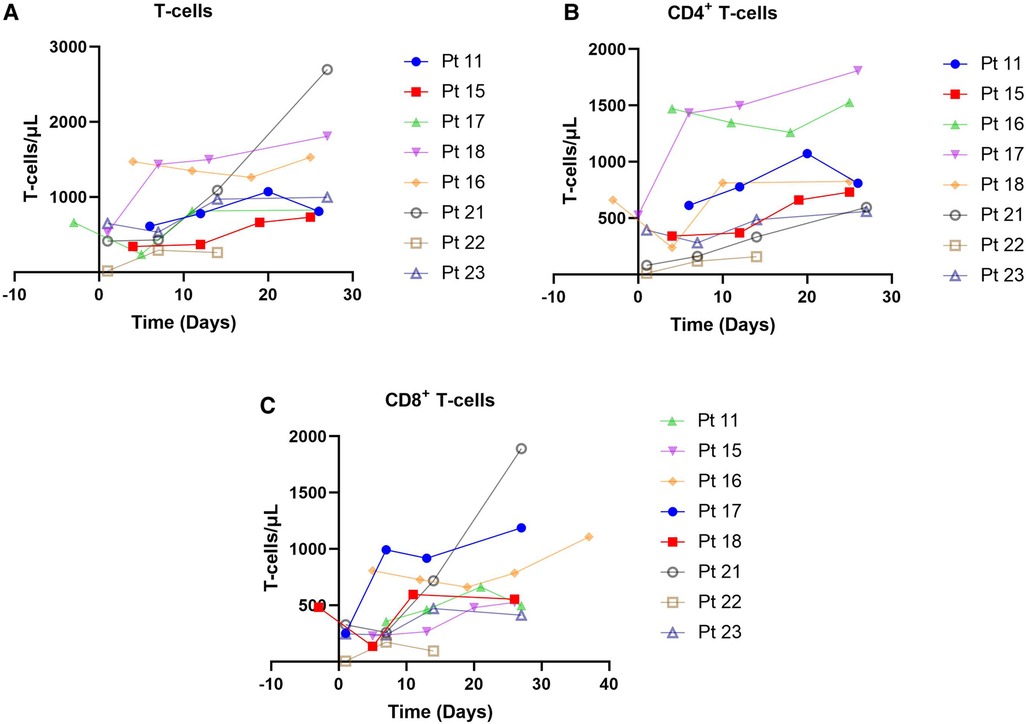
Figure 3. T-cells observed in 8 patients. (A) CD8 + T-cells, chemotherapy-eligible, 7-month follow-up. (B) CD8 + T-cells, chemotherapy-eligible, 2-month follow-up. (C) CD3 + T-cells, chemotherapy-eligible, with 1-month follow-up.
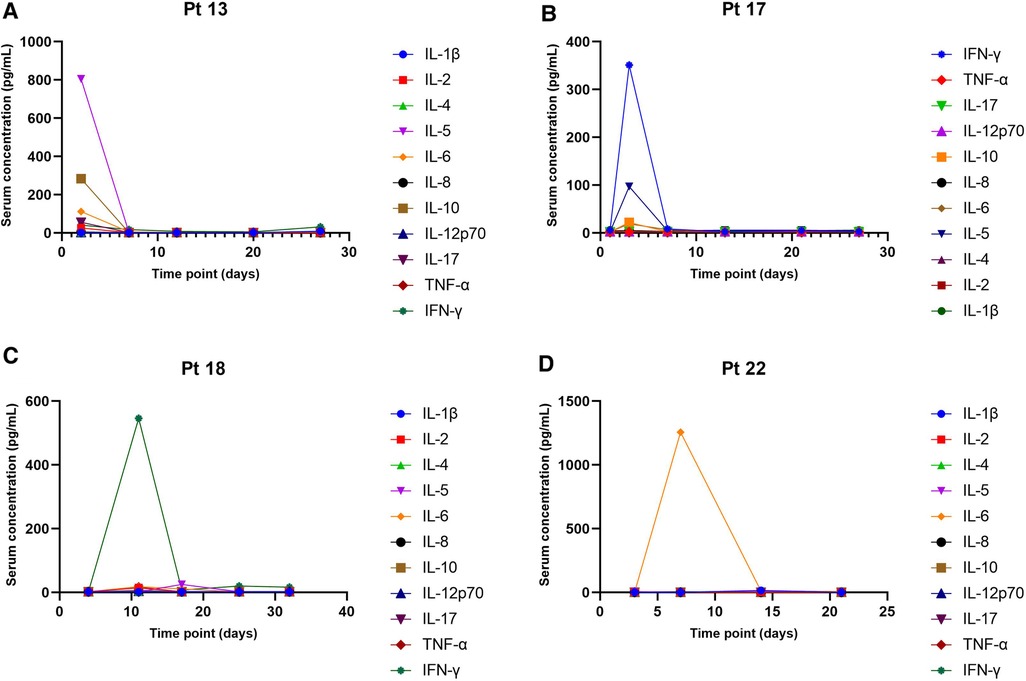
Figure 4. Serum concentrations of cytokines. (A) Patient 13, chemotherapy-eligible, 7-month follow-up. (B) Patient 17, chemotherapy-eligible, 2-month follow-up. (C) Patient 18, chemotherapy-eligible, with 1 month of follow-up. (D) Patient 22, chemotherapy-eligible, with 1 month of follow-up.
In recent years, blinatumomab has been proven to be safe and feasible in clinical trials after approval by authorities in real-world studies among R/R B-ALL (13, 11, 18). The successful experiences in R/R B-ALL has led to an increasingly use of earlier therapy for B-ALL (19, 10). However, blinatumomab for patients with severe chemotherapy-associated toxicities has not been robustly researched. To our knowledge, there are few real-world studies that have assessed the outcomes of blinatumomab in pediatric patients with intensive chemotherapy-associated toxicity (20, 21). In the present study, we evaluated the safety, efficacy, B and T cell responses, and cytokine release of blinatumomab for 23 children with B-ALL based on a large sample size.
In this study, the aim of blinatumomab therapy for B-ALL patients was to supply a “bridge” to HSCT or further therapy, which is required to achieve long-term survival. The outcome here supports findings by previous studies suggesting that such treatment is feasible and safe in pediatric B-lineage ALL with either MRD+ or various complications, including sepsis, pancreatitis, oral mucositis and others. Fewer CRS and neurotoxicity occurred, possibly associated with low tumor load (22). Seizure in one patient was recorded during the first day after the start of blinatumomab infusion.
Probably due to prior chemotherapy, all assessable patients in this study had no or barely detectable B cells in peripheral blood at baseline (23). The observation of low B-cell count at the end of treatment suggested the high cytotoxicity of blinatumomab by depleting new B cells (24). Consistent with previous studies, there is an increase in absolute number of T cells, which most likely results from CD3-engaging bispecific antibodies. Compared with the findings reported in other articles, T-cell activation in this study was much lower (25). This may be related to the low tumor load of the included population, leading to reduced immune activation. However, no depletion of peripheral T-cells was observed at the beginning of therapy as previously reported. A possible explanation for this discrepancy may be that we did not detect peripheral lymphocytes at infusion initiation and baseline in the short duration of the present study. Tregs are known to play an important role in the efficacy of immunotherapy (25). However, the slightly elevated Treg level had no statistical significance in the peripheral blood. This may be the basis for further understanding of the immunopharmacological response of blinatumomab.
Some pediatric ALL patients are intolerant to standard chemotherapy and/or have high-risk prognostic factors, and may develop relapsed/refractory acute lymphoblastic leukemia. Blinatumomab treatment, with only temporary and transient myelosuppression, resulted in greater survival benefit than chemotherapy (26). In recent years, reports moved blinatumomab into the front line of the treatment of childhood B-ALL (27). Elitzur et al. (20) reported 11 patients administered blinatumomab who all successfully bridged to further therapy. Daniel et al. (21) presented 15 pediatric ALL cases with invasive fungal disease (IFD) treated with blinatumomab bridging therapy who received ongoing targeted treatment for ALL whilst recovering from IFD. Our study provides further evidence that blinatumomab is well tolerated in patients with severe chemotherapy-related toxicities.
Since this retrospective study was conducted in a single center, some limitations were unavoidable. The first limitation was the small sample size, even though we included all pediatric B-ALL patients administered blinatumomab in this study. Another limitation was the short-term follow-up time, which was only 9 months. Therefore, long-term follow-up is still needed.
In summary, the current results support the use of the immunotherapeutic drug blinatumomab in the short term among pediatric B-ALL patients with MRD+ or chemotherapy-related toxicities, with good efficacy and safety. As the use of blinatumomab expands, further studies are required to ascertain the long-term efficacy and toxicities of blinatumomab, to identify more details about T cell activation triggered by blinatumomab.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by This study was approved by the Institutional Review Boards of Beijing Children's Hospital ([2022]-E-090-R). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Conception and design: TW, RZ; Methodology and analysis: YW, YL, XW; Collection and assembly of data: YW, YL, JF, PQ, WL, JY, HL, HZ, RZ; Data analysis and interpretation: YW, YL, RZ; Manuscript writing: YL, YW, RZ; Final approval of manuscript: All authors contributed to the article and approved the submitted version.
The authors would like to appreciate the efforts of all those who contributed data to the study. We appreciate the support from patients, families, friends, caregivers, study staff and study investigators for their support in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1034373/full#supplementary-material.
1. Philip A, Pizzo DGP. Principles and practice of pediatric oncology. Philadelphia, USA: Wolters Kluwer Health (2015).
2. Siegel DA, Henley SJ, Li J, Pollack LA, Van Dyne EA, White A. Rates and trends of pediatric acute lymphoblastic leukemia - United States, 2001–2014. MMWR Morb Mortal Wkly Rep. (2017) 66:950–4. doi: 10.15585/mmwr.mm6636a3
3. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. (2015) 373:1541–52. doi: 10.1056/NEJMra1400972
4. Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. (2020) 136:1803–12. doi: 10.1182/blood.2019004043
5. Algeri M, Del Bufalo F, Galaverna F, Locatelli F. Current and future role of bispecific T-cell engagers in pediatric acute lymphoblastic leukemia. Expert Rev Hematol. (2018) 11:945–56. doi: 10.1080/17474086.2018.1540928
6. Winters A, Gore L. Moving immunotherapy into the front line in ALL. Hematol Am Soc Hematol Educ Program. (2019) 2019:209–17. doi: 10.1182/hematology.2019000017
7. Deak D, Pop C, Zimta AA, Jurj A, Ghiaur A, Pasca S, et al. Let's talk about BiTEs and other drugs in the real-life setting for B-cell acute lymphoblastic leukemia. Front Immunol. (2019) 10:2856. doi: 10.3389/fimmu.2019.02856
8. Hoelzer D. Novel antibody-based therapies for acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program. (2011) 2011:243–9. doi: 10.1182/asheducation-2011.1.243
9. Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Sci. (2008) 321:974–7. doi: 10.1126/science.1158545
10. von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. (2016) 34:4381–9. doi: 10.1200/JCO.2016.67.3301
11. Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse B-cell acute lymphoblastic leukemia: a randomized clinical trial. Jama. (2021) 325:843–54. doi: 10.1001/jama.2021.0987
12. Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, et al. Superior overall survival with blinatumomab versus chemotherapy as Pre-transplant consolidation treatment in children with high-risk first-relapse B-cell precursor acute lymphoblastic leukemia (B-ALL): longer follow-up (FU) of a phase 3 randomized controlled trial (RCT). Blood. (2021) 138:1231. doi: 10.1182/blood-2021-145476
13. Brown PA, Ji L, Xu X, Devidas M, Hogan LE, Borowitz MJ, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: a randomized clinical trial. Jama. (2021) 325:833–42. doi: 10.1001/jama.2021.0669
14. Jen EY, Xu Q, Schetter A, Przepiorka D, Shen YL, Roscoe D, et al. FDA Approval: blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clin Cancer Res. (2019) 25:473–7. doi: 10.1158/1078-0432.CCR-18-2337
15. Beneduce G, De Matteo A, Stellato P, Testi AM, Bertorello N, Colombini A, et al. Blinatumomab in children and adolescents with relapsed/refractory B cell precursor acute lymphoblastic leukemia: a real-life multicenter retrospective study in seven AIEOP (associazione italiana di ematologia e oncologia pediatrica) centers. Cancers (Basel). (2022) 14:426. doi: 10.3390/cancers14020426
16. Zheng H. A brief introduction to the hematology oncology center of Beijing Children's Hospital. Pediatr Investig. (2018) 2:207–8. doi: 10.1002/ped4.12105
17. Brown P, Inaba H, Annesley C, Beck J, Colace S, Dallas M, et al. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18:81–112. doi: 10.6004/jnccn.2020.0001
18. Queudeville M, Ebinger M. Blinatumomab in pediatric acute lymphoblastic leukemia— from salvage to first line therapy (A systematic review). J Clin Med. (2021) 10:2544. doi: 10.3390/jcm10122544
19. Queudeville M, Schlegel P, Heinz AT, Lenz T, Döring M, Holzer U, et al. Blinatumomab in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Eur J Haematol. (2021) 106:473–83. doi: 10.1111/ejh.13569
20. Elitzur S, Arad-Cohen N, Barzilai-Birenboim S, Ben-Harush M, Bielorai B, Elhasid R, et al. Blinatumomab as a bridge to further therapy in cases of overwhelming toxicity in pediatric B-cell precursor acute lymphoblastic leukemia: report from the Israeli study group of childhood leukemia. Pediatr Blood Cancer. (2019) 66:e27898. doi: 10.1002/pbc.27898
21. Yeoh DK, Blyth CC, Kotecha RS. Blinatumomab as bridging therapy in paediatric B-cell acute lymphoblastic leukaemia complicated by invasive fungal disease. Br J Haematol. (2022) 198(5):887–92. doi: 10.1111/bjh.18314
22. Baumeister SHC, Mohan GS, Elhaddad A, Lehmann L. Cytokine release syndrome and associated acute toxicities in pediatric patients undergoing immune effector cell therapy or hematopoietic cell transplantation. Front Oncol. (2022) 12:841117. doi: 10.3389/fonc.2022.841117
23. Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. (2012) 119:6226–33. doi: 10.1182/blood-2012-01-400515
24. Haas C, Krinner E, Brischwein K, Hoffmann P, Lutterbuse R, Schlereth B, et al. Mode of cytotoxic action of T cell-engaging BiTE antibody MT110. Immunobiol. (2009) 214:441–53. doi: 10.1016/j.imbio.2008.11.014
25. Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. (2017) 31:2181–90. doi: 10.1038/leu.2017.41
26. Kantarjian HM, Zugmaier G, Brüggemann M, Wood BL, Horst HA, Zeng Y, et al. Impact of blinatumomab treatment on bone marrow function in patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Cancers (Basel). (2021) 13:5607. doi: 10.3390/cancers13225607
Keywords: B-cell acute lymphoblastic leukemia, blinatumomab, pediatric, minimal residual disease, real world
Citation: Wu Y, Li Y, Fan J, Qi P, Lin W, Yang J, Liu H, Wang X, Zheng H, Wang T and Zhang R (2022) Blinatumomab for treating pediatric B-lineage acute lymphoblastic leukemia: A retrospective real-world study. Front. Pediatr. 10:1034373. doi: 10.3389/fped.2022.1034373
Received: 1 September 2022; Accepted: 4 October 2022;
Published: 24 October 2022.
Edited by:
Yang Su, Xuzhou Medical University, ChinaReviewed by:
Sizhou Feng, Chinese Academy of Medical Sciences and Peking Union Medical College, China© 2022 Wu, Li, Fan, Qi, Lin, Yang, Liu, Wang, Zheng, Wang, Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianyou Wang d2FuZ3RpYW55b3VAYmNoLmNvbS5jbg== Ruidong Zhang emhhbmdydWlkb25nQHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Hematology and Hematological Malignancies, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.